Highlight
The Xanthomonas conserved cell–cell quorum-sensing molecule elicits innate immunity in plants. The defence response provoked by DSF is suppressed by the exopolysaccharide xanthan, a quorum-sensing-regulated virulence factor.
Key words: Defence suppressor, elicitor, extracellular polysaccharide, innate immunity, Nicotiana benthamiana, quorum sensing, rice, virulence, Xanthomonas campestris pv. campestris, Xanthomonas oryzae pv. oryzae.
Abstract
Several secreted and surface-associated conserved microbial molecules are recognized by the host to mount the defence response. One such evolutionarily well-conserved bacterial process is the production of cell–cell signalling molecules which regulate production of multiple virulence functions by a process known as quorum sensing. Here it is shown that a bacterial fatty acid cell–cell signalling molecule, DSF (diffusible signal factor), elicits innate immunity in plants. The DSF family of signalling molecules are highly conserved among many phytopathogenic bacteria belonging to the genus Xanthomonas as well as in opportunistic animal pathogens. Using Arabidopsis, Nicotiana benthamiana, and rice as model systems, it is shown that DSF induces a hypersensitivity reaction (HR)-like response, programmed cell death, the accumulation of autofluorescent compounds, hydrogen peroxide production, and the expression of the PATHOGENESIS-RELATED1 (PR-1) gene. Furthermore, production of the DSF signalling molecule in Pseudomonas syringae, a non-DSF-producing plant pathogen, induces the innate immune response in the N. benthamiana host plant and also affects pathogen growth. By pre- and co-inoculation of DSF, it was demonstrated that the DSF-induced plant defence reduces disease severity and pathogen growth in the host plant. In this study, it was further demonstrated that wild-type Xanthomonas campestris suppresses the DSF-induced innate immunity by secreting xanthan, the main component of extracellular polysaccharide. The results indicate that plants have evolved to recognize a widely conserved bacterial communication system and may have played a role in the co-evolution of host recognition of the pathogen and the communication machinery.
Introduction
Plants have evolved the ability to recognize the highly conserved molecular signature characteristic of several secreted and surface-associated microbial molecules and to mount a defence response. These conserved microbial features, known as pathogen-associated molecular patterns (PAMPs) or microbe-associated molecular patterns (MAMPs), act as elicitors; examples are flagellin (Felix et al., 1999), lipopolysaccharide (Newman et al., 2002; Silipo et al., 2005), cold shock protein (Felix and Boller, 2003), and elongation factor Tu (Kunze et al., 2004). The recognition of these elicitors occurs primarily through receptors at the plant cell surface. Recognition of microbial elicitors leads to the innate or basal defence response which restricts pathogen growth in the immediate vicinity of the infected area. However, pathogenic microbes have evolved to suppress or evade the host innate immune response, which is considered to be a pre-condition for their ability to cause disease (for detailed reviews, see Nurnberger et al., 2004; Chisholm et al., 2006; Boller and Felix, 2009). Apart from MAMPs, plants have also developed the ability to recognize endogenous molecules released upon damage caused due to microbial attack, known as damage-associated molecular patterns (DAMPs) (Darvill and Albersheim, 1984; Jha et al., 2007; Aparna et al., 2009).
Cell–cell signalling (quorum sensing; QS) is a widespread phenomenon by which bacteria co-ordinate multiple social behaviours via the production and perception of diverse cell–cell communication molecules (Fuqua et al., 2001; Ng and Bassler, 2009). An increasing body of research now suggests that QS plays a central role in pathogenesis of several bacterial pathogens as it synchronizes production and secretion of several virulence factors such as extracellular polysaccharide (EPS), cell-wall-hydrolysing enzymes, and adhesins, which are beneficial to the pathogen at high cell density (Ng and Bassler, 2009). In general, many Gram-negative bacteria mediate QS via the production of acyl homoserine lactone (AHL), typical of the most well characterized process of QS in Gram-negative bacteria (Fuqua et al., 1994, 2001). In contrast, QS in several Gram-negative bacteria belonging to the genera Xanthomonas and Burkholderia is mediated by the synthesis and perception of a fatty acid signalling molecule (cis-11-methyl-2-dodecenoic acid; Fig. 1A) called DSF (diffusible signal factor) (Deng et al., 2011; Ryan and Dow, 2011). It has been shown that in Xanthomonas and other closely related bacteria, such as Xylella fastidiosa (a pathogen of grapes), synthesis of a DSF family of signalling molecules requires rpfF (regulation of pathogenecity factor F), which encodes DSF synthase (RpfF), a bifunctional crotonase having both dehydratase and thioesterase activities (Wang et al., 2004; He et al., 2010; Bi et al., 2012; Beaulieu et al., 2013). RpfF is involved in the synthesis of the DSF family of signalling molecules which are typically cis-2-unsaturated fatty acids with a chain length varying from 12 to 14 carbons (Deng et al., 2011).
Fig. 1.
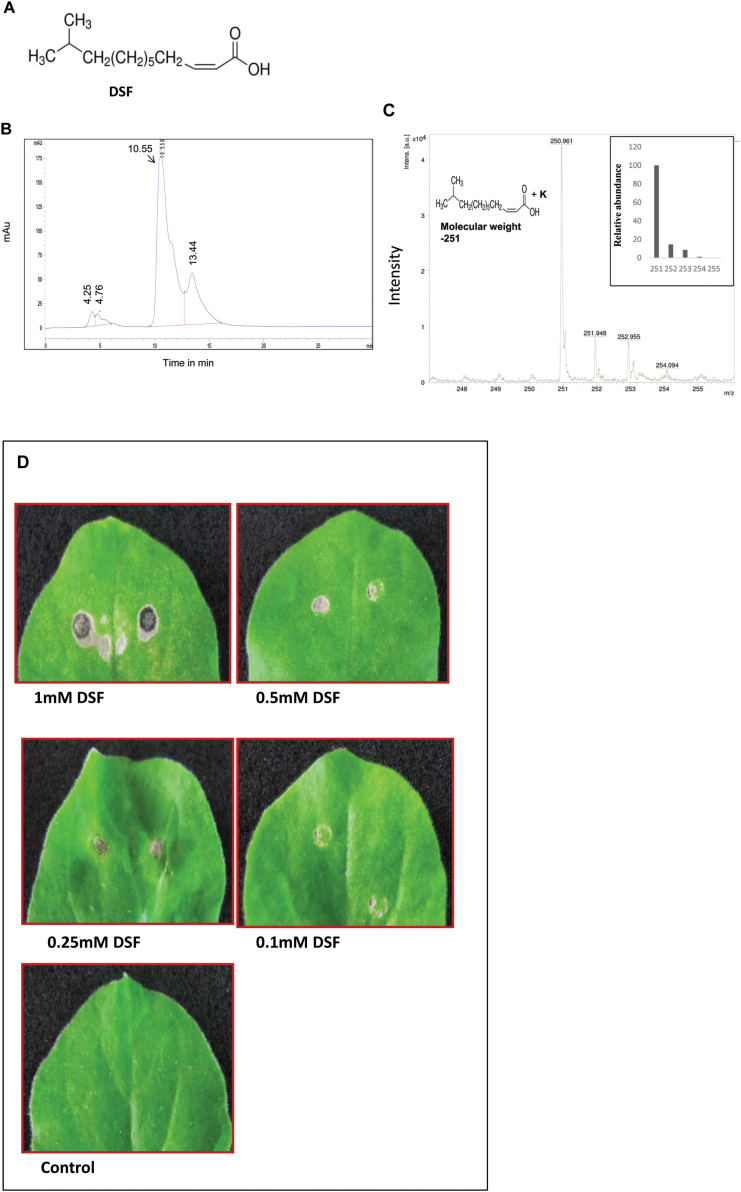
Infiltration of synthetic DSF in N. benthamiana induces HR-like symptoms. (A) Chemical structure of DSF, cis-11-methyl-2-dodecenoic acid. (B and C) Analysis of synthetic DSF by (B) HPLC and (C) MALDI–MS. (B) HPLC separation was achieved with an Agilent C18 (4.6 mm×250 mm×5 μm) column. DSF was eluted with water in methanol (20:80, v/v, with 0.1% formic acid) at a flow rate of 0.8ml min–1 and was detected at 220nm (retention time 10.55) as described in the Materials and methods. (C) Analysis of the MALDI-MS spectrum was done based on quasimolecular ions. Synthetic DSF gave one quasimolecular ion at 251 m/z (inset), which agrees with the calculated mass of a [M+K] + ion. No fragment ions were present at the applied laser energy. (D) Four-week-old N. benthamiana leaves were infiltrated with different concentrations of synthetic DSF. Control: 1% methanol in water. Browning of the infiltrated region and HR-like symptoms were observed 24h post-infiltration.
DSF mediated cell–cell signalling has been reported to play an important role in the virulence of several members belonging to the Xanthomonas group of phytopathogens such as Xanthomonas campestris pv. campestris (Xcc; a pathogen of cruciferous plants; Barber et al., 1997), Xanthomonas oryzae pv. oryzae (Xoo; a pathogen of rice; Chatterjee and Sonti, 2002), Xanthomonas citri subsp. citri (Xac; a pathogen of citrus; Siciliano et al., 2006 ), and Xanthomonas axonopodis pv glycines (Xag; a pathogen of soybean; Thowthampitak et al., 2008). Recent studies indicate that RpfF–DSF mediated cell–cell signalling is widespread in many pathogenic bacteria, including opportunistic human pathogens such as Stenotrophomonas maltophilia and Burkholderia sp. (Deng et al., 2011; Ryan and Dow, 2011). In addition, DSF family signals have been implicated in interspecies and interkingdom signalling, where they exert inhibitory activity against Candida albicans morphological transition, and influence biofilm formation and antibiotic tolerance in Pseudomonas aeruginosa (Boon et al., 2008; Deng et al., 2011; Ryan and Dow, 2011).
Xcc is an important pathogen belonging to the genus Xanthomonas and causes serious disease in cruciferous plants (Mansfield et al., 2012). In Xcc, DSF mediated cell–cell signalling regulates production of several secreted virulence factors such as the EPS xanthan, extracellular cell-wall-hydrolysing enzymes, and glucan (Barber et al., 1997). In Xcc, xanthan and glucan have been shown to play an important role in the disease process, including suppression of the host innate immune response (Yun et al., 2006; Rigano et al., 2007). It has been proposed that Xcc xanthan suppresses the plant defence response, presumably by inhibiting callose deposition (Yun et al., 2006). DSF mediated cell–cell signalling plays a role in the production of an as yet unknown extracellular factor which is involved in suppression of pathogen-induced stomatal closure, a part of the plant innate immune response (Gudesblat et al., 2009).
In this work, it was observed that infiltration of DSF extracts from the cell-free culture supernatant from the wild-type Xcc8004 strain induced hypersensitive response (HR)-like symptoms in Nicotiana benthamiana leaves (Supplementary Fig. S1A available at JXB online). However, extracts from the DSF synthase-deficient mutant of Xcc (rpfF), which is deficient in the production of DSF, exhibited reduced ability to induce HR-like symptoms (Supplementary Fig. S1B). Interestingly, it was also observed that co-inoculation of N. benthamiana leaves with DSF and the wild-type Xcc8004 strain suppressed the HR-like symptoms induced by the wild-type extract alone (Supplementary Fig. S1D). These results prompted further examination of the role of DSF in Xanthomonas–host plant interaction and investigation of whether the DSF signal molecule itself could act as an elicitor of the defence response. Overall, the results clearly indicate that DSF induces plant defence responses such as callose deposition, cell death-associated nuclear fragmentation, and resistance against subsequent infection by pathogenic bacteria. It was also shown that wild-type Xcc can suppress the DSF-induced defence response by the production of the EPS xanthan, a DSF-regulated important virulence factor of X. campestris.
Materials and methods
Bacterial strains and culture conditions
Xcc strains were grown on peptone sucrose agar (PSA) or in PS broth at 28 °C shaking at 200rpm, as described previously (Tsuchiya et al., 1982). Pseudomonas syringae pv. syringae (Pss) strain B728a (Loper and Lindow, 1987) was grown in King’s B (KB) medium or KB agar (King et al., 1954) at 28 °C. Escherichia coli strains were grown in Luria–Bertani (LB) medium at 37 °C. DSF and AHL used in this study were cis-11-methyl-2-dodecenoic acid and 3-oxo-hexanoyl-homoserine lactone (3OC6-HSL; Sigma Aldrich, St. Louis, MO, USA) respectively. AHL and DSF stock solutions were prepared in methanol and stored at –20 °C. Stocks of 3OC6-HSL and DSF were freshly diluted in water and used for plant infiltration and the biosensor assay. The concentrations of antibiotics used were: rifampicin, 50 μg ml–1; rifampin, 100 μg ml–1; spectinomycin, 20 μg ml–1; and tetracycline, 15 μg ml–1.
The purity of the synthetic DSF (Sigma Aldrich) was checked using an Agilent 1100 series high-performance liquid chromatography (HPLC) system (Agilent, USA). Data recording and processing was done using chemstation software (Agilent 1100). Separation was achieved with an Agilent C18 (4.6 mm×250 mm×5 μm) column. DSF was eluted with water in methanol (20:80, v/v, with 0.1% formic acid) at a flow rate of 0.8ml min–1 and was detected at 220nm (retention time 10.55). The final concentration of the DSF injected into the column was 100 μM. For matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) analysis, the synthetic lyophilized DSF sample was redissolved in methanol and then diluted with an appropriate volume of α-cyano-4-hydroxycinnamic acid (HCCA) matrix solution (1:5, v/v). A 1 μl aliquot of the resulting solution was deposited onto a stainless steel target, and the solvent was evaporated under a gentle stream of warm air. The experiment was done on a time-of-flight mass spectrometer (Brukner Daltronics ultraflex III, Germany) equipped with nitrogen laser at 337nm wavelength, 2.5 ns pulse width. After selection of the appropriate site on the target plate by microscopy, the laser light was focused onto the sample–matrix mixture at an angle of 45 ° and a power level of 106–107 W cm–2. Positive ions were extracted by a 5–10 keV acceleration potential, focused by a lens, and mass separated in a reflectron time-of-flight instrument. At the detector, ions were post-accelerated to 20 keV for maximum detection efficiency. The spectrum was acquired using flex control analysis–flex analysis software. Analysis of the spectrum was done based on quasimolecular ions obtained by the MALDI-MS method. Theoretical mass was calculated with unit mass resolution using an isotope distribution calculator and mass spec plotter by Scientific Instrument Services (SIS; http://www.sisweb.com/mstools/isotope.htm). Analysis for this DSF gave one quasimolecular ion at 251 m/z, which agrees with the calculated mass for an [M+K]+ ion. No fragment ions were present at the applied laser energy.
DSF and AHL biosensor assay
For DSF and AHL production assays, Xcc8523 (DSF-deficient rpfF mutant), harbouring the DSF biosensor plasmid pKLN55 (Peng: gfp), and the E. coli AHL biosensor strain, JB524 (Wu et al., 2000), harbouring the Vibrio fischeri luxR and PluxI:gfpmut3*, were grown in PS and LB media respectively, supplemented with appropriate antibiotics as described previously (Newman et al., 2004, 2008; Shepherd and Lindow, 2009). Cultures of biosensor strains were grown until early log phase, and ethyl acetate samples to be assayed for AHL and DSF were added and incubated overnight at 28 °C with shaking. Green fluorescent protein (GFP) fluorescence intensity (excitation 472nm, emission 512nm) was measured using a Varioskan® Flash fluorescence spectrophotometer (Thermo Fisher Scientific, Vantaa, Finland). Raw fluorescence units were normalized for the optical density at 600nm (OD600) values of the biosensor cultures. DSF and AHL concentrations were quantified by comparing normalized fluorescence values with those obtained from standard curves derived from biosensor cells incubated with known concentrations of DSF and AHL standards.
Separation and detection of DSF and AHL on thin-layer chromatography (TLC) plates were done as described previously (Wu et al., 2000; Newman et al., 2008). Ethyl acetate extracts from the culture supernatant of Xcc and PssB728a strains were spotted on C-18 reverse-phase silica TLC plates (Merck, Germany), along with 50 μM synthetic DSF and 3OC6-HSL as standards. TLC plates were resolved with 70% methanol. The plates were later dried and overlaid with a thin layer of 0.7% PS and LB agar containing 108 cells ml–1 of either the DSF indicator strain 8523 (pKLN55) or the E. coli AHL biosensor strain JB524. The plates were then also sprayed with a suspension of the indicator strain as above. Plates were incubated at 28 °C for 48h, and GFP fluorescence was visualized under UV illumination.
Nicotiana benthamiana, Arabidopsis, and rice inoculatons
Nicotiana benthamiana plants were grown in soil in a growth chamber (Conviron, USA) with 70% humidity, 16h of light and 8h dark, and a temperature of 22 °C. For N. benthamiana, 4-week old plants were used for the experiments. Arabidopsis thaliana ecotype Col-0 were grown on a vermiculate and perlite mixture. For A. thaliana, 5-week-old plants were used for the experiments. Rice [susceptible cv. Taichung Native-1 (TN-1)] plants were grown in a growth chamber (Conviron) with a temperature setting of 28 °C/25 °C (day/night) and a light intensity of 350 μmol m–2 s–1. Ten-day-old seedlings were used for callose deposition assay. For infection studies, 30-day-old rice seedlings were used.
Bacterial growth and callose deposition assays for N. benthamiana, Arabidopsis, and rice were done as described previously (Hauck et al., 2003; Yun et al., 2006; Jha et al., 2007; Rigano et al., 2007). All plant inoculations involved a minimum of three replicates. For N. benthamiana, leaves of 4-week-old plants were inoculated by syringe infiltration with Xcc and Pss strains (106 cfu ml–1 in water) alone or co-infiltrated with DSF (100 μM) and xanthan (0.5mg ml–1). For DSF and xanthan treatment experiments, leaves were infiltrated with water (control), synthetic DSF (50 μM to 1mM; for dose–response experiments), and xanthan (0.5mg ml–1). For co-infiltration experiments, 100 μM DSF was infiltrated with xanthan (0.5mg ml–1) or with Xcc strains 8004, 8523 (DSF-deficient rpfF mutant), gumD and gumK (EPS-deficient transposon-induced mutants of Xcc). For xanthan complementation experiments, gumD and gumK mutants were co-infiltrated with either xanthan alone or xanthan+DSF. To monitor growth of Pss strains harbouring either the vector control or the Xcc DSF biosynthetic gene rpfF, leaves were infiltrated with 1×106 cfu ml–1 of bacterial suspension in water with or without exogenous Xcc xanthan (0.5mg ml–1). Three 1cm2 leaf discs from each leaf (three leaves each from two independent experiments) were harvested from the inoculated area at 0, 24, and 48h after inoculation, surface sterilized using sodium hypochlorite (40% solution in water) and 50% ethanol for 1min each, homogenized in 1ml of sterile water using a mortar and pestle, and dilution plated on appropriate antibiotic medium to determine cfu cm–2. For Xcc co-inoculation experiments, the leaves were infiltrated with Xcc8004 (1×106 cfu ml–1) with or without 100 μM DSF or xanthan (0.5mg ml–1). For the pre-infiltration experiment, leaves were syringe infiltrated with either water (control), 100 μM DSF, or DSF+xanthan, 16h prior to inoculation with a 106 cfu ml–1 suspension of the wild-type Xcc8004 strain. To monitor Xcc growth, leaf discs were harvested as described above to determine cfu cm–2. Water soaking-like disease symptoms were monitored at 4 d post-inoculation.
Rice inoculation and resistance assays were done as described previously (Jha et al., 2007). The adaxial surfaces of 10-day-old rice (TN-1) leaves were infiltrated individually with either water or DSF (100 μM) using a 1ml hypodermic syringe without the needle, and examined for callose depositions. For disease resistance assay, the midveins of 30-day-old rice leaves were injected with 50 μl of either DSF (100 μM), cellulase (0.1mg ml–1), or buffer (10mM potassium phosphate buffer, pH 6.0). After 24h, the Xoo wild-type strain (BXO43) was inoculated onto the midvein, 1–2cm above the point of initial injection, by pricking with a needle that had been used to touch a fresh bacterial colony. After 12 d, the leaves were observed for the appearance of visible disease lesions (discoloration of the midvein and surrounding regions). For Arabidopsis, leaves of 5-week-old plants were infiltrated with water (control) or synthetic DSF (50 μM–1mM; for dose–response experiments), or co-infiltrated with 100 μM DSF and xanthan (0.5mg ml–1).
HR assay in N. benthamiana
The leaves were infiltrated with either ethyl acetate extracts isolated from the culture supernatants of Xcc strains [Xcc8004 (wild type), Xcc8523 (DSF-deficient rpfF mutant)] or synthetic DSF (50 μM to 1mM; for dose–response experiments). For HR suppression experiments, either DSF isolated from the culture supernatants or synthetic DSF (50 μM to 1mM) was co-infiltrated with a 1×107 cfu ml–1 suspension of the Xcc8004 wild-type strain in water with a needleless hypodermic syringe. HR-like symptoms (browning of the infiltrated area) were observed 24h after infiltration. To detect cell death, H2O2 accumulation, and autofluorescence, leaves of 4-week-old N. benthamiana plants were infiltrated with 100 μM DSF. Leaves were detached at 24h after DSF infiltration and subjected to observation by staining the leaves with trypan blue, by autofluorescence, and by H2O2 production. Cell death was visualized by lactophenol–trypan blue staining followed by destaining in saturated chloral hydrate as described (Koch and Slusarenko, 1990). Autofluorescence was visualized under a stereo fluorescence microscope (Zeiss Lunar V12; Carl Zeiss, Goettingen, Germany), using an eGFP filter set (excitation 470/40nm, emission 525/50nm). H2O2 was detected by an in situ histochemical staining procedure using 3,3′-diaminobenzidine (DAB) as described previously (Thordal-Christensen et al., 1997). Briefly, the leaves were detached and placed in a solution containing DAB (1mg ml–1; pH 5.5) for 2h at room temperature. The leaves were boiled in 95% ethanol for 2min and stored in distilled water. H2O2 production was visualized as a reddish-brown coloration.
Callose staining
Bacteria or synthetic DSF were infiltrated into N. benthamiana, Arabidopsis, and rice leaves as described above. Callose staining was performed at 18h after inoculation as described by Hauck et al. (2003). After 24h, the leaves were cleared of chlorophyll using alcoholic lactophenol, rinsed in 50% ethanol and then in water, before staining for 2h with 0.02% aniline blue (Sigma Aldrich) in 150mM K2HPO4, pH 9.5. Samples were mounted in 50% glycerol and were visualized by a stereo fluorescence microscope (Zeiss Lunar V12; Carl Zeiss), using a blue filter (excitation wavelength 365nm and emission wavelength >420nm), long band pass (BP), and ×20 objective. The number of callose deposits per 0.5mm2 area surrounding the infiltrated zone were counted using Axio Vision Rel 4.8 image analysis software (Carl Zeiss).
Rice and Arabidopsis root cell death assays
The root cell death assay was carried out as described previously (Jha et al., 2007; Aparna et al., 2009). Arabidopsis and rice root tips, 1–2cm long, were treated with water, 100 μM DSF, and cellulase (0.1mg ml–1). After incubation for 3–4h, roots were washed in 1× phosphate-buffered saline (PBS) and stained with propidium iodide (PI) by vacuum infiltration for 10–15min. The roots were mounted on a microscope slide in 50% glycerol in 1× PBS; 0.3 μm thick longitudinal optical sections were acquired on a Zeiss LSM-510 Meta confocal microscope using a plan ApoChromat ×63/1.4 oil objective and were further projected to obtain the image of 2–3 μm total thickness. An HeNe laser at 543nm excitation and emission >560nm (LP) was used to detect PI internalization. The images were analysed using the Zeiss LSM image examiner software.
Transposon mutagenesis and screening for mutants altered in suppression of DSF-induced HR-like symptoms
Transposon (Tn5) mutagenesis was done in the Xcc8004 wild-type background by introducing the transposon mutagenesis suicidal plasmid pRL27 from E. coli by conjugation as described before (Larsen et al., 2002). Transposon mutants were selected on PSA containing 50 μg ml–1 kanamycin. A total of 3000 colonies from 10 independent matings were screened by co-infiltration with 0.5mM DSF on leaves of 4-week-old N. benthamiana plants with a needleless syringe. HR-like symptoms were scored manually 24h post-inoculation by comparing the leaves treated with DSF alone or co-infiltrated with the wild-type Xcc8004 strain.
Two colonies (SC130 and SC235) were identified that were unable to suppress DSF-induced HR-like symptoms, described in detail in the Supplementary Materials and methods at JXB online. In general, the rest of the 2998 transposon-induced mutants suppressed the DSF-induced HR response and the majority of them exhibited disease symptoms. The sequence of chromosomal DNA flanking the Tn5 insertion site was determined as described previously (Larsen et al., 2002). Briefly, genomic DNA from the mutants was digested with BamHI, circularized, and electroporated into E. coli DH5α λpir cells. Plasmids recovered from DH5α λpir Kmr colonies were sequenced with primers oriented outward from the transposon flanks (Larsen et al., 2002). Sequences obtained from two independent clones were confirmed and analysed by NCBI BLAST X search (Altschul et al., 1997). The transposon insertions in SC130 and SC235 were located at positions corresponding to codon 306 and 83 of gumD (Locus tag: XC_1600; 484 a. a) and gumK (Locus tag: XC_1667; 400 a. a) respectively. gumB and gumK are members of the gum operon of Xanthomonas involved in biosynthesis of the EPS xanthan. To confirm that the phenotype is due to transposon insertion, marker exchange mutagenesis was carried out in the Xcc8004 wild-type background. EPS was isolated and quatitated from the wild type, and transposon-induced and marker exchange mutants by the phenol sulphuric acid method as described previously (Rai et al., 2012). Analysis of EPS in the gumD and gumK mutants indicated that as expected, they were deficient in the production of secreted xanthan.To obtain the gumD and ΔrpfF-gumD double mutant, a 500bp internal fragment of the gumD gene was PCR amplified with primers-Scc15 Pk18 Gum F EcoRI, GCGAATTCGTTGTATTCGGTGATCTGCTTC; and Scc16 PK18 GUM R HindIII, GCAAGCTTGCTCGC CAAGCGGCAACGAGATCCAC, and cloned in the pK18mob plasmid (Schafer et al., 1994). The resulting recombinant plasmid was electroporated individually into competent cells of Xcc8004 wild type and ΔrpfF (Pradhan and Chatterjee, 2014; DSF-deficient deletion mutant) background strains to obtain a Kmr single recombinant which was further confirmed by PCR and sequencing. Integration of pK18mob results in non-polar mutation (Windgassen et al., 2000), as the transcriptional orientations of the lacZ promoter of the pK18mob and the gumD gene fragments were in the same direction so that the mutation caused by the pK18mob plasmid is unlikely to cause any polar effect on the downstream gene. For complementation of the EPS-deficient gumD mutant, the wild-type gumD gene was amplified from the wild-type Xcc8004 strain using primer pairs Scc17 PHM1 F HindIII, GCAAGCTTAGGAGGACAGCT ATGCTTTTGGCAGACTTGAGTAG; and Scc18 PHM1 R Eco RI, GCGAATTCTCAGTACGCGGTCTTCTGTCCGAGC, and cloned in the broad host range pHM1 vector.
Detection of DSF produced by Xcc in planta
To detect in planta production of DSF by Xcc, N. benthamiana leaves were infiltrated with either the wild-type Xcc8004 (pKLN55) or the 8523 (pKLN55) biosensor strain grown in PS medium overnight (12h) at a density of 1×106 cfu ml–1, similar to the inoculation experiments for detecting callose deposition and in planta growth. The wild-type Xcc8004 (pKLN55) exhibited very low GFP fluorescence, indicative of low DSF levels or production at a density of 1×106 cfu ml–1 (Pradhan and Chatterjee, 2014). For estimating DSF levels in planta, the rpfF mutant strain 8523 (pKLN55) (GFP–) was co-infiltrated with different concentrations of DSF (in the range of 10–100 μM). Infiltrated leaves of N. benthamiana were analysed by confocal laser scanning microscopy (CLSM; LSM 510, meta; Carl Zeiss) at 48h post-infiltration using a plan ApoChromat ×100 oil objective. The excitation maximum was at 488nm (argon laser) and the emission maxima were observed in BP 510–530nm (for eGFP fluorescence) and BP 650–710nm (for red autofluorescence of leaf). The mean GFP fluorescence intensity of ~50 bacterial cells of 8523 (pKLN55) was measured and compared with the mean GFP fluorescence intensity of wild-type Xcc8004 (pKLN55) in N. benthamiana leaves at 0, 1, 2, 3, and 4 d post-inoculation.
Pre-infiltration with DSF and subsequent challenge with flg22
Leaves of 4-week-old N. benthamiana plants were pre-infiltrated with 10 μM DSF or 1% methanol (solvent control) for 16h prior to challenge with 100nM flg22 (Genescript, Corp, USA, Cat. no. RP19986) for 18h and stained with aniline blue to visualize callose deposition by epifluorescence microscopy.
RNA gel blot analysis
Total RNA was isolated from N. benthamiana leaves using Trizol (Invitrogen, CA, USA). A 20 μg aliquot of total RNA from each sample was separated on a 1.2% agarose gel containing formaldehyde, and transferred to a Hybond-N+ nylon membrane. The filters were pre-hybridized and hybridized in hybridization buffer: 7% (w/v) SDS, 0.5M phosphate buffer, pH 7.2. Pre-hybridization was performed at 65 °C for 2h. Hybridization was performed overnight with the addition of a denatured 32P-labelled Nb PR-1 probe that was synthesized using the random primer labelling kit (BRIT, Jonaki, India). Membrane washing was performed according to standard protocols. The filters were exposed to storage phosphor screen autoradiography and screened in a BAS 2500 Fuji phosphor imaging system.
Results
The cell–cell signalling molecule diffusible signal factor (DSF) from Xanthomonas induces plant defence response
To understand the effect of DSF on the host plant, synthetic DSF, cis-11-methyl-2-dodecenoic acid (see the Materials and methods), was obtained and examined by HPLC and MALDI-MS analysis (Fig. 1A–C). HPLC and MALDI-MS analysis confirmed that the synthetic DSF was pure. The bioactivity of synthetic DSF was also examined using an Xcc DSF biosensor strain Xcc8523 (pKLN55), a DSF-deficient mutant harbouring the gfp reporter responsive to exogenous DSF (Newman et al., 2004). The addition of synthetic DSF could complement the expression of the DSF-responsive GFP reporter in a dose-dependent manner (Supplementary Fig. S2 at JXB online).
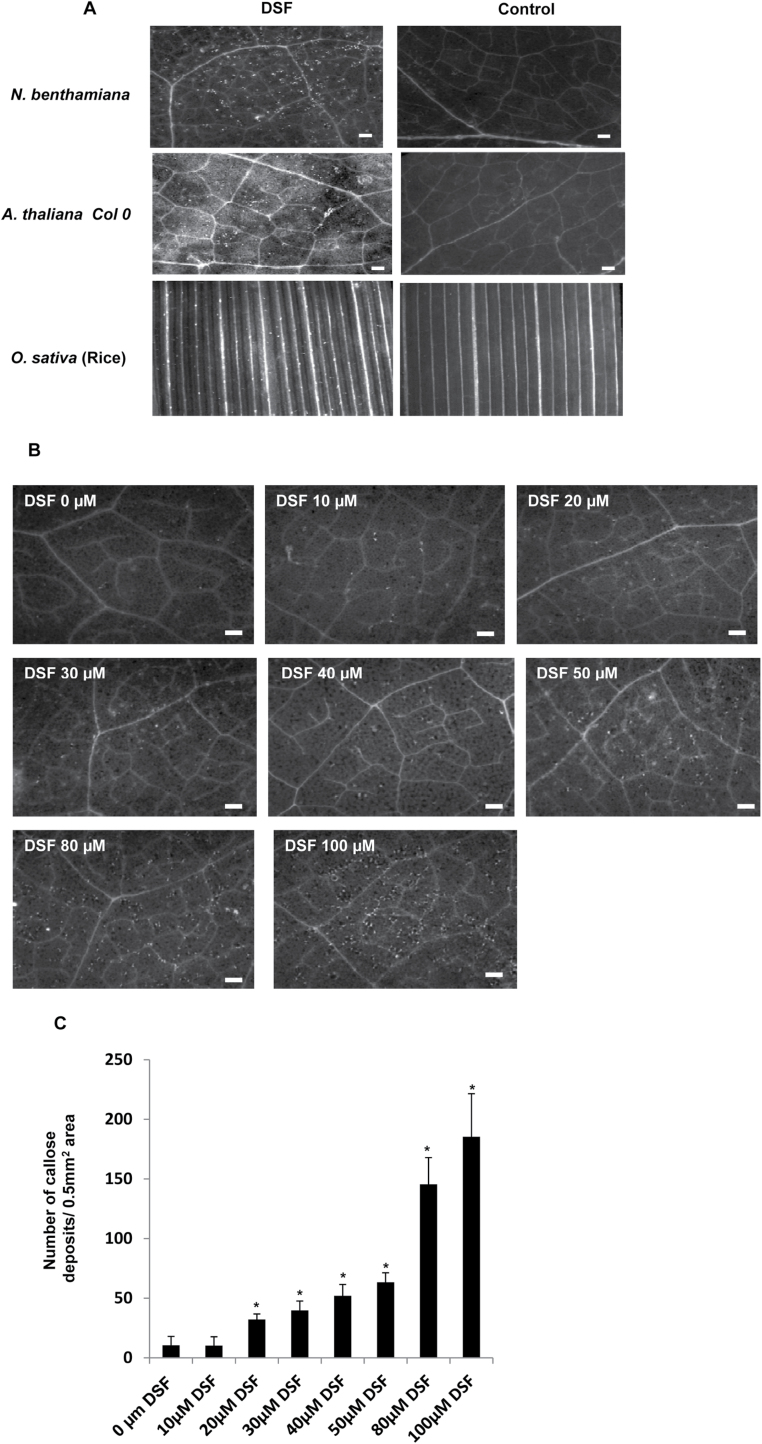
Infiltration of N. benthamiana leaves with synthetic DSF induced browning of the infiltrated region, which appeared similar to HR-like symptoms, in a dose-dependent manner (Fig. 1D). Visible HR-like symptoms (browning) were observed with a concentration of DSF in the range of 0.25–1mM, 24h post-infiltration. Callose deposition has been used as a marker for plant basal defence response and has been reported to play a role in the plant defence in limiting pathogen growth (Bestwick et al., 1995; Adam and Somerville, 1996; Hann and Rathjen, 2007). In order to examine the ability of DSF to induce callose deposition in plants, synthetic DSF was infiltrated in N. benthamiana, Arabidopsis, and rice leaves, at a concentration of 10–200 μM. Callose staining 18h post-inoculation indicated that DSF induced callose deposition in a dose-dependent manner in N. benthamiana, Arabidopsis, and rice (Fig. 2; Supplementary Fig. S3; Supplementary Table S1 at JXB online). It was observed that the N. benthamiana leaves infiltrated with a DSF concentration of ≥20 μM exhibited significantly higher callose deposition compared with those infiltrated with either control (1% methanol in water) or a DSF concentration <10 μM (Fig. 2). This effect was specific to DSF as infiltration of a range of compounds with a related structure, including trans-11-methyl dodecenoic acid, decanoic acid, lauric acid, palmitic acid, myristoleic acid, and palmitoleic acid, on N. benthamiana leaves resulted in very little or no callose deposition (Supplementary Table S2).
Fig. 2.
DSF induces callose deposition. (A) Infiltration of 100 μM synthetic DSF (cis-11-methyl-2-dodecenoic acid) induces callose deposition in N. benthamiana, Arabidopsis thaliana (Col-0), and rice (Oryzae sativa). Callose deposition was visualized 18h post-infiltration by staining the leaves with aniline blue and examination using an epifluorescence microscope. White dots in these pictures are indicative of callose deposition. (B) DSF induced callose deposition in N. benthamiana leaves in a dose-dependent manner. Nicotiana benthamiana leaves were infiltrated with (left to right) 10, 20, 30, 40, 50, 80, and 100 μM DSF, and control (0 μM; 1% methanol in water), and visualized for callose deposition 18h post-infiltration. Scale bars=500 μm. (C) Average number of callose deposits per 0.5mm2. Error bars represent SD values from four leaves of each plant in three independent experiments. Six microscopic fields from each leaf were analysed. *P<0.01, significant differences between the responses to the DSF treatment compared with the control (indicated by 0 μM) as assessed by Student’s t-test.
In a recent study, it was demonstrated that plants pre-treated with a lower concentration of AHL (QS signalling molecule) exhibited an induced defence response (MTI; MAMP-triggered immunity) on subsequent challenge with flg22 (Schenk et al., 2014). To see whether DSF can influence MTI, the callose deposition was examined in plants that were pre-infiltrated with 10 μM DSF or 1% methanol (solvent control) for 16h prior to challenge with 100nM flg22. Interestingly, leaves pre-infiltrated with DSF showed a substantially higher amount of callose deposition compared with leaves pre-infiltrated with solvent control and subsequently challenged with flg22 (Supplementary Fig. S4 at JXB online). It is important to note in this regard that infiltration of 10 μM DSF alone did not induce a significant amount of callose deposition compared with the solvent control (Fig. 2; Supplementary Fig. S4). This suggests that the application of a lower concentration of DSF may prime plants and influence their subsequent defence response.
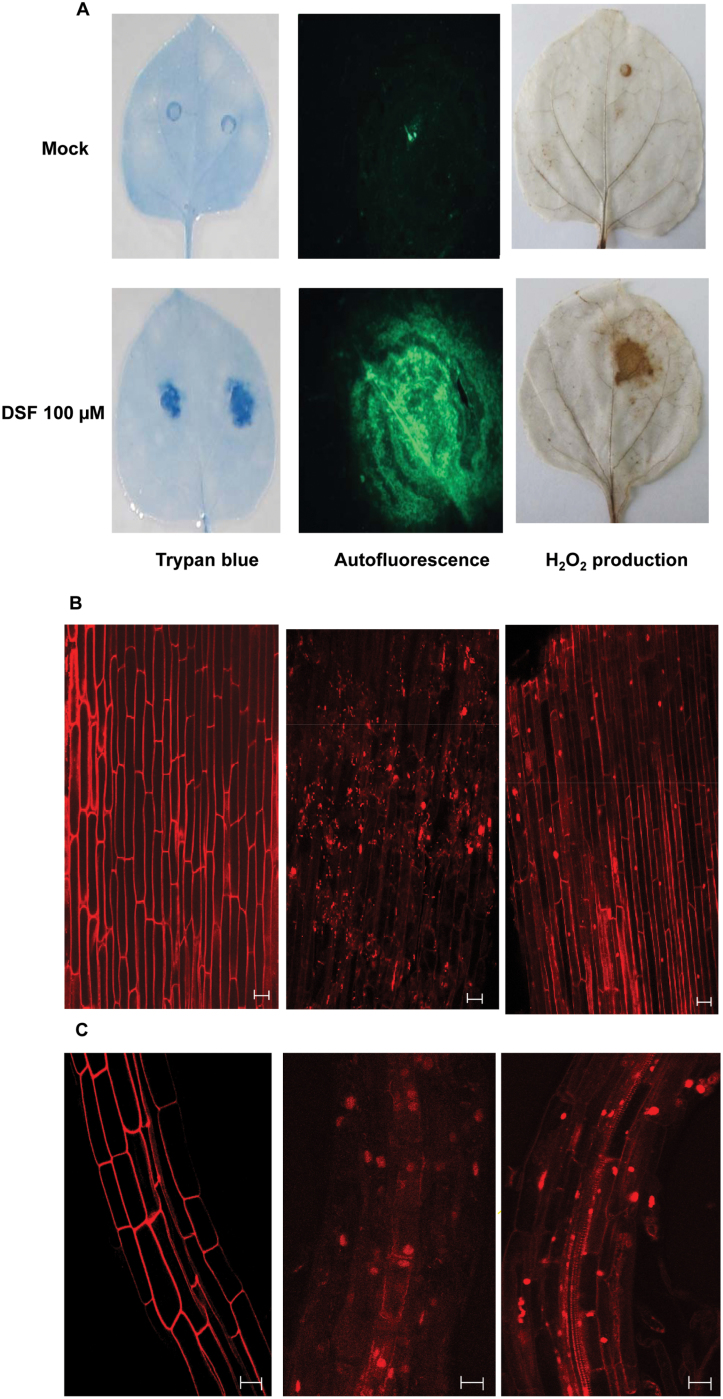
Earlier reports indicated that localized programmed cell death (PCD) reactions are associated with the plant defence response (Pennell and Lamb, 1997). Plant cells undergoing PCD such as the HR-like response exhibit autofluorescence due to deposition of lignin-like compounds and H2O2 accumulation (Stone et al., 2000). Cell death, autofluorescence, and H2O2 accumulation were thus examined in N. benthamiana leaves infiltrated with 100 μM DSF. Examination of leaves infiltrated with DSF indicated a significant increase in trypan blue staining, autofluoresence, and H2O2 accumulation (visualized by DAB), whereas the control treatments did not exhibit these effects (Fig. 3A). It has been demonstrated that rice root cells exhibit PCD upon exposure to cell-wall-hydrolysing enzymes (such as cellulase and lipase), which are potential DAMPs (Jha et al., 2007; Aparna et al., 2009). To assess the ability of DSF to induce cell death, rice and Arabidopsis roots were treated with DSF, stained with PI, and examined by CLSM. Internalization of PI is indicative of cell death and it is excluded by live cells. The control buffer-treated roots exhibited a prominent cell wall-associated autofluorescence but no internalization of PI into the cells (Fig. 3B). Treatment of rice and Arabidopsis roots with 100 μM DSF resulted in intake of PI, indicating compromised integrity of the plasma membrane (Fig. 3B).
Fig. 3.
DSF induced cell death in N. benthamiana, rice, and Arabidopsis. (A) DSF induced cell death in N. benthamiana. Leaves of 4-week old plants were infiltrated with 100 μM DSF. The leaves were detached at 24h after DSF infiltration and subjected to observation by staining the leaves with trypan blue, an indicator of cell death (left); autofluorescence (centre); and H2O2 production (right). H2O2 accumulation was visualized by staining with diaminobenzidine (DAB), a histochemical reagent for in situ detection of H2O2. Six or more leaves were examined for each condition, and representative fields are shown. (B and C) DSF induced cell death in rice and Arabidopsis roots. Isolated roots were treated with DSF, cellulase, and control (buffer-treated) for 16h, stained with propidium iodide (PI), and examined under a confocal microscope. Cellulase (a potential DAMP) from Aspergilus niger was used as a positive control. (B) From left to right, rice root tips, 1–2cm long, from 3- to 4-day-old seedlings were treated with control (methanol in water), 100 μM DSF, and cellulase (0.2mg ml–1) from A. niger. (C) From left to right, Arabidopsis roots treated with water control, DSF (100 μM), and cellulase. Treatment with water control did not induce cell death (intake of PI), whereas treatment with either DSF or cellulase induced cell death. Similar results were obtained in at least three independent experiments. Scale bars=20 μm.
Production of DSF is associated with induced defence response
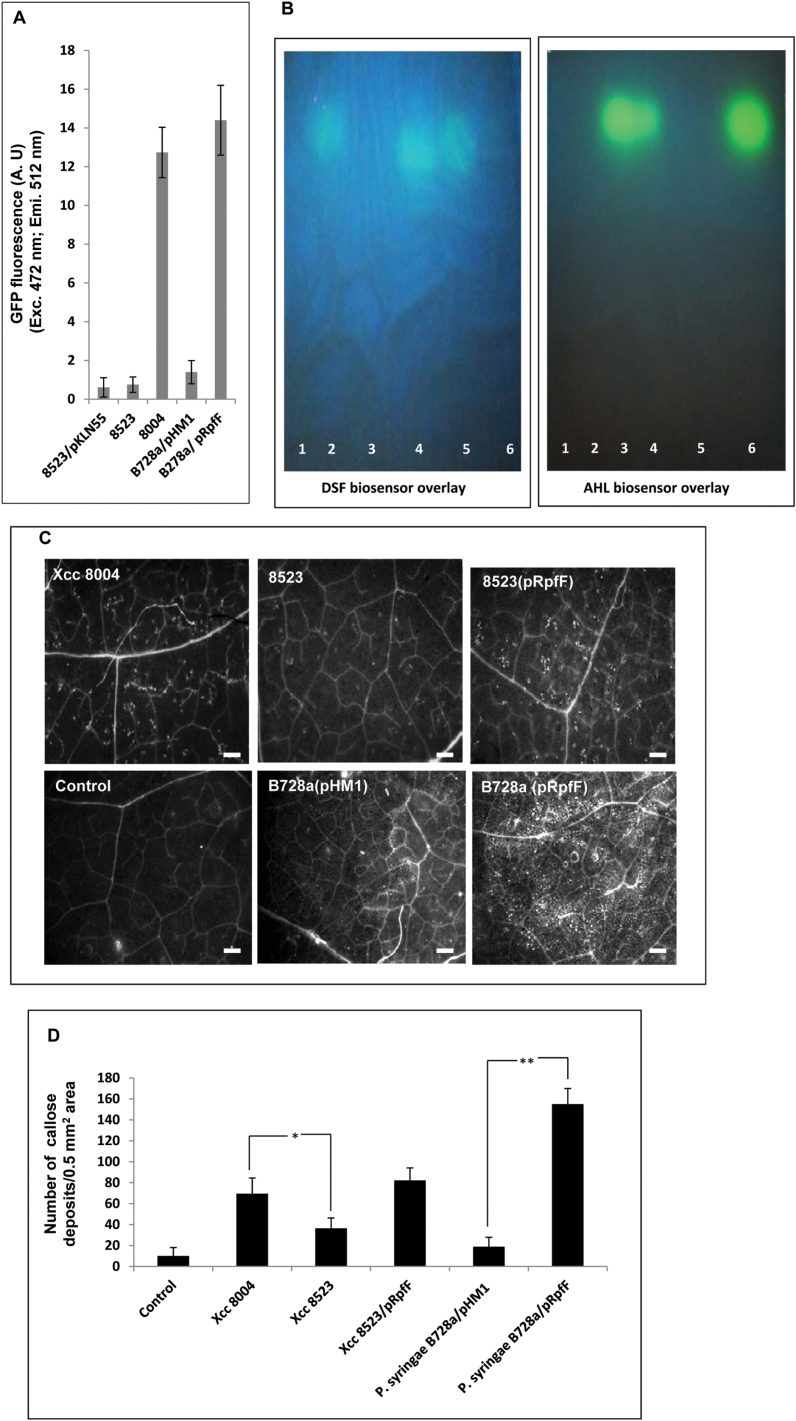
It has been shown previously that callose deposition is required for disease resistance against many pathogens, including Xcc, and enhanced callose deposition is associated with increased resistance response in N. benthamiana to Xcc infection (Bestwick et al., 1995; Hauck et al., 2003; Yun et al., 2006; Rigano et al., 2007). In Xcc, rpfF encodes the DSF synthase (RpfF) which is required for the production of DSF (Fig. 4A, B). In order to investigate whether callose deposition is associated with the production of the cell–cell signalling molecule DSF in Xcc, N. benthamiana leaves were inoculated with a 1×106 cfu ml–1 suspension of wild-type Xcc8004, Xcc8523 (DSF– mutant defective in RpfF), and Xcc8523 (pRpfF) (DSF– mutant harbouring the plasmid-borne wild-type rpfF allele). The number of callose deposits that are elicited by these strains was quantitated following aniline blue staining and epifluorescence microsopy at 24h post-inoculation (Fig. 4C). Higher amounts of callose deposition (~2-fold) were detected in N. benthamiana leaves that were infiltrated with wild-type Xcc8004 compared with Xcc8523 (Fig. 4D). Complementation of Xcc8523 with the wild-type rpfF gene restored callose deposition to the levels that were induced by the wild-type Xcc8004 strain (Fig. 4C, D). To detect DSF levels produced by the wild-type Xcc strain in N. benthamiana leaves, the wild type Xcc8004 (pKLN55) DSF biosensor strain was infiltrated under similar conditions at a density of 1×106 cfu ml–1. At a low cell density (1×106 cfu ml–1), the Xcc DSF biosensor strain exhibited low GFP fluorescence (uninduced) in PS medium (Pradhan and Chatterjee, 2014). Analysis of N. benthamiana leaves by confocal microscopy indicated that the wild-type Xcc produced a significant amount of DSF in planta, as indicated by the induced DSF-responsive GFP fluorescence at 24–48h post-infiltration (Supplementary Fig. S5 at JXB online).
Fig. 4.
Production of DSF is associated with induced callose deposition. (A) Assay for DSF production by different bacterial strains using the Xcc DSF biosensor 8523 (pKLN55) in which the DSF-responsive promoter from the endoglucanase gene is cloned upstream of an eGFP reporter (Peng:GFP). DSF extracted from the cell-free culture supernatant of Xcc8004 (wild type, DSF+); 8523 (DSF–); Pseudomonas syringae pv. syringae PssB728a (pHM1) (wild-type strain harbouring the empty vector); and PssB728a (pRpfF) (Pss harbouring the Xcc DSF synthetic gene rpfF). An increase in GFP fluorescence (represented on the y-axis) compared with the control (extracts from the Xcc8523; rpfF mutant) indicates the amount of DSF produced by different strains. (B) TLC analysis for the production of DSF and AHL in Xcc and PssB728a. TLC was performed with crude ethyl acetate extracts isolated from the culture supernatant of different strains of Xcc and Pss. DSF (left panel) and AHL (right panel) were detected by an overlay of the Xcc DSF indicator strain 8523 (pKLN55) and the E. coli AHL biosensor (JB524) on TLC plates. The photograph was taken with the UV light source oriented from the top of the TLC plates. Column 1, Xcc8523 (DSF-deficient mutant); column 2, Xcc8004 (Xcc wild-type strain); column 3, PssB728a (pHM1); column 4, PssB728a (pRpfF); column 5, synthetic DSF (20 μM cis-11-methyl-2-dodecenoic acid); column 6, synthetic AHL (10 μM N-3-oxo-hexanoyl-dl-homoserine lactone; 3OC6-HSL). Similar results were obtained in three independent experiments. (C) From left to right, callose deposition in N. bethamiana leaves infiltrated with a 1×106 cfu ml–1 suspension of different bacterial strains: wild-type Xcc8004, 8523, 8523 (pRpfF), water control, PssB728a (pHM1), and B728a (pRpfF). Callose deposition was visualized by staining with aniline blue and examined using a stereo fluorescence microscope 24h post-inoculation. White dots in these pictures are indicative of callose deposition. (D) Average number of callose deposits per 0.5mm2 area. Error bars represent SD values from three leaves of each plant from three independent experiments. Six microscopic fields from each leaf were analysed. Differences between the responses to Xcc8523 (DSF– mutant; indicated by *P<0.01) and the P. syringae B728a wild-type strain harbouring the Xcc DSF synthase rpfF (indicated by **P<0.001) compared with the wild-type strains of Xcc8004 and P. syringae B728a were significant as assessed by Student’s t-test.
It has been shown previously that synthesis of DSF in Xanthomonas and other closely related bacteria requires rpfF, which encodes DSF synthase (RpfF), a bifunctional crotonase having both dehydratase and thioesterase activities (Wang et al., 2004; He et al., 2010; Bi et al., 2012; Beaulieu et al., 2013). It has also been demonstrated that recombinant E. coli and Erwinia herbicola expressing RpfF were able to produce the secreted DSF family of signalling molecules (Bi et al., 2012; Beaulieu et al., 2013). It was thus investigated whether the production of DSF in a non-DSF-producing phytopathogen could provoke a defence response-associated callose deposition and reduce its growth in the host plant. The DSF biosynthetic gene rpfF of Xcc was expressed under the control of the Plac promoter in PssB728a, a non-DSF-producing pathogen of snap bean and the model plant N. benthamiana (Loper and Lindow, 1987; Vinatzer et al., 2006). It has been shown that Pss produces the QS signalling AHL, 3OC6-HSL (Quiñones et al., 2004) (Fig. 4A, B). A DSF and AHL biosensor assay, and C18 reverse phase TLC analysis with the ethyl acetate extract from the culture supernatants of the wild-type PssB728a strain harbouring either the Xcc rpfF [B728a (pRpfF)] or the vector control [PssB728a (pHM1)], was performed. As expected, the wild-type PssB728a strain harbouring the vector control [PssB728a (pHM1)] produced 3OC6-HSL and did not produce any detectable DSF, while PssB728a (pRpfF) produced 3OC6-HSL as well as DSF (Fig. 4A, B). The migration pattern of DSF extracted from either the wild-type Xcc8004 or PssB728a (pRpfF) were similar to that of synthetic DSF (Fig. 4B).
Next, N. benthamiana leaves were infiltrated with the PssB728a wild-type strain harbouring either the Xcc rpfF [PssB728a (pRpfF)] or the vector control [PssB728a (pHM1)]. Significantly higher amounts of callose (~6-fold) deposition were detected in B728a (pRpfF) compared with the vector control (Fig. 4C, D). Next, experiments were conducted to determine whether defence responses induced by DSF-producing PssB728a could have an effect on bacterial growth. A bacterial growth assay was carried out in N. benthamiana leaves infiltrated with a 1×106 cfu ml–1 suspension of PssB728a (pRpfF) or vector control. The in planta growth assay indicated that wild-type PssB728a harbouring the Xcc rpfF [PssB728a (pRpfF)] exhibited reduced growth at 24h and 48h after inoculation compared with the vector control, which although smaller (~5- to 6-fold), was still statistically significant (Fig. 5). However, there was not much difference in growth at 4 d post-inoculation (Supplementary Fig. S6 at JXB online).
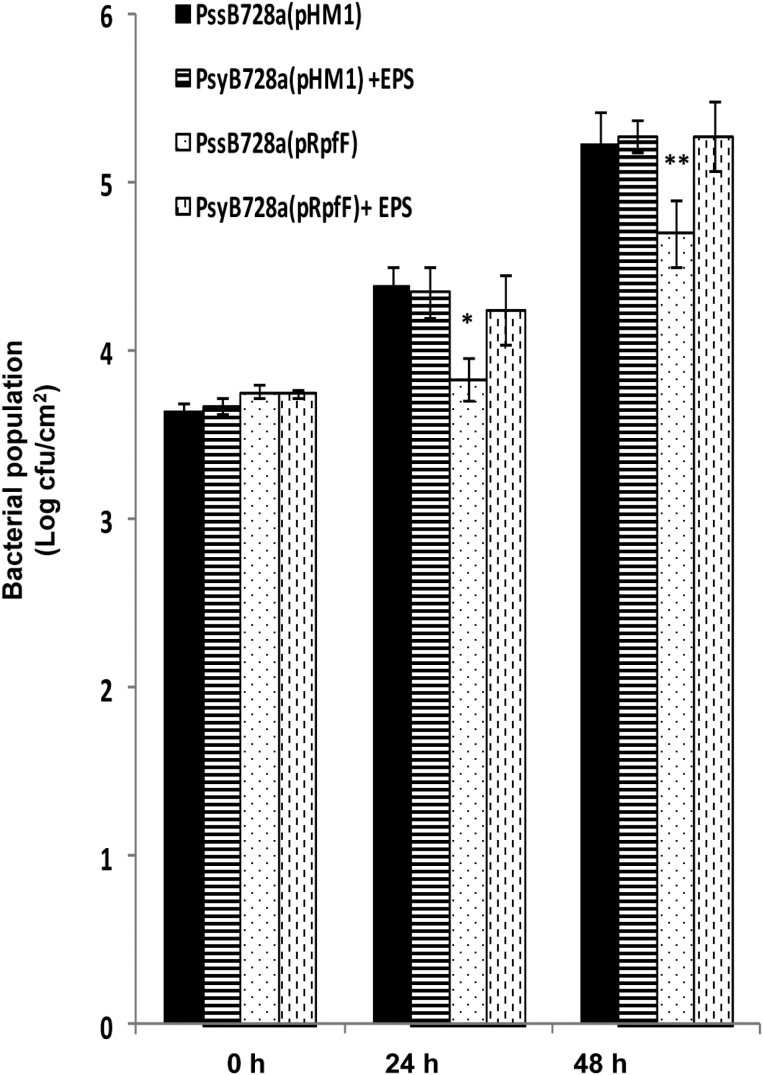
Fig. 5.
Production of DSF in Pseudomonas syringae pv. syringae (Pss) reduces growth in N. benthamiana leaves. Leaves of 4-week-old N. benthamiana were infiltrated with wild-type Pss harbouring the plasmid containing the DSF synthase (pRpfF) or the empty vector (pHM1) alone or co-inoculated with Xcc EPS (xanthan; 0.5mg ml–1). The bacterial population was measured at 0, 24, and 48h post-inoculation from six 1cm2 leaf disc areas around the infiltration zone. Values presented are the average log (cfu cm–1) from six leaves (two independent experiments). A significantly different population of bacteria compared with the wild-type Pss harbouring the empty vector control (pHM1) based on a pairwise Student’s t-test is indicated with either one or two asterisks: *P≤0.05; **P≤0.02.
The extracellular polysaccharide xanthan suppresses the DSF-induced defence response in N. benthamiana
Since it was observed that co-inoculation of the Xcc wild-type strain with DSF suppressed DSF-induced HR-like symptoms in N. benthamiana (Supplementary Fig. S1D at JXB online), further experiments were performed to determine whether co-inoculation of the wild-type Xcc strain with DSF could also suppress DSF-induced callose deposition. Co-infiltration of N. benthamiana leaves with wild-type Xcc8004+DSF resulted in suppression of callose deposition (Fig. 6B, C). Significantly lower amounts of callose deposition were detected in N. benthamiana leaves that were co-infiltrated with Xcc8004+DSF compared with those infiltrated with DSF alone (Fig. 6J).
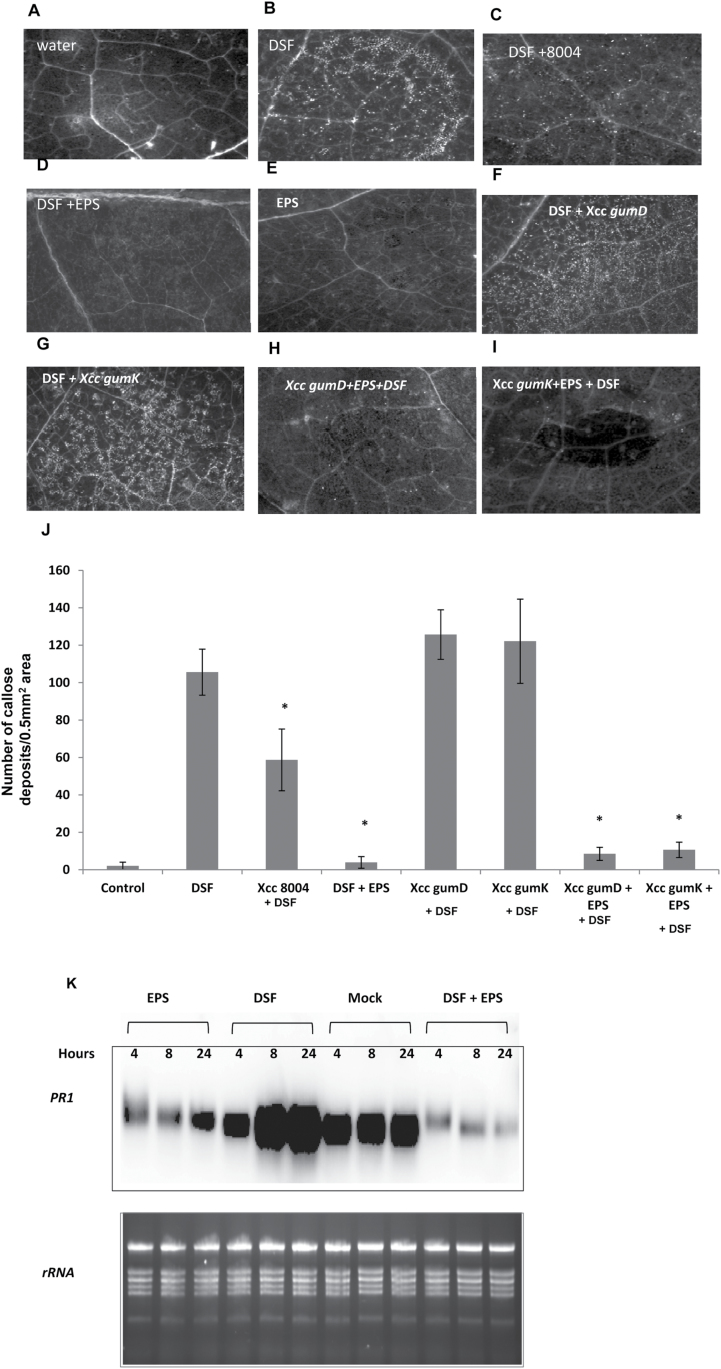
Fig. 6.
DSF-induced callose deposition was suppressed by either the wild-type Xcc8004 or the EPS xanthan. (A–I) Callose deposition in N. benthamiana leaves after inoculation with water control (A), 100 μM DSF (B), and EPS (E; xanthan; 0.5mg ml–1) alone or co-inoculation of DSF with either Xcc8004 (C), EPS (D; xanthan), Xcc gumD (F), Xcc gumK (G), Xcc gumD+EPS (H), or Xcc gumK+EPS (I). Callose deposition was visualized by staining with aniline blue and examined using a stereo fluorescence microscope 24h post-inoculation. For co-inoculation experiments with Xcc strains, a bacterial suspension of 1×106 cfu ml–1 was used. (J) Average number of callose deposits per 0.5mm2 area of N. benthamiana leaves inoculated with control (water) and DSF alone, or co-inoculation of DSF with either Xcc8004, EPS, Xcc gumD amd gumK (Xcc mutants deficient in EPS production), or Xcc gumD and gumK mutants supplemented with EPS (gumD and gumK+ EPS). Error bars represent SD values from three leaves of each plant and three independent experiments. Six microscopic fields from each leaf were analysed. * indicates (P<0.001) significantly lower callose deposits compared with leaves treated with DSF alone as determined by two-tailed Student’s t-test. (K) RNA gel blot analysis of PR-1 gene expression in N. benthamiana leaves infiltrated with water (mock), DSF (100 μM), EPS (xanthan, 0.5mg ml–1) alone or co-infiltration with DSF+EPS. RNA was isolated at the indicated times for RNA gel blot analysis. Data shown are representative of those obtained from three independent experiments.
To gain more insight into DSF–plant interaction and to identify potential suppressor(s) of the DSF-induced defence response, a transposon-induced mutant library of Xcc8004 was screened to isolate mutants altered in their capacity to suppress DSF-induced callose deposition. In this screen, N. benthamiana leaves were co-inoculated with ~3000 transposon-induced mutants from the library (see the Supplementary Materials and methods at JXB online for details) along with DSF, and compared with those infiltrated with DSF alone. Two transposon-induced mutants, gumD (gumD::mTn5) and gumK (gumK::mTn5), that were deficient in their capacity to suppress the DSF-induced callose deposition, were identified (Fig. 6F, G). In Xanthomonas, gumD and gumK are members of the gum operon which consists of 12 genes (gumB–gumM) involved in the synthesis of the EPS xanthan and is highly conserved among Xanthomonas species (Büttner and Bonas, 2010). As expected, the transposon-induced mutants (gumD and gumK) and an insertional mutant gumD::pK18mob exhibited reduced production of extracellular xanthan (Supplementary Fig. S7 at JXB online). Co-infiltration of N. benthamiana leaves with purified commercial xanthan (0.5mg ml–1; Sigma Aldrich: G1235) together with DSF suppressed the DSF-induced callose deposition (Fig. 6D). Interestingly, co-infiltration of xanthan+gumD or gumK mutants suppressed the DSF-induced callose deposition (Fig. 6H–J). Previously it was reported that Xcc in N. benthamiana produces copious amount of the EPS xanthan (14–19mg g–1 as dry weight) at 2–4 d post-infection (Aslam et al., 2008), which agrees with the concentrations of xanthan used for suppression of the DSF-induced defence response.
It has been shown that induced expression of the gene encoding PR-1 in N. benthamiana is associated with the resistance response against Xcc infection (Rigano et al., 2007). To see whether DSF has any effect on PR-1 expression, RNA gel blot analysis was performed with RNA extracted from the N. benthamiana leaves at 4, 8, and 24h after infiltration with either DSF, water (control), xanthan, or xanthan+DSF. In response to control (water), accumulation of PR-1 transcript did not change much during 4–24h after infiltration (Fig. 6K). In contrast, accumulation of PR-1 transcript increased to at least 2-fold more after infiltration with DSF at 8–24h, compared with the response to control treatment. Interestingly, infiltration with xanthan alone or co-infiltration of xanthan+DSF produced a significant reduction in PR-I expression at 4, 8, and 24h (Fig. 6K). This result suggests that DSF induces expression of PR-1 in N. benthamiana while xanthan suppresses PR-1 expression.
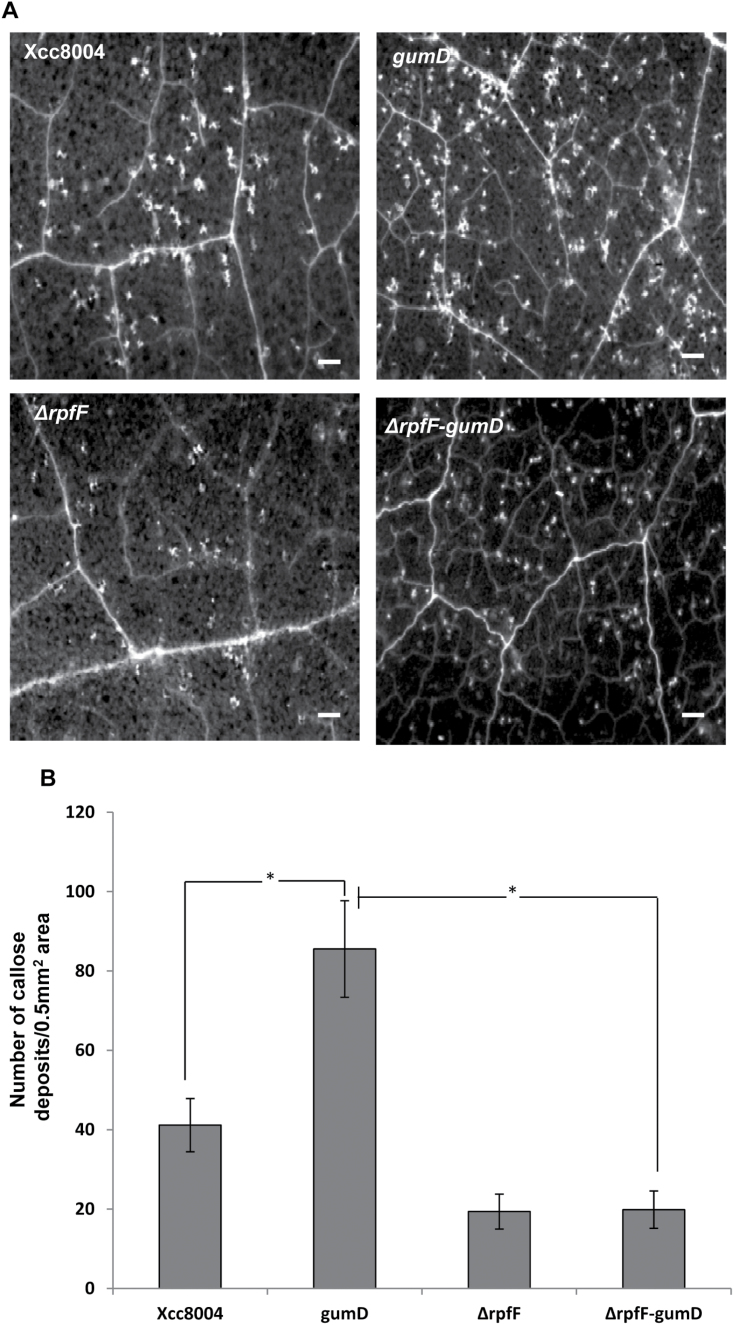
Xanthan has been proposed to be involved in the suppression of the plant defence response and promotion of pathogenesis presumably by suppressing host callose synthesis (Yun et al., 2006). It has been reported earlier that Xcc mutants deficient in EPS production induced higher amounts of callose deposition in N. benthamiana leaves. A higher amount of callose deposition was also observed in N. benthamiana leaves that were infiltrated with the gumD mutant alone compared with those infiltrated with the wild-ype Xcc8004 strain (Fig. 7A, B). To investigate further the interplay between DSF and EPS (xanthan) in Xcc–plant interaction, a ΔrpfF-gumD double mutant was made. The callose deposition assay in N. benthamiana leaves indicated that the ΔrpfF-gumD double mutant induced significantly lower amounts of callose deposits compared with the gumD mutant (Fig. 7A, B). The results indicated that the higher amount of defence response-associated callose deposition induced by the EPS-deficient mutants of Xcc is largely due to the production of DSF. It has been shown that infiltration of 2-deoxy-d-glucose (2DDG), an inhibitor of callose synthesis (Jaffe and Leopold, 1984), could reduce defence response-associated callose deposition induced by Xcc strains in N. benthamiana (Yun et al., 2006). To examine whether the DSF-induced callose deposition can be inhibited by 2DDG, pre-treatment or co-infiltration studies with 2DDG were carried out. The callose deposition assay indicated that either pre- or co-infiltration of 2DDG with DSF significantly reduced the callose deposition in N. benthamiana leaves compared with DSF alone (Supplementary Fig. S8 at JXB online).
Fig. 7.
Callose deposition induced by different strains of Xcc. (A) N. benthamiana leaves were infiltrated with a 1×106 cfu ml–1 suspension of different Xcc strains; Xcc8004 (wild type), gumD (xanthan-deficient mutant), ΔrpfF (DSF-deficient rpfF deletion mutant), and the ΔrpfF-gumD double mutant. Callose deposition was visualized by staining with aniline blue and examined using a stereo fluorescence microscope 24h post-inoculation. White dots in these pictures are indicative of callose deposition. Scale bars=500 μm. (B) Average number of callose deposits per 0.5mm2 area. Error bars represent SD values from three leaves of each plant and three independent experiments. Six microscopic fields from each leaf were analysed. * indicates (P<0.001) significantly different callose deposits induced by the Xcc gumD mutant compared with either the wild-type Xcc8004 strain or the ΔrpfF-gumD double mutant as determined by two-tailed Student’s t-test.
Since Xcc xanthan could suppress DSF-induced callose deposition, next it was determined whether Xcc xanthan could rescue the defence response-associated callose deposition and growth defect exhibited by the wild-type PssB728a expressing the Xcc rpfF [PssB728a (pRpfF)]. Leaves of N. benthamiana were co-infiltrated with wild-type PssB728a harbouring the Xcc rpfF PssB728a (pRpfF) and the empty vector control PssB728a (pHM1) with or without Xcc xanthan. Callose deposition and in planta growth assays indicated that exogenous Xcc xanthan suppressed the induced callose deposition as well as rescued the reduced growth phenotype exhibited by PssB728a (pRpfF) (Fig. 5; Supplementary Fig. S9A–E at JXB online).
Pre-treatment or co-inoculation with DSF inhibits the growth of Xcc8004 and reduces the severity of disease symptoms in N. benthamiana
It has been demonstrated that either pre-treatment or co-inoculation with microbial elicitors (PAMPs or MAMPs) induces disease resistance, which restricts the growth of pathogenic bacteria inoculated subsequently (Jha et al., 2007; Nguyen et al., 2010). To test the proposed role of DSF in inducing the defence response, N. benthamiana leaves were pre-infiltrated with 100 μM DSF 16h before inoculation with 1×106 cfu ml–1 suspensions of the wild-type Xcc8004 strain. Bacterial growth in the zone of infiltration was measured at 0, 24, and 48h post-inoculation (Fig. 8A). The number of wild-type Xcc8004 bacteria recovered from leaves pre-treated with DSF was significantly less (~7-fold) than the number recovered from leaves either pre-treated with water (control) or pre-infiltrated with DSF+xanthan (Fig. 8A).
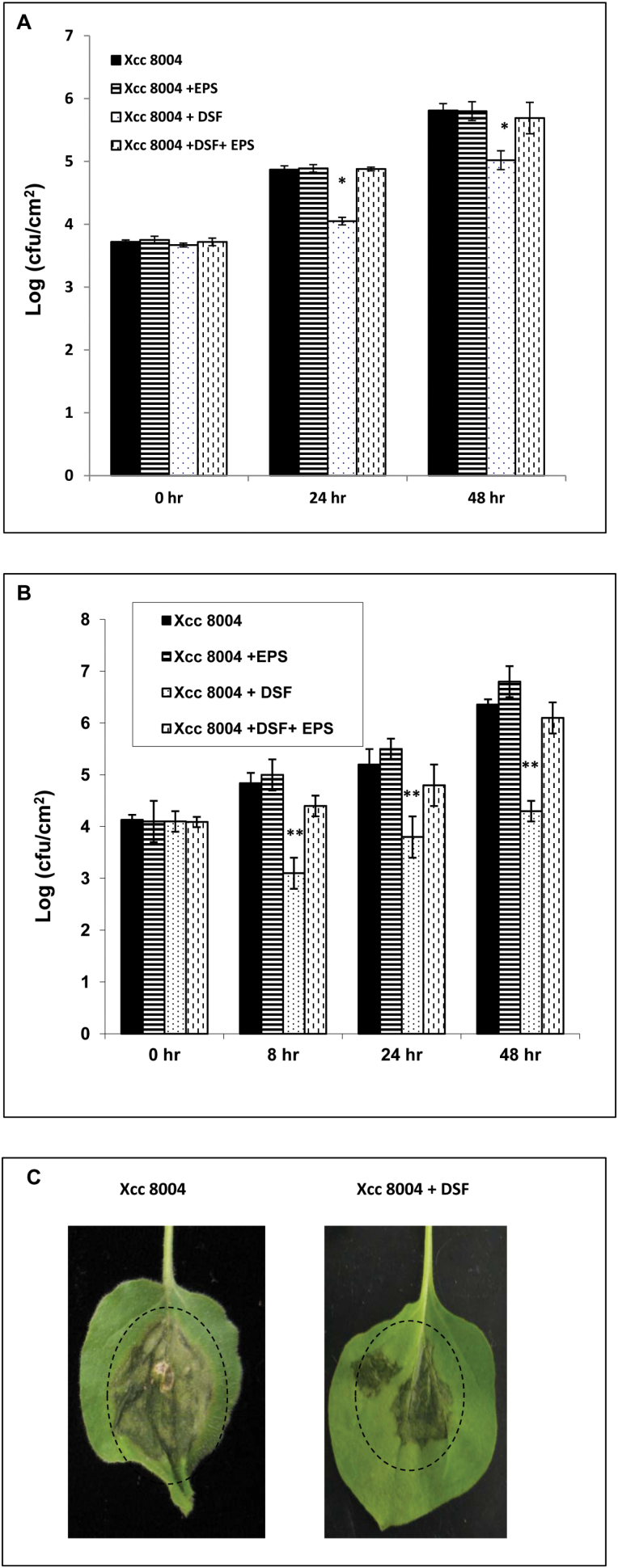
Fig. 8.
Pre-treatment or co-infiltration with DSF inhibits wild-type Xcc8004 growth in N. benthamiana leaves. (A) Leaves of 4-week-old plants were pre-infiltrated with either control buffer (black bars), EPS (xanthan; 0.5mg ml–1), DSF (100 μM), or DSF+EPS (xanthan; 0.5mg ml–1) by syringe infiltration 16h prior to inoculation with a 107 cfu ml–1 suspension of the wild-type Xcc8004 strain. Bacterial populations were measured at 0, 24, and 48h post-inoculation. Values presented are average log (cfu cm–2) from six leaves (three leaves each from two independent experiments). * indicates (P≤0.05) a significantly lower bacterial population in the DSF-pre-inoculated leaves compared with the leaves inoculated with the wild-type Xcc8004 strain alone based on a pairwise Student’s t-test. (B) Bacterial growth assay in N. benthamiana leaves co-infiltrated with the Xcc8004 wild-type strain with either water control (black bars), DSF (100 μM), EPS (xanthan; 0.5mg ml–1), or DSF+EPS (xanthan). The bacterial population was measured at 0, 8, 24, and 48h after inoculation. Values presented are average log (cfu ml–1) from six leaves (three leaves each from two independent experiments). * indicates (P≤0.001) a significantly lower bacterial population in the DSF-co-inoculated leaves compared with the leaves inoculated with wild-type Xcc alone based on a pairwise Student’s t-test. (C) Photographs of representative leaves from the co-inoculation experiments 4 d post-inoculation. N. benthamiana leaves exhibit water soaking-like disease symptoms when inoculated with the Xcc8004 wild-type strain (shown in a dotted circle). Leaves co-inoculated with the Xcc8004 wild-type strain+DSF exhibited less vigorous water soaking symptoms compared with leaves treated with the Xcc8004 strain alone.
Further, to investigate whether co-infiltration of DSF with the wild-type Xcc8004 strain could reduce their growth, N. benthamiana leaves were co-infiltrated with 100 μM DSF and a 106 cfu ml–1 suspension of the wild-type Xcc8004 strain. Bacterial growth in the zone of infiltration was measured at 0, 8, 24, and 48h after inoculation. The number of bacteria recovered from leaves that were co-infiltrated with DSF was significantly less (~15- to 20- fold) as compared with those either infiltrated with water (control) or infiltrated together with DSF and xanthan (Fig. 8B). Interestingly, N. benthamiana leaves co-infiltrated with wild-type Xcc8004 and DSF exhibited reduced disease symptoms (water soaking) compared with leaves inoculated with the wild-type Xcc8004 strain alone (Fig. 8C).
Pre-treatment with DSF induces resistance to Xanthomonas oryzae pv. oryzae in rice
Xoo, a member of the Xanthomonas group of phytopathogens, causes serious disease of rice known as bacterial leaf blight (Niňo-Liu et al., 2006). It has been demonstrated that in Xoo, DSF is required for co-ordination of virulence-associated functions (Chatterjee and Sonti, 2002; Rai et al., 2012). Since DSF mediated cell–cell signalling is conserved among many Xanthomonas, experiments were carried out to determine whether the defence response provoked by DSF would provide resistance against subsequent Xoo infection. Previously, it was demonstrated that pre-treatment of rice leaves with Type II effectors such as cellulase and lipase, which function as potential DAMPs, provides resistance against subsequent Xoo infection (Jha et al., 2007; Aparna et al., 2009). The midveinal regions of rice leaves were injected with DSF, buffer, and cellulase. Approximately 24h later, the leaves were inoculated with the 1×109 cfu ml–1 suspension of the wild-type Xoo (BXO43) strain, 1–2cm above the point of pre-treatment. The wild-type Xoo strain was able to cause lesions, as shown by browning of the midvein and adjacent regions, in the majority of the leaves (80–100%) that had been pre-injected with buffer (Table 1; Supplementary Fig. S10 at JXB online). However, the wild-type Xoo strain was either unable to cause lesions or exhibited lesions of reduced lengths in leaves pre-injected with either DSF or cellulase (Table 1; Supplementary Fig. S10).
Table 1.
Pre-treatment of rice leaves with DSF induces resistance to subsequent Xanthomonas oryzae pv. oryzae infection
| Treatmentsa | Efficiency of infection (%)b | Lesion length (cm)c | ||||
|---|---|---|---|---|---|---|
| Exp 1 | Exp 2 | Exp 3 | Exp 1 | Exp 2 | Exp 3 | |
| Buffer | 80 (8/10) | 83 (10/12) | 100 (20/20) | 7.2±3.7 | 10.2±6.5 | 10.05±4.3 |
| DSF (100 μM) | 50 (5/10) | 66.6 (8/12) | 70 (14/20) | 1.2±1.9* | 2.7±2.6* | 2.7±2.8* |
| Cellulase (1mg ml–1) | 30 (3/10) | 50 (6/12) | 64.2 (9/14) | 1.4±1.1* | 3.3±4.6* | 4.0±4.3* |
a The midvein of rice leaves of 40-day-old susceptible rice plants (TN-1) were pre-injected either with buffer alone or with buffer containing 100 μM DSF or cellulase (1mg ml–1). After 24h, Xanthomonas oryzae pv. oryzae (1×109 cfu ml–1) was inoculated onto the midvein, 1–2cm above the point of initial injection, by prick inoculation. Lesion lengths were measured 12 d post-inoculation.
b The percentage efficiency of infection is presented. Values in parentheses indicate the number of leaves that exhibited infection symptoms compared with the total number of infected leaves.
c Lesion lengths (cm) measured 12 d after inoculation. The lesion length was measured from at least 10 infected leaves from three independent experiments, and the mean values were determined ±SD. A Student’s two-tailed t-test for independent mean was performed in pairwise combinations for each treatment with the control (buffer). *P<0.005 indicates a significant difference in comparison with the buffer treatment.
Discussion
In this study, it was demonstrated that DSF (cis-11-methyl-2-dodecenoic acid), a widely conserved cell–cell signalling molecule from the Xanthomonas group of phytopathogens, induces basal defence response in N. benthamiana, Arabidopsis, and rice. DSF induces a HR-like response, PCD, the accumulation of autofluorescent compounds, including callose deposition, accumulation of H2O2, and expression of the PR-1 gene. Both direct and pre-treatment with DSF induce callose deposition as well as disease resistance against subsequent infection by wild-type Xcc and Xoo, respectively. The results clearly indicated that the infiltration of higher concentrations of DSF (0.2–1mM) results in visible HR-like symptoms. However, at lower concentrations (in the range of 20–100 μM), DSF induces callose deposition without any visible HR-like symptoms (Fig. 2). It is also possible that a certain percentage of DSF infiltrated in the leaf is degraded or metabolized by plant factors, which could be one of the reasons for the fact that a higher concentration of DSF is required to elicit a defence respose compared with other strong elicitors such as bacterial flagellin. However, the in planta DSF production assay using the wild-type Xcc and rpfF mutant (8523) strain infiltrated with standard DSF indicated that the Xcc wild-type strain produces a considerably high amount of DSF at 24–48h post-inoculation (~40–100 μM) (Supplementary Fig. S5 at JXB online).
In a recent study, it was demonstrated that plants pre-treated with a lower concentration of AHL (a QS signalling molecule) exhibited an induced defence response (MTI) on subsequent challenge with flg22 (Schenk et al., 2014). It has been proposed that AHL primes plants for the cell wall-mediated defence response that may be involved in inducing resistance to subsequent bacterial infection. The present results indicate that a low DSF concentration (10 μM) does not induce any visible defence response (callose deposition). However, leaves pre-infiltrated with 10 μM DSF exhibit induced callose deposition on subsequent challenge with flg22 (a potential bacterial MAMP) (Supplementary Fig. S4 at JXB online). It is possible that at an early stage of infection, when the DSF levels are low, it primes plants for cell wall-based defence response and may influence MTI or ETI (elicitor-triggered immunity).
An increasing body of research now indicates that both exogenous and endogenous unsaturated fatty acids play an important role in the plant defence response and can influence plant–microbe interactions (Upchurch, 2008; Kachroo et al., 2001, 2003; Savchenko et al., 2010). Exogenous fatty acids not commonly abundant in plants, such as eicosapentaenoic acid and arachidonic acid, act as potent elicitors of the defence response in solanaceous plants (Bostock et al., 1981; Knight et al., 2001). Arachidonic acid, a cis-unsaturated fatty acid characteristic of oomycete pathogens belonging to the Phytophthora, acts as a potent elicitor of PCD and the defence response in plants at higher concentrations (Knight et al., 2001; Upchurch 2008; Savchenko et al., 2010). It has also been proposed that cis-unsaturation is a critical structural feature for activity of these lipophilic fungal elicitors in inducing the defence response in plants (Bostock et al., 1981, 1992; Bostock, 2005). It is pertinent to note in this regard that the DSF family of signalling molecules has a characteristic cis-unsaturated double bond at the 2-position and has been shown to be a key structural feature for its activity as a QS signalling molecule in the Xanthomonas group of phytopathogens (Wang et al., 2004; Deng et al., 2011). The present results indicated that the ability of DSF to induce the plant defence response is specific, as infiltration of a range of fatty acid compounds including trans-11-methyl dodecenoic acid did not result in visible HR-like symptoms or callose deposition (Supplementary Table S2 at JXB online). It is possible that similar to the DSF-sensing machinery in Xanthomonas phytopathogens, plants might have also evolved a machinery which could recognize the DSF family of signalling molecules containing the characteristic structural feature of a cis-unsaturated double bond.
The present results suggest that the production of DSF in Xcc bacteria is associated with the induced defence response, as the DSF-deficient rpfF mutant of Xcc (Xcc8523) induced reduced amounts of callose deposits in N. benthamiana leaves compared with those infiltrated with either the wild-type Xcc8004 strain or the DSF-deficient mutant strain harbouring the wild-type rpfF allele (Fig. 4C, D). It is possible that the reduced callose deposition induced by the DSF-deficient rpfF mutant may be due to the fact that DSF may positively regulate the production of factors which can induce callose deposition, such as extracellular cell-wall-hydrolysing enzymes. Interestingly, production of DSF in a non-DSF-producing phytopathogen, PssB728a, a pathogen of bean and the model plant N. benthamiana (Loper and Lindow, 1987; Vinatzer et al., 2006), leads to induced callose deposition and reduction of PssB728a growth in N. benthamiana leaves (Figs 4, 5). The DSF synthase RpfF has been reported to be a promiscuous enzyme which can produce closely related members of the DSF family of signalling molecules (Beaulieu et al., 2013). It is also possible that B728a harbouring the Xcc RpfF may produce an altered DSF family of signalling molecules.
It is becoming increasingly evident that during infection, pathogens actively suppress the plant’s PAMP-triggered defence responses. Gram-negative phytopathogens secrete several effector proteins via the type III secretion system (TTSS) which are involved in the suppression of the elicitor-triggered plant defence response (Hauck et al., 2003; DebRoy et al., 2004; Jamir et al., 2004). Apart from effector proteins, several non-proteinaceous effectors from pathogenic bacteria have been reported to play a role in suppression of the plant defence response, such as EPS, glucan, and coronatin (for reviews, see Chisholm et al., 2006; Boller and Felix, 2009). The EPS xanthan is required for virulence and colonization in several members of the Xanthomonas group of phytopathogens. In Xcc, production of EPS is positively regulated by cell–cell signalling mediated by DSF (Barber et al., 1997; Wang et al., 2004). Further, it has been shown that xanthan suppresses the elicitor-induced defence response, presumably by the chelation of extracellular calcium ions and suppression of the plant defence response by inhibiting the callose biosynthesis which is involved in cell wall fortification (Yun et al., 2006; Aslam et al., 2008). The present results suggest that wild-type Xcc is able to suppress the DSF-triggered plant defence response by production of the EPS xanthan (Fig. 6). The Xcc mutants defective in xanthan production induced significantly higher amounts of callose deposits and were unable to suppress DSF-induced callose deposition compared with the wild-type strain (Figs 6, 7). Further, it has been demonstrated that the DSF- and EPS (xanthan)-deficient double mutant (ΔrpfF-gumD) of Xcc induced significantly lower amounts of callose compared with that induced by the EPS-deficient gumD mutant (Fig. 7). These results suggest that the defence responses that are provoked by the EPS-deficient gum mutants are mostly contributed due to production of DSF.
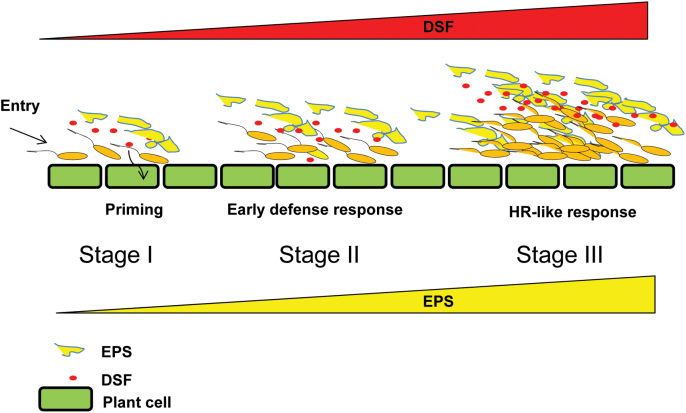
In Xcc, DSF-mediated cell–cell signalling positively regulates the production of several secreted virulence factors such as EPS and cell-wall-degrading hydrolytic enzymes in a density-dependent manner. EPS is required for virulence and is involved in biofilm formation, a social behaviour important for colonization. A model is proposed that elucidates the functional interplay between DSF and EPS in Xanthomonas–plant interaction (Fig. 9). At the initial stage of infection and colonization (stage I), Xcc gains entry through hydathodes or stomata, and colonizes in the xylem vessel. At this stage (low cell density; stage I), the production of DSF and EPS is low. At lower concentrations of DSF (presumably <10 μM), DSF may be involved in priming (sensitization) plants for a cell wall-based defence mechanism, which may influence MTI mediated by MAMPs such as flagellin or lipopolysaccharide. MTI is further suppressed by Type III secretion system effectors. In stage II, there is increase in Xcc cell number, which is associated with increased production of DSF and EPS. An increased DSF level (≥20 μM) induces an early plant defence response (callose deposition), which is suppressed by EPS. At a late stage of infection (stage III), a high level of DSF is produced (50–100 μM) due to further in planta growth of Xcc. This may lead to a further increase in the production of EPS, a virulence-associated factor positively regulated by DSF. A high EPS level can suppress the plant defence response provoked by DSF including early HR-like symptoms.
Fig. 9.
Proposed model for functional interplay between diffusible signalling factor (DSF) and extracellular polysaccharide (EPS) in Xanthomonas–plant interaction. At the initial stage of infection and colonization (stage I), Xcc gains entry through hydathodes or stomata, and colonizes in the xylem vessel. At this stage (at low cell density; stage I), the production of DSF and EPS is low. At lower concentrations of DSF (presumably ≤10 μM), DSF may be involved in priming (sensitization) plants for MTI (MAMP-triggered immunity) mediated by MAMPs such as flagellin or LPS (lipopolysaccharide). MTI is further suppressed by Type III secretion system effectors. At stage II, there is increase in Xcc cell number, which is associated with increased production of DSF and EPS. An increased DSF level (≥20 μM) induces an early plant defence response (callose deposition), which is suppressed by EPS. At a late stage of infection (stage III), there is a further increase in cell density due to growth of Xcc in planta. Due to high cell density, a high level of DSF is produced (50–100 μM). This may lead to a further increase in the production of EPS, a virulence-associated factor positively regulated by DSF. A high EPS level can suppress the plant defence response provoked by DSF including early HR-like symptoms.
Exciting further work and a challenge would be to understand the biochemical and signal transduction pathways that are involved in DSF perception and activation of plant defence responses. It will be also exciting to examine whether cell–cell signalling molecules belonging to different chemical classes from plant pathogens may also contribute to induction of host defence responses.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Induction of the hypersensitive (HR)-like response by DSF isolated from the cell-free culture supernatant of the wild-type Xcc8004 strain by ethyl acetate extraction.
Figure S2. Response of the Xcc DSF biosensor strain to synthetic DSF.
Figure S3. DSF induced callose deposition in N. benthamiana leaves.
Figure S4. Callose deposition in N. benthamiana leaves pre-treated with DSF and subsequently challenged with flg22.
Figure S5. Detection of DSF production in N. benthamiana leaves using the Xcc DSF biosensor strains.
Figure S6. In planta growth of Pss harbouring the Xcc pRpfF.
Figure S7. EPS production assay.
Figure S8. 2-Deoxy-d-glucose (2DDG) inhibits the DSF-induced callose deposition in N. benthamiana leaves.
Figure S9. Callose deposition induced by the P. syringae PssB728a wild-type strain harbouring Xcc rpfF is suppressed by Xcc xanthan.
Figure S10. Pre-treatment of rice leaves with DSF induces resistance against subsequent Xanthomonas oryzae pv. oryzae (Xoo) infection.
Table S1. Infiltration of synthetic DSF into the leaves of rice and Arabidopsis thaliana induces callose deposition.
Table S2. Specificity of DSF in inducing callose deposition in N. benthamiana leaves.
Supplementary Materials and methods
Acknowledgements
We are grateful to S.K. Ray for helpful discussion. AK is the recipient of Junior and Senior Research Fellowships of the Council of Scientific and Industrial Research. NRN and AR were supported by a post-doctoral research fellowship from the Department of Biotechnology (DBT) and Council of Scientific and Industrial Research (CSIR), Government of India. This study was supported by funding to SC from the DBT, Government of India; IYBA; and core funding from the CDFD.
References
- Adam L, Somerville SC. 1996. Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana . The Plant Journal 9, 341–356. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparna G, Chatterjee A, Sonti RV, Sankaranarayanan R. 2009. A cell wall-degrading esterase of Xanthomonas oryzae requires a unique substrate recognition module for pathogenesis on rice. The Plant Cell 21, 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam SN, Newman MA, Erbs G, et al. 2008. Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Current Biology 18, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJG, Slater H, Dow JM, Williams P, Daniels MJ. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Molecular Microbiology 24, 555–566. [DOI] [PubMed] [Google Scholar]
- Beaulieu ED, Ionescu M, Chatterjee S, Yokota K, Trauner D, Lindow S. 2013. Characterization of a diffusible signaling factor from Xylella fastidiosa . MBio 4, e00539–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Bennett MH, Mansfield JW. 1995. Hrp mutant of Pseudomonas syringae pv. phaseolicola induces cell wall alteration but not membrane damage leading to the hypersensitive reaction in lettuce. Plant Physiology 108, 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H, Christensen QH, Feng Y, Wang H, Cronan JE. 2012. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Molecular Microbiology 83, 840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Boon C, Deng Y, Wang LH, He Y, Xu JL, Fan Y, Pan SQ, Zhang LH. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME Journal 2, 27–36. [DOI] [PubMed] [Google Scholar]
- Bostock RM. 2005. Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annual Review of Phytopathology 43, 545–580. [DOI] [PubMed] [Google Scholar]
- Bostock RM, Kuc JA, Laine RA. 1981. Eicosapentaenoic and arachidonic acids from Phytophthora infestans elicit fungitoxic sesquiterpenes in the potato. Science 212, 67–69. [DOI] [PubMed] [Google Scholar]
- Bostock RM, Yamamoto H, Choi D, Ricker KE, Ward BL. 1992. Rapid stimulation of 5-lipoxygenase activity in potato by the fungal elicitor arachidonic acid. Plant Physiology 100, 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D, Bonas U. 2010. Regulation and secretion of Xanthomonas virulence factors. FEMS Reviews 34, 107–133. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Sonti RV. 2002. rpfF mutants of Xanthomonas oryzae pv. oryzae are deficient for virulence and growth under low iron conditions. Molecular Plant-Microbe Interactions 15, 463–471. [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. 2006. Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814. [DOI] [PubMed] [Google Scholar]
- Darvill AG, Albersheim P. 1984. Phytoalexins and their elicitors—a defense against microbial infection in plants. Annual Review of Plant Physiology and Plant Molecular Biology 35, 243–275. [Google Scholar]
- DebRoy S, Thilmony R, Kwack YB, Nomura K, He SY. 2004. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proceedings of the National Academy of Sciences, USA 101, 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wu J, Tao F, Zhang LH. 2011. Listening to a new language: DSF-based quorum sensing in gram-negative bacteria. Chemical Reviews 111, 160–173. [DOI] [PubMed] [Google Scholar]
- Felix G, Boller T. 2003. Molecular sensing of bacteria in plants. The highly conserved RNA binding motif RNP-1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. Journal of Biological Chemistry 278, 6201–6208. [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal 18, 265–276. [DOI] [PubMed] [Google Scholar]
- Fuqua C, Parsek MR, Greenberg EP. 2001. Regulation of gene expression by cell–cell communication: acyl-homoserine lactone quorum sensing. Annual Review of Genetics 35, 4439–4468. [DOI] [PubMed] [Google Scholar]
- Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR LuxI family of cell density-responsive transcriptional regulators. Journal of Bacteriology 176, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA. 2009. Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiology 149, 1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann DR, Rathjen JP. 2007. Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana . The Plant Journal 49, 607–618. [DOI] [PubMed] [Google Scholar]
- Hauck P, Thilmony R, He SY. 2003. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proceedings of the National Academy of Sciences, USA 100, 8577– 8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y-W, Wu J, Cha JS, Zhang LH. 2010. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiology 10, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe MJ, Leopold AC. 1984. Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-d-glucose. Planta 161, 20–26. [DOI] [PubMed] [Google Scholar]
- Jamir Y, Guo M, Oh HS, Petnicki-Ocwieja T, Chen S, Tang X, Dickman MB, Collmer A, Alfano JR. 2004. Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. The Plant Journal 37, 554–565. [DOI] [PubMed] [Google Scholar]
- Jha G, Rajeshwari R, Sonti RV. 2007. Functional interplay between two Xanthomonas oryzae pv. oryzae secretion systems in modulating virulence on rice. Molecular Plant-Microbe Interactions 20, 31–40. [DOI] [PubMed] [Google Scholar]
- Kachroo P, Kachroo A, Lapchyk L, Hildebrand D, Klessig DF. 2003. Restoration of defective cross talk in ssi2 mutants: role of salicylic acid, jasmonic acid, and fatty acids in SSI2-mediated signaling. Molecular Plant-Microbe Interactions 16, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. 2001. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proceedings of the National Academy of Sciences, USA 98, 9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. Journal of Laboratory and Clinical Medicine 44, 301–307. [PubMed] [Google Scholar]
- Knight VI, Wang H, Lincoln J.-E., Lulai EC, Gilchrist DG, Bostock RM. 2001. Hydroperoxides of fatty acids induce programmed cell death in tomato protoplasts. Physiological and Molecular Plant Pathology 59, 277–286. [Google Scholar]
- Koch E, Slusarenko A. 1990. Arabidopsis is susceptible to infection by a downy mildew fungus. The Plant Cell 2, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. 2004The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. The Plant Cell 16, 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RA, Wilson MM, Guss AM, Metcalf WW. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Archives of Microbiology 178, 193–201. [DOI] [PubMed] [Google Scholar]
- Loper JE, Lindow SE. 1987. Lack of evidence for in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology 77, 1449–1454. [Google Scholar]
- Mansfield J, Genin S, Magori S, et al. 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Patholology 13, 614–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KL, Almeida RPP, Purcell AH, Lindow SE. 2004. Cell–cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proceedings of the National Academy of Sciences, USA 101, 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KL, Chatterjee S, Ho KA, Lindow SE. 2008. Virulence of plant pathogenic bacteria attenuated by degradation of fatty acid cell-to-cell signaling factors. Molecular Plant-Microbe Interactions 21, 326–334. [DOI] [PubMed] [Google Scholar]
- Newman M-A, Lahaye ER, Parr A, Daniels MJ, Dow JM. 2002. Prior exposure to lipopolysaccharide potentiates expression of plant defenses in response to bacteria. The Plant Journal 29, 487–495. [DOI] [PubMed] [Google Scholar]
- Ng W-L, Bassler BL. 2009. Bacterial quorum-sensing network architectures. Annual Review of Genetics 43, 197–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HP, Chakravarthy S, Velásquez AC, McLane HL, Zeng L, Nakayashiki H, Park DH, Collmer A, Martin GB. 2010. Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana . Molecular Plant-Microbe Interactions 23, 991–999. [DOI] [PubMed] [Google Scholar]
- Niňo-Liu DO, Ronald PC, Bogdanove AJ. 2006. Xanthomonas oryzae pathovars: model pathogens of a model crop. Molecular Plant Pathology 7, 303–324. [DOI] [PubMed] [Google Scholar]
- Nurnberger T, Brunner F, Kemmerling B, Piater L. 2004. Innate immunity in plants and animals: striking similarities and obvious differences. Immunology Reviews 198, 249–266. [DOI] [PubMed] [Google Scholar]
- Pennell RI, Lamb C. 1997. Programmed cell death in plants. The Plant Cell 9, 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan BB, Chatterjee S. 2014. Reversible non-genetic phenotypic heterogeneity in bacterial quorum sensing. Molecular Microbiology 92, 557–569. [DOI] [PubMed] [Google Scholar]
- Quiñones B, Pujol CJ, Lindow SE. 2004. Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae . Molecular Plant-Microbe Interactions 17, 521–531. [DOI] [PubMed] [Google Scholar]
- Rai R, Pradhan BB, Ranjan M, Chatterjee S. 2012. Atypical regulation of virulence associated functions by a diffusible signal factor in Xanthomonas oryzae pv. oryzae . Molecular Plant-Microbe Interactions 25, 789–801. [DOI] [PubMed] [Google Scholar]
- Rigano LA, Payette C, Brouillard G, et al. 2007. Bacterial cyclic beta-(1, 2)-glucan acts in systemic suppression of plant immune responses. The Plant Cell 19, 2077–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RP, Dow JM. 2011. Communication with a growing family: diffusible signal factor (DSF) signaling in bacteria. Trends in Microbiology 19, 145–152. [DOI] [PubMed] [Google Scholar]
- Savchenko T, Walley JW, Chehab EW, Xiao Y, Kaspi R, Pye MF, Mohamed ME, Lazarus CM, Bostock RM, Dehesh K. 2010. Arachidonic acid: an evolutionarily conserved signaling molecule modulates plant stress signaling networks. The Plant Cell 22, 3193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutumicum . Gene 145, 69–73. [DOI] [PubMed] [Google Scholar]
- Schenk ST, Hernández-Reyes C, Samans B, et al. 2014. N-Acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. The Plant Cell 26, 2708–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RW, Lindow SE. 2009. Two dissimilar N-acyl-homoserine lactone acylases of Pseudomonas syringae influence colony and biofilm morphology. Applied and Environmental Microbiology 75, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano F, Torres P, Sendin L, Bermejo C, Filippone P, Vellice G, Ramallo J, Castagnaro A, Vojnov A, Marano MR. 2006. Analysis of the molecular basis of Xanthomonasaxonopodis pv. citripathogenesis in Citrus limon . Electronic Journal of Biotechnology 9, 199. [Google Scholar]
- Silipo A, Molinaro A, Sturiale L, Dow JM, Erbs G, Lanzetta R, Newman MA, Parrilli M. 2005. The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris . Journal of Biological Chemistry 280, 33660–33668. [DOI] [PubMed] [Google Scholar]
- Stone JM, Heard JE, Asai T, Ausubel FM. 2000. Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants. The Plant Cell 12, 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. The Plant Journal 11, 1187–1194. [Google Scholar]
- Thowthampitak J, Shaffer BT, Prathuangwong S, Loper JE. 2008. Role of rpfF in virulence and exoenzyme production of Xanthomonas axonopodis pv. glycines, the causal agent of bacterial pustule of soybean. Phytopathology 98, 1252–1260. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Mew TW, Wakimoto S. 1982. Bacteriological and pathological characteristics of wild types and induced mutants of Xanthomonas campestris pv. oryzae . Phytopathology 72, 43–46. [Google Scholar]
- Upchurch RG. 2008. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnology Letters 30, 967–977. [DOI] [PubMed] [Google Scholar]
- Vinatzer BA, Teitzel GM, Lee MW, Jelenska J, Hotton S, Fairfax K, Jenrette J, Greenberg JT. 2006. The type III effector repertoire of Pseudomonas syringae pv. syringae B728a and its role in survival and disease on host and non-host plants. Molecular Microbiology 62, 26–44. [DOI] [PubMed] [Google Scholar]
- Wang LH, He Y, Gao Y, et al. 2004. A bacterial cell–cell communication signal with cross-kingdom structural analogues. Molecular Microbiology 51, 903–912. [DOI] [PubMed] [Google Scholar]
- Windgassen M, Urban A, Jaeger KE. 2000. Rapid gene inactivation in Pseudomonas aeruginosa . FEMS Microbiology Letters 193, 201–205. [DOI] [PubMed] [Google Scholar]
- Wu H, Song Z, Hentzer M, et al. 2000. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa . Microbiology 146, 2481–2493. [DOI] [PubMed] [Google Scholar]
- Yun MH, Torres PS, El Oirdi M, Rigano LA, Gonzalez-Lamothe R, Marano MR, Castagnaro AP, Dankert MA, Bouarab K, Vojnov AA. 2006. Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiology 141, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.