Highlight
H2S could help plants coping with iron deficiency through increasing phytosiderophore accumulation and secretion, regulating expression of genes related to iron homeostasis and sulphur metabolism, and consequently enhancing the photosynthesis of maize seedlings.
Keywords: Chlorophyll, chloroplast ultrastructure, hydrogen sulphide (H2S), iron-deficient, photosynthesis, phytosiderophore, Zea mays.
Abstract
Hydrogen sulphide (H2S) is emerging as a potential molecule involved in physiological regulation in plants. However, whether H2S regulates iron-shortage responses in plants is largely unknown. Here, the role of H2S in modulating iron availability in maize (Zea mays L. cv Canner) seedlings grown in iron-deficient culture solution is reported. The main results are as follows: Firstly, NaHS, a donor of H2S, completely prevented leaf interveinal chlorosis in maize seedlings grown in iron-deficient culture solution. Secondly, electron micrographs of mesophyll cells from iron-deficient maize seedlings revealed plastids with few photosynthetic lamellae and rudimentary grana. On the contrary, mesophyll chloroplasts appeared completely developed in H2S-treated maize seedlings. Thirdly, H2S treatment increased iron accumulation in maize seedlings by changing the expression levels of iron homeostasis- and sulphur metabolism-related genes. Fourthly, phytosiderophore (PS) accumulation and secretion were enhanced by H2S treatment in seedlings grown in iron-deficient solution. Indeed, the gene expression of ferric-phytosiderophore transporter (ZmYS1) was specifically induced by iron deficiency in maize leaves and roots, whereas their abundance was decreased by NaHS treatment. Lastly, H2S significantly enhanced photosynthesis through promoting the protein expression of ribulose-1,5-bisphosphate carboxylase large subunit (RuBISCO LSU) and phosphoenolpyruvate carboxylase (PEPC) and the expression of genes encoding RuBISCO large subunit (RBCL), small subunit (RBCS), D1 protein (psbA), and PEPC in maize seedlings grown in iron-deficient solution. These results indicate that H2S is closely related to iron uptake, transport, and accumulation, and consequently increases chlorophyll biosynthesis, chloroplast development, and photosynthesis in plants.
Introduction
Iron is an essential nutrient for various cellular and physiological processes in plants. It functions as a component of many important enzymes and proteins that are involved in fundamental biochemical processes, such as respiration, photosynthesis, oxygen transport, and so on (Graziano and Lamattina, 2007). However, iron is often unavailable and difficult for crops to assimilate due to the low solubility of its oxidized form. Therefore, iron deficiency often occurs in plants and leads to interveinal chlorosis, impairment of chlorophyll biosynthesis and chloroplast development, and further affects the photosynthetic apparatus establishment (Ramirez et al., 2011). Thereby, iron availability is directly correlated with plant productivity.
Iron abundance in plant cells is tightly regulated by iron uptake, translocation, and recycling. Under iron-deficient conditions, plants have evolved two separate strategies for iron acquisition. In strategy I, plants respond to iron deprivation by at least three steps including acidification of the rhizosphere by an H+-ATPase, reduction of Fe(III) to Fe(II) by ferric-chelate reductase, and uptake of Fe(II) by iron transporters. In contrast, all gramineous plants such as barley and wheat have a distinct strategy (strategy II) for iron acquisition, which is different from that of non-grass species (Curie and Briat, 2003; Walker and Connolly, 2008; Morrissey and Guerinot, 2009). In strategy II, iron acquisition includes (i) biosynthesis of phytosiderophores (mugineic acids, MAs) inside the roots; (ii) secretion of phytosiderophores (PSs) to the rhizosphere; (iii) solubilization of insoluble iron in soils by chelation of PSs; and (iv) uptake of the ferric-phytosiderophore complex by the roots (Ma, 2005; Ueno et al., 2009). However, strategies I and II are not sufficient to support the iron requirement for plant development when iron availability is under a threshold level, thus stress symptoms become evident in iron-deficient plants.
Gramineous plants secrete Fe chelators called mugineic acids family PSs from their roots via transporter of MAs (TOM1) to solubilize Fe in the rhizosphere. Gramineous plants then take up Fe as Fe(III)-MA complexes from the rhizosphere through the action of yellow stripe1 (YS1) transporter at the plasma membrane (Curie et al., 2001). The biosynthetic pathway for MAs in gramineous plants has been elucidated (Mori, 1999). Methionine, which is a precursor of MAs, is converted to 2′-deoxymugineic acid (DMA) via a series of reactions (Mori, 1999). There are many genes involved in these reactions including NAS1, NAS2, DMAS1 etc. (Higuchi et al., 1999). TOM1, which is a major facilitator superfamily (MFS) antiporter, was identified as an efflux transporter of DMA in rice (Nozoye et al., 2011). TOM1 expression is strongly induced in Fe-deficient roots (Nozoye et al., 2011). Similarly, YS1 expression levels increase in both shoots and roots in Fe-deficient Zea mays plants (Ueno et al., 2009). Expression of the ferric-chelate reductase genes AtFRO2, LeFRO1, and PsFRO1 is induced by iron starvation in Arabidopsis, tomato, and pea, respectively (Robinson et al., 1999; Connolly et al., 2003; Li et al., 2004; Wu et al., 2005). Besides, IRT1 encoding an iron-regulated transporter—a high-affinity iron uptake system in roots—was found to be the major route for iron entering the cell, and its expression obviously increased in low versus sufficient iron supply (Connolly et al., 2002; Vert et al., 2002). Moreover, iron binding protein (IBP) is ubiquitous in nature because iron transport and delivery to cells for utilization and storage are essential functions in all organisms. Moreover, IBP is also vital in sequestering iron where an over-abundance can quickly lead to oxidative stresses (Strickler-Dinglasan et al., 2006).
Recently, many studies have revealed that H2S can act as a signalling molecule similar to nitric oxide (NO) and carbon monoxide (CO) in animals at lower concentrations and can participate in various biological processes (Wang, 2002; Yang et al., 2008). Interestingly, some studies have focused on the physiological function of H2S in plants. For instance, it was reported that H2S promoted wheat seed germination, alleviated oxidative damage against copper stress, counteracted chlorophyll loss, and alleviated oxidative damage due to osmotic stress in sweet potato seedling leaves (Zhang et al., 2008; Zhang et al., 2009). Furthermore, boron toxicity, salinity toxicity, and aluminium toxicity were alleviated by H2S (Wang et al., 2010; Zhang et al., 2010; Wang et al., 2012; Chen et al., 2013). In addition, a low concentration of H2S can promote growth of the embryonic root of Pisum sativum and act as a regulator of flower senescence (Li et al., 2010; Zhang et al., 2011). Besides, H2S induces stomatal closure and participates in the abscisic acid (ABA)-dependent signalling pathway by regulating ATP-binding cassette (ABC) transporter in guard cells (García-Mata and Lamattina, 2010). A previous study also showed that H2S enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings (Chen et al., 2011).
It is known that, H2S is endogenously generated during the metabolism of l-cysteine by the enzymes cystathionine β-synthase and cystathionine γ-lyase in plants (Hughes et al., 2009). Indeed, H2S is thought to be released from cysteine via a reversible O-acetyl-l-serine(thiol)lyase (OAS-TL) reaction in plants (Riemenschneider et al., 2005a ; Birke et al., 2015). Moreover, the uptake of H2S is largely dependent on its rate of metabolism into cysteine by OAS-TL and subsequent assimilation into other organic sulphur compounds (De Kok et al., 1998, 2002; Durenkamp et al., 2007). Therefore, H2S is an important compound involved in plant sulphur metabolism. It is noteworthy that S supply could help plants cope with the Fe shortage (Astolfi et al., 2004, 2010, 2012; Zuchi et al., 2012). For instance, Astolfi et al. (2010) showed that barley exhibited a positive correlation between the S nutritional status of the plant and its capability of coping with Fe deficiency. Moreover, one of the first responses to Fe deficiency in strategy II plants is the extrusion of PSs in the root rhizosphere in order to chelate and solubilize Fe3+ (Astolfi et al., 2010, 2012). PSs are derived from nicotianamine that is synthesized from three molecules of S-adenosyl-methionine, thus representing another possible junction between Fe and S metabolism. Under sulphur-deficiency conditions, the release of PSs is reduced (Astolfi et al., 2006a, 2010, 2012); however, the question is,whether H2S as a sulphur compound or a signalling molecule plays a key role in response to Fe deficiency in plants.
Although iron uptake and translocation are controlled by many components which have been recently characterized at the molecular level (Schmidt, 1999), it is not known how signalling molecules are involved in the response of plants to iron deficiency. Recent studies have shown that NO, as a bioactive free radical molecule, regulates iron metabolism in plants (Graziano et al., 2002; Murgia et al., 2002). Likewise, some evidence has been provided that CO, as an endogenous gaseous molecule, may play an important role in improving plant adaptation to iron deficiency (Kong et al., 2010). However, it is still not clear whether a low concentration of H2S, similar to that of NO and CO, is involved in the regulation of iron assimilation and availability in plants.
In this study, compelling data are presented that reveal a novel effect of H2S in plant biology, more specifically on iron nutrition. The results support the idea that H2S is closely related to iron uptake, transport, and accumulation, and consequently increases chlorophyll biosynthesis, chloroplast development, and photosynthesis in plants.
Materials and methods
Plant growth and treatments
Seeds of maize (Zea mays L. cv Canner) were first sterilized in 75% ethanol for 3min and then in 10% sodium hypochlorite solution for an additional 10min followed by washing with distilled water and germinating in a soil:vermiculite (1:1) mixture for 5 d. Five-day-old seedlings were transferred to plastic pots (two seedlings per pot) filled with 0.75 l of nutrient solution. The nutrient solution had the following composition: 5.25mM KNO3, 7.75mM Ca(NO3)2, 4.06mM MgSO4, and 1.0mM KH2PO4; micronutrients: 46 μM H3PO4, 9.18 μM MnSO4, 5.4 μM ZnSO4, 0.3 μM CuSO4, and 2.0 μM Na2MoO4. Iron was supplied as 50 μM Fe(III)-EDTA or in different concentrations ranging from 10 to 250 μM. Plants were grown in a controlled growth chamber with a light/dark regime of 15/9h, relative humidity of 80%, temperature of 21/27 °C and a photosynthetically active radiation (PAR) of 190 μmol m−2 s−1.
NaHS was used as exogenous H2S donor as described by Hosoki et al. (1997). Seedlings were divided into four groups for further treatment. In the first group, 5-d-old seedlings were pre-treated with various concentrations of NaHS (0, 10, 100, 500, 1000 μM) for 8 d and then were grown in nutrient solution containing 50 μM Fe(III)-EDTA for 12 d. In the second group, in order to distinguish the possible roles of H2S, HS-, Na+, or other sulphur-containing components in iron acquirement, a series of chemicals—Na2S, Na2SO4, Na2SO3, NaHSO4, NaHSO3, NaAC, cysteine (Cys), glutathione (GSH), and methionine (Met) were used as the controls of NaHS. In the third group, 5-d-old seedlings were pre-treated with the optimal concentration of NaHS for 8 d and then were grown in nutrient solution containing 0, 25, 50, 75, 100, 250 μM Fe(III)-EDTA for 12 d. In the fourth group, seedlings were pre-treated with the optimal concentration of NaHS for 8 d and then were grown in nutrient solution with 1 μM Fe(III)-EDTA (–Fe) and with 50 μM Fe(III)-EDTA (+Fe) for 12 d. All above of solutions were changed every 4 d.
Chlorophyll quantification
Chlorophyll content was measured according to Lichtenthaler (1987). Maize leaves (0.2g of fresh weight) were powdered with liquid nitrogen, and pigments were extracted using four volumes of 80% (v/v) aqueous acetone until complete bleaching; the content of total chlorophyll was then calculated from the absorbance of leaf chlorophyll extracts at 470, 646, and 663nm.
Iron quantification
The samples (leaves, stems, and roots) were washed three times with distilled deionized water and then dried at 70 °C for 48h. The samples with weight ranging from 50 to 100mg were placed into the digestion vessels, mixed with 5ml of concentrated HNO3 (65–68%), and digested in microwave digestion system (CEM Inc., Mars-V). The solution was finally diluted to a certain volume with distilled deionized water. The content of Fe was analysed by inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer Inc., Elan DRC-e) (Chen L. et al., 2010).
Determination of endogenous H2S, GSH, and non-protein thiols content
Endogenous H2S was measured by the formation of methylene blue from dimethy-p-phenylenediamine in H2SO4 according to the method described by Sekiya et al. (1982) and Chen et al. (2011) with some modifications. Reduced GSH was estimated using a kit of GSH reagent (Jiancheng Bioengineering Institute, Nanjing, China) according to the method described by Chen et al. (2013). The total content of non-protein thiols (NPTs) in maize seedlings was measured according the Del Longo et al. (1993) with minor modifications.
Measurement of PSs content in root tips
PSs content in root tips was determined according to the method of Reichman and Parker (2007) and Zuchi et al. (2012). Maize seedlings were removed from nutrient solution and root tips (about 10mm) were washed, collected, and then homogenized to a fine powder with liquid nitrogen. Distilled water (100 °C) was added to aliquots of the powdered tissue and homogenates were incubated for 10min at 80 °C. Insoluble material was removed by 10min centrifugation in a microliter centrifuge at 12000rpm and the pellet was then re-extracted with boiling water as described above. After a further centrifugation, the supernatant was used for determination of PSs content in root tips using the Fe-binding assay revised by Reichman and Parker (2007).
Collection of root exudates and determination of PSs release
PSs release from maize seedling roots was analysed by determining PSs content in root washing. Maize seedlings were removed from the nutrient solution and the roots were washed two times for 1min in deionized water. Root systems were submerged into 200ml deionized water for 6h with continuous aeration. PSs content in root washing was determined using the Fe-binding assay revised by Reichman and Parker (2007).
Transmission electron microscopy
Leaves were cut into 0.5×1mm pieces and immediately fixed with 2.5% glutaraldehyde (in 0.1M sodium phosphate buffer, pH 7.0) at room temperature for 4h. After three 20min rinses, the samples were post-fixed with 1% OsO4 in the same buffer for another 4h, followed by three buffer rinses. Samples were dehydrated in an acetone series, embedded in Spurr’s resin, and sectioned with a Leica EM UC6 ultramicrotome (Leica Microsystems GmbH, Wetzlar, Germany). The ultrathin sections (70–90nm) were stained with uranyl acetate and lead citrate. A Philips CM 100 transmission electron microscopy (TEM) (Philips, Eindhoven, Netherlands) at 80kV was used for ultrastructure imaging of chloroplast. At least three seedlings and more than 30 individual chloroplasts were observed for each treatments by the method of Chang et al. (2008).
Leaf gas exchange measurements
Light-response and CO2-response curves of photosynthesis (Pn) were measured using a portable photosynthesis system (Li-6400, Li-Cor, Lincoln, NE, USA) on the fourth fully developed leaf of the seedlings. All measurements were conducted in the morning (9:00–11:30) to avoid high temperatures and the air vapour pressure deficit in the afternoon. Light was supplemented using a LED light system. The measurement was carried out on at least six leaves for all treatments in 380μl l−1 CO2 at room temperature (25°C). The apparent dark respiration (Rd), light compensation point (Lcp), light saturation point (Lsp), apparent quantum yield (AQE), and maximal net photosynthetic rate (Pmax) were calculated by modelling the response of leaf Pn to PAR by a non-rectangular hyperbola, as described by Prioul and Chartier (1977).
where θ is the convexity. The Pn was modelled as a function of intercellular CO2 concentration (Ci). This application fits a model curve described by the rectangular hyperbola (Olsson and Leverenz, 1994):
where Pn is the assimilation rate, CE is carboxylation efficiency, Ci is intercellular CO2 concentration, Pmax is the assimilation at saturating CO2 and Re is the respiratory processes (dark and light). Experimental data are fitted by first obtaining initial estimates of CE and Re values using linear regression over the lower part of the curve and estimating Pmax from the largest value. Subsequently a least-squares fit was obtained and values for CE, Re, and Pmax were presented.
SDS-PAGE and western blot analysis
Leaf samples (0.5g) were ground in liquid N2 with a mortar and pestle. Total protein was extracted with a buffer containing 50mM phosphate buffer solution (pH7.5), 2% β-mercaptoethanol, 100mM EDTA, 1% PVPP (w/v), and 1% TritonX-100 (v/v). After 15min centrifugation (4 °C, 15000 g), the upper phase was transferred to a new centrifuge tube. Two volumes of Tris-saturated phenol (pH 8.0) were added and then the mixture was further vortexed for 30min. Proteins were precipitated by adding five volumes of ammonium sulphate saturated-methanol, and incubated at −20 °C for at least 4h. After centrifugation as described above, the protein pellets were re-suspended and rinsed with ice-cold methanol followed by washing with ice-cold acetone twice, and spun down at 15000 g for 10min at 4 °C after each washing. Finally the washed pellets were air-dried and recovered in the lysis buffer containing 62.5mM Tris-HCl (pH 6.8), 2% SDS (v/v), 10% glycerol (v/v), and 2% β-mercaptoethanol (v/v). Protein concentrations were quantified using the Bradford assay (Bradford, 1976).
For western blot analysis, proteins (20 μg from each sample) were separated by SDS-PAGE using 12% (w/v) acrylamide gels according to Laemmli (1970) and electrophoretically transferred to polyvinylidene difluoride (PVDF) membrane for 50min. The membrane was blocked overnight with western blocking buffer (TIANGEN, China). The protein blot was probed with a primary antibody of ribulose-1,5-bisphoshate carboxylase (RuBISCO) large subunit (RuBISCO LSU) (AS03 037-200, Agrisera, Sweden) and phosphoenolpyruvate carboxylase (PEPC) (AS09 458, Agrisera, Sweden) at dilutions of 1:5000 and 1:1000, respectively for 4h at room temperature with agitation. Then the blot was washed three times in PBS with Tween-20 (PBST) solution containing 50mM Tris-HCl (pH 8.0), 150mM NaCl, 0.05% Tween-20 (v/v), followed by incubation with the secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, Abcam, UK; 1:5000 dilution) for 2h at room temperature. β-actin (1:5000; Santa Cruz, CA, USA) was used as an internal control. The blots were finally washed as above and developed with SuperSigmal West Pico Chemiluminescent Substrate (Pierce, USA) according to the manufacturer’s instructions. Images of the blots were obtained using a CCD imager (FluorSMax, Bio-Rad, USA). The Quantity One software (Bio-Rad, Hercules, CA, USA) was used to determine the optical density value. The comparative optical density value was used to determine the relative amount of protein expression, with the expression of β-actin used as an internal control.
Total RNA extraction, reverse transcription and quantitative real-time PCR (qRT-PCR)
Leaves and roots (0.5g) were frozen and ground in liquid nitrogen with 2% polyvinylpyrrolidone and extracted with 0.5ml of RNA purification reagent (Invitrogen Inc., CA, USA) by following the manufacturer’s procedure. The RNA concentration was determined by using anultraviolet spectrophotometer (Cary 50, Varian, USA) and RNA integrity was detected by 1% agarose gel electrophoresis. Total RNAs were reverse-transcribed into first-strand cDNAs with M-MLV reverse transcriptase (TaKaRa, Dalian, China). A 10-μl real-time PCR reaction contained the following: 0.6 μl of forward and reverse primers including iron homeostasis-related genes, sulphur metabolism-related genes, and photosynthesis-related genes (Supplementary Table S1, available at JXB online), 1 μl of cDNA (equivalent to 10ng of mRNA), and 5 μl of Faststart Universal SYBR Green Master (ROX, Mannheim, Germany). Amplification and detection of dsDNA synthesis of these genes were performed respectively using the PCR conditions as described in Supplementary Table S2, available at JXB online. Three independent replicates were performed for each sample. The comparative threshold cycle (Ct) method was used to determine the relative amount of gene expression. Actin2 was used as an internal control. The mRNA transcriptional abundance value of genes was expressed as 2−ΔΔCt (Livak and Schmittgen, 2001).The LightCycler 480II Real-Time PCR system (Roche, Bern, Switzerland) was used to run qRT-PCR.
Statistical analysis
For gas exchange measurements, at least six leaves were used. For physiological and biochemical analyses, at least three replicates were included. Statistical significance was tested by one-way or two-way ANOVA with SPSS 13.0 (SPSS Inc., Chicago, IL, UA), and results are expressed as the mean values ± standard error. Post-hoc comparisons were tested using the Tukey test at a significance level of P<0.05.
Results
H2S rather than other sulphur-containing derivatives significantly inhibits the chlorophyll loss in iron-deficient maize plants
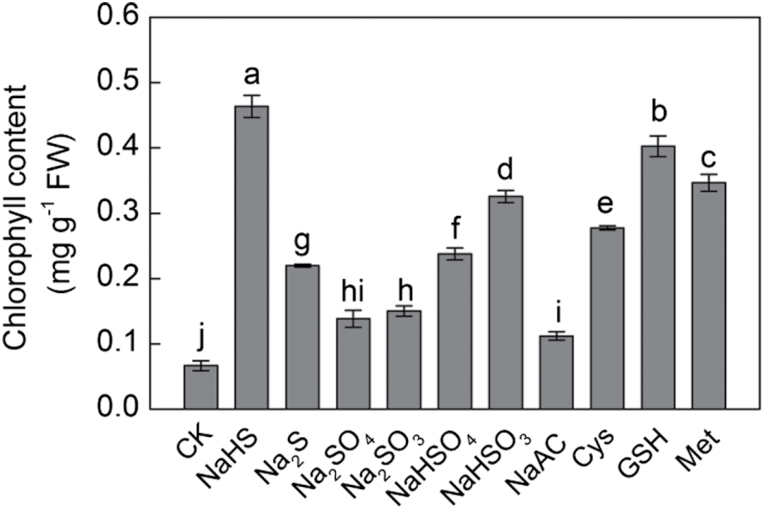
The first step in studying the role of H2S in regulating plant iron assimilation, was to examine its effect on chlorophyll content in maize seedling leaves. In order to distinguish the role of H2S from that of other sulphur-containing derivatives and sodium, a series of sulphur- and sodium-containing chemicals including NaHS, Na2S, Na2SO4, Na2SO3, NaHSO4, NaHSO3, NaAC, Cys, GSH, and Met were used to treat maize seedlings under –Fe conditions. After treatment for 20 d, Na+ or other sulphur-containing compounds, which were used as controls of NaHS, did not cause as great an increase in chlorophyll content as NaHS (Fig. 1). It was also observed that the leaf chlorophyll content of seedlings treated with 100 μM NaHS was much higher than that of other treatments. In addition, the chlorophyll content under GSH treatment increased, but not by as much as the NaHS-induced increases. These results showed that H2S rather than other sulphur-containing compounds or sodium was responsible for the increase in chlorophyll content in iron-deficient maize plants. Therefore, NaHS was used as a donor of H2S in subsequent experiments.
Fig. 1.
H2S but not other sulphur- or sodium-containing compounds derived from NaHS contribute to increased chlorophyll content in iron-deficient maize plants. Maize seedlings were pre-treated with different sulphur-containing compounds for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA (–Fe) for 12 d. Data are presented as means ± SE. Columns labelled with different letters indicate significant differences at P<0.05.
H2S induces greening and seedling growth in iron-deficient maize plants
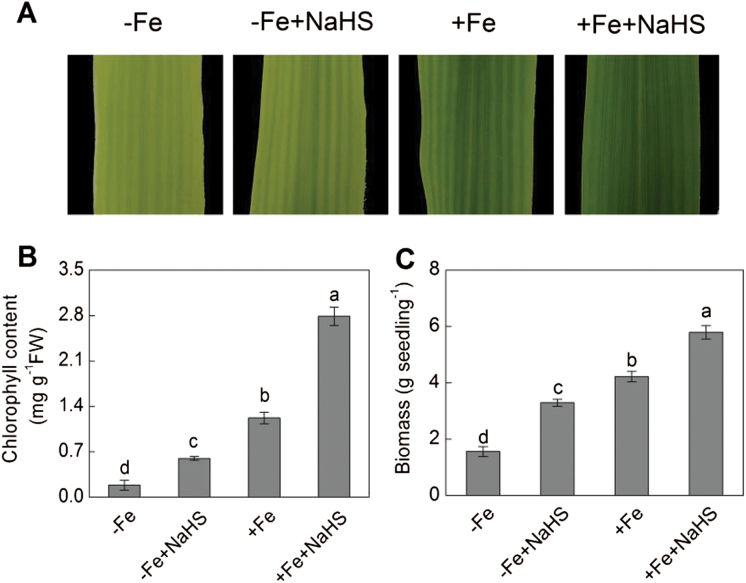
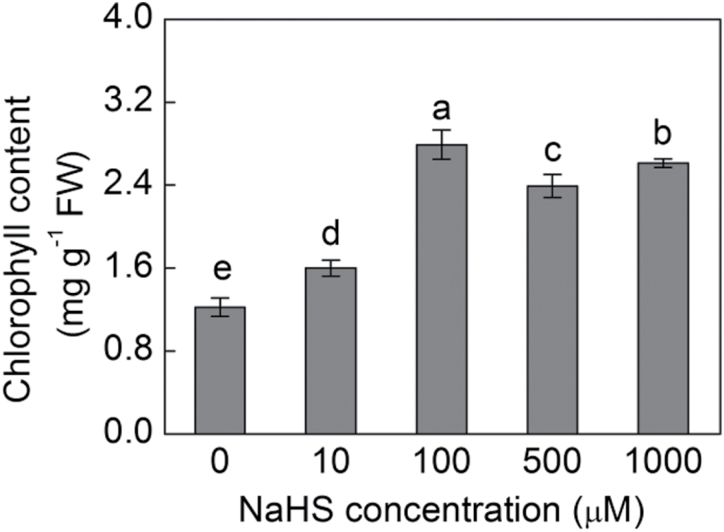
Interveinal yellowing or chlorosis of the leaves is a distinguishable symptom associated with iron deficiency. Therefore, it was investigated whether exogenous H2S could improve leaf greening in maize plants growing under –Fe or +Fe conditions. Maize plants grown under –Fe conditions showed significant yellowing in the young leaves; however, under +Fe conditions, the young leaves became more healthy to some extent. Importantly, the yellowing leaves could be largely reversed by the addition of 100 μM NaHS, a H2S donor (Fig. 2A). The H2S-induced leaf greening was estimated by measuring chlorophyll content. With NaHS treatment, 3.24-fold and 2.28-fold increases were achieved in the maize leaves under –Fe and +Fe conditions compared with their control plants, respectively (Fig. 2B). Moreover, Fig. 2C showed that biomass under –Fe+NaHS was 2.12-fold higher than that of –Fe alone. Meanwhile, a 137% increase was also found under +Fe+NaHS than that of +Fe alone. In addition, it was found that H2S mediated the increase in chlorophyll content in a dose-dependent manner. For example, the chlorophyll content in +Fe plants treated with various concentrations of NaHS (10–1000 μM) were significantly higher than that of control plants, in which the optimal concentration of NaHS was 100 μM (Fig. 3).
Fig. 2.
Effect of NaHS on phenotype (A), chlorophyll content (B), and biomass (C) of iron-deficient maize plants. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d. Data are presented as means ± SE. Columns labelled with different letters indicate significant differences at P<0.05. –Fe, 1 µM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe. A colour version of this figure is available at JXB online.
Fig. 3.
Effect of various NaHS concentrations on chlorophyll content in a dose-dependent manner in iron-deficient maize plants. Maize seedlings were pre-treated with 0, 10, 100, 500, and 1000 µM NaHS for 8 d and then grown hydroponically in a nutrient solution containing 50 µM Fe(III)-EDTA for 12 d. Chlorophyll content in the fourth leaves was measured. Data are presented as means ± SE. Columns labelled with different letters indicate significant differences at P<0.05.
H2S-mediated chlorophyll increase is dependent on iron concentration
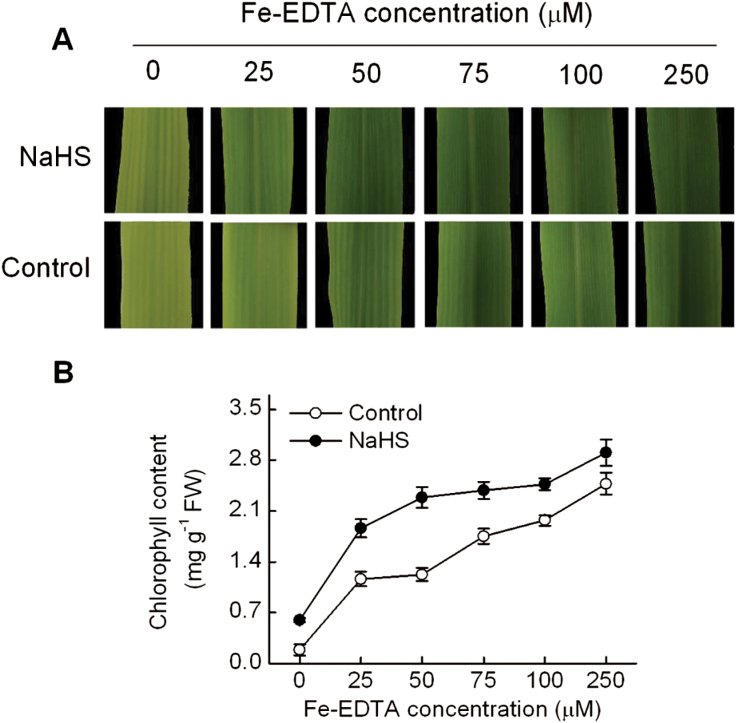
To assess the relationship between H2S-mediated greening and iron availability, chlorophyll content of maize plants grown at different Fe(III)-EDTA concentrations (0–250 μM) plus 100 μM NaHS was analysed. Figure 4A shows representative leaves of plants grown in the above conditions. H2S prevented iron deficiency-induced chlorosis even in plants growing in the absence of iron by increasing the chlorophyll content three-fold compared with that of the control plants (Fig. 4B). Chlorophyll content in untreated maize leaves markedly increased with increasing concentrations of iron in the nutrient solution. Thus, between 0 and 250 μM Fe-EDTA, chlorophyll content increased from 0.18 to 2.48mg g−1 fresh weight. In H2S-treated plants, chlorophyll content increased from 0.59 to 2.90mg g−1 fresh weight for the same range of iron concentrations (Fig. 4B). In fact, the chlorophyll content of 100 μM Fe-EDTA-treated plants without H2S treatment (control plants) was the same level as that of plants grown in 25 μM Fe-EDTA treated with H2S. Plants grown under severe iron deficiency showed slow growth and usually fail to complete their vegetative cycle, however, H2S-treated plants exhibited normal development under –Fe conditions (Supplementary Fig. S1, available at JXB online).
Fig. 4.
NaHS enhances the content of chlorophyll in maize seedlings grown under different Fe(III)-EDTA supplies. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown hydroponically in a nutrient solution with 0, 25, 50, 75, 100, or 250 µM Fe(III)-EDTA for 12 d.(A) Representative photographs of the fourth leaves of plants grown at different Fe(III)-EDTA concentrations. (B) Chlorophyll content in the fourth leaves. Data are presented as means ± SE. A colour version of this figure is available at JXB online.
H2S treatment increases iron accumulation in maize plants
To evaluate whether H2S promotes an increase in iron accumulation inside the plant, total iron content in maize plants was estimated by ICP-MS. The iron concentration in the H2S-treated plants was significantly higher than that of the controls (the plants without H2S treatment) except for stems grown under 50 μM Fe-EDTA (Table 1). For example, more than a two-fold increase in H2S-treated roots of maize plants grown under 50 μM Fe-EDTA conditions was found. Indeed, iron concentration in H2S-treated leaves increased by 21% compared with the controls. Overall, H2S-treated leaves and roots have higher iron content, suggesting that H2S functions mainly through improving iron uptake and availability inside the leaf.
Table 1.
Iron content in leaves, stems, and roots of maize plants
Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d.
| –Fe | –Fe+NaHS | +Fe | +Fe+NaHS | |
|---|---|---|---|---|
| Fe content (mg g−1 DW) | ||||
| Leaves | 0.137±0.016 a | 0.166±0.006 b | 0.180±0.001 c | 0.218±0.002 d |
| Stems | 0.174±0.002 a | 0.209±0.013 b | 0.222±0.012 b | 0.215±0.010 b |
| Roots | 0.424±0.020 a | 0.481±0.005 b | 1.115±0.046 c | 2.270±0.038 d |
Data are presented as means ± SE.
Different letters indicate significant differences at P<0.05 from different treatments.
DW, dry weight; –Fe, 1 μM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe.
H2S regulates the expression of Fe homeostasis-related genes to iron acquisition in maize plants
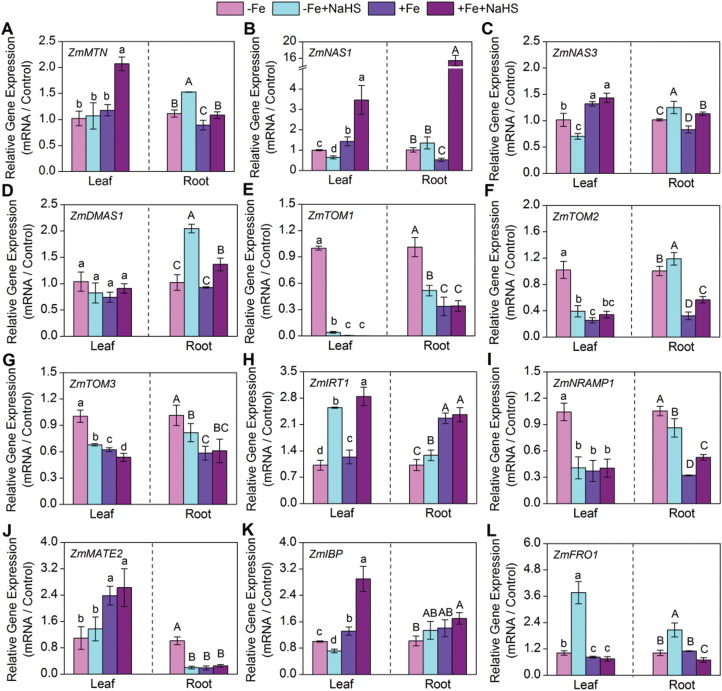
To investigate the physiological role of H2S on iron bioavailability, the expression of Fe homeostasis-related genes—those that encode enzymes involved in the MAs biosynthesis pathway, including the methionine cycle, as well as those for transcription factors and transporters involved in Fe homeostasis—was measured. Firstly, the gene expression of ZmMTN was markedly elevated in roots under H2S-treated seedlings (Fig. 5A). In addition, the expression levels of those genes involved in MAs biosynthesis including ZmNAS1, ZmNAS3, and ZmDMAS1 were significantly induced by H2S under –Fe and +Fe conditions in roots of maize seedlings, but this function of H2S was not marked in leaves (Fig. 5B–D). Besides, transporter genes with similarities to ZmTOM1 and ZmTOM3 were induced in roots and leaves under the –Fe condition, but this induced expression was decreased due to the application of NaHS (Fig. 5E, G). In contrast, the expression of ZmTOM2 was obviously higher in H2S-treated seedling roots than that of the controls (Fig. 5F). Moreover, the gene expression of ZmIRT1 in leaves was up-regulated profoundly under H2S treatment, but 50 μM Fe-EDTA itself rather than NaHS treatment promoted the gene expression of ZmIRT1 in roots (Fig. 5H). The expression levels of ZmNRAMP1 and ZmMATE2 in roots were induced by –Fe, but H2S could significantly inhibit this increase under the –Fe condition (Fig. 5I, J). Here, the gene expression of ZmIBP in +Fe+NaHS-treated maize leaves was increased 2.2-fold compared with the controls (+Fe), but the expression of this gene did not change significantly after NaHS treatment in roots (Fig. 5K). Finally, 3.7-fold and 2.0-fold increases of the gene expression of FRO1 were found in H2S-treated leaves and roots of maize grown in –Fe solution compared with their controls, respectively. However, there was no obvious change between +Fe and +Fe+NaHS-treated maize leaves (Fig. 5L).
Fig. 5.
Gene expression of ZmMTN (A), ZmNAS1 (B), ZmNAS3 (C), ZmDMAS1 (D), ZmTOM1 (E), ZmTOM2 (F), ZmTOM3 (G), ZmIRT1 (H), ZmNRAMP1 (I), ZmMATE2 (J), ZmIBP (K), and ZmFRO1 (L) of maize leaves (left column) and roots (right column) with different treatments. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d. The relative mRNA level of each gene was normalized to the mRNA of Zmactin2. Data are presented as means ± SE of three replicates. Columns labelled with different letters indicate significant differences at P<0.05. –Fe, 1 µM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe. A colour version of this figure is available at JXB online.
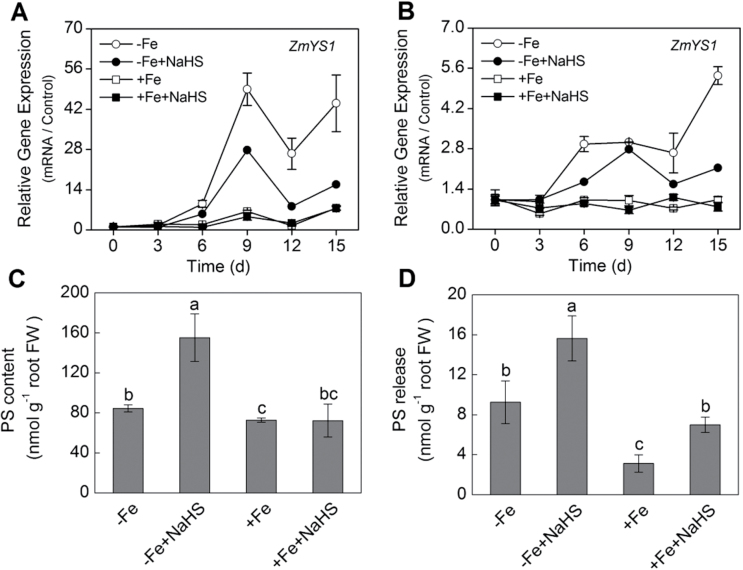
H2S regulates the transcript abundance of ZmYS1 and PSs secretion and content in maize seedling roots
To improve understanding of how H2S influences the response to Fe deficiency, the expression of ZmYS1 in maize seedlings was analysed. The expression of ZmYS1 in leaves and roots significantly increased with length of Fe-deficient treatment (Fig. 6A, B). The transcript abundance of ZmYS1 in leaves after 9 d of Fe-deficient treatment increased 50-fold (Fig. 6A). Similarly, this gene expression in roots also increased by six-fold at 15 d after Fe-deficient treatment (Fig. 6B). However, ZmYS1 transcript abundance in leaves and roots of maize seedlings treated with NaHS were significantly lower than those without NaHS treatment at each time point. In contrast to –Fe treatment, there was no significant difference between +Fe and +Fe+NaHS treatment in both leaves and roots of maize seedlings (Fig. 6A, B).
Fig. 6.
Time-dependent gene expression of ZmYS1 in maize leaves (A) and roots (B). PSs accumulation in roots (C) and PSs release (D) of maize seedlings. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 15 d. The relative mRNA level of each gene was normalized to the mRNA of Zmactin2. Data (A, B) are presented as means ± SE of three replicates. Data (C, D) are presented as means ± SE of four replicates. Columns labelled with different letters indicate significant differences at P<0.05. –Fe, 1 µM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe.
It is well known that secretion of PSs has been described as a physiological response to Fe deficiency in strategy II plants. After NaHS treatment, both the PSs accumulation in root tips and the rate of PSs exudation were evaluated. As shown in Fig. 6C, PSs accumulation in NaHS-treated roots was higher by up to two-fold compared with those without NaHS-treated roots under –Fe conditions, whereas no significant change was found between +Fe and +Fe+NaHS-treated roots. Similar to PSs accumulation, PSs release was also significantly induced by –Fe and NaHS (Fig. 6D). For instance, around a 1.7-fold increase of PSs release was observed in NaHS-treated maize roots under –Fe conditions. The same positive effect of NaHS on PSs release was confirmed in +Fe-treated maize roots.
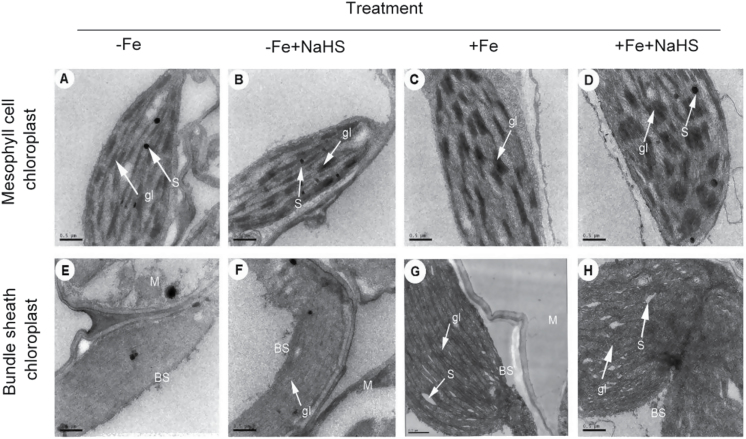
Effect of H2S on chloroplast ultrastucture in iron-deficient maize plants
Electron micrographs of mesophyll cells from iron-deficient maize plants revealed plastids with few photosynthetic lamellae and with some rudimentary grana, displaying typical features of thylakoid disorganization induced by iron deprivation (Fig. 7A). In contrast, when iron-deficient plants were treated with H2S, mesophyll chloroplasts appeared completely developed and the numbers of normal grana stacking and grana lamellae were significantly increased (Fig. 7B, D). For instance, the number of grana stacking in H2S-treated seedlings increased by 27% and 47% compared with that in the controls under –Fe or +Fe conditions by quantitative analysis, respectively (Supplementary Table S3, available at JXB online). Moreover, there was a two-fold increase in the number of grana lamellae both under –Fe+H2S and +Fe+H2S conditions compared with their controls (Supplementary Table S3, available at JXB online). In bundle sheath chloroplasts of iron-deficient plants, electron micrographs also revealed important differences between H2S-treated and control plants. Control plants (1 μM Fe-EDTA) had chloroplasts with no detectable starch granules and grana stacking, whereas chloroplast of H2S-treated plants showed slight grana stacking (Fig. 7F). However, under 50 μM Fe-EDTA treatment, pronounced starch granules and grana stacking was observed in chloroplasts. Furthermore, when treated with H2S, the number of starch granules and grana stacking increased slightly (Fig. 7G, H).
Fig. 7.
TEM analysis of the effect of NaHS on mesophyll cell (A–D) and bundle sheath (E–H) chloroplast ultrastructure of maize leaves.(A, E), –Fe; (B, F), –Fe+NaHS; (C, G), +Fe; (D, H), +Fe+NaHS; gl, grana lamella; S, starch; BS, bundle sheath cell; M, mesophyll cell. Bar = 0.5 µm.
Effect of H2S on endogenous H2S, GSH, and NPTs content in iron-deficient maize plants
A high accumulation of endogenous H2S in maize seedling leaves and roots caused by exogenously applied NaHS was observed under –Fe or +Fe conditions (Table 2). Meanwhile, to study further whether exogenously applied NaHS has an effect on thiol redox modification of proteins involved in iron homeostasis, GSH and NPTs content was measured. As shown in Table 2, NaHS treatment resulted in different degrees of increases in GSH and NPTs content in roots and leaves under –Fe or +Fe conditions.
Table 2.
Effects of H2S donor NaHS and iron on endogenous H2S, NPTs, and GSH content in leaves and roots of maize seedlings
Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d.
| Treatment | Endogenous H2S content (μmol g−1 FW) | NPTs (μmol g−1 FW) | GSH (nmol g−1 FW) | |||
|---|---|---|---|---|---|---|
| Leaf | Root | Leaf | Root | Leaf | Root | |
| −Fe | 0.022±0.005 a | 0.032±0.007 A | 1.58±0.13 a | 8.23±0.32 A | 162.8±29.6 a | 407.7±1.95 A |
| −Fe+NaHS | 0.069±0.006 b | 0.064±0.005 B | 1.92 0.27 a | 10.66±0.69 B | 372.1±27.5 b | 534.3±48.4 B |
| +Fe | 0.025±0.004 a | 0.023±0.001 A | 2.75±0.31 b | 8.35±0.61 A | 378.3±7.71 b | 476.2±34.4 B |
| +Fe+NaHS | 0.108±0.006 c | 0.100±0.015 C | 4.12±0.27 c | 11.30±1.53 B | 619.2±97.6 c | 510.3±42.4 B |
Data are presented as means ± SE.
Different letters indicate significant differences at P<0.05 from different treatments.
–Fe, 1 μM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe.
FW, fresh weight.
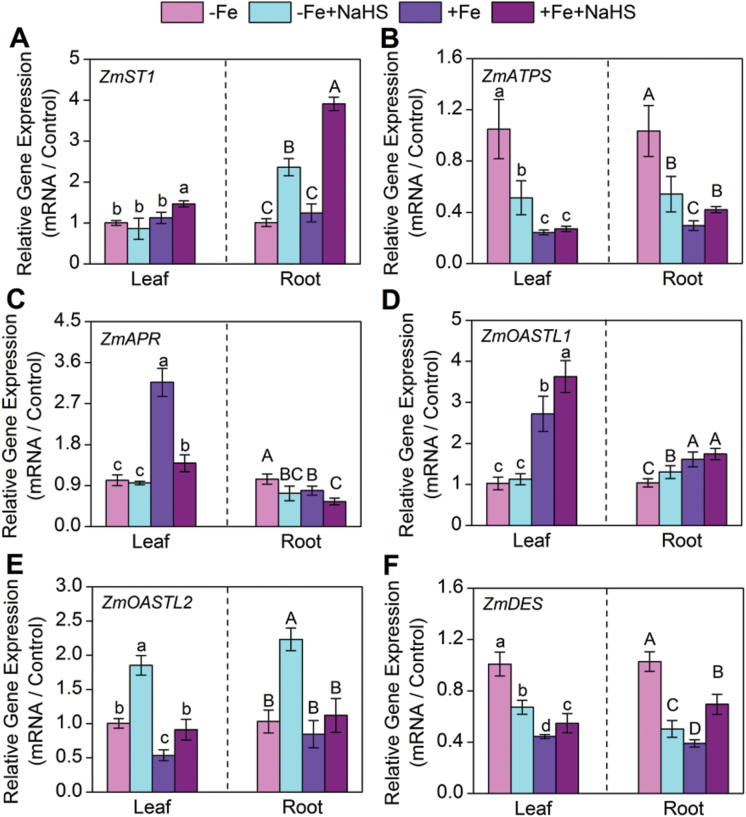
H2S regulates the expression of sulphur metabolism-related genes in iron-deficient maize plants
Under NaHS treatment, the amount of ZmST1 transcript encoding a high-affinity sulphate transporter in maize seedling roots increased by 134% and 215% under –Fe and +Fe conditions, respectively (Fig. 8A). However, in leaves, there was no obvious change in the amount of ZmST1. In contrast, exogenously applied NaHS could significantly down-regulate the amount of ZmATPS transcript under iron-deficient maize seedling leaves and roots (Fig. 8B). Similarly, the amount of ZmAPR transcript in iron-deficient maize roots also was decreased (Fig. 8C). Interestingly, the amount of ZmOASTL1 and ZmOASTL2 transcripts encoding O-acetyl-L-serine(thiol)lyase was significantly increased under the –Fe+NaHS condition compared with that of the –Fe condition in maize seedling roots (Fig. 8D, E). Moreover, the expression of ZmOASTL2 gene in –Fe or +Fe maize seedling leaves was up-regulated by exogenously applied NaHS (Fig. 8E). On the contrary, under the –Fe condition NaHS also decreased significantly the amounts of ZmDES transcript encoding cysteine desulfhydrase in leaves and roots, but under the +Fe condition, its expression was up-regulated by NaHS in leaves and roots (Fig. 8F).
Fig. 8.
Gene expression of ZmST1 (A), ZmATPS (B), ZmAPR (C), ZmOASTL1 (D), ZmOASTL2 (E), and ZmDES (F) of maize leaves (left column) and roots (right column) with different treatments. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d. The relative mRNA level of each gene was normalized to the mRNA of Zmactin2. Data are presented as means ± SE of three replicates. Columns labelled with different letters indicate significant differences at P<0.05. –Fe, 1 µM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe. A colour version of this figure is available at JXB online.
Effect of H2S on photosynthesis in iron-deficient maize plants
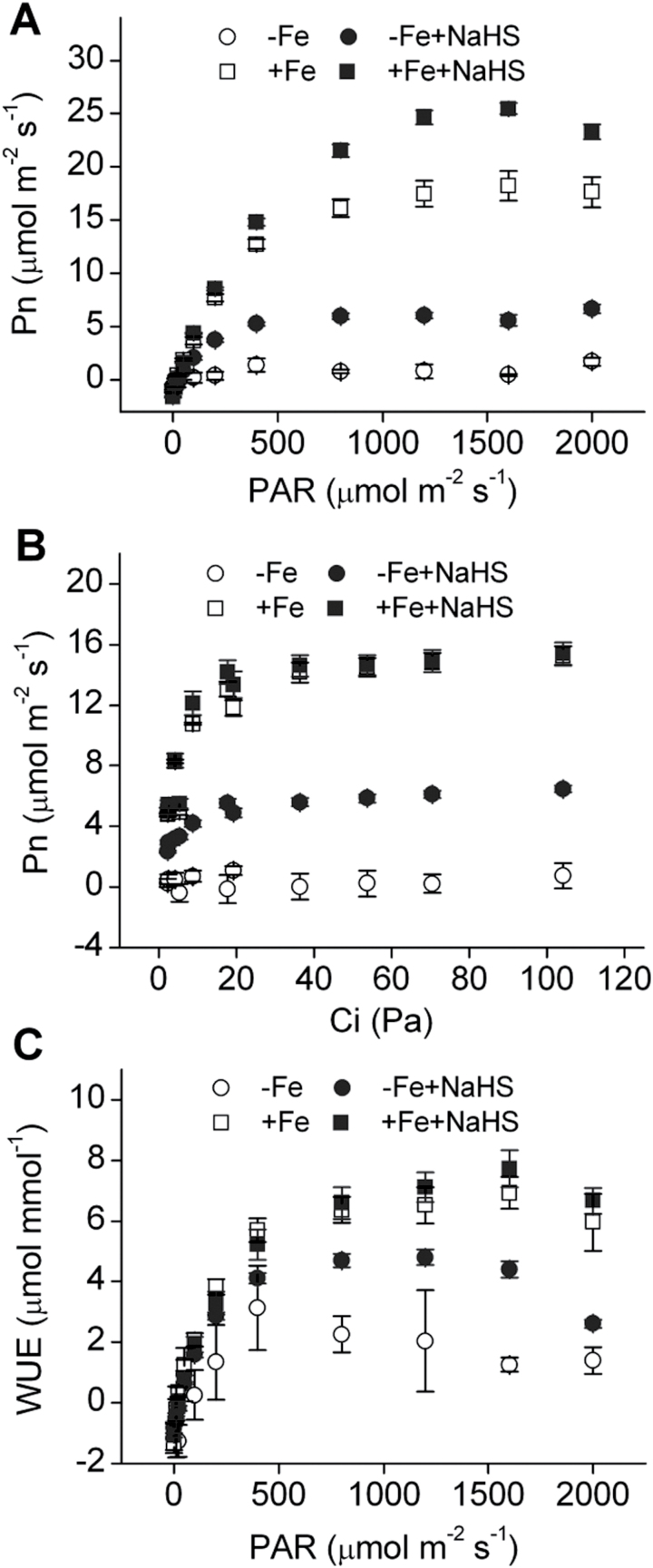
Iron deficiency causes a marked reduction in photosynthesis. Therefore, photosynthesis was further measured in iron-deficient maize plants. Light-response curves and CO2-response curves indicated that Pn was obviously higher in NaHS-treated maize plants than in the controls under –Fe or +Fe conditions (Fig. 9A, B). Similarly, water use efficiency (WUE) was also increased in NaHS-treated maize plants, particularly under the –Fe condition (Fig. 9C). In addition, the Rd, Lcp, Lsp, AQE, and Pmax of maize plants were calculated by modelling the response of leaf Pn to PAR by a non-rectangular hyperbola and the CE was acquired by a rectangular hyperbola (Table 3). Rd, Lsp, AQE, Pmax, and CE were notably higher under NaHS treatment than their controls. Meanwhile, these parameters were obviously higher under +Fe treatment compared with those under –Fe treatment, except for Lcp. Collectively, these results suggested that maize photosynthetic characteristics were significantly affected by NaHS treatment.
Fig. 9.
Light-response curve (A), Intercellular CO2-response curve (B), and WUE-response curve (C) of maize plants grown under iron-deficient conditions. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d. Data are presented as means ± SE. –Fe, 1 µM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe.
Table 3.
Effects of H 2 S donor NaHS and iron on AQE, Rd, Lcp, Lsp, Pmax, and CE of maize seedlings
| Variables | –Fe | –Fe+NaHS | +Fe | +Fe+NaHS |
|---|---|---|---|---|
| AQE | 0.014±0.002a | 0.038±0.004 b | 0.051±0.003 c | 0.050±0.001 c |
| Rd (μmol CO2 m−2 s−1) | 0.440±0.255 a | 0.989±0.052 b | 0.887±0.159 b | 1.040±0.050 b |
| Lcp (μmol m−2 s−1) | 34.80±9.14 a | 26.43±1.87 a | 17.16±2.17 b | 20.73±0.84 c |
| Lsp (μmol m−2 s−1) | 214.5±80.4 a | 226.7±16.2 a | 416.7±46.7 b | 562.3±7.86 c |
| Pmax (μmol CO2 m−2 s−1) | 2.74±0.71 a | 7.48±0.51 b | 20.20±1.78 c | 27.17±0.66 d |
| CE (mol m−2 s−1) | 0.036±0.024 a | 0.730±0.184 b | 2.819±0.055 c | 5.686±1.082 d |
Data are presented as means ±SE.
Different letters indicate significant differences at P<0.05 from different treatments.
–Fe, 1 μM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe.
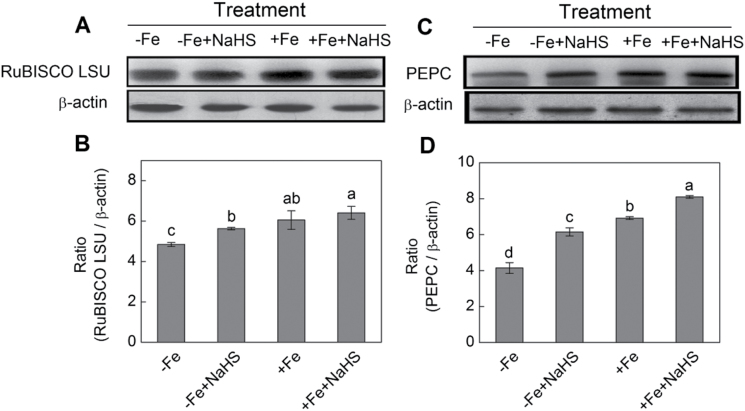
Effect of H2S on the protein expression of RuBISCO LSU and PEPC in iron-deficient maize plants
As shown in Fig. 10A, B, after quantification and normalization to β-actin, the protein expression of RuBISCO LSU showed a slight increase in the leaves of maize grown in the –Fe condition after NaHS treatment. However, there was no significant effect of NaHS on RuBISCO LSU protein expression under high iron supply conditions. Meanwhile, it was also found that the protein expression of PEPC was significantly enhanced by H2S compared with that of their controls (Fig. 10C, D).
Fig. 10.
Western blot analysis of RuBISCO LSU (A) and PEPC (C) of maize plants. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d. Relative expression level is shown as the ratio of RuBISCO LSU:β-actin (B) and PEPC:β-actin (D) using Quantity One software. Data are presented as means ± SE. Columns labelled with different letters indicate significant differences at P<0.05. –Fe, 1 µM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe.
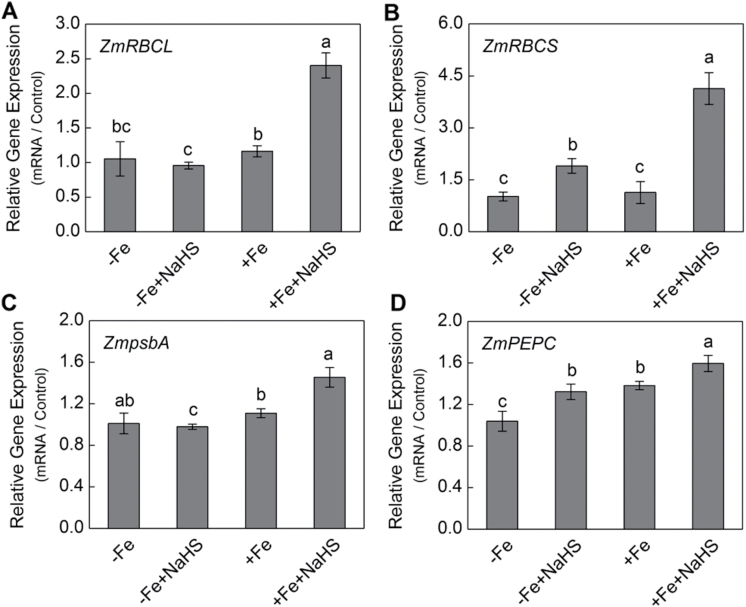
Effect of H2S on ZmRBCL, ZmRBCS, ZmpsbA, and ZmPEPC gene expression in iron-deficient maize plants
Iron deficiency causes a marked reduction in the accumulation of chloroplastic proteins and mRNAs. Two-fold higher gene expression of ZmRBCL was detected in NaHS-treated maize than that of the control under +Fe conditions, although there was no obvious change between NaHS-treated maize and control plants under –Fe conditions (Fig. 11A). Similarly, under NaHS-treatment conditions, the relative gene expression of ZmRBCS in leaves of maize under –Fe and +Fe conditions was two-fold and four-fold higher compared with their controls, respectively (Fig. 11B). In addition, under +Fe conditions, ZmpsbA expression increased by 31% in H2S-treated plants compared with the control (Fig. 11C). Moreover, ZmPEPC expression increased by 28% and 16% in H2S-treated plants both under –Fe and +Fe conditions compared with their controls, respectively (Fig. 11D).
Fig. 11.
Gene expression of ZmRBCL (A), ZmRBCS (B), ZmpsbA (C), and ZmPEPC (D) of maize leaves. Maize seedlings were pre-treated with 100 µM NaHS for 8 d and then grown in a nutrient solution containing 1 µM Fe(III)-EDTA or 50 µM Fe(III)-EDTA for 12 d. The relative mRNA level of each gene was normalized to the mRNA of Zmactin2. Data are presented as means ± SE of three replicates. Columns labelled with different letters indicate significant differences at P<0.05. –Fe, 1 µM Fe; –Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 1 µM Fe; +Fe, 50 µM Fe; +Fe+NaHS, seedlings were pre-treated with 100 µM NaHS and then treated with 50 µM Fe.
Discussion
It is well known that a high concentration of iron is required to maintain the structural and functional integrity of thylakoid membranes in chloroplasts, because more than 80% of leaf iron is located in the chloroplast (Terry and Abadia, 1986; Kim and Guerinot, 2007). Chlorophyll constitutes the major component of chloroplasts and is positively correlated with photosynthesis and leaf iron concentration. In the present study, the effects of H2S on the expression of genes and proteins related to photosynthesis, sulphur metabolism, and iron acquisition were investigated in maize plants. The results showed that exogenous H2S is closely related to iron uptake, transport, and accumulation, and consequently promotes chlorophyll biosynthesis, chloroplast development, and photosynthesis in plants.
Under iron-deficient conditions, H2S increased the chlorophyll content in maize leaves by three-fold over control plants, achieving a similar chlorophyll level to that found in maize plants grown under iron-sufficient conditions. H2S-mediated chlorophyll increase was accompanied by the accumulation of transcripts of genes including ZmpsbA of PSII, ZmPEPC, ZmRBCL, and ZmRBCS. Previous reports showed that levels of these gene transcripts were reduced under iron deficiency and would be recovered after iron supply (Spiller et al., 1987). The results presented here are consistent with observations by other researchers showing that NO and CO improve iron availability in plants (Graziano et al., 2002; Kong et al., 2010).
H2S regulates chlorophyll biosynthesis and chloroplast development in iron-deficient plants
Previous studies have indicated that iron deficiency could cause an interveinal yellowing of the leaves, the disruption of chloroplast ultrastructure, and degradation of chloroplast protein components (Terry, 1980; Winder and Nishio, 1995; Thoiron et al., 1997). Indeed, several steps involved in photosynthetic pigment synthesis and chloroplast ultrastructure establishment are dependent on iron availability (Briat et al., 2007). In the present study, maize seedlings under iron-deficient conditions displayed severe chlorotic characteristics with an interveinal yellowing and a low concentration of chlorophyll (Figs 1–4). Similarly, a typical phenotype of chlorosis in Arabidopsis and Chlamydomonas reinhardtii grown in an iron-deficient solution has been reported, which resulted in the reduction of photosynthetic rate (Briat et al., 2007; Kong et al., 2010). However, exogenously applied H2S donor NaHS rather than other sulphur- or sodium-containing compounds increased the chlorophyll content in iron-deficient maize seedlings (Fig. 1), which is consistent with the observations by other researchers that H2S affects chlorophyll content (Zhang et al., 2009; Chen et al., 2011). Moreover, the ultrastructure of chloroplasts from mesophyll cells was severely altered under iron deficiency conditions. As reported previously, chloroplasts exhibited few photosynthetic lamellae and granain iron-deficient mesophyll cells (Fig. 7A, C) (Stocking, 1975; Thoiron et al., 1997; Muneer et al., 2014). Interestingly, chloroplasts from mesophyll cells of H2S-treated plants developed extensive grana and increased the number of grana lamellae as well (Fig. 7B, D). Similarly, Muneer et al. (2014) reported that Fe-deficient results in a reduction of chloroplast number, and the disintegration of the lamellar network inside the chloroplast, most severely in the absence of sulphur. However, the context of their experiments was slightly different; for example, here, changes in the number of chloroplasts under –Fe and +Fe conditions were not assessed. Moreover, this study focused on the signalling function of exogenously applied NaHS in terms of Fe-deficiency coping strategies. These results showed that H2S, as a signalling molecule, could promote the biogenesis of chloroplasts by increasing the number of grana lamellae and the biosynthesis of chlorophyll, which will lead to an enhancement of photosynthesis in iron-deficient maize plants.
H2S increases iron accumulation by changing of iron homeostasis-related genes and ZmYS1 expression, PSs secretion, and PSs content in iron-deficient plants
Some iron homeostasis-regulated genes play a crucial role in iron uptake and translocation in plants (Hell and Stephan, 2003). Results presented here showed that NaHS improved significantly iron accumulation in plant tissues under iron-deficient conditions (Table 1). These results are in agreement with previous studies that revealed CO could increase iron accumulation in both Arabidopsis root and leaf tissues and Chlamydomonas reinhardtii cells (Kong et al., 2010). Therefore, it was concluded that H2S can confer iron homeostasis through either intracellular iron mobility or accumulation in plants.
The analysis of iron homeostasis-related genes expression would allow a better understanding of iron deficiency-induced chlorosis and the role of H2S in iron acquisition and chloroplast development. In this respect, results showed that NaHS could regulate the expression of ZmMTN, ZmNAS1, ZmNAS3, and ZmDMAS1 in roots of iron-deficient maize seedlings and then increase the methionine and nicotianamine (NA) contents for coping with iron deficiency (Fig. 5A–D). Similarly, the expression of ZmTOM1, ZmTOM2, and ZmTOM3 also were induced by iron deficiency, but exogenously applied NaHS could attenuate the increase of iron deficiency-induced expression (Fig. 5E–G). Besides, previous studies showed that once Fe(III) is reduced to Fe(II) and transported into plants, iron-regulated transporter (IRT) is responsible for iron uptake in roots (Vert et al., 2001; Vert et al., 2002). Research presented here indicated that the abundance of ZmIRT1 was up-regulated by H2S in leaves or roots of maize plants grown under –Fe and +Fe conditions, suggesting that H2S may mediate iron acquisition through the activation of ZmIRT1 gene expression in iron-stressed plants (Fig. 5H). Similarly, some studies have demonstrated that NO and CO can significantly enhance the gene expression of IRT1 in iron-deficient plants (Graziano and Lamattina, 2007; Kong et al., 2010). In plants, IBP (ferritin) is a large polymeric protein that can store up to 4000 atoms of iron within its protein shell and be capable of releasing iron when required (Briat et al., 1999). Ferritins are mainly located in chloroplast and accumulate when iron is sufficiently available (Briat et al., 1999). Here, the expression abundance of ZmIBP (IBP) was also found to be up-regulated by H2S in leaves or roots of maize plants grown under –Fe and +Fe conditions (Fig. 5K). A previous study also indicated that NO promoted accumulation of both ferritin mRNA and protein in Arabidopsis (Murgia et al., 2002). Indeed, results presented here showed that ZmFRO1 gene expression in leaf and root of maize plants grown under the –Fe condition was up-regulated by H2S treatment. However, under the +Fe condition, no obvious regulation effect of H2S was found (Fig. 5L). Graziano and Lamattina (2007) also found that NO could up-regulate the mRNA expression of LeFRO1 in tomato grown under –Fe conditions, but had almost no effect on this gene expression in tomato grown under high iron levels. Likewise, Chen W. et al. (2010) have also reported that both auxin (NAA) and NO could enhance the expression of AtFRO2 in Arabidopsis grown under iron-deficient conditions, but almost no effect under high levels of iron. Moreover, a previous study has reported that there is a positive correlation between FRO1 gene expression and ferric-chelate reductase activity in iron-deficient roots (Connolly et al., 2003). Interestingly, under –Fe conditions, the expression of ZmFRO1 and ZmIRT1 in maize leaves and roots are increased by addition of NaHS. These results are consistent with Zuchi et al. (2009), who reported that Fe deficiency and S-sufficiency caused increases in the expression levels of LeIRT1 and LeFRO1 in tomato seedlings. However, it was noteworthy that the expression of ZmFRO1, ZmIBP, and ZmIRT1 in –Fe plants is very similar to that of +Fe plants, especially in leaves, although these genes are generally induced by Fe deficiency. Moreover, Paolacci et al. (2014) reported that the expression of SlIRT1 and SlFRO1 was strongly up-regulated by iron deprivation in roots of tomato seedlings. However, in the present study, the expression of ZmIRT1 was down-regulated by iron deprivation in leaves and roots of maize seedlings.These results are contradictory with previous studies. The following explanations are presented: Firstly, the expression of FRO1, IBP, and IRT1 mainly functioned in dicotyledons and their expression levels were up-regulated under –Fe conditions (Meiser et al., 2011; Nishida et al., 2011). However, there was little focus on these gene expressions in gramineous plants, such as maize. Therefore, it can be speculated that the response of FRO1, IBP, and IRT1 gene expression is different between dicotyledons and gramineous plants. Secondly, previous studies showed that 50 μM Fe-EDTA represents iron-insufficient conditions for the growth of maize seedlings (Stocking, 1975; Graziano et al., 2002). Thirdly, different plants respond differently to the effects of iron concentration, which has a close relationship with plant growth environment and the time of iron treatment. Fourthly, Gonzalo et al., (2011) showed that the gene expression of FRO2 and IRT1 was up-regulated after 5 d of iron-shortage, but their expression level was down-regulated after 6 d of iron-shortage compared with iron-sufficient conditions in Prunus plants. These results concur with those presented here relating to gene expression of ZmFRO1, ZmIBP, and ZmIRT1 in iron-deficient maize plants, which have a close relationship with the plant growth environment, time of iron treatment, and the concentration of iron treatment. Finally, importantly, Graziano and Lamattina (2007) reported that in leaves, LeFRO1 expression does not depend on the iron status, there being no changes between iron-deficient and iron-sufficient conditions. This result confirmed that the regulation of LeFRO1 in leaves is different from that in roots.
Iron acquisition in gramineous plants is characterized by secretion of chelating compounds such as PSs; PSs and Fe(III) then form a complex [Fe(III)-PS], which is subsequently transported by a specific Fe(III)-PS transporter in plant roots (Astolfi et al., 2012). Results presented here showed that NaHS could enhance iron assimilation through increasing PSs content and PSs secretion in roots of maize under –Fe and +Fe conditions (Fig. 6C, D). Moreover, PSs biosynthesis requires methionine, therefore the effect of NaHS on Fe acquisition could be presumably due to an enhanced assimilation of sulphur and its subsequent incorporation into methionine in order to sustain an increased production of PSs and NA. The most well characterized Fe-PS transporter is the maize oligo peptide transporter (OPT) family membrane, ZmYS1. ZmYS1 is expressed in roots in response to iron deficiency and re-supplying iron decreases the expression of ZmYS1 (Morrissey and Guerinot, 2009). In the present study, although NaHS increased PSs content and PSs secretion in roots of maize plants under –Fe conditions (Fig. 6C, D), the expression level of ZmYS1 was reduced by NaHS treatment in roots of maize plants (Fig. 6B). Besides, a baseline for ZmYS1 gene expression in iron-deficient maize seedling roots after the NaHS treatment and before Fe treatment was established. Results showed that ZmYS1 gene expression was significantly decreased after exogenously applied NaHS compared with the controls under iron-deficient conditions. Interestingly, when 1 μM Fe was added to the solution, ZmYS1 gene expression also was decreased (Supplementary Fig. S2, available at JXB online). Similarly, Astolfi et al. (2010) reported that HvYS1 transcript abundance was progressively decreased when the S- and Fe-deficient plants were supplied with both nutrients. One possible explanation for this disagreement is that the decreased expression level of ZmYS1 prompted NaHS to alleviate the symptoms of iron deficiency and negating the need for an increase in the expression of this transporter. On the other hand, the enhancement of PSs secretion and PSs accumulation in root by NaHS treatment could increase the mobility of Fe(III) by the form of PS-Fe(III) in plant root and rhizosphere. Taken together, these results suggested that H2S is a necessary signal molecule for the expression of iron homeostasis-related genes and PSs secretion and accumulation in maize plants under –Fe and +Fe conditions.
H2S could regulate sulphur-containing metabolites and sulphur metabolism-related genes expression to cope with iron deficiency in maize seedlings
Sulphate assimilation is an energy demanding process and supplying H2S would directly feed into cysteine and GSH synthesis. Previous studies have shown that H2S exposure generally results in an increased content of water-soluble NPT compounds including GSH and cysteine in shoot, and in some species, an increase in the sulphate content of the shoot has been observed (Schutz et al., 1991; Durenkamp and De Kok, 2004; Riemenschneider et al., 2005b ; Durenkamp et al., 2007). In this study, a high accumulation of endogenous H2S in maize seedling leaves and roots caused by exogenously applied NaHS was observed under –Fe or +Fe conditions (Table 2). Meanwhile, NaHS treatment resulted in different degrees of increases in GSH and NPTs contents in roots and leaves under –Fe or +Fe conditions. These results indicated that exogenously applied NaHS was not only directly fed into cysteine and GSH synthesis by regulating sulphur metabolism-related enzyme activities and gene expression, but also increased the content of endogenous H2S in plants. Interestingly, a previous paper also showed that externally applied NaHS led to increases in the internal concentration of H2S, the contents of GSH, NPTs and cysteine in S. oleracea leaves by affecting the enzyme activities of OAS-TL and l-cysteine desulphydrase (LCD) (Chen et al., 2011). The pathway of sulphur assimilation in plants is divided into two reaction sequences: sulphate reduction and cysteine synthesis. Sulphate reduction is preceded by the uptake of sulphate from the soil into plant by specific sulphate transporters (Buchner et al., 2004). Within the plant, sulphate is firstly activated by ATP sulphurylase (ATPS), and the resulting adenosine-5′-phosphosulphate (APS) is reduced in a two-step reaction via sulphite to sulphide by APS reductase (APR) and sulphide reductase (SiR). Sulphide is then incorporated into OAS by OAS-(thiol)lyase (OAS-TL) to form cysteine, which serves as a sulphur source for all organic molecules containing reduced sulphur, e.g. GSH, protein, cofactors, and vitamins (Takahashi et al., 2011). Therefore, expression of the sulphur metabolism-related genes was analysed in iron-deficient maize seedling leaves and roots. Results showed that under –Fe or +Fe conditions, exogenously applied NaHS could affect the expression levels of sulphate transporter 1 (ST1) (ZmST1) gene and sulphate reduction-related genes including ZmATPS and ZmAPR in maize seedlings (Fig. 8A–C). Moreover, the amounts of the transcript of ZmOASTL1 and ZmOASTL2 encoding O-acetyl-L-serine(thiol)lyase were significantly increased under –Fe+NaHS conditions compared with –Fe conditions in maize seedling roots (Fig. 8D, E). The expression of ZmOASTL2 gene in –Fe or +Fe maize seedling leaves was up-regulated by exogenously applied NaHS (Fig. 8E). These results were consistent with previous studies. For instance, Chen et al. (2011) reported that exogenously applied NaHS could up-regulate the expression of OASTL gene in S. oleracea seedling leaves. Birke et al. (2015) reported that H2S exposure down-regulated the expression levels of ATPS1, APR2, and DES1, but up-regulated the expression levels of S-sulphocysteine synthase (CS26) in tomato seedling leaves. Therefore, it was concluded that H2S as a signalling molecule could cope with iron deficiency through increasing sulphur-containing metabolites including GSH and NPTs and regulating the expression level of sulphur metabolism-related genes in maize seedlings. Interestingly, Astolfi et al. (2004, 2006b , 2010, 2012) reported that improved sulphur nutrition status enables plants to better cope with Fe deficiency. However, unlike previous studies, this study mainly focused on the signalling function of H2S in improving adaptation of maize seedlings to iron deficiency.
H2S-improved photosynthesis (by increasing the protein and gene expression of photosynthetic enzyme) is dependent on iron availability
Iron deficiency can cause a decrease of chlorophyll (chlorosis) and then lead to the reduction of leaf photosynthetic rate (Larbi et al., 2006). Moreover, other studies showed that the efficiencies of light absorption, PSII and RuBISCO carboxylation were significantly reduced under iron-deficient conditions (Stocking, 1975; Larbi et al., 2006). Besides, chlorotic leaves caused by iron deficiency exhibited lower stomatal apertures and lower photosynthetic rates, finally resulting in the reduction of WUE (Larbi et al., 2006). In the present study, light-response curves and CO2-response curves indicated that photosynthetic rate was significantly enhanced by H2S under –Fe and +Fe conditions, suggesting that H2S promotes photosynthesis through increasing iron acquisition in iron-stressed plants (Fig. 9A, B). Besides, WUE was also obviously enhanced by H2S (Fig. 9C). Meanwhile, further analysis of photosynthetic features revealed that Pmax was also significantly higher in NaHS-treated seedlings grown under –Fe and +Fe solution than that in their controls (Table 3). This result is important for clarifying the effect of H2S on photosynthesis by enhancing iron acquisition, because a higher Pmax allows seedlings to acquire a higher potential to assimilate carbon dioxide. AQE reflects the light use efficiency of plants at low light intensities. This data showed that AQE had an obvious increase in NaHS-treated maize plants grown in –Fe solution compared with the control. These results indicated that NaHS could enhance the light use efficiency of maize plants under –Fe conditions at a lower light intensity. Lsp represents the capacity of plants to use the maximal light. The results showed that Lsp was higher in NaHS-treated maize plants grown in +Fe solution than that in the control, but there was no obvious change in the –Fe solution, indicating that NaHS can increase the maximal light use efficiency of maize plants grown in +Fe solution under a high light intensity. Importantly, CE has been considered to play a major role in the improvement of photosynthesis (Pell et al., 1992; Farage and Long, 1999). Data presented here showed that CE was significantly increased by H2S in maize plants grown in –Fe and +Fe solutions, suggesting that H2S could induce the enhancement in carboxylation process through increasing iron acquisition in iron-deficient plants (Table 3).
RuBISCO and PEPC are key enzymes controlling photosynthetic carbon fixation in plants, and the level of activated RuBISCO and PEPC are closely related to the Lsp, CE, and the rate of photosynthetic carbon assimilation (Lin and Hsu, 2004). The present results showed that the protein expression of RuBISCO LSU and PEPC were significantly increased by H2S in maize plants grown under –Fe and +Fe conditions compared with their controls, suggesting that the improvement in photosynthesis with NaHS treatment might be a result of an increase in RuBISCO and PEPC levels (Fig. 10). Besides, results presented here showed that H2S-mediated chlorophyll increase and the enhancement of photosynthetic rate were accompanied by the accumulation of transcripts encoding the D1 protein of PSII (ZmpsbA), RuBISCO large subunit (ZmRBCL) and small subunit (ZmRBCS), and PEPC (Fig. 11). Previous studies showed that the levels of these transcripts were reduced during iron deficiency and recovered after iron supply (Spiller et al., 1987). Similarly, Graziano et al. (2002) also reported that NO-mediated chlorophyll increase was accompanied by the accumulation of transcripts encoding both the D1 protein of PSII and the RuBISCO large subunit. Taken together, all the evidence presented here indicates that the positive effect of H2S on leaf photosynthesis and chlorophyll biosynthesis occurs mainly through stimulating the transcription of ZmRBCL, ZmRBCS, ZmPEPC, and ZmpsbA and enhancing the accumulation of RuBISCO and PEPC protein in maize grown under iron-deficient conditions.
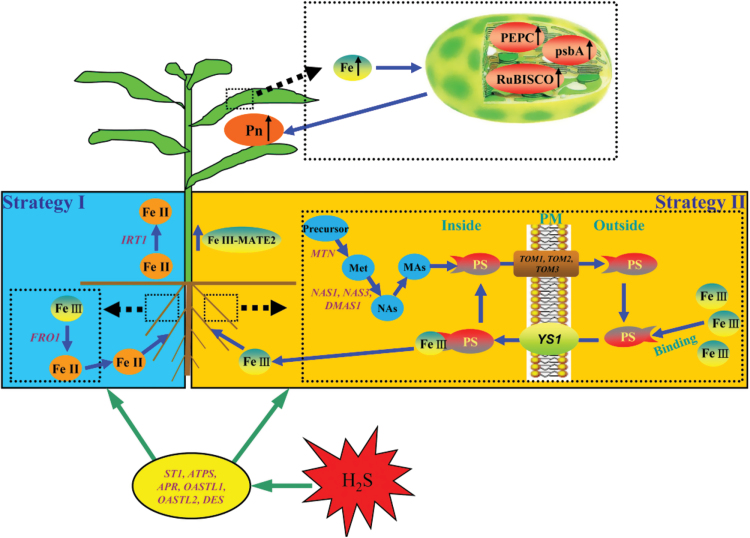
Pathway of H2S-improved adaptation of maize seedlings to iron deficiency
Based on results shown here and current knowledge about the mechanisms of plant response to iron deficiency, a signalling pathway by which H2S improves adaptation of maize seedlings to iron deficiency has been proposed. As shown in Fig. 12, H2S could regulate the expression of sulphur metabolism-related genes and further promote iron mobility and uptake through mainly the mechanism of strategy II—including increasing the secretion of PSs and PSs content in the roots of maize seedlings and changing the mRNA expression abundance of ZmYS1 and iron homeostasis-related genes. Besides, it was hypothesized that iron uptake and availability have some relation with the mechanism of strategy I in maize seedlings. For instance, under iron-deficient conditions, H2S could regulate the mRNA expression abundance of ZmFRO1 and ZmIRT1 in roots and leaves of maize seedlings. Taken together, these two strategies can increase iron uptake and availability, which will further promote chlorophyll content, chloroplast development, photosynthesis-related gene expression, and photosynthesis in maize seedlings.
Fig. 12.
A schematic model for H2S-improved adaptation of maize seedlings to iron deficiency. ATPS, ATP sulphurylase; APR, APS reductase; DES, Cys desulfhydrase; DMAS1, deoxymugineic acid synthase 1; FRO1, ferric-chelate reductase 1; MATE2, multi-drug and toxin efflux 2; MTN, S-adenosyl homocysteine nucleosidase; NAS1/3, nicotianamine synthase 1/3; NAs, nicotianamine; OASTL1/2, O-acetyl-L-serine(thiol)lyase 1/2; psbA, D1 protein; ST1, sulphate transporter 1; TOM1/2/3, transporter of MAs 1/2/3; YS1, yellow stripe 1. A colour version of this figure is available at JXB online.
In summary, results of this study further show that H2S is closely related to iron uptake, transport, and availability, consequently increasing chlorophyll biosynthesis, chloroplast development, and photosynthesis. Further studies are needed to understand and unveil the possible multiple pathways of H2S-regulated iron availability in plants. This report opens a new window for the study for H2S in plant nutrient availability, and adds fresh knowledge for the improvement of crop growth and production.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Sequences of forward and reverse primers used in qRT-PCR for gene expression analysis in maize seedlings.
Table S2. Procedures of dsDNA synthesis used in qRT-PCR for gene expression analysis in leaves of maize plants.
Table S3. TEM analysis of the effect of NaHS on the numbers of grana stacks and grana lamellae in mesophyll cell chloroplast ultrastructure of maize leaves.
Fig. S1. Effect of NaHS on biomass of iron-deficient maize plants during the period of growth.
Fig. S2. Time-dependent gene expression of ZmYS1 in iron-deficient maize roots after NaHS treatment.
Acknowledgments
We are grateful to Bing-Bo Li and Li-Na Yin for assistance in experiments. This study was financially supported by the Natural Science Foundation of China (NSFCNo 30930076, 31260057, 30770192, 30670317), the Foundation of the Chinese Ministry of Education(20070384033, 209084), the Program for New Century Excellent Talents in Xiamen University(NCETXMU No X07115), Zhejiang Provincial Natural Science Foundation (LY13C160014), the foundation of doctoral scientific research in Northwest A&F University (2013BSJJ090), and West Light PhD Project Foundation of the Chinese Academy of Sciences, Chinese Universities Scientific Fund.
References
- Astolfi S, Zuchi S, Cesco S, Varanini Z, Pinton R. 2004. Influence of iron nutrition on sulphur uptake and metabolism in maize (Zea mays L.) roots. Soil Science and Plant Nutrition 50, 1079–1083. [Google Scholar]
- Astolfi S, Zuchi S, Cesco S, Sanità di Toppi L, Pirazzi D, Badiani M, Varanini Z, Pinton R. 2006. a . Iron deficiency induces sulfate uptake and modulates redistribution of reduced sulfur pool in barley plants. Functional Plant Biology 33, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Astolfi S, Cesco S, Zuchi S, Neumann G, Roemheld V. 2006. b .Sulfur starvation reduces phytosiderophores release by iron-deficient barley plants. Soil Science and Plant Nutrition 52, 43–48. [Google Scholar]
- Astolfi S, Zuchi S, Hubberten HM, Pinton R, Hoefgen R. 2010. Supply of sulphur to S-deficient young barley seedlings restores their capability to cope with iron shortage. Journal of Experimental Botany 61, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi S, Zuchi S, Neumann G, Cesco S, di Toppi LS, Pinton R. 2012. Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation. Journal of Experimental Botany 63, 1241–1250. [DOI] [PubMed] [Google Scholar]
- Birke H, De Kok LJ, Wirtz M, Hell R. 2015. The role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis. Plant and Cell Physiology 56, 358–367. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Briat JF, Lobréaux S, Grignon N, Vansuyt G. 1999. Regulation of plant ferritin synthesis: how and why. Cellular and Molecular Life Sciences 56, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briat JF, Curie C, Gaymard F. 2007. Iron utilization and metabolism in plants. Current Opinion in Plant Biology 10, 276–282. [DOI] [PubMed] [Google Scholar]
- Buchner P, Stuiver CEE, Westerman S, Wirtz M, Hell R, Hawkesford MJ, De Kok LJ. 2004. Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleraceaas affected by atmospheric H2S and pedospheric sulfate nutrition. Plant Physiology 136, 3396–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-S, Li Y-H, Chen L-T, et al. 2008. LZF1, a hy5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. The Plant Journal 54, 205–219. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu F-H, Wang W-H, Zheng C-J, Lin G-H, Dong X-J, He J-X, Pei Z-M, Zheng H-L. 2011. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. Journal of Experimental Botany 62, 4481–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang W-H, Wu F-H, You C-Y, Liu T-W, Dong X-J, He J-X, Zheng H-L. 2013. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant and Soil 362, 301–318. [Google Scholar]
- Chen L, Wu F-H, Liu T-W, Chen J, Li Z-J, Pei Z-M, Zheng H-L. 2010. Soil acidity reconstruction based on tree ring information of a dominant species Abies fabri in the subalpine forest ecosystems in southwest china. Environmental Pollution 158, 3219–3224. [DOI] [PubMed] [Google Scholar]
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ. 2010. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis . Plant Physiology 154, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Fett JP, Guerinot ML. 2002. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. The Plant Cell 14, 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML. 2003. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiology 133, 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat J-F, Walker EL. 2001. Maize yellow stripe1 encodes a membrane protein directly involved in Fe (III) uptake. Nature 409, 346–349. [DOI] [PubMed] [Google Scholar]
- Curie C, Briat JF. 2003. Iron transport and signaling in plants. Annual Review of Plant Biology 54, 183–206. [DOI] [PubMed] [Google Scholar]
- De Kok LJ, Stuiver CEE, Stulen I. 1998. Impact of atmospheric H2S on plants. In: De Kok LJ, Stulen I, eds. Respondes of plant metabolism to air pollution and global change . Leiden: Backhuys Publishers, 51–63. [Google Scholar]
- De Kok LJ, Castro A, Durenkamp M, Stuiver CEE, Westerman S, Yang L, Stulen I. 2002. Sulphur in plants physiology. Proceedlings NO. 500 . York: International Fertiliser Society, 1–26. [Google Scholar]
- Del Longo OT, Gonzalez CA, Pastori GM, Trippi VS. 1993. Antioxidant defences under hyperoxygenic and hyperosmotic conditions in leaves of two lines of maize with differential sensitivity to drought. Plant and Cell Physiology 34, 1023–1028. [Google Scholar]
- Durenkamp M, De Kok LJ. 2004. Impact of pedospheric and atmospheric sulphur nutrition on sulphur metabolism of Allium cepa L., a species with a potential sink capacity for secondary sulphur compounds. Journal of Experimental Botany 55, 1821–1830. [DOI] [PubMed] [Google Scholar]
- Durenkamp M, De Kok LJ, Kopriva S. 2007. Adenosine 5′-phosphosulphate reductase is regulated differently in Allium cepa L. and Brassica oleracea L. upon exposure to H2S. Journal of Experimental Botany 58, 1571–1579. [DOI] [PubMed] [Google Scholar]
- Farage PK, Long SP. 1999. The effects of O3 fumigation during leaf development on photosynthesis of wheat and pea: An in vivo analysis. Photosynthesis Research 59, 1–7. [Google Scholar]
- García-Mata C, Lamattina L. 2010. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytologist 188, 977–984. [DOI] [PubMed] [Google Scholar]
- Gonzalo MJ, Moreno MA, Gogorcena Y. 2011. Physiological responses and differential gene expression in Prunus rootstocks under iron deficiency conditions. Journal of Plant Physiology 168, 887–893. [DOI] [PubMed] [Google Scholar]
- Graziano M, Beligni MV, Lamattina L. 2002. Nitric oxide improves internal iron availability in plants. Plant Physiology 130, 1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. 2007. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. The Plant Journal 52, 949–960. [DOI] [PubMed] [Google Scholar]
- Hell R, Stephan UW. 2003. Iron uptake, trafficking and homeostasis in plants. Planta 216, 541–551. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S. 1999. Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiology 119, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. 1997. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and Biophysical Research Communications 237, 527–531. [DOI] [PubMed] [Google Scholar]
- Hughes MN, Centelles MN, Moore KP. 2009. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radical Biology and Medicine 47, 1346–1353. [DOI] [PubMed] [Google Scholar]
- Kim SA, Guerinot ML. 2007. Mining iron: Iron uptake and transport in plants. FEBS Letters 581, 2273–2280. [DOI] [PubMed] [Google Scholar]
- Kong WW, Zhang LP, Guo K, Liu ZP, Yang ZM. 2010. Carbon monoxide improves adaptation of Arabidopsis to iron deficiency. Plant Biotechnology Journal 8, 88–99. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Larbi A, Abadía A, Abadía J, Morales F. 2006. Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynthesis Research 89, 113–126. [DOI] [PubMed] [Google Scholar]
- Li D, Xiao Z, Liu L, Wang J, Song G, Bi Y. 2010. Effects of exogenous hydrogen sulfide (H2S) on the root tip and root border cells of Pisum sativum . Chinese Bulletin of Botany 45, 354–362. [Google Scholar]
- Li L, Cheng X, Ling H-Q. 2004. Isolation and characterization of Fe(III)-chelate reductase gene LeFRO1 in tomato. Plant Molecular Biology 54, 125–136. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymology 148, 350–382. [Google Scholar]
- Lin M-J, Hsu B-D. 2004. Photosynthetic plasticity of Phalaenopsis in response to different light environments. Journal of Plant Physiology 161, 1259–1268. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔct method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Ma JF. 2005. Plant root responses to three abundant soil minerals: Silicon, aluminum and iron. Critical Reviews in Plant Sciences 24, 267–281. [Google Scholar]
- Meiser J, Lingam S, Bauer P. 2011. Posttranslational regulation of the iron deficiency basic Helix-Loop-Helix transcription factor FIT is affected by iron andnitric oxide. Plant Physiology 157, 2154–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. 1999. Iron acquisition by plants. Current Opinion in Plant Biology 2, 250–253. [DOI] [PubMed] [Google Scholar]
- Morrissey J, Guerinot ML. 2009. Iron uptake and transport in plants: The good, the bad, and the ionome. Chemical Reviews 109, 4553–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneer S, Lee BR, Kim KY, Park SH, Zhang Q, Kim TH. 2014. Involvement of sulphur nutrition inmodulating iron deficiency responses in photosynthetic organelles of oilseed rape (Brassica napus L.). Photosynthesis Research 119, 319–329. [DOI] [PubMed] [Google Scholar]
- Murgia I, Delledonne M, Soave C. 2002. Nitric oxide mediates iron-induced ferritin accumulation in Arabidopsis. The Plant Journal 30, 521–528. [DOI] [PubMed] [Google Scholar]
- Nishida S, Tsuzuki C, Kato A, Aisu A, Yoshida J, Mizuno T. 2011. AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana . Plant and Cell Physiology 52, 1433–1442. [DOI] [PubMed] [Google Scholar]
- Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. 2011. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. Journal of Biological Chemistry 286, 5446–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Leverenz JW. 1994. Non-uniform stomatal closure and the apparent convexity of the photosynthetic photon flux density response curve. Plant, Cell & Environment 17, 701–710. [Google Scholar]
- Paolacci AR, Celletti S, Catarcione G, Hawkesford MJ, Astolfi S, Ciaffi M. 2014. Iron deprivation results in a rapid but not sustained increase of the expression of genes involved in iron metabolism and sulfate uptake in tomato (Solanum lycopersicum L.) seedlings. Journal of Integrative Plant Biology 56, 88–100. [DOI] [PubMed] [Google Scholar]
- Pell EJ, Eckardt N, Enyedi AJ. 1992. Timing of ozone stress and resulting status of ribulose bisphosphate carboxylase/oxygenase and associated net photosynthesis. New Phytologist 120, 397–405. [Google Scholar]
- Prioul JL, Chartier P. 1977. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: A critical analysis of the methods used. Annals of Botany 41, 789–800. [Google Scholar]
- Ramirez L, Simontacchi M, Murgia I, Zabaleta E, Lamattina L. 2011. Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: A well equipped team to preserve plant iron homeostasis. Plant Science 181, 582–592. [DOI] [PubMed] [Google Scholar]
- Reichman SM, Parker DR. 2007. Critical evaluation of three indirect assays for quantifying phytosiderophores released by the roots of Poaceae . European Journal of Soil Science 58, 844–853. [Google Scholar]
- Riemenschneider A, Riedel K, Hoefgen R, Papenbrock J, Hesse H. 2005. a . Impact of reduced O-Acetylserine(thiol)lyase isoform contents on potato plant metabolism. Plant Physiology 137, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemenschneider A, Nikiforova V, Hoefgen R, De Kok LJ, Papenbrock J. 2005. b . Impact of elevated H2S on metabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiology and Biochemistry 43, 473–483. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. 1999. A ferric-chelate reductase for iron uptake from soils. Nature 397, 694–697. [DOI] [PubMed] [Google Scholar]
- Schmidt W. 1999. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytologist 141, 1–26. [Google Scholar]
- Schutz B, De Kok LJ, Rennenberg H. 1991. Thiol accumulationand cysteine desulfhydrase activity in H2S-fumigated leaves and leafhomogenates of cucurbit plants. Plant and Cell Physiology 32, 733–736. [Google Scholar]
- Sekiya J, Schmidt A, Wilson LG, Filner P. 1982. Emission of hydrogen sulfide by leaf tissue in response toL-cysteine. Plant Physiology 70, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller SC, Kaufman LS, Thompson WF, Briggs WR. 1987. Specific mRNA and rRNA levels in greening pea leaves during recovery from iron stress. Plant Physiology 84, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking CR. 1975. Iron deficiency and the structure and physiology of maize chloroplasts. Plant Physiology 55, 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler-Dinglasan PM, Guz N, Attardo G, Aksoy S. 2006. Molecular characterization of iron binding proteins from Glossina morsitans morsitans (diptera: Glossinidae). Insect Biochemistry and Molecular Biology 36, 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Kopriva S, Giordano M, Saito K, Hell R. 2011. Sulfurassimilation in photosynthetic organisms: molecular functions andregulations of transporters and assimilatory enzymes. Annual of Review Plant Biology 62, 157–184. [DOI] [PubMed] [Google Scholar]
- Terry N. 1980. Limiting factors in photosynthesis. I. Use of iron stress to control photochemical capacity in vivo . Plant Physiology 65, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N, Abadia J. 1986. Function of iron in chloroplasts. Journal of Plant Nutrition 9, 609–646. [Google Scholar]
- Thoiron S, Pascal N, Briat JF. 1997. Impact of iron deficiency and iron re-supply during the early stages of vegetative development in maize (Zea mays L.). Plant, Cell & Environment 20, 1051–1060. [Google Scholar]
- Ueno D, Yamaji N, Ma JF. 2009. Further characterization of ferric-phytosiderophore transporters ZmYS1 and HvYS1 in maize and barley. Journal of Experimental Botany 60, 3513–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Briat J-F, Curie C. 2001. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. The Plant Journal 26, 181–189. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat J-F, Curie C. 2002. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. The Plant Cell 14, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EL, Connolly EL. 2008. Time to pump iron: Iron-deficiency-signaling mechanisms of higher plants. Current Opinion in Plant Biology 11, 530–535. [DOI] [PubMed] [Google Scholar]
- Wang B-L, Shi L, Li Y-X, Zhang W-H. 2010. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativusL.) seedlings. Planta 231, 1301–1309. [DOI] [PubMed] [Google Scholar]
- Wang R. 2002. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? The FASEB Journal 16, 1792–1798. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li L, Cui W, Xu S, Shen W, Wang R. 2012. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant and Soil 351, 107–119. [Google Scholar]
- Winder TL, Nishio JN. 1995. Early iron deficiency stress response in leaves of sugar beet. Plant Physiology 108, 1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Li L, Du J, Yuan Y, Cheng X, Ling H-Q. 2005. Molecular and biochemical characterization of the Fe(III) chelate reductase gene family in Arabidopsis thaliana . Plant and Cell Physiology 46, 1505–1514. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. 2008. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science 322, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hu L-Y, Hu K-D, He Y-D, Wang S-H, Luo J-P. 2008. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. Journal of Integrative Plant Biology 50, 1518–1529. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ye Y-K, Wang S-H, Luo J-P, Tang J, Ma D-F. 2009. Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regulation 58, 243–250. [Google Scholar]
- Zhang H, Tan Z-Q, Hu L-Y, Wang S-H, Luo J-P, Jones RL. 2010. Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. Journal of Integrative Plant Biology 52, 556–567. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu S-L, Zhang Z-J, Hu L-Y, Jiang C-X, Wei Z-J, Liu J, Wang H-L, Jiang S-T. 2011. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biology and Technology 60, 251–257. [Google Scholar]
- Zuchi S, Cesco S, Varanini Z, Pinton R, Astolfi S. 2009. Sulphur deprivation limits Fe-deficiency responses in tomato plants. Planta 230, 85–94. [DOI] [PubMed] [Google Scholar]
- Zuchi S, Cesco S, Astolfi S. 2012. High S supply improves Fe accumulation in durum wheat plants grown under Fe limitation. Environmental and Experimental Botany 77, 25–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.