Abstract
Artemisia is an important genus of Iranian flora whose potent anti-proliferative effect has been demonstrated previously on human cancerous cell lines. In the current study, further fractionation was carried out on the petroleum ether extract of A. aucheri and their cytotoxic effects were evaluated on three human cancer cell lines. Cell viability was determined by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assay. Real time polymerase chain reaction (RT-PCR) was used to evaluate the expression of apoptotic related genes. Activation of caspases and detection of intracellular doxorubicin (DOX) accumulation were evaluated using a spectrophotometer. Mitochondrial membrane potential (MMP) was measured using flow cytometry. The fraction NO-7 (F7) of petroleum ether extract showed the highest anti-proliferative effect, especially against SKNMC cells. Therefore, we focused on a description of the cytotoxic mechanism of the most potent fraction on SKNMC cells. The results indicated that F7 was able to induce apoptosis through MMP disruption, activation of caspases and increament of proapoptotic genes Bax and Smac/DIABLO. Moreover, our observation indicated that F7 is able to increase the cytotoxicity of DOX in SKNMC cells. The combination of F7+DOX significantly increased the intracellular accumulation of DOX. These results indicated that F7 induces apoptosis in SKNMC cells. Moreover, it might enhance the antitumor activity of DOX, through modulating the activity of multidrug resistant cancer cells and inducing apoptosis.
Keywords: Artemisia aucheri, Apoptosis, Doxorubicin, Human carcinoma cell line
INTRODUCTION
Higher plants play an essential role in the treatment of various types of cancers. The anticancer effects of these plants are attributd to compounds such as Vinca alkaloids, Taxus diterpenes, Camptotheca alkaloids and Podophyllum lignans, as well as modified related compounds (1). Artemisia aucheri Boiss. which is called “Dermaneyekoohi” in Persian language is one of the 34 Artemisia species growing wildly in Iran (2). Phytochemical investigation of the species resulted in isolation of six highly oxygenated geraniol derivatives (3). The first study on the composition of the essential oil of A. aucheri grown in Iran has revealed the presence of camphor (45.5%) and 1,8-cineole (14.3%) as the main components (4). Volatiles from the aerial parts of A. aucheri were also identified as verbenone (21.5%), camphor (21.0%) 1,8-cineole (8.3%) and trans-verbenol (8.1%) (5). Another study by a different method of extraction resulted in the identification of 1,8-cineol (22.8%), chrysanthenone (18.16%), α-pinene (8.33%), and mesitylene (7.41%) as the main constituents (6). In vitro anti-fungal (7,8), in vitro and in vivo anti-leishmanial (9,10), as well as antimicrobial activities (11) have been reported for different extracts or essential oil. Additionally, wound healing (12), hypocholesterolemic and anti-atherosclerotic effects (13,14,15,16) of A. aucheri have been proved. Based on another study, A. aucheri can be a potential candidate species for artemisinin overproduction (17). Dichloromethane extract of A. aucheri has shown a significant antimalarial activity using cell free β-hematin formation assay (18). Numerous experimental studies have demonstrated that some Asteraceae species have anti-tumor activity. Flavonoids, sesquiterpene lactones, lignans, acetylenes, triterpenes or glycolipids may be responsible for anti-proliferative effect of Asteraceae (19,20). In our previous study, we demonstrated that petroleum ether extract of A. aucheri has potent anti-proliferative effect on human cancerous cell lines (20). Therefore, in the current study further fractionation of petroleum ether extract of A. aucheri was carried out and their cytotoxic effects were evaluated on human cancer cell lines. The apoptosis-induction capacity rather than necrosis induction is accepted as a key feature of a potential anti-tumor drug (21). In view of the importance of apoptotic cell death as a key feature of a potential anti-tumor drug, in the next set of experiments, the apoptotic potentials of the most potent fraction was investigated. In recent years much efforts have been directed towards the identification of the agents that are able to sensitize cancer cells to conventional anti-cancer drugs such as doxorubicin (DOX) (22,23). Therefore, in this study, potent fraction from petroleum ether extract of A. aucheri was evaluated for its possible effects on enhancement of DOX cytotoxicity.
MATERIALS AND METHODS
Reagents and chemicals
Silica gel 60 (0.040- 0.063 mm) was purchased from Merck (Germany) and all solvents used for extraction (petroleum ether 40-60) and fractionation (n-heptane and ethyl acetate) were from Caledon and Scharlau (Spain). Doxorubicin (DOX), 3-(4,5-dimethylthiazol-2yl)-2,5 - diphenyltetrazolium bromide (MTT), rhodamine 123, and caspases activity detection kit were procured from Sigma Aldrich (St Louis, MO, USA). Cell culture medium, penicillin–streptomycin solution, and fetal bovine serum (FBS) were purchased from Gibco (Gibco, Grand Island, NY, USA). RNA isolation kit with high purity was purchased from Roche (Mannheim, Germany). Real time polymerase chain reaction (RT-PCR) kit was supplied by Invitrogen (Carlsbad CA). BCA protein assay kit obtained from Pierce (Pierce, Bonn, Germany). All tissue culture wells were from Becton Dickinson (USA).
Plant material
Aerial parts of A. aucheri were collected from Chahar-Bagh region (Golestan province, Iran) in September 2011. Sample was identified by Mr. S. A. Hosseini (Agricultural and Natural Resources Research Center of Golestan Province, Gorgan, Iran) using morphological examination in comparison to voucher specimen (No. 2383).
Preparation of the extract and fractions
The dried-powdered aerial parts (400 g) of A. aucheri were extracted with petroleum ether (40-60), (maceration with ca. 3 × 4 L of the solvent). The extract was filtrated and dried using rotary evaporator at reduced pressure below 45 °C to yield 4.31 g of the extract. The extract was subjected to a vacuum liquid chromatography system (silica gel) with n-heptane containing increasing amounts of ethyl acetate (0%, 5%, 10%, 20%, 40%, 60%, 80%, and 100%). The procedure was followed by using pure acetone as the eluting solvent to give nine fractions namely F1 to F9.
Assessment of cell proliferation
SKNMC, MCF-7 and A2780 were obtained from Pasteur Institute (Tehran, Iran) and maintained at 37 °C in a humidified atmosphere (90%) containing 5% CO2. Cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM-F12) with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin.
SKNMC, MCF-7 and A2780 cells were seeded in triplicate in 96-well tissue culture plates (15 × 103 cells/well) and incubated overnight. Cells were treated with different concentrations of the obtained fractions (64, 128, 192, 256, and 320 μg/ml) for 24 h. To examine the effect of most potent fraction on DOX-induced cytotoxicity, one day after seeding, different concentrations of DOX and selected fraction were added to the cells. After 24 h of incubation, the medium was removed and 0.1 mg/well of MTT were added to the cells, and plates were further incubated for 3 h at 37 °C. The formazan crystals were solubilized in 0.1 ml of dimethyl sulfoxide and the optical density (OD570) was measured using a microplate reader (BioTek Instruments, USA). Cell viability was calculated using following formula:
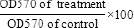
IC50 values were calculated by plotting the log10 of percent cell viability versus drug concentrations.
Measurement of mitochondrial membrane potential
Mitochondrial dysfunction has been shown to participate in the induction of apoptosis (24). In this study mitochondrial membrane potential (MMP) was measured using rhodamine 123 fluorescent dye. Depolarization of MMP during cell apoptosis results in the loss of rhodamine 123 from the mitochondria and a decrease in intracellular fluorescence intensity (25). Cells were seeded in triplicate in 6-well tissue culture plates (700 × 103 cells/well), incubated overnight, and then treated with different concentrations of the selected fraction for 24 h. Next, cells were incubated with rhodamine 123 for 30 min at 37 °C. The fluorescence was measured by flow cytometery using a Partec™ cytometer (Gemany) with standard Argon laser for 488 nm excitation and 520 nm band pass (FL1) filter.
Caspase activity assays
Caspase-3, 8 and 9 activity assays were carried out using the sigma colorimetric caspase kit. This assay is based on the ability of the active enzyme to cleave the chromophore from the enzyme substrate, Ac-DEVD-pNA (for caspase-3), Ac-IETD-pNA (for caspase-8), and Ac-LEHD-pNA (for caspase-9) in equal amounts of cell protein. The cells were seeded in quadruplicate in 6-well tissue culture plates (700 × 103 cells/well), incubated overnight, and then treated with different concentrations of the selected fraction for 24 h. Next, the cells (7 × 105) were harvested and lysed in 70 μl of the cell lysis buffer included with the kit, and protein concentrations were equalized for each condition. Subsequently, 10 μl of cell lysate was combined with an equal amount of substrate reaction buffer containing caspase-3, 8 and 9 colorimetric substrates. This mixture was incubated for 2 h at 37 °C, and the absorbance was then measured using a plate reader (BioTek, H1M).
Analysis of apoptosis–related gene expression
Total RNA were extracted from SKNMC cells (7 × 105) pretreated with IC50 concentration of the most potent fraction using high pure isolation kit (Roche, Mannheim, Germany) according to the manufacture instructions. Quality and quantity of total RNA was assessed using spectrophotometer (NanoDrop™ 2000, USA) and samples stored at -80 °C until the use. The primers used in this study were selected from previously published studies (26,27). The performances of all primer pairs were tested in a primer concentration gradient experiment to determine the optimal reaction conditions. Thermal cycler program was run as follows: 15 min at 50 °C for cDNA synthesis, 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C to denature the DNA, and 45 s at 60 °C to anneal and extend the template. Melting curve analysis was performed to ascertain specificity by continuous acquisition from 65 °C–95 °C with a temperature transient rate of 0.1 °C/s. All reactions were performed in triplicate in a Corbett system (Australia). The value obtained for the target gene expression were normalized to β-actin and analyzed by the relative gene expression -ΔΔCT method where -ΔΔCT=(CT target–CT β-actin) Unknown – (CT target – CT β-actin) Calibrator.
Measurement of intracellular doxorubicin accumulation
For evaluation of DOX accumulation, SKNMC cells were cultured on 6-well plate for 24 h to achieve approximately 80% confluence. DOX (33 µM) was added to the experiment wells. After incubation for 1 h, the culture medium containing DOX was discarded. Then medium with different concentration of F7 were added to the wells. After incubation for another 1 h, the cells were lysed with 1% Triton X-100. For the measurement of DOX fluorescence using micro plate reader, excitation and emission filters were set at 480 and580 nm, respectively.
Statistical analysis
Each experiment was repeated at least three times and the results were presented as the mean ± S.E.M. One-way analysis of variance (ANOVA) followed by Tukey's test was used to compare the differences between the means. A probability value of P<0.05 was considered to be statistically significant.
RESULTS
Antiproliferative effects of the fractions
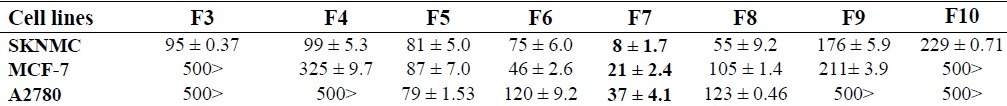
Results of MTT assay for detection of antiproliferative effects of the fractions are listed in Table 1. F7 fraction proved to be an outstandingly potent cytostatic agent, especially against SKNMC cells as demonstrated by its IC50 value. Other fractions were effective against SKNMC, MCF-7, and A2780 cells with rank order potencies of 7 >8 >6 >5 >3 >4 >9, 7 >6 >5 >8 >9 >4, and 7 >5 > 6>8, respectively. Therefore, in subsequent experiments F7 was adopted for identification of mechanisms of action on SKNMC cell line.
Table 1.
IC50 (Mean ± S.E.M) concentrations (μg /ml) of fractions in three human cancer cell lines.
Effect of F7 on mitochondrial membrane potential
To characterize the changes in mitochondrial events induced by F7 treatments, the collapse of MMP in SKNMC cells was monitored with the rhodamine 123. The result indicated that F7 was able to significantly decrease MMP (71.57% ± 0.51 P<0.001) in SKNMC human neuroblastoma cell line (Fig. 1).
Fig. 1.
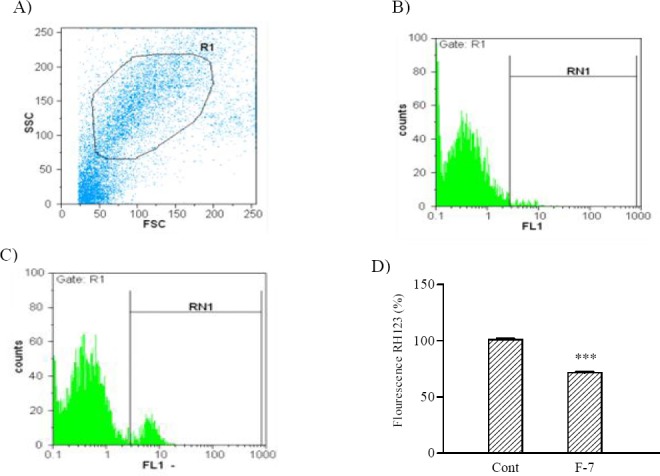
Effect of F7on MMP alteration in SKNMC cells. A; Dot plot in arbitrary unit on a linear scale. Each dot represents the Forward-scattered light (FSC) and Side-scattered light (SSC) value for a single SKNMC cells. B; Untreated cells, C; Cells treated with indicated concentration of F7 for 24 h. D; Column bar graph of mean cell florescent for rhodamine 123. Data are expressed as the mean ± S.E.M of three separate experiments. #P<0.05 vs. Control, *P<0.05 vs. doxorubicin treated cells.
Effect of F7 on caspases activity
As shown in Fig. 2, 24 h treatment with IC50 concentration of F7 caspase-3 activation in SKNMC cells was increased significantly (P<0.001). To determine which apoptotic pathway is activated by F7, we evaluated the activation of caspase-8 and 9, the apical proteases in extrinsic and intrinsic pathways, respectively (28). F7 was able to increase activities of caspase -8 (22.7 ± 3.2%, P<0.05) and caspase-9 (19.2 ± 1.82%, P<0.05) in SKNMC cells indicating that the F7 fraction induces apoptosis through both intrinsic and extrinsic pathways (Fig. 2).
Fig. 2.
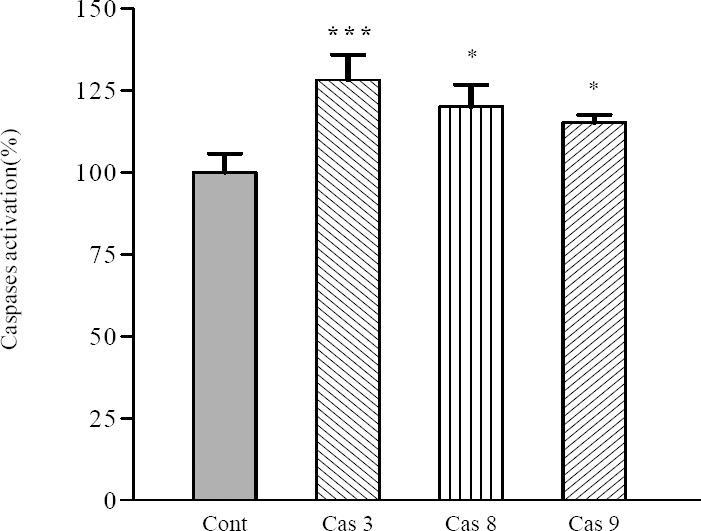
Involvement of activation of caspases in the induction of apoptosis on SKNMC cells. Cells were incubated with IC50 concentration of the F7at 8 μg/ml and harvested at 24 h and cell lysates were assayed using microplate reader for activation caspases. Differences were compared with the control. Data are presented as mean ± S.E.M. *P<0.05, and ***P<0.001 vs. control.
Effect of F7 on expression level of some critical genes involved in apoptosis
We found that F7 significantly increased Baxm RNA expression in SKNMC cell line (P<0.001). However, induction of apoptosis by F7 was not accompanied by an increase in mRNA levels of antiapoptotic Bcl-2 gene. Subsequently the mRNA expression of Bax and Smac/DIABLO were measured. The results demonstrated that 24 h treatment with F7 significantly increased Smac/DIABLO at the level of mRNA expression in SKNMC cells (P<0.001) (Fig. 3).
Fig. 3.
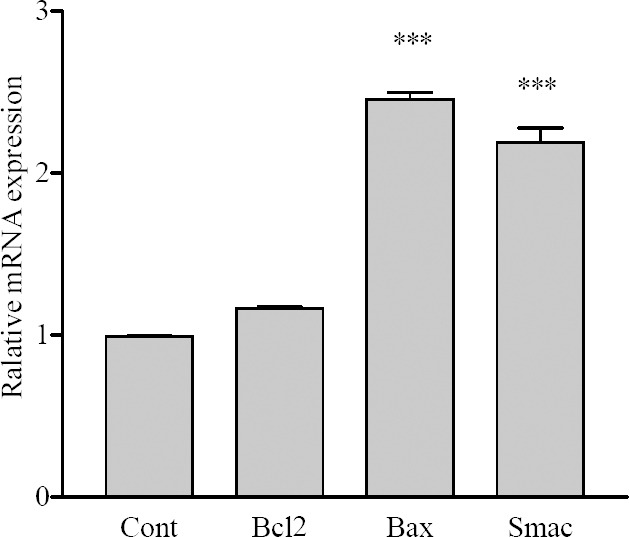
The effect of F7on expression of apoptotic-related genes on SKNMC cells. Normalization relative to b-actin was performed. Levels of mRNA are expressed relative to the control. Mean ± S.E.M values derived from three independent experiments. ***P<0.001 vs. control.
Effect of F7 on doxorubicin-induced cytotoxicity in SKNMC cells
IC50 of DOX was determined to be 5.25 µM. The combinatorial effects of DOX and plant extract fraction F7 were tested at 8, 16, 32, and 64 µg/ml of F7 with 1, 5, and 10 µM of DOX, on SKNMC cells. As shown in Fig. 4, pretreatment with F7 shifted the concentration–response curve to the lower IC50 values in a dose-dependent manner. Moreover, morphological assessment by an inverted microscope showed that after 24 h treatment of the cells with a mixture of IC50 concentrations of F7 and DOX in comparison with DOX alone, number of cells was greatly decreased, moderate cytoplasmic granulations were noticeable and a large number of cells became rounded and started to detach from the flasks (Fig. 4b A–D).
Fig. 4.
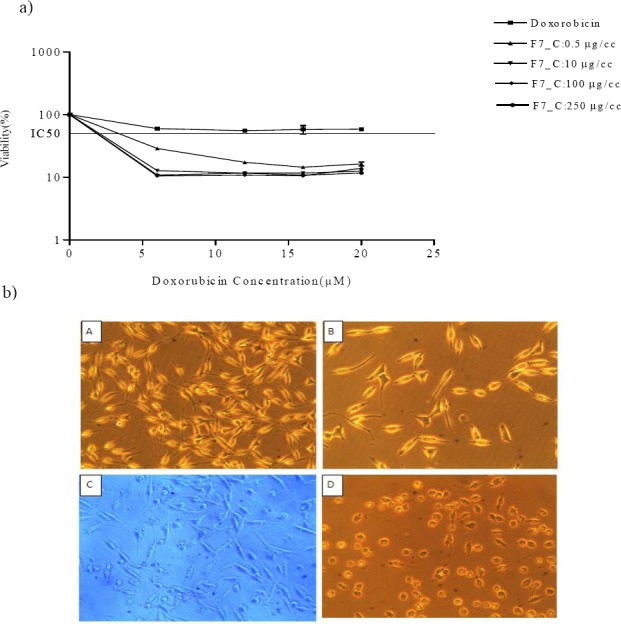
The effect of F7 on doxorubicin-induced cytotoxicity in SKNMC cells. a; Cells were treated with 0.5-250 μg/ml concentrations of F7 in combination with indicated concentrations of doxorubicin for 24 h. IC50 values were obtained by plotting the log10 of the percentage of proliferation values versus drug concentration. Results are mean ± S.E.M. b; Light microscopic images of SKNMC cells show morphological changes after various treatments (40 × magnification). A; Untreated cells, B; cells treated with doxorubicin, C; cells treated with F7, D; cells treated with doxorubicin plus F7.
Effect of F7 on doxorubicin induced apoptosis
In order to determine the mechanism involved in cytotoxic effects of F7 and DOX combinations, caspases activity and MMP were analyzed. As shown in Fig. 5a, the obtained results revealed that DOX significantly increased caspase-3, 8 and 9 activation in SKNMC cells. Moreover, combination of F7 and DOX induced caspse-3 and 8, which were significantly (P<0.01) higher than DOX treated cells. When the combination effect of F7 and DOX on MMP were tested, it was found that F7 potentiated the effect of DOX on MMP in SKNMC cells (Fig. 5b)
Fig. 5.
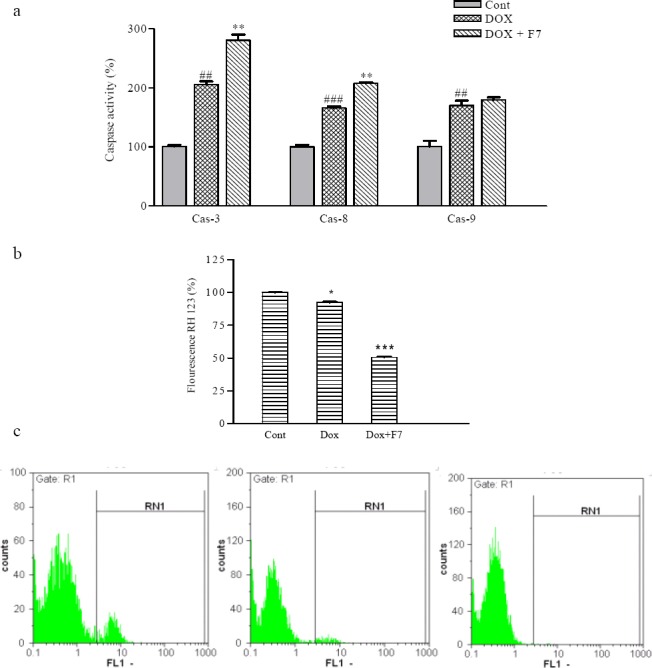
The effect of F7on a; caspases activity, b and c; on mitochondrial membrane potential in the presence and absence of doxorubicin in SKNMC cells ##P<0.01 and ###P<0.001 vs. control, *P<0.05, and **P<0.01, ***P<0.001 vs. doxorubicin-treated cells.
Determination of intracellular doxorubicin accumulation
To examine whether F7 enhanced DOX potency is a result of its inhibition of any other or undefined drug transporters, which may recognize DOX as a substrate, we determined the effects of F7 on cellular uptake of DOX as a substrate of P-glycoprotein/MRPs in SKNMC cell lines. The results showed that F7 at 80, 160, and 250 µg/ml inhibited DOX efflux efficiently as compared to the control in SKNMC cell line (P<0.01) (Fig. 6). This result indicate that F7 in the presence of DOX, have inhibitory effect on the activity of MDR transporters.
Fig. 6.
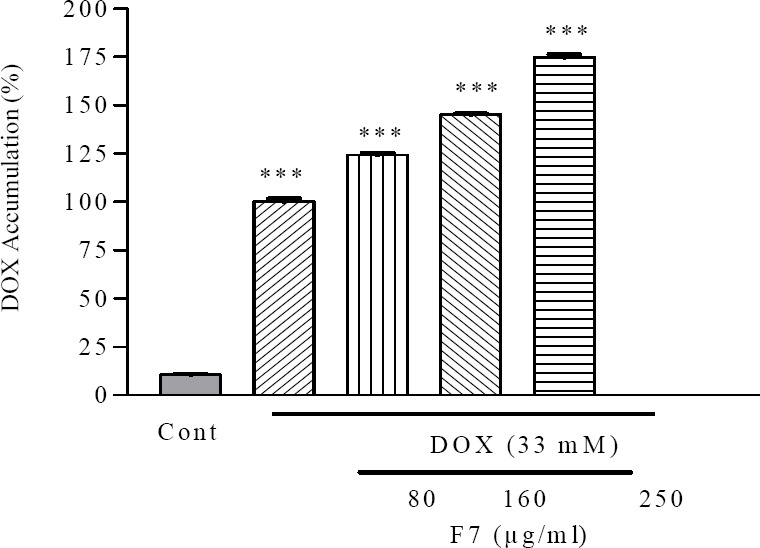
The effect of F7 on doxorubicin accumulation in SKNMC cells. Cells were treated with 80, 160 and 250 μg/ml of F7 in combination with indicated concentrations of doxorubicin. Data are presented as mean ± S.E.M. ###P<0.001 vs. control, **P<0.01 vs. doxorubicin-treated cells.
DISCUSSION
The aim of the current study was to assess antiproliferative effect of different fractions of petroleum ether extract of A. aucheri in three human carcinoma cell lines. In the present project, F7 from A. aucheri petroleum ether extract was evaluated for its possible effects on the enhancement of DOX cytotoxicity in SKNMC cells.
The apoptosis-induction capacity rather than necrosis induction is accepted as a key feature of a potential antitumor drug (21). Accordingly, the apoptotic potentials of the most cytotoxic fraction F7 were investigated using well-characterized apoptosis markers. The mitochondria is an integral part of the apoptotic machinery; therefore, we analyzed the most important proteins involved in mitochondrial pathway of apoptosis (Bax and Bcl-2) in the most sensitive cell line SKNMC (29). Cell survival in the early phases of apoptotic cascade depends mostly on the balance between the pro-apoptotic and anti-apoptotic proteins of Bcl-2 family (30). Next the mRNA expression of Smac/DIABLO was measured. Smac/DIABLO antagonizes inhibitor of apoptosis proteins to relieve their inhibitory effects on caspases (26). F7 induced apoptosis accompanied by upregulation of proapototic genes Bax and Smac/DIABLO leading to a decrease in mitochondrial membrane potential. Activation of caspase cascade is critical in the initiation of apoptosis in various biological systems (28). Our results showed that F7 was able to increase caspase -3 in SKNMC cell. To determine which apoptotic pathway is activated by F7, we evaluated the activation of caspase-8 and 9, the apical proteases in extrinsic and intrinsic pathways, respectively (29). This result identified that apoptosis induced by F7 was probably occurred through both intrinsic mitochondrial and extrinsic pathways of apoptosis. Moreover, we demonstrated that this fraction enhances the cytotoxic effect of DOX on SKNMC cells. We further investigated the role of apoptosis in the observed enhancement of the cytotoxic efficacy of DOX. In our study, as have been previously reported (30), apoptosis induced by DOX occurred through caspase-8 and caspase-9, the mediators of extrinsic and intrinsic pathways, respectively. Combination of F7 with DOX increased significantly caspase- 8 activations. For better evaluation of apoptosis we assayed changes in mitochondrial membrane potential. The obtained results showed that treatment with F7 and DOX potentiates the fluorescence intensity decreased compared with DOX. In our experiment treatment with F7 and DOX was also associated with the potentiation of the downstream apoptosis signaling pathways finally increasing the effect or caspase-3, a key mediator of apoptosis in mammalian cells. Previous studies have shown that numerous plants of the genus Artemisia have cytotoxic effect on cancer cell line (31,32). Induction of apoptosis in human lung cancer cell line through mitochondrial dependent pathway has been reported for a sesquiterpene coumarin ether and two other new sesquiterpenes isolated from the cultured hairy roots of A. annua (33,34,35). Lung and colon cancer cell lines showed sensitivity to isoscopoletin from A. argyi and artemisinin from A. annua (36). Cha and coworkers reported the ability of the essential oil from A. capillaris to induce apoptosis in human oral cancer cells (37). Among nine fractions from A. sacrorum, the CH2Cl2 fraction possessed the highest cytotoxicity against HepG2, HT-29, and MCF-7 cells (38). The findings of another study suggested the presence of some secondary metabolites in CH2Cl2 extract of A. turanica which is in charge of cytotoxity against human leukemic cancer cell lines (39). The multidrug resistance (MDR) of tumor cells to chemotherapeutic agents is a major problem in the clinical treatment of cancer. Many drugs used in cancer treatment are MDR's substrates (40). MDR's inhibitors derived from plants may prove to be efficacious when administered in combination with commonly used chemotherapeutic drugs (22,23). To study the effects of F7 on the activity of MDR transporters, accumulation of DOX was assayed. The results showed that F7 significantly increased the intracellular accumulation of DOX. These effects indicate that F7 might enhance the antitumor activity of DOX through modulating the activity of MDRs and inducing the apoptosis.
CONCLUSION
In the present study we found that F7 was more cytotoxic than other fractions especially in SKNMC cell line. More detailed studies showed that this fraction is able to induce apoptosis through extrinsic and intrinsic pathways. Furthermore, F7 potentiated cytotoxicity induced by DOX through apoptosis in SKNMC cells. Our results also revealed that selected fraction significantly enhanced the intracellular accumulation of DOX, indicating the inhibition MDRs mediated drug efflux.
Taking together these findings proved the presence of some phytochemicals responsible for the observed effects. Further analytical experiments on the most active fraction of petroleum ether extract of A. aucheri as well as structure elucidation of purified compounds should be performed.
ACKNOWLEDGMENT
This study was financially supported by the Research Council of Kermanshah University of Medical Sciences, Kermanshah, I.R. Iran.
REFERENCES
- 1.Itokawa H, Morris-Natschke SL, Akiyama T, Lee KH. Plant-derived natural product research aimed at new drug discovery. J Nat Med. 2008;62:263–280. doi: 10.1007/s11418-008-0246-z. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian V. Tehran: Farhang Moaser Publishers; 1998. A dictionary of iranian plant names; pp. 56–58. [Google Scholar]
- 3.Rustaiyan A, Bamonieri A, Raffatrad M, Jakupovica J, Bohlmann F. Eudesmane derivatives and highly oxygenated monoterpenes from Iranian Artemisia species. Phytochemistry. 1987;26:2307–2310. [Google Scholar]
- 4.Mohammadpoor S, Yari M, Rustaiyan A, Masoudi S. Chemical constituents of the essential oil of Artemisia aucheri Boiss a species endemic to Iran. J Essent Oil Res. 2002;14:122–123. [Google Scholar]
- 5.Sefidkon F, Jalili, A, Mirhaji T. Essential oil composition of three Artemisia spp from Iran. Flavour Frag J. 2002;17:150–152. [Google Scholar]
- 6.Hashemi P, Abolghasemi MM, Fakhari AR, Ebrahimi SN, Ahmadi S. Hydrodistillation-solvent microextraction and GC-MS identification of volatile components of Artemisia aucheri. Chromatographia. 2007;66:283–286. [Google Scholar]
- 7.Amin G, Dehmoobed-Sharifabadi A, Salehi Surmaghi MH, Yasa N, Aynechi Y, Emami M, et al. Screening of Iranian plants for antifungal activity: Part 1. Daru. 2002;10:34–37. [Google Scholar]
- 8.Nabigol A, Farzaneh M. In vitro antifungal activity of some plant essential oils on postharvest pathogens of strawberry fruit. ActaHortic. 2010;858:305–310. [Google Scholar]
- 9.Sharif M, Ziaei H, Azadbakht M, Daryani A, Ebadattalab A, Rostami M. Effect of methanolic extracts of Artemisia aucheri and Camelli asinensis on Leishmania major (in vitro) Turk J Med Sci. 2006;36:365–369. [Google Scholar]
- 10.Rostami M, Daryani A, Azadbakht M, Nahrevanian H, Sharif M. Evaluation of anti-leishmanial efficacy by in vivo administration of herbal extract Artemisia auchery on leishmania major in Balb/c mice. Pharmacol online. 2009;2:1136–1144. [Google Scholar]
- 11.Asghari G, Jalali M, Sadoughi E. Antimicrobial activity and chemical composition of essential oil from the seeds of Artemisia aucheri Boiss. Jundishapur J Nat Pharm Prod. 2012;7:11–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Allahtavakoli M, Allahtavakoli M, Asad FAB, Mahmoudi M, JafariNaveh H, Tavakolian V, et al. Effect of hydro-alcoholic extract of Artemisia aucheri on healing of skin wound in rat. J Mazandaran Univ Med Sci. 2010;20:70–76. [Google Scholar]
- 13.Jafari Dinani N, Asgari S, Madani H, Mahzouni P, Naderi GHA. Effect of Artemisia aucheri extract on atherogenic lipids and atherogenesis in hypercholesterolemic rabbits. J Med Plants. 2007;6:20–28. [Google Scholar]
- 14.Asgary S, Jafari Dinani N, Madani H, Mahzouni P. Ethanolic extract of Artemisia aucheri induces regression of aorta wall fatty streaks in hypercholesterolemic rabbits. Pharmazie. 2008;63:394–397. [PubMed] [Google Scholar]
- 15.JafariDinani N, Asgari S, Madani H, Naderi GHA, Mahzouni P. Effect of Artemisia aucheri on regression of atherosclerotic plaque in rabbits. J Med Plants. 2009;8:72–79. [Google Scholar]
- 16.JafariDinani N, Asgary S, Madani H, Naderi GHA, Mahzouni P. Hypo-cholesterolemic and anti-atherosclerotic effect of Artemisia aucheri in hyper cholesterolemic rabbits. Pak J Pharm Sci. 2010;23:321–325. [PubMed] [Google Scholar]
- 17.Hosseini R, Yazdani N, Garoosi GA. The presence of amorpha-4, 11-diene synthase, a key enzyme in artemisinin production in ten Artemisia species. Daru. 2011;19:332–337. [PMC free article] [PubMed] [Google Scholar]
- 18.Mojarrab M, Shiravand A, Delazar A, HeshmatiAfshar F. Evaluation of in vitro antimalarial activity of different extracts of Artemisia aucheri Boiss. and A. armeniaca Lam and fractions of the most potent extracts. Sci World J. doi: 10.1155/2014/825370. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh NP, Ferreira JFS, Park JS, Lai HC. Cytotoxicity of ethanolic extracts of Artemisia annua to molt-4 human leukemia cells. Planta Med. 2011;77:1788–1793. doi: 10.1055/s-0030-1271157. [DOI] [PubMed] [Google Scholar]
- 20.Ghazi-Khansari M, Mojarrab M, Ahmadi F, Hosseinzadeh L. The antiproliferative effects of petroleum ether extract of Artemisia aucheri on the human cancerous cell lines. J Rep Pharm Sci. 2013;2:61–66. [Google Scholar]
- 21.Réthy B, Zupkó I, Minorics R, Hohmann J, Ocsovszki I, Falkay G. Investigation of cytotoxic activity on human cancer cell lines of arborinine and furanoacridones isolated from Rutagraveolens. Planta Med. 2007;73:41–48. doi: 10.1055/s-2006-951747. [DOI] [PubMed] [Google Scholar]
- 22.S Methanolic extract of Teucrium polium potentiates the cytotoxic and apoptotic effect of vincristin, vinblastin and doxorubicin against a panel of cancerous cell lines. Exp Oncol. 2008;30:133–138. [PubMed] [Google Scholar]
- 23.Qiang Zhang, Dongzhi Wei, Jianwen Liu. In vivo reversal of doxorubicin resistance by [2] -epigallocatechingallate in a solid human carcinoma xenograft. Cancer Lett. 2004;208:179–186. doi: 10.1016/j.canlet.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadi F, Derakhshandeh K, Jalalizadeh M, Mostafaie A, Hosseinzadeh L. Encapsulation in PLGA-PEG 5% enhances 9- Nitrocamptothecin mediated cytotoxicity to human ovarian carcinoma cell line through apoptosis pathway. Res Pharm Sci. 2015;2:161–168. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Ruan Y, Chen Q, Li S, Wang Q, Cai J. Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca2+ concentration. Eur J Pharmacol. 2010;650:41–47. doi: 10.1016/j.ejphar.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 26.HL, Liu PS, Zheng JF, Zhang PH, Zhou LG, Xin G, et al. Transfection of Smac/DIABLO sensitizes drug-resistant tumor cells to TRAIL or paclitaxel-induced apoptosis in vitro. Pharmacol Res. 2007;56:483–492. doi: 10.1016/j.phrs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Porichi O, Nikolaidou ME, Apostolaki A, Tserkezoglou A, Arnogiannaki N, Kassanos D, et al. BCL-2, BAX and P53 Expression profiles in endometrial carcinoma as studied by Real-time PCR and immunohistochemistry. Anticancer Res. 2009;29:3977–3982. [PubMed] [Google Scholar]
- 28.Shokoohinia Y, Hosseinzadeh L, Moieni-Arya M, Mostafaie A, Mohammadi MMA. Osthole attenuates doxorubicin-induced apoptosis inPC12 cells through inhibition of mitochondrial dysfunctionand ROS production. Bio Med Res Inter. 2014 doi: 10.1155/2014/156848. ID 156848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseinzadeh L, Khorand A, Aliabadi A. Discovery of 2-phenyl-n-(5-(trifluoromethyl)-1,3,4-thiadiazol- 2-yl) acetamide derivatives as apoptosis inducers via the caspase pathway with potential anticancer activity. Arch Pharm (Wienheim) 2013;346:812–818. doi: 10.1002/ardp.201300180. [DOI] [PubMed] [Google Scholar]
- 30.Hosseinzadeh L, Behravan J, Mosaffa F, Bahrami GH, Bahrami M, Karimi GH. Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food Chem Toxicol. 2011;49:1102–1109. doi: 10.1016/j.fct.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Emami A, Zamani Taghizadeh Rabe SH, Ahi A. Study on toxic effects of Artemisisa spp. fractions from Iran on human cancer cell lines. J Zanjan Univ Med Sci. 2010;18:58–67. [Google Scholar]
- 32.Taghizadeh Rabe SZ, Mahmoudi M, Ahi A, Emami SA. Anti-proliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharma Biol. 2011;49:962–969. doi: 10.3109/13880209.2011.559251. [DOI] [PubMed] [Google Scholar]
- 33.Zhai DD, Supaibulwatana K, Zhong JJ. Inhibition of tumor cell proliferation and induction of apoptosis in 95-D lung cancer cells by Drimartol A from hairy root cultures of Artemisia annua. Lat Am J Pharm. 2010;29:1159–1165. doi: 10.1016/j.phymed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Zhai DD, Supaibulwatana K, Zhong JJ. Inhibition of tumor cell proliferation and induction of apoptosis in human lung carcinoma 95-D cells by a new sesquiterpene from hairy root cultures of Artemisia annua. Phytomedicine. 2010;17:856–861. doi: 10.1016/j.phymed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhai DD, Jin HZ, Zhong JJ. A new sesquiterpene from hairy root culture of Artemisia annua. Chinese Chem Lett. 2010;21:590–592. [Google Scholar]
- 36.McGovern PE, Christofidou-Solomidou M, Wang W, Dukes F, Davidson T, El-Deiry WS. Anticancer activity of botanical compounds in ancient fermented beverages (review) Int J Oncol. 2010;37:5–14. doi: 10.3892/ijo_00000647. [DOI] [PubMed] [Google Scholar]
- 37.Cha JD, Moon SE, Kim HY, Cha IH, Lee KY. Essential oil of Artemisia capillaris induces apoptosis in KB cells via mitochondrial stress and caspase activation mediated by MAPK-stimulated signaling pathway. J Food Sci. 2009;74:75–81. doi: 10.1111/j.1750-3841.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 38.Piao GC, Li YX, Yuan HD, Jin GZ. Cytotoxic fraction from Artemisia sacrorum Ledeb. against three human cancer cell lines and separation and identification of its compounds. Nat Prod Res. 2012;26:1483–1491. doi: 10.1080/14786419.2011.565473. [DOI] [PubMed] [Google Scholar]
- 39.Tayarani-Najaran Z, Sareban M, Gholami A, Emami SA, Mojarrab M. Cytotoxic and apoptotic effects of different extracts of Artemisia turanica Krasch. on K562 and HL-60 cell lines. Sci World J. 2013 doi: 10.1155/2013/628073. ID 628073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Wijngaarden J, van Beek E, van Rossum G, van der Bent C, Hoekman K, van der Pluijm G, et al. Celecoxib enhances doxorubicin-induced cytotoxicity in MDA-MB231 cells by NF-kB-mediated increase of intracellular doxorubicin accumulation. Eur J Cancer. 2007;43:433–442. doi: 10.1016/j.ejca.2006.09.010. [DOI] [PubMed] [Google Scholar]


