Highlight
NH4 + protects tomato plants against Pseudomonas syringae by triggering putrescine, ABA, and H2O2 accumulation. H2O2 derived from NH4 + mild chronic stress induces ABA-dependent signalling pathways required for the establishment of systemic acquired acclimation.
Key words: ABA, H2O2, induced resistance, NH4+ nutrition, putrescine, systemic acquired acclimation.
Abstract
NH4 + nutrition provokes mild toxicity by enhancing H2O2 accumulation, which acts as a signal activating systemic acquired acclimation (SAA). Until now, induced resistance mechanisms in response to an abiotic stimulus and related to SAA were only reported for exposure to a subsequent abiotic stress. Herein, the first evidence is provided that this acclimation to an abiotic stimulus induces resistance to later pathogen infection, since NH4 + nutrition (N-NH4 +)-induced resistance (NH4 +-IR) against Pseudomonas syringae pv tomato DC3000 (Pst) in tomato plants was demonstrated. N-NH4 + plants displayed basal H2O2, abscisic acid (ABA), and putrescine (Put) accumulation. H2O2 accumulation acted as a signal to induce ABA-dependent signalling pathways required to prevent NH4 + toxicity. This acclimatory event provoked an increase in resistance against later pathogen infection. N-NH4 + plants displayed basal stomatal closure produced by H2O2 derived from enhanced CuAO and rboh1 activity that may reduce the entry of bacteria into the mesophyll, diminishing the disease symptoms as well as strongly inducing the oxidative burst upon Pst infection, favouring NH4 +-IR. Experiments with inhibitors of Put accumulation and the ABA-deficient mutant flacca demonstrated that Put and ABA downstream signalling pathways are required to complete NH4 +-IR. The metabolic profile revealed that infected N-NH4 + plants showed greater ferulic acid accumulation compared with control plants. Although classical salicylic acid (SA)-dependent responses against biotrophic pathogens were not found, the important role of Put in the resistance of tomato against Pst was demonstrated. Moreover, this work revealed the cross-talk between abiotic stress acclimation (NH4 + nutrition) and resistance to subsequent Pst infection.
Introduction
Plants employ diverse constitutive and inducible defence strategies to counteract colonization by microbial pathogens (Spoel and Dong, 2012). One of the earliest cellular responses following elicitation of pathogen-associated molecular patterns (PAMPs) is the oxidative burst produced by NADPH oxidases, cell wall peroxidases, or polyamine oxidases (Yoda et al., 2006). In addition to the classical salicylic acid (SA) and jasmonic acid (JA)/ethylene defence pathways, plant immunity to microbial pathogens is regulated by distinct pathways related to nitrogen (N) compounds such as amino acids and polyamines (PAs) (Takahashi and Kakehi, 2010; Zeier, 2013). PAs, including putrescine (Put), spermidine (Spd), and spermine (Spm), are positively charged small metabolites implicated in physiological processes, including organogenesis, embryogenesis, floral initiation and development, leaf senescence, pollen tube growth, and fruit development and ripening (Tiburcio et al., 2014). PAs are synthesized from amino acids by decarboxylation of ornithine or arginine by ornithine decarboxylase (ODC) or arginine decarboxylase (ADC), respectively (Walters, 2003). In addition to free PAs, some PAs are conjugated to hydroxycinnamic acids, and the products of PA oxidation participate in the response to abiotic and biotic stresses (Tiburcio et al., 2014). Oxidation of PAs by copper amine oxidases (CuAOs) contributes to the regulation of PA homeostasis and generates catabolic products with biological functions. CuAOs are homodimeric enzymes with high affinity for oxidizing the primary amino groups of Put and cadaverine, and lower affinity for Spd and Spm (Moschou et al., 2012). It is commonly accepted that H2O2 produced by CuAO in Put oxidation has an important role in stress-induced cell wall stiffening, in stomatal movement, and in programmed cell death (Angelini et al., 2008). Although several studies have demonstrated a role for PAs in protection against abiotic stresses (Bouchereau et al., 1999; Kasinathan and Wingler, 2004), little is known about how they act under conditions of biotic stress.
Plants are frequently exposed to a myriad of biotic and abiotic stresses that can act in succession or simultaneously. Being sessile organisms, plants have developed sophisticated acclimation and defence mechanisms to cope with different stress situations (Bray et al., 2000; Boyko and Kovalchuk, 2011; Reddy et al., 2011). These can be activated in the initial tissue exposed to stress as well as in systemic tissues that have not yet been exposed to the stress. Looking at the source of the stimulus, several kinds of induced resistance can be distinguished. If the induced resistance is achieved by treatment with a chemical or natural compound, this phenomenon is known as priming. Priming enables cells to respond to low levels of a stimulus in a more rapid and robust manner than is found in non-primed cells (Conrath, 2011). Thus, when plants are primed and subsequently challenged by pathogens or abiotic stresses, they show a faster and/or stronger activation of defence responses (Prime-A-Plant Group et al., 2006). Recently, it has been demonstrated that hexanoic acid (Hx) induces resistance in Solanum lycopersicum and Arabidopsis thaliana plants against Botrytis cinerea (Vicedo et al., 2009; Kravchuk et al., 2011) and against Pseudomonas syringae pv tomato DC3000 (Pst) (Schalschi et al., 2013) as well as in citrus plants against the fungus Alternaria alternata (Llorens et al., 2013). The activation of defence or acclimation mechanisms in systemic non-challenged tissues is normally termed systemic acquired resistance (SAR)—if the local stimulus is induced by viruses, bacteria, or fungi—or systemic acquired acclimation (SAA)—if the initial stimulus is an abiotic stress situation—and serves an important role in preventing further infection or damage to the entire plant (Baxter et al., 2014). Recent studies have demonstrated that the reactive oxygen species (ROS) wave functions as a general priming signal in plants, warning systemic tissues what is happening is a localized abiotic stress stimulus (Mittler et al., 2011). Upon abiotic stress, SAA is mediated by temporal–spatial interactions of the ROS wave with hormone or amino acid signals activated in systemic tissues (Suzuki et al., 2013). Specifically, recent reports demonstrated that acclimation-induced cross-tolerance in tomato plants is largely attributed to respiratory burst oxidase homologue (RBOH1)-dependent H2O2 production at the apoplast, which may subsequently activate MPK1/2 to induce stress responses (Zhou et al., 2014). In recent years there has been evidence to suggest that plants with increased activation of response mechanisms by acclimation to abiotic stimuli can respond better to biotic stress, although little is known about the mechanisms underlying this type of induced resistance. Mild chronic stress situations can also boost plant stress resistance through induction of acclimation responses. Factors such as light, temperature, drought, mineral concentrations, and biotic infection are all capable of causing extensive damage to plants as well as inducing short- and long-term acclimation responses (Gordon et al., 2013).
NH4 + is a fundamental substrate for amino acids, nucleic acids, alkaloids, and polysaccharides, as well as for secondary metabolites such as PAs (Bagh et al., 2004), in all living organisms (von Wirén and Merrick, 2004). The downstream molecular events produced by NH4 + nutrition have been extensively studied and are related mainly to cell wall stability and biosynthesis, carbon metabolism and energy, primary N metabolism, phytohormones, and signalling molecules (Ariza et al., 2013). However, NH4 + is toxic to cells when it is present at high concentrations in the soil or the nutrient solution because it causes the so-called ‘ammonium syndrome’. This may include leaf chlorosis, lower plant yield production and root/shoot ratio, lower cation content, acidification of the rhizosphere, and changes in several metabolite levels, such as amino acids or organic acids (Britto and Kronzucker, 2002; Bittsánszky et al., 2015). Despite this, recent studies regarding the possible effect of NH4 + nutrition as an inducer indicate that NH4 + or one of its assimilation products (e.g. glutamine or glutamate) may serve as a stress signal and, in NH4 +-grown plants, operate metabolic pathways that induce the accumulation of ROS (Misra and Gupta, 2006). Specifically, Fernández-Crespo et al. (2012) demonstrated that NH4 + nutrition confers protection against subsequent salt stress by reducing Cl– uptake and decreasing its toxicity by priming accumulation of ABA and PAs, and by enhancing the basal content of H2O2 and proline in citrus plants. The authors concluded that NH4 + nutrition triggers mild chronic stress, which may account for the noted stress-induced morphogenetic responses (SIMRs) as part of a general acclimation strategy. The induction of the ‘acclimation stage’ leads to better adaptation to subsequent salt stress. Moreover, Fernández-Crespo et al. (2014) demonstrated that H2O2 and the manipulation of the antioxidant machinery act as intermediaries between mild stress induced by NH4 + nutrition and the development of the acclimation stage.
In this work, the effectiveness of NH4 + nutrition as an inducer of resistance against a biotic stress was tested, selecting for this purpose the pathogen Pst. Evidence is provided that NH4 + nutrition induces resistance against Pst in tomato plants, and assays were performed to determine the mode of action. It was concluded that NH4 + nutrition provokes mild toxicity in tomato plants, inducing H2O2 accumulation, which acts as a signal that can activate SAA and thus impart resistance to subsequent biotic stress. Moreover, the importance of Put and ABA downstream signalling pathways in NH4 +-induced resistance (NH4 +-IR) against Pst infection was demonstrated.
Materials and methods
Plant material, growth conditions, and nutrition treatments
Four-week-old tomato plants (Solanum lycopersicum Mill. cv. Ailsa Craig) were germinated in vermiculite in a growth chamber under the following environmental conditions: light/dark cycle of 16/8h, temperature of 24/18 °C, light intensity of 200 μmol m–2 s–1, and relative humidity of 60%. Seeds were irrigated twice a week with distilled water. Seedlings were irrigated for 3 weeks with Hoagland solution (Hoagland and Arnon, 1950) (control plants) or with Hoagland solution lacking N complemented with 20mM KNO3 – (NO3 – plants), or 2, 5, or 8mM NH4 + [(NH4)2SO4] (N-NH4 + plants). Then, K2SO4 and CaSO4 were added to compensate for the absence of K+ and Ca2+. The pH of the nutrient solution was adjusted to 6.0 with 1mM KOH.
Tomato genotypes used in the study were wild-type Ailsa Craig, Moneymaker, and Castlemart. The authors are grateful to Jonathan Jones (John Innes Centre, Norwich, Norfolk, UK) for seeds of the SA-deficient NahG tomato plant in the background Moneymaker, and to G. Howe (Michigan State University, East Lansing, MI, USA) for the JA pathway mutant def1 in the background Castlemart. The ABA pathway mutant used was the ABA-deficient mutant flacca in the background Ailsa (LA3613), which was provided by the Tomato Genetics Resource Center (TGRC), University of California, Davis, CA, USA.
Treatments with Put and PA biosynthesis inhibitors (DFMA and DFMO)
The chemical PA biosynthesis inhibitors difluoromethylarginine (DFMA) and difluoromethylornithine (DFMO), obtained from Dr Altabella, Centre for Research in Agricultural Genomics (CRAG), were dissolved in water and an inhibitor solution containing 2mM DFMA and 5mM DFMO was produced. Four treatments were performed, with the inhibitor solution was applied directly to each pot during the week prior to Pst inoculation. For Put treatment, 4-week-old plants were treated with 0.5mM Put or mock solution (water) using foliar sprays applied 48h before Pst infection. Tomato plants were maintained in the same culture conditions and inoculated as described above.
Pseudomonas syringae and Botrytis cinerea bioassays
Pst was grown in King’s B (KB) medium (King et al., 1954) at 28 °C. Rifampicin was added to KB medium at a concentration of 50mg ml–1. The coronatine-less strain of Pst (CmaA) (COR–) (Brooks et al., 2005) was grown in KB medium with rifampicin (50mg ml–1) and kanamycin (25mg ml–1). For inoculation, Pst was grown in KB medium at 28 °C for 24h. Bacterial suspensions were adjusted to 5×105 colony-forming units (cfu) ml–1 in sterile MgSO4 (10mM) containing 0.01% of the surfactant Silwet L-77 (Osi Specialties, Danbury, CT, USA), as described previously (Katagiri et al., 2002). Pathogen inoculation was performed by dipping the third and fourth leaves into the bacterial suspension. The disease rate was scored at 72h post-inoculation (hpi) by determining the percentage of dark-brown spots on the leaf surface. For molecular and hormonal analyses, the samples were taken at 3, 24, and 48 hpi. At least three samples for colony counting and 20 samples for disease rate scoring were taken for each treatment over a 3 d period. Each experiment was independently conducted at least three times.
Botrytis cinerea CECT2100 was routinely cultured on potato dextrose agar at 24 °C. The B. cinerea spores were collected from 10- to 15-day-old cultures with sterile water containing 0.01% (v/v) Tween-20, which was then filtered, quantified with a haemacytometer, and adjusted at 1×106 ml–1.
Biomass, chlorophyll content, and photosynthetic rate (A N)
Four-week-old tomato plants (control and N-NH4 + plants) were collected and dried in an oven during 2 d at 65 ºC. Dried plant tissues were weighed, and the dry weight (DW) of 10 plants was obtained and expressed as biomass.
The chlorophyll level of the leaves of 4-week-old tomato plants was measured using a chlorophyll meter (SPDA; Minolta, Tokyo, Japan). Three measurements were taken per leaf on each side of the central vein, with 10 plants per treatment. The three SPAD readings taken on one leaf for each of the 10 plants per treatment were averaged to represent one observation. The results were obtained as SPAD values (arbitrary units). Although the equations used to convert the SPAD value into the chlorophyll concentration have been obtained for other species such as Quercus serrata (Hoshino, 1996), there is no such equation known for tomato plants to date.
For the net photosynthetic rate (A N) an LCpro+ portable infrared gas analyser (ADC BioScientific Ltd., Hoddesdon, UK) was utilized under ambient CO2 and humidity. Supplemental light was provided by a PAR lamp at 1000 μmol m−2 s−1 photon flux density and air flow was set at 150 μmol mol−1. After instrument stabilization, measurements were taken on three mature leaves (from an intermediate position on the stem) in each of the 10 replicate plants.
H2O2 determination, microscopy analysis, and quantification
Samples of 10 leaves were collected for 3′,3-diaminobenzidine (DAB) staining at 3, 24. and 48 hpi. Leaves were cut and put immediately in 1mg ml–1 DAB at pH <3 for 24h in the dark and were subsequently destained in 96% ethanol and rehydrated in distilled water. DAB staining intensities were quantified in micrographies by the number of dark-brown DAB pixels in relation to the total pixels corresponding to plant material using the GIMP program (version 2.6.12).
H2O2 accumulation in leaves was quantified using the xylenol orange method (N.H. Kim et al., 2013). Ten leaf discs (0.5cm2) were floated on 1ml of distilled water for 1h, centrifuged for 1min at 12 000 g, and 100 μl of supernatant was immediately added to 1ml of xylenol orange assay reagent. The mixture was incubated for 30min at room temperature. A standard curve for H2O2 was generated from measurements obtained from a serial dilution of 100nM to 100mM of H2O2. H2O2 was quantified by measuring the A 560 using a spectrophotometer.
Analysis of gene expression by quantitative real-time polymerase chain reaction (qRT-PCR)
Gene expression analysis by qRT-PCR was performed using RNA samples extracted from leaf tissue using the E.Z.N.A.® Plant RNA Kit (www.omegabiotek.com), according to the manufacturer’s instructions. Tomato leaf tissue samples for RNA isolation were collected at 3 and 48 hpi. Leaf tissue from 10 plants each of the NH4 +-treated and untreated plants was collected. A total of 1.5 μg of total RNA was digested using 1U of RNase-free DNase (Promega; http://www.promega.com) in 1 μl of DNase buffer and up to 10 μl of Milli-Q water, and was incubated for 30min at 37 ºC. After the incubation, 1 μl of RQ1 DNase stop buffer was added, and the solution was incubated again at 65 ºC for 10min to inactivate the DNase. Highly pure RNA was used for the reverse transcription reaction which was performed according to the instructions for the Omniscript reverse transcriptase kit (QIAGEN; http://www.qiagen.com/). The reaction mixture was incubated at 37 ºC for 60min. Forward and reverse primers (0.3 μM) were added to 12.5 μl of QuantiTect™ SYBR Green PCR reaction buffer (QIAGEN), as were 2 μl of cDNA and Milli-Q sterile water up to a total reaction volume of 25 μl. Quantitative PCR was carried out using the Smart Cycler II instrument (Cepheid; http://www.cepheid.com). A list of the primers used in the qRT-PCR is shown in Supplementary Table S1 available at JXB online. Levels of EF1α gene expression were used as an internal housekeeping control. Melting curve analysis was performed at the end of the PCR to confirm the purity of the amplified products.
The amplification efficiency for each primer pair was calculated using serial cDNA dilutions. Differences in cycle numbers during the linear amplification phase between samples from treated and untreated plants were used to determine differential gene expression. At least three independent experiments were performed to confirm the results. In each experiment, three biological replicates were used to generate means and determine the statistical significance.
Stomatal aperture analysis
Tomato plants were maintained in the same culture conditions and treated as described above. The third and fourth leaves were inoculated with Pst collected from the plants at 1 and 3 hpi, and placed on glass slides with the adaxial epidermis in contact with dental resin. The dental resin mould was filled with nail polish to create a cast that was examined by light microscopy (Scalschi et al., 2013). For stomatal aperture analysis, images of random regions were taken using a Leica IRB microscope equipped with a LeicaDC300F camera (Leica Microsystems CMS GmbH, Wetzlar, Germany). The stomatal aperture was analysed using the Eclipse-Net software of the Laboratory imaging program (http://www.laboratory-imaging.com). Approximately 50 stomata from each leaflet were measured.
Chromatographic analysis
For hormonal analysis, fresh material was frozen in liquid N, ground, and freeze dried. Fresh tissue (0.5g) was immediately homogenized in 2.5ml of ultrapure water, and a mixture of internal standards ([2H6]ABA, [2H4]SA, dihydrojasmonic acid, and propylparaben) was added at 100ng ml–1 prior to extraction. After extraction, a 20 μl aliquot was injected directly into the high-performance liquid chromatography (HPLC) system. For PA analysis, fresh material was frozen in liquid N. Before extraction, according to the method described by Sánchez-López et al. (2009), a mixture of internal standards containing [13C4]putrescine and 1,7-diamineheptane was added. A 20 μl aliquot of this solution was directly injected into the HPLC system.
Analyses of hormone and PA samples were carried out using a Waters Alliance 2690 HPLC system (Milford, MA, USA) with a nucleosil ODS reversed-phase column (100 mm×2mm, i.d. 5 μm; Scharlab, Barcelona, Spain; http://www.scharlab.com). The chromatographic system was interfaced to a Quatro LC (quadrupole–hexapole–quadrupole) mass spectrometer (Micromass; http://www.micromass.co.uk). The MASSLYNX NT software version 4.1 (Micromass) was used to process the quantitative data from calibration standards and plant samples.
Statistical analysis
Statistical analysis was carried out using a one-way analysis of variance in the Statgraphics-plus software of Windows V.5 (Statistical Graphics Corp., Rockville, MD, USA). The means are expressed with standard errors and compared using a Fisher’s least-significant difference test at the 95% confidence interval. All of the experiments were repeated at least three times.
Results
NH4 + nutrition enhances tomato resistance against Pst infection
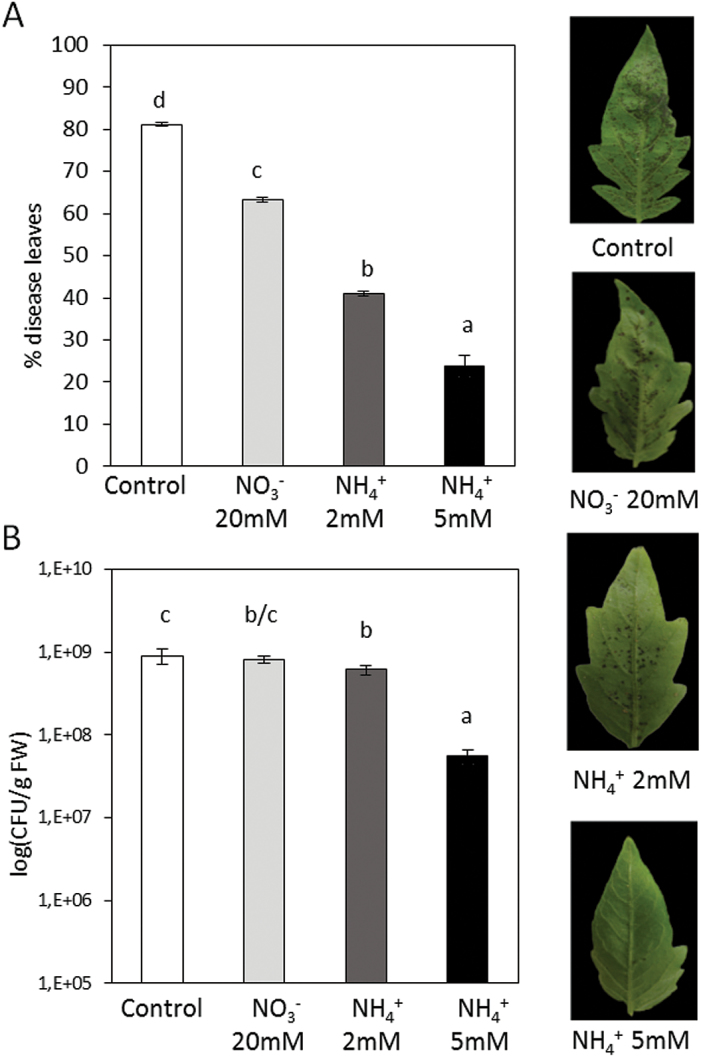
To investigate the role of NH4 + nutrition in induced resistance against Pst, 4-week-old tomato plants of S. lycopersicum were germinated in vermiculite under growth chamber-controlled conditions. Seeds were irrigated twice weekly with Hoagland complete solution (control plants) or with Hoagland solution lacking N completed with different NO3 – and NH4 + concentrations [20mM KNO3 –, N-NO3 – plants; and 2, 5, and 8mM (NH4)2SO4, N-NH4 + plants]. All plant groups were inoculated with Pst by dipping leaves into the bacterial suspension. Statistically significant reductions in disease symptoms (Fig. 1A) and in the size of the bacterial population (Fig. 1B) were found at 72 hpi under all N nutrition conditions. This reduction was more pronounced in plants grown with 5mM NH4 +, demonstrating that the inducer effect of NH4 + nutrition is concentration dependent. This direct correlation between NH4 + concentration and Pst resistance is lost at high concentrations of this ion since clear toxicity symptoms were observed in tomato plants grown with 8mM NH4 + (data not shown). To gain further insight into the biochemical and molecular mechanisms related to NH4 +-mediated resistance to Pst, the 5mM nutrition condition was selected for use in this study. This concentration was also tested against a necrotrophic pathogen, B. cinerea, and a similar effect was observed (Supplementary Fig. S2 at JXB online).
Fig. 1.
Effect of different N treatments on the resistance of tomato plants to Pst. Four-week-old tomato plants grown with different NO3 – and NH4 + concentrations were inoculated by dipping them in a bacterial suspension of Pst at 5×105 cfu ml−1. At 72 hpi, the disease rating was scored by measuring the percentage of infected leaves in relation to the total number of analysed leaves (A) and by recounting of bacterial populations by plating in agar–KB medium (B). The photograph shows a representative picture of disease symptoms in the different N treatments. Data show the average of three independent experiments of a pool of 10 plants per experiment ±SE. Letters indicate statistically significant differences (P<0.05; least-significant difference test). (This figure is available in colour at JXB online.)
NH4 + nutrition induces mild toxicity, the first step of the acclimation stage
NH4 + is a paradoxical nutrient ion because, despite being a major N source for many metabolic reactions, it can provoke toxic symptoms in sensitive species. In this study, the influence of NH4 + nutrition on plant growth, chlorophyll content, and photosynthetic rate (A N) (Table 1) was analysed. As expected, plants grown with NH4 + as the sole source of N showed a 27% reduction in their growth, when compared with control plants. Moreover, it was observed that NH4 + nutrition provoked a significant increase in chlorophyll content and induced A N inhibition in tomato plants.
Table 1.
Effect of NH4 + nutrition on growth, chlorophyll content, and photosynthetic rate (AN)
| Treatments | Biomass (g DW) | Cholorophyll content (SPAD units) | A N (μmol m−2 s−1) |
|---|---|---|---|
| Control | 0.480±0.117 b | 37.573±0.578 b | 21.565±0.841 b |
| NH4 + | 0.345±0.031 a | 43.300±0.716 a | 13.543±0.663 a |
Put accumulation is required for NH4 +-IR against Pst
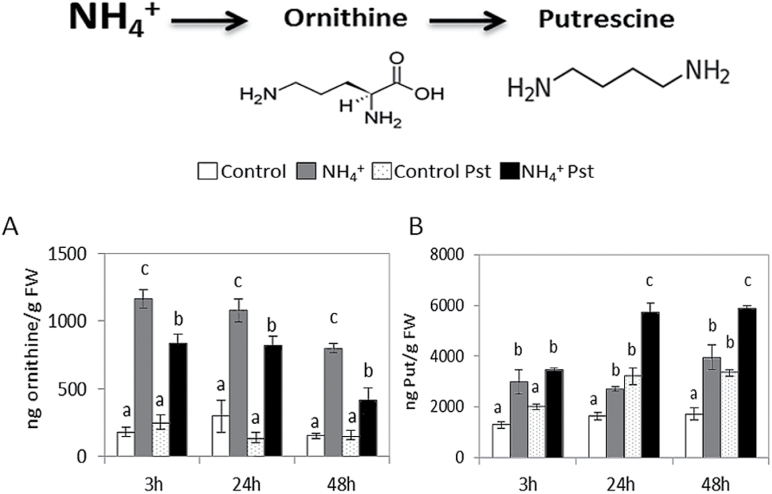
To determine whether NH4 + nutrition affects the PA content, ornithine, Put, Spm, and Spd levels were determined at 3, 24, and 48 hpi in control and N-NH4 + plants (Fig. 2; Supplementary Fig. S1 at JXB online). N-NH4 + plants displayed higher basal levels of ornithine and Put content when compared with control plants. However, upon infection, no changes in ornithine content were observed in control plants, while infected N-NH4 + plants showed a marked decrease in ornithine content, which was apparently transformed into Put. Regarding Put accumulation, although both control and N-NH4 + plants displayed an increase in Put content at 24 and 48 hpi, the increase was higher in infected N-NH4 + plants (Fig. 2). No changes were observed in the Spm and Spd content under any experimental condition (Supplementary Fig. S1). Collectively, these results suggest the involvement of PA metabolism, specifically of Put, in NH4 +-IR.
Fig. 2.
PA content in control and N-NH4 + tomato plants following Pst infection. Four-week-old tomato plants were grown under control conditions or with 5mM NH4 +, and inoculated by dipping them in a bacterial suspension of Pst at 5×105 cfu ml−1. Leaves were collected at various time points, and ornithine (A) and Put (B) levels were determined in freeze-dried material by HPLC–MS. Data show the average of three independent experiments of a pool of 10 plants per experiment ±SE. Letters indicate statistically significant differences (P<0.05; least-significant difference test).
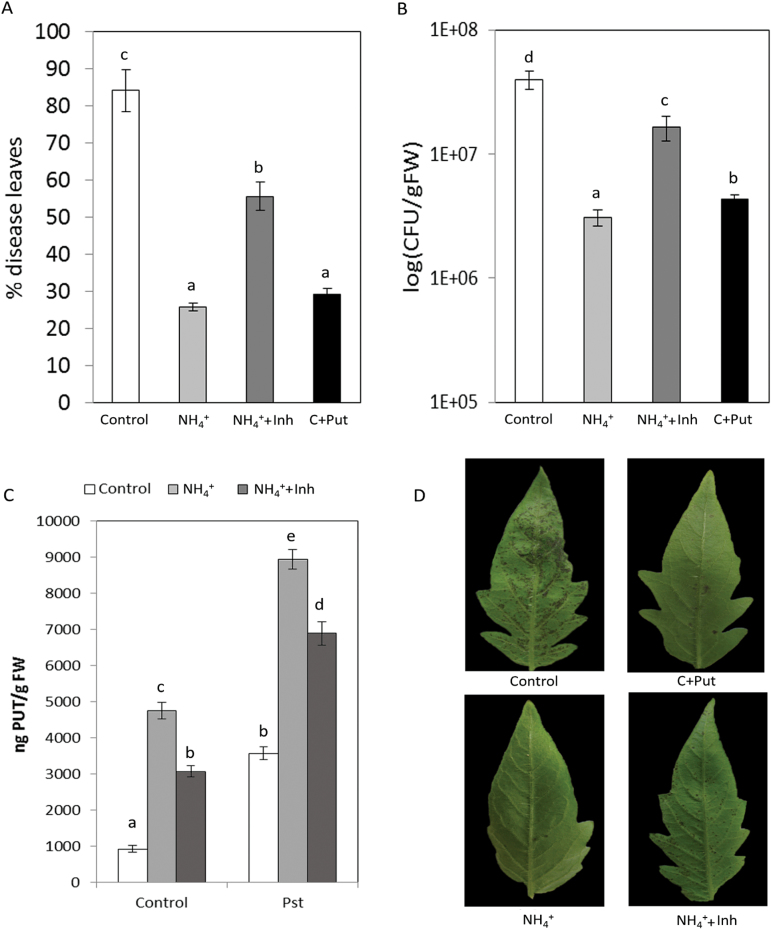
To assess the importance of Put accumulation, DFMA and DFMO were used as irreversible inhibitors of ADC and ODC enzymes, respectively (Fallon and Phillips, 1988), with the purpose of reducing cellular Put accumulation induced by NH4 + nutrition (Fig. 3). As expected, N-NH4 + plants treated with inhibitor solution displayed a reduction of 35.3% in Put content in the absence of Pst (Fig. 3C). Surprisingly, N-NH4 + plants treated with the inhibitor solution were more susceptible to Pst when compared with untreated N-NH4 + plants (Fig 3A, B, D). This shows that reduced Put accumulation reverts the resistance phenotype induced by NH4 + nutrition, revealing an important role for Put accumulation. To confirm the effect of Put on Pst infection, control plants were treated with 0.5mM Put by foliar spray 48h before infection. It was observed that, in control plants, Put induced statistically significant reductions in disease symptoms (Fig. 3A) and bacterial population size (Fig. 3B). These experiments demonstrate that Put accumulation is a crucial event in the resistance of tomato plants to Pst.
Fig. 3.
Influence of Put accumulation on NH4 +-IR against Pst. Plants were grown as described in Fig. 2 and, 1 week prior to inoculation, N-NH4 + plants were treated with inhibitor solution (containing DFMO and DFMA; NH4 ++Inh) and control plants were treated with 0.5mM Put 48h before infection (C+Put), and inoculated by dipping them in a bacterial suspension of Pst at 5×105 cfu ml−1. The disease rating was scored by measuring the percentage of infected leaves in relation to the total number of analysed leaves (A) and by recounting of bacterial populations by plating in agar–KB medium (B) at 72 hpi. The Put level in control, NH4 +, and NH4 ++Inh plants was analysed at 48 hpi (C). The photograph shows a representative picture of disease symptoms in control, NH4 +, NH4 ++Inh, and C+Put tomato leaves at 72 hpi (D). Data show the average of three independent experiments of a pool of 10 plants per experiment ±SE. Letters indicate statistically significant differences (P<0.05; least-significant difference test). (This figure is available in colour at JXB online.)
Oxidative burst induced by NH4 + nutrition enhances the resistance against Pst
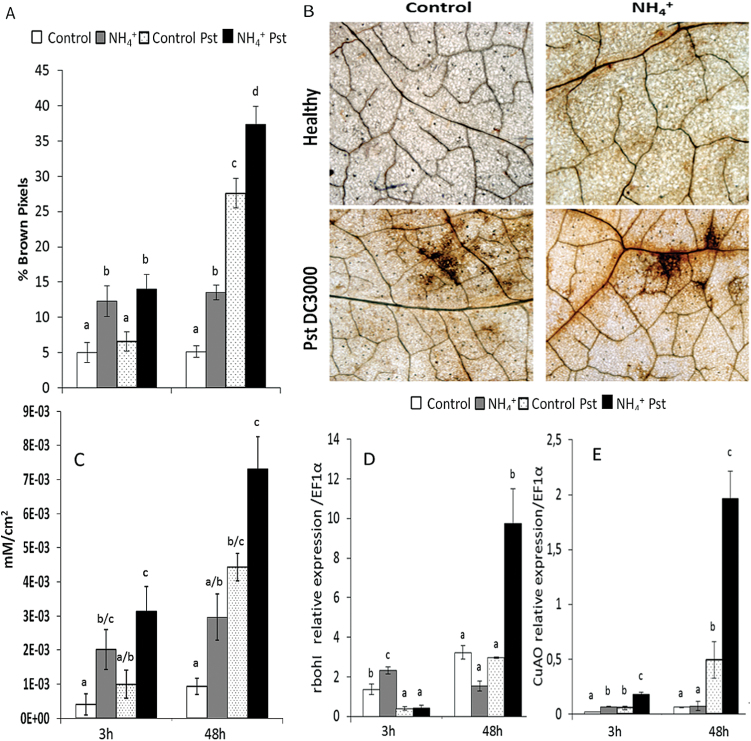
Early accumulation of ROS is one of the first biochemical responses of the plant to pathogen attack. To clarify how NH4 + nutrition affects cellular oxidative burst, and to determine its relationship to NH4 +-IR against Pst, H2O2 accumulation was evaluated by means of DAB staining (Fig. 4). H2O2 accumulation was examined at 3 and 48 hpi in control and N-NH4 + plants by digital quantification of DAB intensity (Fig 4A–C). This analysis revealed that N-NH4 + plants showed higher basal levels of H2O2 accumulation than control plants. Upon infection with Pst, no changes in H2O2 accumulation were observed at 3 hpi. However, at 48 hpi, Pst induced strong oxidative bursts in control and N-NH4 + plants, although the highest levels of H2O2 accumulation occurred in the latter. These results indicate that cellular oxidative stress could have an important role in NH4 +-IR against Pst. Thus, respiratory burst oxidase rboh1 and CuAO gene expression was analysed at 3 and 48 hpi by qRT-PCR (Fig 4D, E). In N-NH4 + plants, higher rboh1 mRNA accumulation was observed at 3h in the absence of Pst. At 48 hpi, N-NH4 + plants displayed strong rboh1 induction when compared with control plants. Similar results to those obtained for rboh1 were observed in the analysis of CuAO.
Fig. 4.
Effect of NH4 + nutrition on the oxidative burst upon Pst infection. Plants were grown and inoculated as described in Fig. 2. H2O2 accumulation was visualized by DAB staining. Quantification was performed by determining the number of brown pixels on digital photographs of leaves at 3 and 48 hpi (A). Representative photographs were taken of H2O2 accumulation in control and N-NH4 + plants in the absence of pathogens, as well as 48 hpi (B). Data show average values ±SE (n=20) of the relative number of brown or yellow pixels per photograph. H2O2 concentrations were quantified by xylenol orange analysis (C). Total RNA was isolated from leaves at 3 and 48 hpi and was converted into cDNA and subjected to a qRT-PCR analysis. The relative level of (D) rboh1 and (E) CuAO was analysed in the control and N-NH4 + plants. The results were normalized to the EF1α gene expression measured in the same samples. Letters indicate significant differences between treatments at each time point (P<0.05; least-significant difference test). (This figure is available in colour at JXB online.)
NH4 + nutrition induces changes in the hormonal profile of tomato plants
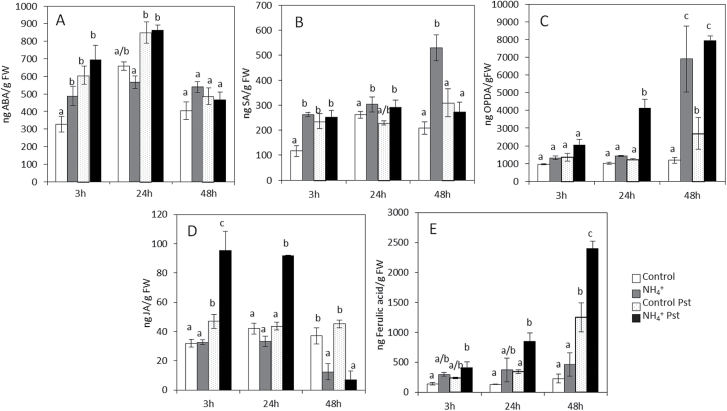
To determine whether the main signalling pathways are involved in NH4 +-IR, the levels of hormones and ferulic acid were analysed simultaneously in control and N-NH4 + plants at 3, 24, and 48 hpi (Fig. 5). N-NH4 + plants displayed higher basal ABA and SA levels compared with control plants. On infection with Pst, no changes in ABA and SA content were observed in treated plants during the experiment. In the oxylipin pathway, a significant increase was observed in the 12-oxophytodienoic acid (OPDA) content of the infected N-NH4 + plants at 24 and 48 hpi compared with the control plants. A fast increase in the JA level in response to Pst at 3 hpi was found in the N-NH4 + condition, whereas no changes were found in control and infected plants. These results showed that NH4 + nutrition induces fast activation of oxylipin pathways in tomato plants, which leads to significant OPDA accumulation. Despite that, an abolished production of JA-Ile levels was observed in these plants (data not shown). Moreover, an increase in the ferulic acid content was observed at 48 hpi, which was higher in the infected N-NH4 + plants.
Fig. 5.
Hormonal profile in control and the N-NH4 + tomato plants upon Pst infection. Plants were grown and inoculated as described in Fig. 2. Leaves were collected at different time points, and ABA (A), SA (B), OPDA (C), JA (D), and ferulic acid (E) levels were determined by HPLC–MS. The concentration of the hormones was determined in all samples by normalizing the chromatographic area for each compound with the fresh weight of the corresponding sample. Data show the average of three independent experiments of a pool of 20 plants per experiment ±SE. Letters indicate statistically significant differences at each time point (P<0.05; least-significant difference test).
NH4 +-IR against Pst involves ABA signalling pathways
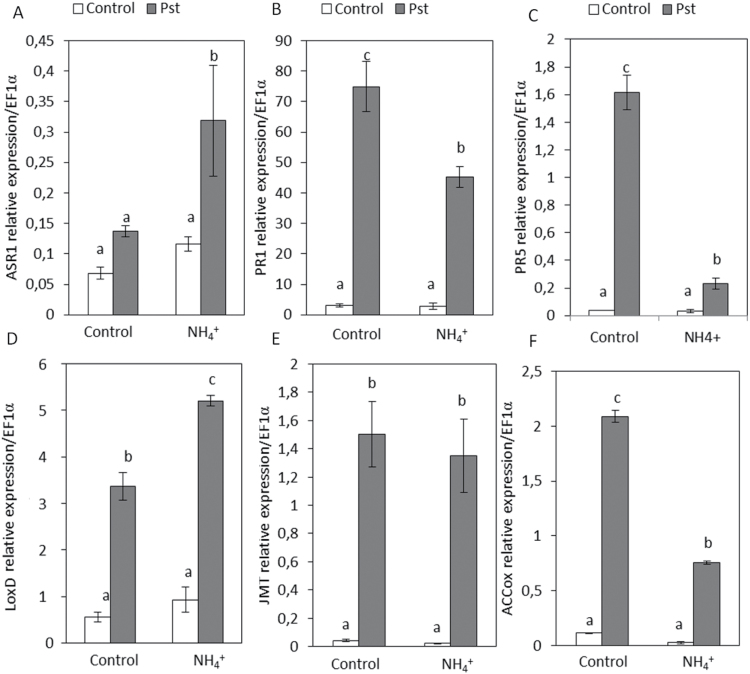
To confirm the changes in hormonal balance produced by NH4 + nutrition, the expression patterns of marker genes for ABA (Asr1), SA (PR1 and PR5), JA (LoxD, JMT), and ethylene (ACCOx) signalling pathways were analysed in the control and N-NH4 + plants at 48 hpi (Fig. 6). NH4 + nutrition induces Asr1 mRNA accumulation at 48 hpi, although an increase in ABA levels was not observed at this time point. However, in the absence of infection, N-NH4 + plants displayed higher basal levels of ABA at early time points. This initial ABA accumulation and the strong induction of Asr1 suggest that the ABA pathway has an important role in NH4 +-IR. Regarding SA pathways, although N-NH4 + plants showed higher basal SA levels compared with control plants, upon infection no significant increase in SA content was observed. However, control and N-NH4 + plants displayed larger increases in PR1 and PR5 mRNA accumulation in response to Pst infection, being more pronounced in control infected plants. The expression patterns of marker genes related to oxylipin pathways revealed that Pst infection induces LoxD expression at 48 hpi, but its expression was higher in N-NH4 + plants. Regarding JMT expression, both control and N-NH4 + plants displayed an increase in mRNA accumulation at 48 hpi without significant changes between treatments. Moreover, the analyses were extended to the ACCox marker gene related to the ethylene pathway and it was observed that, in control and N-NH4 + plants, Pst infection enhances ACCox mRNA accumulation, although this was higher in control plants. Based on these results, it can be concluded that the higher induction of Asr1 and LoxD genes points to the fact that NH4 +-IR might be due to the activation of ABA and JA defence signalling pathways.
Fig. 6.
Gene expression profile of plant defence pathways in control and N-NH4 + tomato plants upon Pst infection. Plants were grown and inoculated as described in Fig. 2. The expression of genes representing key components of ABA (Asr1) (A), SA (PR1 and PR5) (B and C), JA (LoxD and JMT) (D and E), and ethylene (ACCOx) (F) signalling pathways were analysed in cDNA from leaves of control and N-NH4 + plants at 48 hpi. The results were normalized to the EF1α gene expression measured in the same samples. Data show the average of three independent experiments of a pool of 10 plants per experiment ±SE. Letters indicate statistically significant differences (P<0.05; least-significant difference test).
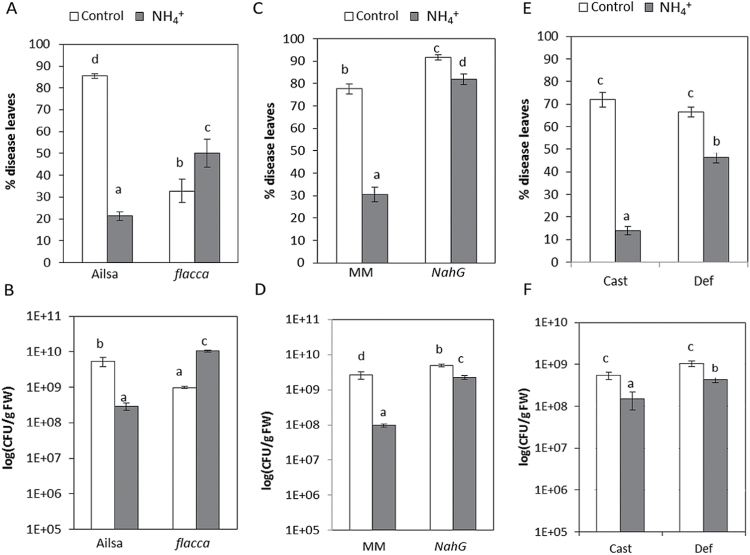
To gain further insight into the mechanisms behind NH4 +-IR against Pst, tomato mutants impaired in the SA, ABA, or JA pathways were analysed (Fig. 7). NH4 + nutrition did not protect ABA-deficient mutant flacca plants against Pst (Fig, 7A, 7B). N-NH4 + flacca plants displayed significant increases in disease symptoms, as well as in the size of the bacterial population, compared with control flacca plants, indicating a requirement for this hormonal pathway in NH4 +-IR. Transgenic NahG plants displayed a basal susceptibility to Pst due to their reduced SA levels. NahG plants displayed intact NH4 +-IR, which supports the theory that SA does not play an important role in NH4 +-IR (Fig. 7C, D). The mutant def1, which is deficient in JA biosynthesis and acts downstream of OPDA formation, showed intact NH4 +-IR (Fig. 7E, F). This result supports the possible implication of oxylipin molecules upstream of OPDA in NH4 +-IR.
Fig. 7.
Influence of ABA, SA, and JA signalling pathways on NH4 +-IR against Pst. Four-week-old tomato mutants impaired in these signalling pathways were grown under control and NH4 + treatments and inoculated by dipping in a bacterial suspension of Pst. The disease rating was scored for wild-type tomato plants of Ailsa Craig (Ailsa), Moneymaker (MM), and Castlemart (Cast) and their respective ABA-impaired mutant flacca (A, B), SA-impaired mutant NahG (C, D), and JA-impaired mutant def1 (E, F) at 72 hpi. Data show the average of three independent experiments of a pool of 10 plants per experiment ±SE. Letters indicate statistically significant differences (P<0.05; least-significant difference test).
NH4 + nutrition induces basal stomatal closure in tomato plants
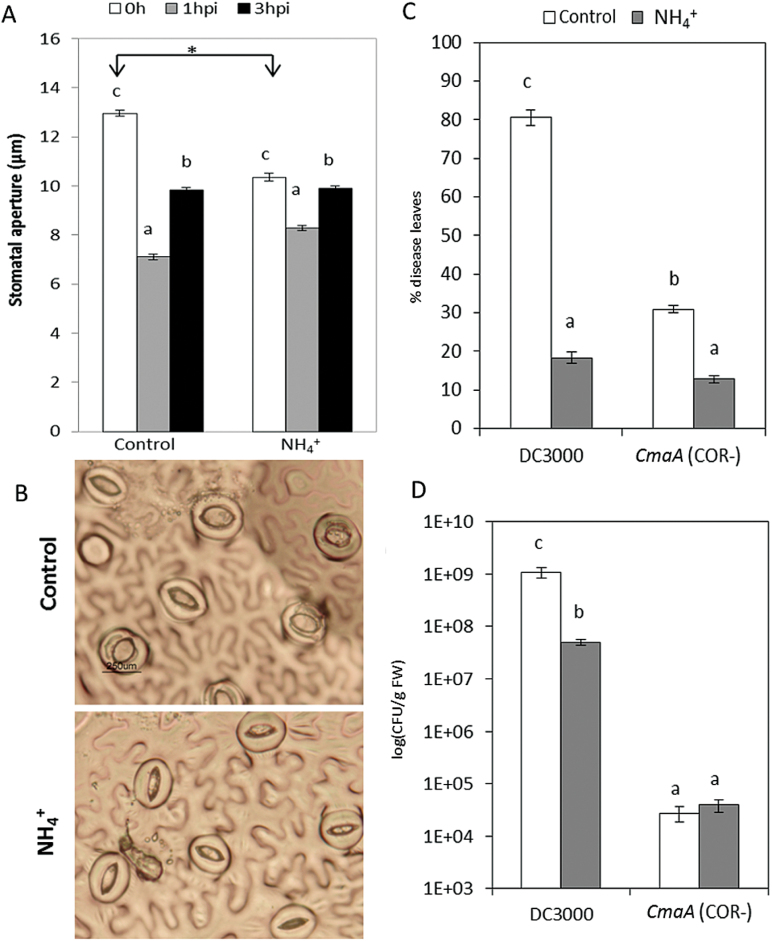
A cellular response induced upon PAMP recognition is the closure of stomata within a few hours to restrict pathogen spread to other plant tissues. Therefore, in order to invade the apoplast and cause disease, Pst produces the bacterial phytotoxin COR to impede stomatal closure and/or trigger stomatal reopening. To study the possible effect of NH4 + on this process, stomatal aperture was measured at 0, 1, and 3 hpi in control and N-NH4 + plants (Fig. 8). In both treatments, it was observed that plants closed their stomata to restrict pathogen entry into the apoplast at 1 hpi. Moreover, at 3 hpi, both control and N-NH4 + plants showed stomatal reopening, probably induced by COR, without significant changes between treatments (Fig. 8A). Curiously, N-NH4 + plants displayed basal stomatal closure when compared with control plants (Fig. 8B) which might contribute to the NH4 +-IR against Pst. Thus, the relevance of the virulence factor COR, a JA-Ile mimic, on NH4 +-IR was further tested. Control and N-NH4 + plants were infected with the Pst strain cmaA, which lacks COR (COR–). A reduction in bacterial growth was observed in plants infected with the mutant strain. Under NH4 + nutrition, a statistically significant reduction in disease symptoms was observed when compared with control plants (Fig. 8C). Although no significant changes were found in the size of the bacterial population (Fig. 8D), the reduction in disease symptoms and the normal reopening at 3 hpi in N-NH4 + plants revealed that this nutrition does not directly interfere with COR action.
Fig. 8.
NH4 + treatment induces basal stomatal closure, and NH4 +-IR against Pst is independent of the COR toxin effect. Tomato plants were grown, treated, and inoculated as described in Fig. 2. Stomatal apertures were analysed ‘in situ’ in leaflets of control and N-NH4 + plants at 0, 1, and 3 hpi (A). Results are means ± SE (n>50 stomata). Representative photographs of basal stomatal closure induced by NH4 + treatment (B) were taken. Tomato plants were infected by dipping them in a bacterial suspension of Pst and the coronatine-less strain of Pst (CmaA COR–). The disease rating was scored by measuring the percentage of infected leaves (C) and by recounting of bacterial populations (D) at 72 hpi. Data show the average of three independent experiments of a pool of 10 plants per experiment ±SE. Letters indicate statistically significant differences (P<0.05; least-significant difference test). (This figure is available in colour at JXB online.)
Discussion
The role of NH4 + as an essential macronutrient and signalling molecule has been extensively studied (Castaings et al., 2011), but its impact on plant defence responses is still unclear. In this study, the complex relationship between SAA mechanisms and the NH4 +-IR against Pst was clarified by observing that changes in the N nutrition status, specifically NH4 + applied as the sole N source, resulted in an increased resistance against Pst in tomato plants. NH4 + nutrition protects tomato plants in a concentration-dependent manner, and this resistance is especially important for plant growth under conditions of 5mM NH4 +. Extensive studies investigating the underlying mechanisms of NH4 + toxicity have been reported in plants, but how plants acclimated to high levels of NH4 + are able to induce the mechanisms of resistance against subsequent stress situations more efficiently is poorly understood. Plants accumulate PAs to compensate for the lack of some cations other than NH4 +, besides serving as a sink for excess NH4 + to reduce toxicity (Gerendás et al., 1997). Although long known for their implication in abiotic stress responses, there has been little investigation of defence responses of PAs during pathogen infection. In this work, it was observed that N-NH4 + plants displayed a higher basal content of ornithine and Put. Upon infection, N-NH4 + plants showed a marked decrease in ornithine content, which was apparently transformed into Put. Interestingly, N-NH4 + tomato plants displayed more susceptibility to Pst when plants were treated with the inhibitors DFMA and DFMO, and Put content was reduced. Moreover, Put treatment induces resistance against this pathogen in control tomato plants, revealing the importance of PAs, specifically Put, in the resistance of tomato plants to a biotic stress. According to these findings, it can be confirmed that signalling derived from changes to the ornithine pool and its conversion in Put, leading to high Put accumulation, are directly linked to NH4 + nutrition and are key events in NH4 +-IR against Pst. S.H. Kim et al. (2013) observed that an adc2 knock-out mutant displayed reduced Put content, reduced expression of PR1, and enhanced susceptibility against Pst. Disease susceptibility of the adc2 mutant was recovered by the addition of exogenous Put, revealing its direct impact on resistance against this pathogen. Another common response to the toxic effect of NH4 + nutrition is ROS accumulation and the modification of the redox cell state (Patterson et al., 2010). Recent studies identified plastid retrograde signalling-derived responses as key factors in plants against NH4 + stress (Li et al., 2012, 2013). These authors propose that, under NH4 + stress, the chloroplast receives the stress signal (mediated by ROS) and activates retrograde signalling pathways, recruiting downstream ABA signalling to regulate the expression of NH4 +-responsive genes in the nucleus and prevent NH4 + toxicity. In this study, the mild toxic effect of NH4 + nutrition on tomato plants was confirmed since growth retardation, increased chlorophyll content, reduced photosynthetic rate, and basal H2O2 accumulation were observed in N-NH4 + plants. The plastid retrograde signalling induced by H2O2 accumulation is probably related to the expression of nuclear genes to prevent NH4 + toxicity. The activation of this defensive pathway might induce the establishment of SAA in leaves of tomato plants, allowing them to better withstand a subsequent Pst infection. This hypothesis is in accordance with previous studies, in which it was demonstrated that NH4 + nutrition in citrus plants triggers mild chronic stress, induces H2O2 accumulation, and acts as a signal, which primes plant defence responses by stress imprinting and confers protection against subsequent salt stress (Fernández-Crespo et al., 2012, 2014). Although little is known about how prior exposure of plants to abiotic stress improves their capacity to respond effectively to biotic stress, recent studies suggest that ROS are closely associated with this adaptive process. For example, high light exposure induced ROS accumulation, and this signal is required for the SAA of plants, enhancing tolerance of plants to Pst, indicating that there is cross-talk between abiotic stress acclimation and pathogen responses (Karpinski et al., 2013). For this reason, it was concluded that H2O2 accumulation derived from mild chronic NH4 + stress acts as a signal and primes plant defence responses, probably mediated by the activation of downstream plastid retrograde signalling. The signalling derived from the chloroplast could be related to the establishment of SAA in tomato plants, enhancing the resistance against subsequent Pst infection.
To investigate further the role of ROS in the SAA induced by NH4 + nutrition, rboh1 and CuAO gene expression as H2O2 producers was analysed. As expected, NH4 + nutrition induced mRNA accumulation of both genes in tomato plants. RBOHs are required for the initiation and self-propagation of systemic signals by H2O2 accumulation to generate a ‘ROS wave’ (Mittler et al., 2011). Suzuki et al. (2013) demonstrated that SAA of plants to heat stress was correlated with activation of the ROS wave and the transient accumulation of ABA in systemic tissues, and these responses were suppressed in a mutant lacking RBOHD. According to these findings, the higher basal induction of rboh1, accompanied by the higher basal H2O2 accumulation induced by NH4 + nutrition, might be the key event in the acclimation stage induced in tomato plants. As for CuAO, it is commonly accepted that H2O2 produced as a catabolite by CuAO action is involved in ABA-induced stomatal closure in Vicia faba (An et al., 2008). Therefore, H2O2 produced by CuAO and RBOH1 could have a dual role; first it may play an important role in ABA-mediated basal stomatal closure, which was found in N-NH4 + tomato plants, and, secondly, the H2O2 wave could be used as an amplifier for signalling related to SAA mechanisms. N-NH4 + plants displayed a strong oxidative burst, probably mediated by the highest induction of the rboh1 and CuAO gene. This response which leads to limited pathogen spread might play a key role in NH4 +-IR against Pst.
To understand the effect of NH4 + nutrition on defence, signalling pathways were analysed. The hormonal profile revealed that NH4 + nutrition induces higher basal levels of SA in tomato plants, but upon Pst infection no significant changes in SA content between treatments were found. PR1 and PR5 gene expression is less induced in the infected NH4 + plants, and NahG plants displayed intact NH4 +-IR. This evidence supports the hypothesis that SA signalling pathways are not the main pathways required for NH4 +-IR against Pst. Regarding oxylipin pathways, a faster and stronger accumulation of JA in N-NH4 + plants at 3 hpi was observed. However, throughout the infection, decreased JA levels occurred accompanied by a higher increase in OPDA content and a strong induction of LoxD in N-NH4 + plants. The role of JA and OPDA in the NH4 +-IR was tested with JA-deficient def1 plants impaired in JA as well as OPDA accumulation, and an intact resistance against Pst was shown, revealing the possible implication of oxylipins upstream of OPDA in the NH4 +-IR against Pst. Regarding the phenylpropanoid pathway, infected NH4 + plants displayed higher accumulation of ferulic acid, which points to the implication of this pathway in NH4 +-IR. It is probable that shikimate derived from tyrosine competes with SA accumulation synthesized via phenylalanine ammonia lyase, and, thus, significant changes in free SA as well as reduced induction of PR1 and PR5 in N-NH4 + plants were not observed.
As expected, N-NH4 + plants displayed higher basal ABA accumulation and, on infection, NH4 + nutrition primed the induction of Asr1. To clarify the role of ABA-dependent signalling pathways, the effect of NH4 + nutrition on the responses of ABA-deficient flacca mutants was analysed. It was demonstrated that flacca NH4 +-treated plants were more susceptible to Pst, and displayed impaired NH4 +-IR expression. The fact that NH4 +-IR was absent in flacca supports the idea that an intact ABA signalling pathway is required, at least in part, in NH4 +-IR against Pst. ABA has also emerged as a complex modulator of plant defence responses, since it can function as a positive or negative regulator, depending on the plant–pathogen interaction analysed (Ton et al., 2009). Specifically, ABA was found to be a key regulator of the pathogen-mediated stomatal closure (Melotto et al., 2006). One mechanism of Pst pathogenesis is to produce the effector COR necessary for stomatal reopening in the process of bacterial infection (Melotto et al., 2006). To confirm the role of NH4 + nutrition in the stomatal movement on Pst infection, the stomatal aperture was analysed in treated and untreated plants at 0, 1, and 3 hpi. As expected, it was observed that bacteria induced closure of stomata at 1 hpi, and stomata reopened in a COR-dependent manner at 3 hpi in control and N-NH4 + plants (Tsai et al., 2011). However, N-NH4 + plants displayed more closed stomata than control plants, probably due to the higher basal ABA accumulation. These findings reveal that NH4 + does not have a direct effect on stomatal movement induced by COR, but the basal stomatal closure observed in NH4 + plants was sufficient to reduce the entry of bacterial into the mesophyll and reduce the disease symptoms. In addition to its role in stomatal movement, ABA and its role in PA homeostasis has been widely studied. Toumi et al. (2010) showed that ABA induced PA accumulation and secretion into the apoplast, where they were oxidized by CuAOs producing H2O2. Based on these findings, it seems clear that activation of ABA-dependent signalling pathways mediated by NH4 + nutrition may activate PA biosynthesis and catabolism. For this reason, it is concluded that ABA could act as a positive regulator of NH4 +-IR since it was able to strengthen Put biosynthesis, which has a direct effect on the resistance to Pst, and induced PA catabolism by enhancing CuAO activity, causing H2O2 accumulation and therefore provoking basal stomatal closure.
Collectively, these results indicate that NH4 + nutrition provokes mild chronic stress that leads to the activation of SAA responses that prime tomato defence pathways and induce resistance against subsequent biotic stress. NH4 + nutrition enhanced H2O2 accumulation, which acts as a signal to induce ABA-dependent responses, reducing the NH4 + toxicity. These ABA-dependent signalling pathways induce PA biosynthesis and catabolism, which in turn enhance H2O2 accumulation, favouring stress signal amplification. Moreover, NH4 + nutrition induces Put accumulation, and it was demonstrated that compromising Put accumulation provokes increased susceptibility to Pst. Conversely, the basal stomatal closure observed in N-NH4 + plants was probably produced by H2O2 derived from enhanced CuAO activity induced by ABA-mediated NH4 + responses. The basal stomatal closure observed in NH4 + plants may reduce the entry of bacteria into the mesophyll, reducing the diseased symptoms, favouring the NH4 +-IR. Besides these functions, it is speculated that H2O2 acts as a signal of NH4 + stress and primes plant defence responses against subsequent pathogen infection. Although classical SA-dependent responses against biotrophic pathogens were not found, other defence mechanisms were observed related to the resistance of these lifestyle pathogens, namely strong and fast oxidative bursts, probably mediated by induction of rboh1 and CuAO genes, which directly reduce the spread of pathogens in the plant (Fig. 9). The study of the mechanism of action of NH4 + as an inducer of resistance showed that N-NH4 + plants displayed basal responses related to the establishment of the acclimation state in systemic leaves through SAA-related mechanisms. This activation allows the plant to trigger more efficient and effective specific responses to prevent pathogen spread, and therefore induce resistance to disease. The NH4 +-IR mediated by SAA mechanisms is especially important because it has been shown to be effective in other species against abiotic stress (Fernandez-Crespo et al., 2012) and against two different lifestyle pathogens, Pst and B. cinerea. The study of events underlying this kind of induced resistance, which is effective against biotic and abiotic stress, provides knowledge necessary to exploit this phenomenon in the context of sustainable agriculture.
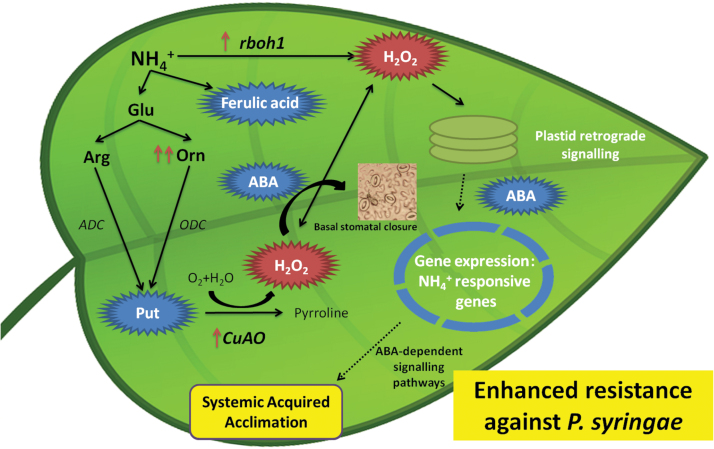
Fig. 9.
Model of achievement of SAA induced by NH4 + nutrition. Tomato plants grown in NH4 + as a sole N source develop different responses to relieve the mild toxicity effect. These responses play an important role in achieving SAA and in the resistance against Pst. A common response against NH4 + nutrition is the accumulation of PAs to compensate for the lack of some cations other than NH4 +, besides serving as a sink for excess NH4 + to reduce the toxicity. Here, basal metabolic changes induced by NH4 + nutrition were observed, specifically Put, ABA, and ferulic acid accumulation. Another response against the mild toxic effect of NH4 + nutrition is ROS accumulation and, consequently, the modification of the redox cell state. This modification occurs by the basal induction of CuAO and rboh1 genes observed in N-NH4 + plants. Moreover, it was found that basal stomatal closure is probably produced by H2O2 derived from enhanced CuAO activity, induced by ABA-mediated NH4 + responses. The chloroplast receives the stress signal (mediated by ROS) and activates retrograde signalling pathways, recruiting downstream ABA signalling to regulate the expression of NH4 +-responsive genes in the nucleus and prevent NH4 + toxicity. The activation of this defensive pathway might induce the establishment of SAA in tomato plant leaves, allowing them to better withstand a subsequent Pst. (This figure is available in colour at JXB online.)
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Spm and Spd content in control and N-NH4 + tomato plants during Pst infection.
Figure S2. Effect of NH4 + treatment on tomato plants infected with B. cinerea.
Table S1. Primer sequences.
Acknowledgements
This work was supported by grants from the Spanish Ministry of Science and Innovation (AGL2013-49023-C3-2-R). The authors are grateful to the Serveis Centrals d’Instrumentació Científica (SCIC) from Universitat Jaume I (UJI, Castellón, Spain).
References
- An Z, Jing W, Liu Y, Zhang W. 2008. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba . Journal of Experimental Botany 59, 815–825. [DOI] [PubMed] [Google Scholar]
- Angelini R, Tisi A, Rea G, Chen MM, Botta M, Federico R, Cona A. 2008. Polyamine oxidase involvement in wound-healing. Plant Physiology 146, 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza I, Asensioa AC, Zamarrenob AM, Garcia-Minab JM, Aparicio-Tejoa PM, Moran JF. 2013. Changes in the C/N balance caused by increasing external ammonium concentrations are driven by carbon and energy availabilities during ammonium nutrition in pea plants: the key roles of asparagine synthetase and anaplerotic enzymes. Physiologia Plantarum 148, 522–537. [DOI] [PubMed] [Google Scholar]
- Bagh K, Hiraoki T, Thorpe TA, Vogel HJ. 2004. Nitrogen-15 NMR studies of nitrogen metabolism in Picea glauca buds. Plant Physiology and Biochemistry 42, 803–809. [DOI] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N. 2014. ROS as key players in plant stress signaling. Journal of Experimental Botany 65, 1229–1240. [DOI] [PubMed] [Google Scholar]
- Bittsánszky A, Pilinszky K, Gyulai G, Komives T. 2015. Overcoming ammonium toxicity. Plant Science 231, 184–190. [DOI] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. 1999. Polyamines and environmental challenges: recent development. Plant Science 140, 103–125. [Google Scholar]
- Boyko A, Kovalchuk I. 2011. Genome instability and epigenetic modification—heritable responses to environmental stress? Current Opinion in Plant Biology 14, 260–266. [DOI] [PubMed] [Google Scholar]
- Bray EA, Bailey-Serres J, Weretilnyk E. 2000. Responses to abiotic stresses. In: Buchanan BB, Gruissem W, Jones RL, eds. Biochemistry and molecular biology of plants . Rockville, MD: American Society of Plant Physiologists, 1158–1249. [Google Scholar]
- Britto DT, Kronzucker HJ. 2002. NH4 + toxicity in higher plants: a critical review. Journal of Plant Physiology 159, 567–584. [Google Scholar]
- Brooks DM, Bender CL, Kunkel BN. 2005. The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Molecular Plant Pathology 6, 629–640. [DOI] [PubMed] [Google Scholar]
- Castaings L, Marchive C, Meyer C, Krappa A. 2011. Nitrogen signalling in Arabidopsis: how to obtain insights into a complex signalling network. Journal of Experimental Botany 62, 1391–1397. [DOI] [PubMed] [Google Scholar]
- Conrath U. 2011. Molecular aspects of defence priming. Trends in Plant Science 16, 524–531. [DOI] [PubMed] [Google Scholar]
- Fallon KM, Phillips R. 1988. Polyamines in relation to growth in carrot cell cultures. Plant Physiology 88, 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Crespo E, Camañes G, Garcia-Agustin P. 2012. Ammonium enhances resistance to salinity stress in citrus plants. Journal of Plant Physiology 169, 1183–1191. [DOI] [PubMed] [Google Scholar]
- Fernández-Crespo E, Gómez-Pastor R, Scalschi L, Llorens E, Camañes G, García-Agustín P. 2014. NH4 + induces antioxidant cellular machinery and provides resistance to salt stress in citrus plants. Trees 28, 1693–1704. [Google Scholar]
- Gerendás J, Zhu Z, Bendixen R, Ratcliffe RG, Sattelmacher B. 1997. Physiological and biochemical processes related to ammonium toxicity in higher plants. Zeitschrift für Pflanzernährung und Bodenkunde 160, 239–251. [Google Scholar]
- Gordon MJ, Carmody M, Albrecht V, Pogson B. 2013. Systemic and local responses to repeated HL stress-induced retrograde signaling in Arabidopsis. Frontiers in Plant Science 3, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347, 1–32. [Google Scholar]
- Hoshino D. 1996. Seasonal changes in leaf chlorophyll and stem-diameter growth of major tree species in a secondary broadleaf forest, central Japan . Masters Thesis, Nagoya Univeristy. [Google Scholar]
- Karpinski S, Szechynska-Hebda M, Wituszynska W, Burdiak P. 2013. Light acclimation, retrograde signalling, cell death and immune defences in plants. Plant, Cell and Environment 36, 736–744. [DOI] [PubMed] [Google Scholar]
- Kasinathan V, Wingler A. 2004. Effect of reduced arginine decarboxylase activity on salt tolerance and on polyamine formation during salt stress in Arabidopsis thaliana. Physiologia Plantarum 121, 101–107. [DOI] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY. 2002. The Arabidopsis thaliana– Pseudomonas syringae interaction. The Arabidopsis Book 1, e0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Kim BS, Hwang BK. 2013. Pepper arginine decarboxylase is required for polyamine and γ-aminobutyric acid signaling in cell death and defense response. Plant Physiology 162, 2067–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim SH, Yoo SJ, Min KH, Nam SH, Cho BH, Yang KY. 2013. Putrescine regulating by stress-responsive MAPK cascade contributes to bacterial pathogen defense in Arabidopsis. Biochemical and Biophysical Research Communications 437, 502–508. [DOI] [PubMed] [Google Scholar]
- King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of phycocyanin and fluorescein. Journal of Laboratory and Clinical Medicine 44, 301–307. [PubMed] [Google Scholar]
- Kravchuk Z, Vicedo B, Flors V, Camañes G, González-Bosch C, García-Agustín P. 2011. Priming for JA-dependent defenses using hexanoic acid is an effective mechanism to protect Arabidopsis against B. cinerea . Journal of Plant Physiology 168, 359–366. [DOI] [PubMed] [Google Scholar]
- Li B, Li Q, Xiong L, Kronzucker HJ, Krämer U, Shi W. 2012. Arabidopsis plastid metalloprotease AMOS1/EGY1 integrates with ABA signaling to regulate global gene expression in response to ammonium stress. Plant Physiology 160, 2040–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Kronzucker HJ, Shi W. 2013. Molecular components of stress-responsive plastid retrograde signaling networks and their involvement in ammonium stress. Plant Signaling Behaviour 8, e23107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens E, Fernandez-Crespo E, Vicedo B, Lapeña L, García-Agustín P. 2013. Enhancement of the citrus immune system provides effective resistance against Alternaria brown spot disease. Journal of Plant Physiology 170, 146–154. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980. [DOI] [PubMed] [Google Scholar]
- Misra N, Gupta AK. 2006. Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. Journal of Plant Physiology 163, 11–18. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends in Plant Science 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Moschou PN, Wu J, Cona A, Tavladoraki P, Angelini R, Roubelakis-Angelakis KA. 2012. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. Journal of Experimental Botany 63, 5003–5015. [DOI] [PubMed] [Google Scholar]
- Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA. 2010. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant, Cell and Environment 33, 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime-A-Plant Group, Conrath U Beckers GJ et al. . 2006. Priming: getting ready for battle. Molecular Plant-Microbe Interactions 19, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Reddy AS, Ali GS, Celesnik H, Day IS. 2011. Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. The Plant Cell 23, 2010–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-López J, Camañes G, Flors V, Vicent C, Pastor V, Vicedo B, Cerezo M, García-Agustín P. 2009. Underivatized polyamine analysis in plant samples by ion pair LC coupled with electrospray tandem mass spectrometry. Plant Physiology and Biochemistry 47, 592–598. [DOI] [PubMed] [Google Scholar]
- Scalschi L, Camañes G, Fernandez-Crespo E, Lapeña L, González-Bosch C, García-Agustín P. 2013. Hexanoic acid is a resistance inducer that protects tomato plants against Pseudomonas syringae by priming the jasmonic acid and salicylic acid pathways. Molecular Plant Pathology 14, 342–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Dong X. 2012. How do plants achieve immunity? Defence without specialized immune cells. Nature Reviews Immunology 12, 89–100. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, et al. 2013. Temporal–spatial interaction between ROS and ABA controls rapid systemic acclimation in plants. The Plant Cell 9, 3553–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kakehi J. 2010. Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Annals of Botany 105, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio AF, Altabella T, Bitrián M, Alcázar R. 2014. The roles of polyamines during the lifespan of plants: from development to stress. Planta 240, 1–18. [DOI] [PubMed] [Google Scholar]
- Ton J, Flors V, Mauch-Mani B. 2009. The multifaceted role of ABA in disease resistance. Trends in Plant Science 14, 310–317. [DOI] [PubMed] [Google Scholar]
- Toumi I, Moschou PN, Paschalidis KA, Bouamama B, Ben Salem-Fnayou A, Ghorbel AW, Mliki A, Roubelakis-Angelakis KA. 2010. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. Journal of Plant Physiology 167, 519–525. [DOI] [PubMed] [Google Scholar]
- Tsai C, Singh P, Chen C, Thomas J, Weber J, Mauch-Mani B, Zimmerli L. 2011. Priming for enhanced defence responses by specific inhibition of the Arabidopsis response to coronatine. The Plant Journal 65, 469–479. [DOI] [PubMed] [Google Scholar]
- Vicedo B, Flors V, de la O Leyva M, Finiti I, Kravchuk Z, Real MD, García-Agustín P, González-Bosch C. 2009. Hexanoic acid-induced resistance against Botrytis cinerea in tomato plants. Molecular Plant-Microbe Interactions 22, 1455–1465. [DOI] [PubMed] [Google Scholar]
- von Wirén N, Merrick M. 2004. Regulation and function of ammonium carriers in bacteria, fungi and plants. Topics in Current Genetic 9, 95–120. [Google Scholar]
- Walters D. 2003. Polyamines and plant disease. Phytochemistry 64, 97–107. [DOI] [PubMed] [Google Scholar]
- Yoda H, Hiroi Y, Sano H. 2006. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiology 142, 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J. 2013. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant, Cell and Environment 36, 2085–2103. [DOI] [PubMed] [Google Scholar]
- Zhou J, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ. 2014. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. Journal of Experimental Botany 65, 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.