Abstract
Context:
Autism is a serious behavioral disorder among young children that now occurs at epidemic rates in developing countries like India. We have used tract-based spatial statistics (TBSS) of diffusion tensor imaging (DTI) measures to investigate the microstructure of primary neurocircuitry involved in autistic spectral disorders as compared to the typically developed children.
Objective:
To evaluate the various white matter tracts in Indian autistic children as compared to the controls using TBSS.
Materials and Methods:
Prospective, case-control, voxel-based, whole-brain DTI analysis using TBSS was performed. The study included 19 autistic children (mean age 8.7 years ± 3.84, 16 males and 3 females) and 34 controls (mean age 12.38 ± 3.76, all males). Fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) values were used as outcome variables.
Results:
Compared to the control group, TBSS demonstrated multiple areas of markedly reduced FA involving multiple long white matter tracts, entire corpus callosum, bilateral posterior thalami, and bilateral optic tracts (OTs). Notably, there were no voxels where FA was significantly increased in the autism group. Increased RD was also noted in these regions, suggesting underlying myelination defect. The MD was elevated in many of the projections and association fibers and notably in the OTs. There were no significant changes in the AD in these regions, indicating no significant axonal injury. There was no significant correlation between the FA values and Childhood Autism Rating Scale.
Conclusion:
This is a first of a kind study evaluating DTI findings in autistic children in India. In our study, DTI has shown a significant fault with the underlying intricate brain wiring system in autism. OT abnormality is a novel finding and needs further research.
Keywords: Autism, diffusion tensor imaging, India, tract-based spatial statistics
INTRODUCTION
Autism is a neurodevelopmental disorder with a range of clinical presentations, now classified in a broader class of disease called autism spectrum disorders (ASDs), which includes autism, Asperger syndrome, and pervasive developmental disorder-not otherwise specified (PDD-NOS). Impaired social interaction, associated with verbal and nonverbal communication deficits and stereotyped behaviors are the most common clinical sign.[1]
Autism is a growing problem worldwide and possesses a greater healthcare and economic burden to the developing nations like India. Research is on rise in the last decade on understanding various aspects (e.g., pathophysiology, epidemiology etc.) of this condition. Clinicians and researchers worldwide are being challenged by the unique nature of this condition. India is no way behind, research is more focused in-silo in major institutions under various disciplines (e.g., neurology, psychiatry, neuroradiology, pediatrics, epidemiology etc.) across the country.
Neuroimaging plays an integral role in complimenting the clinical research, multiple studies have been conducted to correlate the association between the imaging and clinical findings which would in turn benefit the early diagnosis and treatment. To date, the most dramatic changes occur when the children receive early and intensive behavioral intervention.[2]
Diffusion tensor imaging (DTI) is an advanced magnetic resonance imaging (MRI) technique which studies the microstructural integrity of white matter tracts in the brain. Although numerous studies have been published in the literature based on DTI analysis of autistic individuals worldwide, there is no path-breaking large scale study conducted on Indian population till date to study the nature of this entity in this ethnic group.
Our study is an attempt to analyze this complex entity affecting the Indian population. We hypothesized that white matter located between the brain regions known to be involved in social cognition would be structurally and functionally abnormal in autistic individuals.
MATERIALS AND METHODS
This study was conducted at a national level tertiary care center, with dedicated mental health and neuroscience service post approval by the Institutional Board of Ethics. In total, 19 autistic children (diagnosed by Diagnostic and Statistical Manual of Mental Disorders Fourth Edition [DSM-IV] criteria) and 34 typically developing controls were included in the study.
Patients who visited the child and adolescent psychiatric outpatient department and diagnosed with autism spectral disorders and fulfilled the inclusion criteria were included in this study. Childhood Autism Rating Scale (CARS) autism severity rating scorings were performed and categorized into mild, moderate and severely autistic. As a part of the assessment, all children underwent MRI imaging of the brain to rule out structural and morphological pathologies.
Inclusion criteria for cases
DSM-IV diagnosis of autistic disorder and pervasive development disorder - not otherwise specified (PDD NOS) excluding Rett's disorder and childhood disintegrative disorder
Age group - 2 years to 20 years; any gender.
Inclusion criteria for controls
No present or past history or family history of any form of psychiatric illness, no significant medical history, normal developmental history, no learning disabilities with average intelligent quotient (IQ)
Age group - 2 years to 20 years; any gender.
Exclusion criteria for cases and controls
Lack of appropriate consent: For minor patients, consent was obtained from all legal guardians/caretakers
Known case of any known syndrome
Medically unfit for sedation/anesthesia
Any contraindications to MRI, such as metallic implants, cochlear implants, pacemakers.
Data acquisition
Cases
Imaging was performed with 3 Tesla MRI (Acheiva, Philips) using an 8 channel dedicated head coil as per the standard operating procedure under anesthesia, if required. The MRI scans were obtained by first performing a three-plane localizer scan, followed by an axial turbo spin-echo T2-weighted scan (TR/TE 3270/80 ms, flip angle 90°, field of view (FOV) 250 mm, acquisition voxel size 0.89/1.21/5.00 mm, slice thickness/gap 5 mm/1 mm); T2-weighted fluid attenuation and inversion recovery sense (TR/TE 11000/120 MS, flip angle 90°, FOV 250 mm, ACQ voxel size 0.96/1.79/5.00 mm, slice thickness/gap 5 mm/1 mm). Three-dimensional magnetization prepared rapid gradient echo volumetric acquisition of the whole-brain were acquired (TR/TE = 8.3/3.8 ms, sense factor = 1.5, flip angle = 8°, FOV = 256, acquisition voxel size 1/1/1 mm). DTI was done in 32 directions (DWISE, TR/TE = 8987/61.2 ms, no of slices 70, FOV 224 mm, acquisition voxel size 2/2/2 mm, slice thickness/gap = 2 mm/0 mm, no of directions 32, b values = 0, 1000). Total scan time was 35 min. All the children with autism required some form of sedation for the acquisition of MRI data under the monitoring by a neuroanesthesiologist. There were no anesthesia-related complications.
Controls
MRI data (DTI) in typically developing children were collected from the already existing bank of healthy controls. These children were assessed in detail by a psychiatry resident in terms of the family history of psychiatric illness, developmental milestones, IQ and socioeconomic background.
Data analysis
Data analysis was carried out using FMRIB (Functional Magnetic Resonance Imaging of the Brain) Analysis Group, Oxford University, UK. Library tools (www.fmrib.ox.ac.uk/fsl) version 4.1.6. Raw DTI images were preprocessed using eddy current correction, to correct for distortions due to the gradient directions applied.
Fractional anisotropy (FA) and mean diffusivity (MD) maps were generated using DTI fit, part of FMRIB's Diffusion Toolbox (http://www.fmrib.ox.ac.uk/fsl/fdt) that fits a diffusion tensor model at each voxel. The largest axis (λ1) or Eigen value is denoted as axial diffusivity (AD). The two minor axes (λ2 and λ3) are averaged to compute radial diffusivity (RD). The average of all three Eigen values is measured as MD.
Tract-based spatial statistics with statistical analysis of diffusion tensor imaging data
The FA output images were used as an input for tract-based spatial statistics (TBSS), a voxel wise approach for analysis of FA data.[3] All subject's FA data were aligned into a common space using FMRIB's nonlinear image registration tool. The mean FA image was generated and thinned to create a mean FA skeleton, which represents the centers of all tracts common to the group. Each subject's aligned FA data were then projected onto this skeleton, and the resulting data fed into voxel wise general linear modeling cross-subject statistics. We used a threshold of 0.2 for creation of mean FA skeleton. A voxel- by-voxel permutation nonparametric test (5000 permutations) was used to assess the group-related differences using threshold-free cluster enhancement, which avoids using an arbitrary threshold for the initial cluster formation.[4] For statistical inference, the results were observed at family wise error corrected P < 0.0125 (0.05 divided by 4 for Bonferroni correction) across space. In addition to FA data, MD, AD, and RD were also compared using TBSS in an analogous fashion. The analysis was performed to compare between the ASD group and matched controls. The age, gender, and total intracranial volume were used as nuisance variable/covariates of no interest in the statistical design matrix for the TBSS analysis.
To investigate the relationship between structural changes and clinical variables in the ASD group, a series of stepwise linear regressions in the TBSS design model were carried out to test the predictive power of structural changes for changes in clinical scores. The clinico-radiological correlation was done between the DTI indices (FA and MD) and the CARS sub-scores.
Statistical methods used for clinical variables
The following assumptions on data were made: (1) Dependent variables should be normally distributed, (2) Samples drawn from the population should be random; cases of the samples should be independent.
Analysis of covariance has been used to find the significance of study parameters on a continuous scale between two groups (inter-group analysis) on metric parameters. Levene's test for homogeneity of variance has been performed to assess the homogeneity of variance. Statistical software: The Statistical software namely statistical analysis system (SAS) 9.2 (SAS Inc., Cary, NC, USA), statistical package for the social sciences (SPSS) 15.0 (SPSS Inc. Chicago, IL, USA), Stata 10.1 (StataCorp LP, College Station, Tx, USA), MedCalc 9.0.1 (MedCalc Software bvba, Ostend, Belgium), Systat 12.0 (Cranes Software Inc., Bangalore, India), and R environment ver. 2.11.1 (free online software) were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs, tables etc. Results on continuous measurements are presented on mean ± standard deviation (minimum-maximum) and results on categorical measurements are presented in number (%). Significance was assessed at 5% level of significance.
RESULTS
The study included 19 autistic children with age ranging from 3 to 19 years (mean age 8.7 ± 3.84 years, 16 males and 3 females) and 34 age-matched controls with age ranging from 7 to 18 years (mean age 12.38 ± 3.76 years, all males). Of the 19 autistic children, 4 (21%) were born by cesarian section, others by normal delivery. Three children (15.7%) were preterm with a history of neonatal jaundice in 2 (10.5%) of them.
The most common presenting symptom was poor social interaction, stereotyped behavior and poor eye contact (93.5%). Three children (15.7%) presented with global developmental delay. In addition, 11/19 (57.8%) cases also had the co-morbid attention deficit hyperactive disorder (ADHD) and some form of mental retardation as well. 5/19 (26.3%) cases had a history of seizures and were on antiepileptic medications. Urine for abnormal metabolites and Tandem mass spectroscopy were negative for all cases.
CARS scoring for the cases ranged from 18.5 to 48 (mean 34.21 ± 7.057). Although, CARS score below 30 are generally considered outside the ASD spectrum, these children (n = 5/19, age range of 4–6 years, all males) were diagnosed as autistic based on the DSM-IV diagnostic criteria.
There were no significant conventional MRI abnormalities, except for the mild diffuse paucity of cerebral white matter in two children (10.5%). The handedness of all the ASD children's could not be assessed as many were in the smaller age group and had not attained fine motor grip. The data on handedness among controls was not available.
Tract-based spatial statistics (P < 0.00125 false discovery rate corrected)
The autism group had significant reductions of FA bilaterally involving numerous associations, commissural, and projection tracts [Figures 1 and 2]. Affected association tracts include left inferior fronto-occipital fasciculus (IFOF), left inferior longitudinal fasciculus (ILF), and left uncinate fasciculus (UNF). Commissural fibers includes the entire corpus callosum (CC), and right forceps minor (RT FMIN). Projection tracts included the bilateral anterior thalamic radiations (RT > LT) and right corticospinal tract (CST). Bilateral posterior thalami also showed the significantly reduced FA. Also noted a peculiar finding of significantly reduced FA along the bilateral optic tracts (B/L OTs). Notably, there were no voxels where FA was significantly increased in the ASD group. Significantly increased RD was noted in left ILF (LT ILF), CC, bilateral fornices, RT FMIN, bilateral CST and B/L OT [Figure 3]. The MD was also significantly elevated in the LT ILF, bilateral UNF, body of corpus callosum (BCC), left FMIN (LT FMIN), bilateral anterior thalamic radiations, bilateral CSTs and B/L OTs [Figure 4]. Subtle areas of increased AD were noted in the right ILF, right superior corona radiate and posterior BCC [Figure 5].
Figure 1.
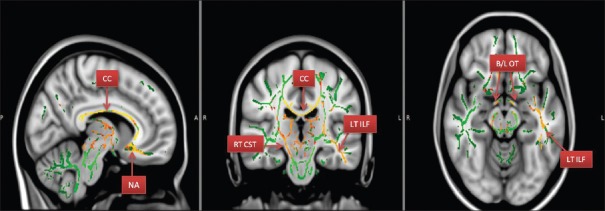
Areas with reduced FA- fractional anisotropy (CC – Corpus callosum; nucleus accumbens area [NA]; RT CST – Right corticospinal tract; LT ILF – Left inferior longitudinal fasciculus; B/L OTs – Bilateral optic tracts)
Figure 2.
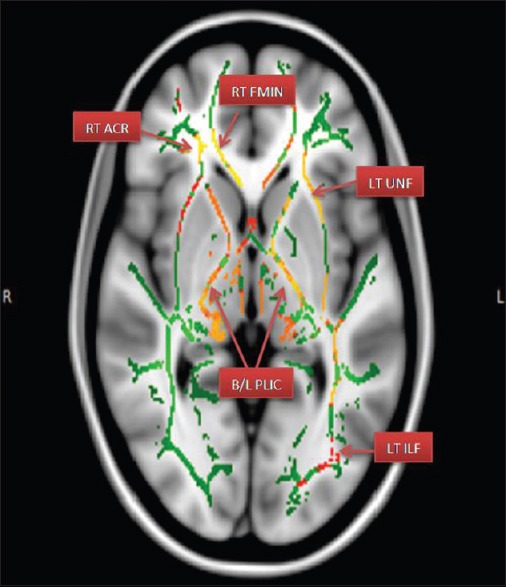
Areas of reduced FA – Fractional anisotropy. (RT ACR – Right anterior corona radiate; RT FMIN – Right forceps minor; LT UNF – Left uncinated fasciculus; B/L PLIC – Bilateral posterior limbs of internal capsules)
Figure 3.
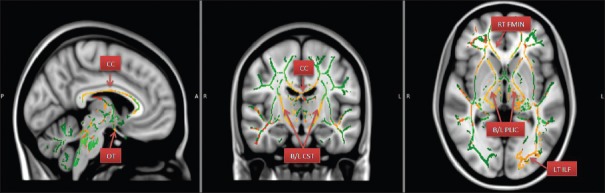
Areas of increased RD – Radial diffusivity. (CC – Corpus callosum; OTs – Optic tracts; B/L CST – Bilateral corticospinal tracts; RT FMIN – Right forceps minor; B/L PLIC – Bilateral posterior limbs of internal capsules; LT ILF – Left inferior longitudinal fasciculus)
Figure 4.
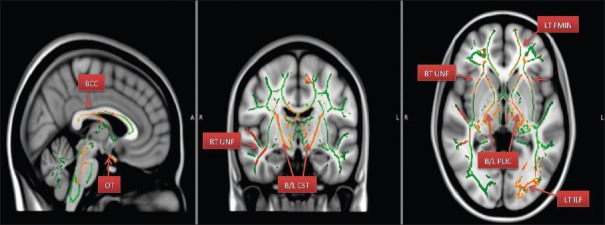
Areas of increased MD – Mean diffusivity. (BCC – Body of corpus callosum; LT ILF – Left inferior longitudinal fasciculus; B/L CST – Bilateral corticospinal tracts; LT FMIN – Left forceps minor; RT UNF – Right uncinated fasciculus; B/L PLIC – bilateral posterior limbs of internal capsule)
Figure 5.
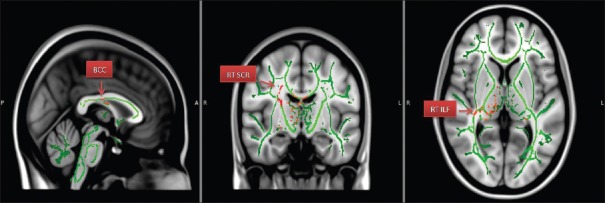
Areas of increased AD – Axial diffusivity. (BCC – Body of corpus callosum; RT SCR – Right superior corona radiate; RT ILF – Right inferior longitudinal fasciculus)
DISCUSSION
ASD is the term used for the neurodevelopmental disorders of empathy that includes autism, Asperger syndrome, atypical autism, and PDD-NOS. ASDs are a syndrome complex characterized by serious impairments in social communication and reciprocal of social interaction, with restricted and repetitive behaviors and impairments of imaginary thought.[1]
Epidemiological research reports a dramatic increase in the rates of autism during the last decades with a prevalence of 5 to 10 cases of classic autism per 10,000 to even 60–65 per 10,000 cases when including the entire spectrum.[5]
Microstructural changes of primary neurocircuitry have been observed in autistic spectral disorders. Various advanced structural and functional imaging techniques have been used in the past to study this entity.
DTI is a relatively new technique that provides us with more direct information about the white matter connections and the integrity of communication pathways.[6] This approach is even suitable for use in very young children under sedation enabling the characterization of the early development of white matter tracts.
DTI enables us to study the various parameters such as FA, AD, and radial diffusivity (RD). FA is considered to reflect fiber density, axonal diameter, and myelination in WM.[7,8] AD represents the direction in which the water diffusion is highest, and is typically affected by cellular debris buildup, breakdown of axonal structure, disordered microtubule arrangement, aggregation of filaments, and expansion of extracellular space.[9]
RD is the average value of the two small Eigen values and is considered to reflect the diffusivity, orthogonal to the axonal bundles and to be mainly affected by the myelin in WM.[10]
Our study included 19 children diagnosed with ASD in the age group of 3 to 19 years, most of them being boys (16/19). Our study group was heterogeneous as they included children with co-existing ADHD, seizure disorder as well as mental retardation. Also, we could not procure the exact age-matched controls as it was difficult to gather healthy children of such young age for MRI scan.
TBSS demonstrated the multiple areas of markedly reduced FA involving the nucleus accumbens area, left IFOF, LT ILF, left UNF, entire CC, RT FMIN, bilateral anterior thalamic radiations (RT > LT), and right CST. Bilateral posterior thalami also showed significantly reduced FA. Also noted was a peculiar finding of significantly reduced FA along B/L OT. Notably, there were no voxels where FA was significantly increased in the ASD group. Significantly increased RD was noted in LT ILF, CC, bilateral fornices, RT FMIN, bilateral CST and B/L OT, suggesting myelination defect.[11] The MD was also significantly elevated in the LT ILF, bilateral UNF, BCC, LT FMIN, bilateral anterior thalamic radiations, bilateral CSTs and B/L OTs. We also noted that there were no significant changes in the AD in these regions, indicating that there is no significant axonal injury.[12] A sub-analysis between the FA values and CARS autism rating scales showed no significant correlation.
Our finding of abnormal FA and RD involving B/L OTs is not described in literature till date to the best of our knowledge. A literature search revealed that ASDs are over-represented in the visually impaired population, with the prevalence estimates as high as 1 case of autism in every 4 visually impaired persons.[13]
Recently a meta-analysis by C. Fink and M. Borchert put forth the hypothesis that a potential common underlying neurological mechanism lies between children with optic nerve hypoplasia (ONH) and ASD.[14] They concluded that the prevalence of autistic-like behaviors in children with ONH is striking and opined that further research should be directed toward deciphering the underlying common pathogenic mechanism. The presence of DTI abnormalities in the optic nerve in our patients further strengthens this hypothesis. Keeping aside the OT abnormality, our study is almost in accordance with many similar recent studies.[15,16,17,18]
To summarise, as compared to other similar studies we found many common tracts showing abnormal DTI findings; for example the CC, forceps minor, IFOF, and UNF which forms a part of major networks relating to several behaviors that are recognized as core deficits in autism. These findings support the conception of autism as a connectivity disorder. The variability of findings might be due to underlying diversity in autism research, and particularly imaging studies, as well as due to the age-related differences.
Limitations of our study
Modest sample size and heterogeneous group of ASD children, most of them have significant co-morbidities such as ADHD, mental retardation, and seizures. Because of the small individual sample size intra-group analysis was not attempted. We did not pursue to procure accurately age-matched controls because of the ethical issues involved in imaging younger healthy children under sedation. This may have a significant implication for our data interpretation, as we are aware of age-related DTI/connectivity changes in developing/growing human brains. This remains a serious limitation of this original research.
Future studies may be attempted to include pure autism cases with no significant co-morbidities. Comparison among the ASDs with genetic correlation and longitudinal studies will also be useful in understanding this condition better.
CONCLUSION
Our prospective study in a small population (n = 19) of children with ASD under 20 years of age demonstrated significant abnormalities in various white matter tracts by TBSS analysis of DTI data as compared to the typically developed healthy controls. We found the reduced FA and increased RD in many of the white matter tracts, with little effect on the AD, suggestive of myelination defects rather than axonopathy. A sub-analysis was carried out to check the correlation of these DTI metrics with CARS scoring, which failed to demonstrate any significant correlation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gillberg C, Coleman M. Autism and medical disorders: A review of the literature. Dev Med Child Neurol. 1996;38:191–202. doi: 10.1111/j.1469-8749.1996.tb15081.x. [DOI] [PubMed] [Google Scholar]
- 2.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price S, Paviour D, Scahill R, Stevens J, Rossor M, Lees A, et al. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson's disease. Neuroimage. 2004;23:663–9. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 5.O’Hare A. Autism spectrum disorder: Diagnosis and management. Arch Dis Child Educ Pract Ed. 2009;94:161–8. doi: 10.1136/adc.2008.150490. [DOI] [PubMed] [Google Scholar]
- 6.Wing L. Autistic spectrum disorders. Br Med J. 1996;312:327. doi: 10.1136/bmj.312.7027.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Hüppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–97. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz ED, Cooper ET, Fan Y, Jawad AF, Chin CL, Nissanov J, et al. MRI diffusion coefficients in spinal cord correlate with axon morphometry. Neuroreport. 2005;16:73–6. doi: 10.1097/00001756-200501190-00017. [DOI] [PubMed] [Google Scholar]
- 10.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 11.Song SK, Sun SW, Cross AH, Le TQ, Armstrong R. Increased radial diffusivity: A demyelination marker. Proc Int Soc Magn Reson Med. 2004;11:723. [Google Scholar]
- 12.Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: A quantitative pixelwise analysis. J Neurosci. 2009;29:2805–13. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown R, Hobson RP, Lee A, Stevenson J. Are there – Autistic-like features in congenitally blind children? J Child Psychol Psychiatry. 1997;36:693–703. doi: 10.1111/j.1469-7610.1997.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 14.Fink C, Borchert M. Optic nerve hypoplasia and autism: Common features of spectrum diseases. J Vis Impair Blind. 2011;105:334–8. [Google Scholar]
- 15.Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161:334–40. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- 16.Ameis SH, Fan J, Rockel C, Voineskos AN, Lobaugh NJ, Soorya L, et al. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: A diffusion tensor imaging study. PLoS One. 2011;6:e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, et al. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb Cortex. 2010;20:2103–13. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, et al. Abnormal white matter integrity in young children with autism. Hum Brain Mapp. 2011;32:534–43. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]


