Abstract
The prognosis for patients with glioblastoma remains poor with current treatments. Although platinum based drugs are sometimes offered at relapse, their efficacy in this setting is still disputed. In this study, we use convection-enhanced delivery (CED) to deliver the platinum-based drugs (cisplatin, carboplatin, and Lipoplatin™-liposomal formulation of cisplatin) directly into the tumor of F98 glioma-bearing rats that were subsequently treated with γ radiation (15 Gy). CED increased by factors varying between 17 and 111, the concentration of these platinum-based drugs in the brain tumor compared to intra-venous (i.v.) administration, and by 9- to 34-fold, when compared to intra-arterial (i.a.) administration. Furthermore, CED resulted in a better systemic tolerance to platinum drugs compared to their i.a. injection. Among the drugs tested, carboplatin showed the highest maximum tolerated dose (MTD). Treatment with carboplatin resulted in the best median survival time (MeST) (38.5 days), which was further increased by the addition of radiotherapy (54.0 days). Although the DNA-bound platinum adduct were higher at 4 h after CED than 24 h for carboplatin group, combination with radiotherapy led to similar improvement of median survival time. However, less toxicity was observed in animals irradiated 24 h after CED-based chemotherapy. In conclusion, CED increased the accumulation of platinum drugs in tumor, reduced the toxicity, and resulted in a higher median survival time. The best treatment was obtained in animals treated with carboplatin and irradiated 24 h later.
Keywords: Convection-enhanced delivery, Lipoplatin™, glioblastoma, platinum-based chemotherapy, radiation therapy, routes of drug administration
Introduction
Glioblastoma (GBM) is the most aggressive primary brain tumor in adults. The prognosis of patients with glioblastomas remains dismal. A substantial number of clinical trials involving platinum-based antineoplastic drugs (cisplatin, carboplatin) for glioma have been carried out [1–6]. Whereas cisplatin showed no or limited improvements in patients with glioblastoma [1, 2], carboplatin, especially delivered via an intra-arterial route, has demonstrated the drug’s efficacy to improve median survival [3–6]. However, toxicities associated with the systemic administration of platinum-based antineoplastic agents were consistently observed. For example, the notorious nephrotoxicity, gastrointestinal toxicity were associated with cisplatin [7], while carboplatin treatments to myelosuppression and ototoxicity [8].
The low anti-tumor efficacy and high systemic toxicity of platinum-based antineoplastic drugs may be due to one simple reason: the non-optimal administration of platinum-based antineoplastic drugs. The blood-brain barrier limits macromolecular and polar molecules, like these platinum-based antineoplastic drugs, from entering the tumor sites, resulting in low anti-tumor activity [9]. Therefore, to overcome these limitations, convection-enhanced delivery (CED) was developed by Dr. R. Hunt Bobo in 1994 [10]. CED allows the delivery of chemotherapy drugs at a high concentration, directly into the tumor and thus reduces systemic toxicity [11].
In the last decade, the teams of Elleaume and Barth have innovatively and extensively studied convection-enhanced delivery (CED) of cisplatin and carboplatin in glioma bearing animals. They reported an improvement of median survival time (MeST) or even cure of glioma bearing rats when combined with radiation therapy [12–17]. Additionally, a phase I clinical trial showed that it was safe and feasible to deliver carboplatin by CED to treat glioblastoma [18]. However, due to the large diversity of the techniques, animal models, and/or drug dosages used in different studies [12, 13, 19, 20], it is unclear if the CED of one particular platinum-based antineoplastic agent is better than the others. Moreover, it is paramount to evaluate the liposomal formulations of platinum drugs and compare their performance to that of free platinum drugs, particularly since it has been reported that liposomal chemotherapy drugs delivered by CED have longer retention times in the tumor area, and can further extend survival [21]. Huo T. et al have studied the toxicity of Lipoplatin™ delivered by CED in non-tumor bearing rat brain. Acute neurotoxicity with haemorrhage was observed [22]. They also showed an acute neurotoxicity with cisplatin. However, at a tolerated dose, a significant increase of survival or even cure in F98 glioma-bearing Fisher rats were observed when treated with cisplatin [12, 15]. These results suggest that the direct injection by CED of platinum drug in brain tumor can optimize the treatment by reducing toxicity to healthy brain and increasing the anti-tumor effect.
Because of its inherent delivery mechanistic, CED evidently bears the risks of neurotoxicity from direct injection into the brain, when compared to other delivery strategies. Furthermore, depending on the infusion volume, nature of the drugs, and infusion rate, an injected drug may not always be able to reach the infiltrative peripheral area of glioma [23]. Thus, the survival times of glioma bearing rats treated with platinum drugs delivered by different administration routes should also be compared. In addition, platinum-based antineoplastic drugs have long been considered as radiosensitizers. Our previous in vitro and in vivo studies showed that the amount of DNA-platinum adducts varies over time, and can be associated with the efficiency of chemo-radiation therapy, with the highest yields of DNA-platinum adducts conferring the greatest concomitant effect [24, 25]. Consequently, survival was also investigated by irradiation at different times after CED. In the present study, we thereby evaluated the efficacy CED using cisplatin, carboplatin, or Lipoplatin™, with the addition of radiotherapy. The optimal time between injection of the drug and irradiation of the tumor that lead to best anti-tumoral effect was also determined. We also compared these results with previous experiments done with i.v., i.a., or BBBD delivery of the same agents in F98 glioma-bearing rats.
Methods and Materials
Chemicals
Cisplatin and carboplatin were obtained from Hospira (Saint-Laurent, QC). Lipoplatin™ (liposomal formulation of cisplatin) was generously provided by Dr. Teni Boulikas, (Regulon Inc, Athens, Greece).
Cell lines and animal model
F98 rat glioblastoma cells were used as their infiltrative and radioresistent properties are similar to the pattern of human glioblastoma [26]. These cells are also syngeneic with Fischer rats. The cell line F98 was purchased from American type culture collection (ATCC) and tested negative for the Rat antibody production (RAP) test by Charles River Laboratories. Male Fischer rats and Lewis rats weighing from 210 to 225 g were purchased from Charles River Laboratories International, Inc. (Wilmington, MA). The experimental protocol was approved by the institutional ethical committee and complied with the regulations of the Canadian Council on Animal Care (protocol # 329-13B).
F98 glioblastoma cells implantation in Fischer rats
The implantation method has been described by Mathieu et al [27]. Briefly, five microliter Dulbecco’s Modified Eagle Medium (DMEM) without fetal bovine serum (FBS) containing 10,000 F98 cells were prepared and implanted into the right caudate nucleus (1 mm anterior, 3 mm right of the bregma, and 6 mm deep) of the brain in 5 min.
CED procedure
CED was performed 10 days after implantation of F98 cells, at the same injection point using a 33 Ga Hamilton syringe (Hamilton Company, Reno, NV). Before infusion, the burr was filled with bone wax, and the needle was inserted to a depth of 6.5 mm, retained there 5 min and then, withdrawn to 6 mm, where drugs were infused at an infusion rate of 0.5 μl μl/min for 20 min. After infusion, the needle was left for 5 min and then withdrawn during 6 min. This procedure reduced backflow and increased convection volume.
Radiotherapy with Gamma Knife
Depending on the treatment group, either 4 h or 24 h after CED, rats were anesthetized and mounted on a home-made frame [28] and treated with 15 Gy of radiation from a Gamma Knife PERFEXION (Elekta Instruments AB, Norcross, GA). The Gamma Knife was chosen because of its high precision to irradiate tumors implanted in rats. Only a single dose of radiation was delivered to rats with the Gamma Knife because it is easier to evaluate the concomitant effect of chemotherapy and radiation with a single dose as well as to compare our previous results [29, 30] with those reported in the present study.
Drug distribution volume
CED of a green colorant (10 μl) containing tartrazine and Brilliant Blue FCF was performed into the normal brains of Lewis rats to model the distribution of agents introduced by CED into healthy tissue. The Lewis rats were used only to optimize the CED procedure. Brains were sliced through the injection point, 2 mm before and after the injection point and photos were taken. (Fig. 1) The Rat brain and the colorant distribution was reconstructed using FIJI software [31].
Fig. 1.
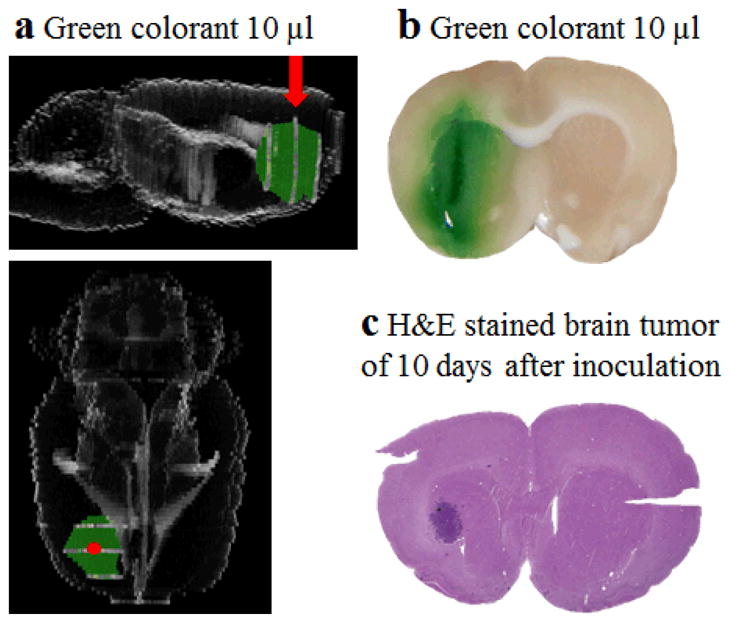
Distribution of green colorant after injection by CED in rat brain. (a) Side view and upper view of 3D reconstruction of rat brain and green colorant (red arrow and red dot indicate injection point). (b) Distribution of green colorant from the injection site 30 min after CED. (c) H&E staining of brain tumor 10 days after inoculation of F98 cells.
Maximum tolerated dose
The maximum tolerated dose (MTD) of cisplatin, carboplatin, and Lipoplatin™ were determined via through a traditional 3 + 3 design [32]. Rats were followed for 10 days after CED, during which observation time, those animals unable to feed or groom, or lethargic, were considered to exhibit drug toxicity.
Tissue platinum concentration and DNA-platinum adducts quantification
Due to the lower survival benefits and similar antitumor mechanism of cisplatin relative to carboplatin, only carboplatin was chosen for quantification of DNA-platinum adducts. It was administered by CED at 10 days after implantation of the F98 cells. After periods of 4 h, 24 h, or 48 h, rats were euthanized, their brains were extracted, and a 2 mm slice at the injection point was cut by a brain matrix. The tumor area and normal brain tissue beside the tumor area were cut and weighed. To determine the total platinum concentration in tissue, the tissues were digested as described in the section “Drug distribution volume” and platinum quantified by ICP-MS. The concentration of drug was calculated in μg platinum per g tissue. For DNA-platinum adducts quantification, DNA were extracted by the phenol/chloroform method [33] and quantified by spectrophotometry. The concentration of drug was calculated in ng platinum per μg DNA.
Survival study
Treatment groups were planned as follows: control (CED of 5% dextrose), radiotherapy alone (CED of 5% dextrose plus radiotherapy), chemotherapy alone (CED of cisplatin, carboplatin, Lipoplatin™), chemotherapy plus radiotherapy (CED of cisplatin, carboplatin, Lipoplatin™ plus radiotherapy). At least 8 rats per group were used. The rats’ weight, mobility, coordination, and grooming were monitored and assessed on a daily basis. The rats were euthanized when the rats lost more than 30% of their initial weight or one of the monitored indices reached a score of 1/10. After anesthesia, 4% paraformaldehyde (PFA) was infused through intra cardiac. Craniotomy was performed to remove the brain that was kept for hematoxylin and eosin (H&E) staining.
Statistical analysis
Two-way ANOVA was used to assess the differences in the amount of DNA-bound platinum in different tissues at different time post-CED. Survival study was analyzed by Kaplan–Meier survival curves and the significance between groups was analyzed by log rank test with GraphPad prism 6, (GraphPad Software, Inc., San Diego, CA). A P-value under 0.05 was considered as statistically significant. Outliers of the survival data were analyzed using box plots with fences [34], with the following calculation: lower inner fence: Q1 - 1.5*IQ, upper inner fence: Q3 + 1.5*IQ (Q1, Q3 are 25th and 75th percentiles, IQ is interquartile range, equals to (Q3 - Q1)). Data smaller than the lower inner fence or larger than the upper inner fence were defined as outliers and were removed.
Results
Validation of CED procedure
The CED procedure was optimized and validated using a green colorant as the infusate to assess volume distribution within the brain parenchyma. Different infusion protocols were compared with respect to diffusion volume and level of backflow. Once determined, the optimal infusion protocol, described in the method section, was used throughout this study. Distribution of the green colorant into the brain under these conditions is shown in (Fig. 1a and b). The green color was uniformly distributed around the caudate nucleus area. The distribution volume of 37.7 mm3 was calculated assuming that the distribution is an ellipsoid with semi-axes lengths a, b, and c measured as 1.5 mm, 2 mm, and 3 mm. Mathieu et al [27] have previously shown that tumor volume is 15.9 ± 7.0 mm3 at 10 days after inoculation. Thus, the distribution volume of the drug is expected to sufficiently cover that of tumor. This has been further confirmed by comparing the green color distribution slice (Fig. 1b) to H&E slices of brain tumor (Fig. 1c).
Maximum tolerated dose (MTD) and tumor uptake
Due to the steep dose-response curve of chemotherapy and the aggressive growth pattern of glioblastoma, to achieve a maximum anti-tumor effect, it is necessary to employ the highest dose of chemotherapy drugs that avoids intolerable toxicity [35]. Thus, MTDs of these drugs administered by CED were evaluated in the F98 glioma bearing Fischer rats through a dose escalation protocol. Initial doses were chosen with reference to the existing literature [13, 19]. Signs of lethargy, groom or feeding cessation, were considered as evidence of intolerable toxicity.
As expected, cisplatin showed the highest toxicity (i.e., the lowest MTD) (Table 1). However, the toxicity was not reduced by incorporating cisplatin into liposomes (Lipoplatin™). Regarding carboplatin, this non-encapsulated drug was better tolerated than cisplatin and Lipoplatin™. The neuropathologic evaluation showed that Lipoplatin™ induced focal hemorrhage, necrosis, and loss of glial cells only in the needle track. (Fig. S3). Similar changes were observed in carboplatin treated brains (Fig. S4).
Table 1.
MTD of platinum-based agents
| Drugsa | MTD |
|---|---|
| Cisplatin | 7.5 μg |
| Lipoplatin™ | 7.5 μg |
| Carboplatin | 25 μg |
Drugs (10 μL) were administered by CED
The concentration of platinum-drugs in the F98 tumor was then determined at their respective MTD. CED increased by 17 to 202 times the concentration of these platinum-based drugs in brain tumor compared to the concentrations produced via i.v. injection, and by 3.6- to 59-fold when compared to the i.a. introduction (Table 2).
Table 2.
Tumor uptake of platinum-based drugs
| Drugs | Tumor uptake (μg Pt/g tissue)
|
|||
|---|---|---|---|---|
| CED | i.v.29 | i.a.b 29 | i.a. + BBBDc 29 | |
| Cisplatin | 9.34 ± 5.33 | 0.45 ± 0.19 | 1.03 ± 0.24 | -- |
| Lipoplatin™ | 5.56 ± 0.75 | 0.32 ± 0.03 | 0.47 ± 0.26 | 1.55 ± 0.62 |
| Carboplatin | 16.1 ± 2.00 | 0.15 ± 0.04 | 0.47 ± 0.24 | 0.99 ± 0.62 |
Concentration of platinum drugs was determined 24 h after their injection by the different routes in F98 glioma bearing rats.
MeST of F98 glioma bearing rats after CED administration of platinum drugs
The MeST was measured in Fischer rats bearing the F98 glioma tumor that were treated either with chemotherapy alone or in combination with radiotherapy (Table 3, Supplementary material Fig. S1). For the groups receiving chemotherapy alone, CED administrations of all the drugs tested at their respective MTD significantly improve the median survival time, relative to the control group.
Table 3.
Median survival time of F98 glioma bearing Fischer rats
| Treatmenta | MSTd (days) | MeSTc (days) | Range (days) | P valuef |
|---|---|---|---|---|
| 5% Dextrose | 22.9 ± 2.1 | 23.5 | 19–25 | |
| 5% Dextrose + 15 Gyb | 31.8 ± 5.2 | 30.0 | 26–35 | P=0.0002 |
| 7.5 μg Cisplatinc | 36.1 ± 6.6 | 35.5 | 25–46 | P<0.0001 |
| 7.5 μg Cisplatin + 15 Gy | 39.3 ± 4.5 | 39.0 | 32–45 | P=0.0002 |
| 7.5 μgLipoplatin™ | 27.0 ± 2.0 | 26.5 | 25–31 | P=0.0004 |
| 25 μg Carboplatin | 40.9 ± 6.0 | 38.5 | 31–47 | P<0.0001 |
| 25 μg Carboplatin + 15 Gy | 53.1 ± 9.6 | 54.0 | 39–65 | P<0.0001 |
Injection volume = 10 μL.
Radiotherapy was delivered 24 h after chemotherapy.
Drugs were tested at their respective MTD.
Mean Survival Time
Median Survival Time
Compared to 5% Dextrose
Cisplatin was the most neurotoxic among the drugs tested. Nevertheless, when injected by CED at its MTD, cisplatin largely improved the MeST of the Fischer rats (35.5 days compared to 23.5 days for the control group). Its therapeutic effect was however mostly lost following its incorporation in a liposomal formulation (Lipoplatin™) which led to a MeST only 3 days better than the control group.
Among the drugs tested, carboplatin led to the best improvement in the MeST for treated animals, which was 15 days longer than the control group.
The MeST was then assessed in animals treated with the platinum drugs plus radiation (15 Gy) delivered 24 h post-CED. The effectiveness of Lipoplatin™ plus radiation was not assayed because this drug when injected alone, caused only a slight increase in the MeST of the animals. Treatment with cisplatin plus radiation, resulted in modest improvement of the median survival time, 3.5 days longer than the animal groups treated with chemotherapy alone. On the other hand, the addition of radiation therapy to carboplatin resulted in a MeST of 54 days, 15.5 days longer than the interval observed in the group of animals treated only with carboplatin.
Median survival time and neurotoxicity measured at 4 and 24 h between CED and radiotherapy
The amount of platinum bound to DNA during radiotherapy can greatly affect the effectiveness of anti-cancer treatment [36]. This was assessed by measuring the accumulation of platinum bound to DNA in tumor and normal brain tissue at 4, 24 and 48 h post-CED for carboplatin. First, we determined the total amount of platinum in the entire tumor, and found that the highest levels for each drug were observed at 4 h post-CED after which, levels gradually decreased (Fig. 2a). Regarding the normal brain tissue, the amount of platinum bound to DNA was smaller and varied much less with time (Fig. 2b).
Fig. 2.

Accumulation of carboplatin in F98 tumor. (a) Kinetic of carboplatin uptake after CED in F98 glioma bearing Fischer rats. (b) Quantification of carboplatin bound to DNA of F98 tumor and normal brain measured after CED administration. All data points are the average of three measurements.
Based on this kinetic study, it was decided to compare median survival times when irradiating the brain tumor at 4 and 24 h after CED, which correspond to a maximal and lower binding of platinum drugs to DNA, respectively (Table 4). However, no significant difference in survival was found for carboplatin group (Table 4).
Table 4.
Survival time and acute toxicity of F98 glioma bearing Fischer rats treated with radiotherapy at 4 h or 24 h after CED of platinum-based drugs.
| dextrose + GK 4 h | dextrose + GK 24 h | carboplatin + GK 4 h | carboplatin + GK 24 h | |
|---|---|---|---|---|
| MeST (days) | 30.5 | 30.0 | 54.5 | 54.0 |
|
| ||||
| Number of rats tested | 8 | 8 | 11 | 8 |
| Number of rats manifesting symptoms of toxicity | 0 | 0 | 4 | 3 |
| Number of rats euthanized due to intolerable toxicity | 0 | 0 | 3 | 0 |
It is noteworthy that a larger number of rats treated with carboplatin showed symptoms of acute toxicity when radiotherapy was performed at 4 h after CED (Table 4). For the group receiving radiotherapy at 4 h after CED of carboplatin, 4 rats manifested symptoms of toxicity, and 3 of them had to be euthanized due to intolerable toxicity (such as unable to feed or groom, or lethargic). A delay of 24 h between carboplatin injection by CED and tumor irradiation reduced the toxicity. Only 3 rats manifested symptoms of toxicity and no rat was euthanized for excessive toxicity.
Discussion
CED drug administration to treat glioma bearing animals has been previously studied for some platinum-based antineoplastic drugs [12–17, 19, 20]. However, those studies were performed using various CED parameters in different animal model settings, and neglected to study any liposomal formulations of the platinum drugs. Therefore, it is difficult to compare the relative effectiveness of all platinum compounds delivered by CED across different studies. In the present study, cisplatin, carboplatin, and Lipoplatin™ were administered by CED in F98 glioma bearing Fischer rats. Here, the MeST of the animals after treatment was used to assess the therapeutic efficacy and to compare the effectiveness of CED with results previously reported with i.v., i.a. and i.a. plus BBBD delivery.
The toxicity of these drugs was assessed by determining their MTD. The lowest MTD (highest toxicity) was obtained with cisplatin, a result in accordance with previous studies that also reported its important toxicity in the F98 glioma rat model [30]. Nevertheless, when employed at its MTD, cisplatin injected by CED was well tolerated by the Fischer rats. More importantly, the second best MeST was obtained when cisplatin was combined to radiotherapy. Lipoplatin™, a liposomal formation, was specifically designed to reduce the toxicity of cisplatin [37]. Studies in the rat model after i.v. injection, confirmed the advantage of this liposomal formulation in reduce the toxicity of cisplatin [37]. This encouraging pre-clinical result led to a phase I study treating different cancer types with Lipoplatin™, those studies also demonstrated the reduced toxicity of Lipoplatin™ [38]. In our animal model of GBM, CED injection directly into the tumor did not demonstrate this benefit of Lipoplatin™ since similar MTD were measured for cisplatin and Lipoplatin™. On the other hand, Huo T. et al have shown the neurotoxicity of Lipoplatin™ when delivered in non-tumor bearing rat brain [22]. In their study, acute hemorrhage in the brain was found outside the needle track. In our study, the toxicity was assessed in animals bearing tumor and toxicities such as focal hemorrhage and necrosis were observed but were limited to the injection site (Fig. S3). Moreover, the anticancer efficiency of Lipoplatin™ was lower than cisplatin as a shorter MeST was found. These results contrast with those obtained with these two drugs when they were injected either by i.v. or i.a. in the same GBM model, as part of another study by our group (Table 5, Fig. S2) [29]. A MeST of 17 days was measured in the group treated with cisplatin injected i.v., whereas it was only 13 days when an i.a. infusion was used. An important toxicity was suspected, as the non-treated group survived longer (MeST of 23.5 days). This severe toxicity of cisplatin was diminished by the use of its liposomal formulation Lipoplatin™ resulting in a MeST of 24 days for i.v. that was further improved to 30 days by i.a. injection (Table 5). In our previous studies, we had already observed that carboplatin was well tolerated in the F98 Fischer rat models when administered either by i.v. or i.a. [29]. This trend continued with CED, as its MTD was 3.3-fold higher (less toxic) than that was measured for cisplatin. Furthermore, a longer MeST was measured in the treated animals with carboplatin, and the best concomitant effect was obtained by combining this platinum drug with radiotherapy. This important anti-tumor effects of carboplatin are in agreement with those reported in the same animal model by Rousseau et al [13]. Our median survival times of rats treated with carboplatin were generally shorter than those reported by the teams of Elleaume and Barth. For example, MeST of 45 days and 43 days were obtained respectively by Rousseau J. et al [13] and Yang W. et al [20], while only 38.5 days was observed in our study when treated with carboplatin. The differences may be due to the following reasons. 1) The experimental design for the implantation of cancer cells is different (i.e., we implanted 10, 000 cells and started CED at day 10, while in the teams of Elleaume and Barth have implanted 1,000 cells, with CED usually starting at day 13). 2) The doses of the drugs administered were different (i.e., in the study of Rousseau J. et al [13], 20 μg of carboplatin was injected (MeST of 55 days), whereas we used 25 μg of carboplatin (MeST of 38.5 days)). Their study also showed that 40 μg of carboplatin lead to a shorter MeST of 32 days. This suggests that the optimal dose of carboplatin still needs to be determined. 3) The end points used to determine the survival of the animals are different and subjective. This could lead to a different evaluation of the survival time. Nevertheless, their studies and ours lead to the same conclusion that CED of carboplatin was efficient to treat glioma bearing Fischer rats. Taken together, these results support the view that CED significantly improves the treatment of GBM by reducing the systemic toxicity of platinum-based drugs and by increasing their anticancer efficacy, relative to results obtained following i.v and i.a. injections. The liposomal formulations of cisplatin, which reduced their toxicity when injected by i.v. and i.a do not appears useful for the CED procedure. Direct injection of these drugs into tumor minimises the unnecessary exposure of normal tissue to these agents. Higher efficiency of CED delivered treatments is due to consequent higher tumor uptake of platinum drugs.
Table 5.
Survival time of F98 glioma bearing Fischer rats treated with platinum-based drugs through different administration routes
| Drugs | Median survival time (days)
|
|||
|---|---|---|---|---|
| CED | i.v.33 | i.a.b33 | i.a. + BBBDc33 | |
| 5% Dextrose | 23.5 | 22.5 (P=0.7679)a | 22.0 (P=0.2323) | 27.0 (P=0.0272) |
| 5% Dextrose + 15 Gy | 30.0 | 30.0 (P=0.5355) | 36.5 (P=0.0825) | 36.0 (P=0.0030) |
| Cisplatin | 35.5 | 17.0 (P<0.0001) | 13.0 (P<0.0001) | -- |
| Cisplatin + 15 Gy | 39.0 | -- | -- | -- |
| Lipoplatin™ | 26.5 | 24.0 (P=0.0022) | 30.0 (P=0.2310) | 29.0 (P=0.0979) |
| Lipoplatin™ + 15 Gy | -- | 29.0 | 36.0 | 33.5 |
| Carboplatin | 38.5 | 23.5 (P<0.0001) | 29.5 (P=0.0011) | 33.5 (P=0.0078) |
| Carboplatin + 15 Gy | 54.0 | 31.0 (P<0.0001) | 46.0 (P=0.1254) | 36.0 (P=0.0158) |
P values were calculated by comparing to the same treatment but by CED routes of delivery.
i.a. delivery through the right carotid artery
BBBD is achieved by infusing of 25% mannitol before i.a. injection of platinum-based drugs
The major anti-tumor effect of platinum-based drugs is associated with the production of DNA damage by forming DNA-platinum adducts [39]. A concomitant effect is obtained when a tumor is treated with a platinum drug and radiotherapy [24]. This radiosensitizing effect was indeed associated with the formation of a larger number of DNA single- and double-strand breaks [40] and potentially, other damages. It is therefore expected that optimization of the anti-cancer treatment would be obtained when the tumor is irradiated at the post-injection time corresponding to the maximum accumulation of platinum drugs in the DNA. This hypothesis has been validated in mice bearing HCT116 colorectal tumor. The best tumor growth delay was measured when radiotherapy was delivered at the post-injection time corresponding to the greatest amount of platinum bound to DNA [25]. On the other hand, when drugs are injected by CED in the F98 tumor of rats, the amount of carboplatin bound to DNA of the tumor varied less with time. A slow decrease in the amount of carboplatin bound to tumor DNA was found between 4 h and 24 h after drugs administration. Consequently, their radiosensitizing effects were less affected by the post-administration time and similar median survival times were measured, and it was easier to reach the concomitance effect with radiotherapy. On the other hand, the concomitant effect of platinum drugs with radiation has resulted in less neurotoxicity when radiotherapy was performed 24 h after drugs administration. We hypothesize that this reduction of neurotoxicity was caused by the lower amount of DNA-bound platinum in normal brain tissue found at 24 h post-injection compared to 4 h post-injection. It is also interesting to note that the amount of carboplatin bound to DNA decreased more rapidly with time in normal brain tissues. Therefore, the observed lower level of neurotoxicity could be linked to a reduction in the level of damage induced by the concomitant treatment of normal brain tissue. Alternatively, the CED procedure obviously involves injecting directly in the tumor a certain volume of the drug solution thereby increasing volume in the extracellular space, and potentially triggering intracranial hypertension; acute cerebral edema caused by radiotherapy may be more important after an interval of 4 h than at 24 h. Thus, although the amount of DNA-platinum adducts was higher at 4 h after CED, we found it was preferable to perform radiotherapy at least 24 h after CED procedure.
In humans, tumor sizes are usually much larger than in the rat model used here; the CED diffusion volume may not encompass the entire tumor, but rather be limited to a smaller volume close to the infusion point. Multiple CED injections might be required to reach the periphery and the infiltrative area of tumor. Other factors such as the safety and feasibility of treating the peritumoral area of the brain [41], the entry points for catheter placement [42] should be considered.
Conclusion
CED increased the accumulation of platinum drugs in the tumors, reduced the toxicity, and resulted in a higher median survival time. The best treatment was obtained in animals treated with carboplatin and irradiated 24 h later. Lipoplatin™ does not show superior antitumor effect over free platinum drugs when delivered by CED in F98 glioma bearing rats.
Supplementary Material
Acknowledgments
Funding: This work was supported by Canadian Institutes of Health Research (grant # MOP 81356). David Fortin, Léon Sanche and Benoit Paquette are members of the Centre de Recherche du CHUS supported by the Fonds de la Recherche du Québec en Santé.
We would like to thank Dr. T. Boulikas for generously providing Lipoplatin™, Dr. A-M Crous-Tsanaclis for her assistance in reviewing the histological samples, and Dr. A.D. Bass for helpful suggestions and corrections.
Footnotes
Conflict of Interest: none
Supplementary material is available online at Investigational New drugs.
References
- 1.Fountzilas G, Karavelis A, Makrantonakis P, et al. Concurrent radiation and intracarotid cisplatin infusion in malignant gliomas: a feasibility study. Am J Clin Oncol. 1997;20:138–142. doi: 10.1097/00000421-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Grossman SA, O’Neill A, Grunnet M, et al. Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncol. J Clin Oncol. 2003;21:1485–91. doi: 10.1200/JCO.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 3.Roci E, Cakani B, Brace G, et al. Platinum-based Chemotherapy in Recurrent High-grade Glioma Patients: Retrospective Study. Med Arch. 2014;68:140. doi: 10.5455/medarh.2014.68.140-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortin D, Morin P-A, Belzile F, et al. Intra-arterial carboplatin as a salvage strategy in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2014;119:397–403. doi: 10.1007/s11060-014-1504-4. [DOI] [PubMed] [Google Scholar]
- 5.Fortin D, Desjardins A, Benko A, et al. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the Sherbrooke experience. Cancer. 2005;103:2606–2615. doi: 10.1002/cncr.21112. [DOI] [PubMed] [Google Scholar]
- 6.Warnick RE, Prados MD, Mack EE, et al. A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neurooncol. 1994;19:69–74. doi: 10.1007/BF01051050. [DOI] [PubMed] [Google Scholar]
- 7.Hiesiger EM, Green SB, Shapiro WR, et al. Results of a randomized trial comparing intra-arterial cisplatin and intravenous PCNU for the treatment of primary brain tumors in adults: Brain Tumor Cooperative Group trial 8420A. J Neurooncol. 1995;25:143–154. doi: 10.1007/BF01057758. [DOI] [PubMed] [Google Scholar]
- 8.Aquino VM, Fort DW, Kamen BA. Carboplatin for the treatment of children with newly diagnosed optic chiasm gliomas: a phase II study. J Neurooncol. 1999;41:255–259. doi: 10.1023/a:1006149809479. [DOI] [PubMed] [Google Scholar]
- 9.Acton Q. Cellular Structures—Advances in Research and Application. 2013. 2013. p. 17. [Google Scholar]
- 10.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allard E, Passirani C, Benoit J-P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–2318. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Biston M-C, Joubert A, Adam J-F, et al. Cure of Fisher Rats Bearing Radioresistant F98 Glioma Treated with cis-Platinum and Irradiated with Monochromatic Synchrotron X-Rays. Cancer Res. 2004;64:2317–2323. doi: 10.1158/0008-5472.CAN-03-3600. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau J, Boudou C, Barth RF, et al. Enhanced survival and cure of F98 glioma-bearing rats following intracerebral delivery of carboplatin in combination with photon irradiation. Clin cancer Res. 2007;13:5195–5201. doi: 10.1158/1078-0432.CCR-07-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rousseau J, Barth RF, Moeschberger ML, Elleaume H. Efficacy of intracerebral delivery of Carboplatin in combination with photon irradiation for treatment of F98 glioma-bearing rats. Int J Radiat Oncol Biol Phys. 2009;73:530–6. doi: 10.1016/j.ijrobp.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau J, Barth RF, Fernandez M, et al. Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J Neurooncol. 2010;98:287–95. doi: 10.1007/s11060-009-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barth RF, Yang W, Huo T, et al. Comparison of intracerebral delivery of carboplatin and photon irradiation with an optimized regimen for boron neutron capture therapy of the F98 rat glioma. Appl Radiat Isot. 2011;69:1813–6. doi: 10.1016/j.apradiso.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Huo T, Barth RF, et al. Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J Neurooncol. 2011;101:379–90. doi: 10.1007/s11060-010-0272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White E, Bienemann A, Taylor H, et al. A phase I trial of carboplatin administered by convection-enhanced delivery to patients with recurrent/progressive glioblastoma multiforme. Contemp Clin Trials. 2012;33:320–31. doi: 10.1016/j.cct.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Tange Y, Kondo A, Egorin MJ, et al. Interstitial continuous infusion therapy in a malignant glioma model in rats. Childs Nerv Syst. 2009;25:655–662. doi: 10.1007/s00381-008-0805-3. [DOI] [PubMed] [Google Scholar]
- 20.Yang W, Barth RF, Huo T, et al. Radiation therapy combined with intracerebral administration of carboplatin for the treatment of brain tumors. Radiat Oncol. 2014;9:25. doi: 10.1186/1748-717X-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito R, Krauze MT, Noble CO, et al. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro Oncol. 2006;8:205–214. doi: 10.1215/15228517-2006-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo T, Barth RF, Yang W, et al. Preparation, Biodistribution and Neurotoxicity of Liposomal Cisplatin following Convection Enhanced Delivery in Normal and F98 Glioma Bearing Rats. PLoS One. 2012;7:e48752. doi: 10.1371/journal.pone.0048752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito R, Bringas JR, McKnight TR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64:2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 24.Tippayamontri T, Kotb R, Paquette B, Sanche L. Synergism in concomitant chemoradiotherapy of cisplatin and oxaliplatin and their liposomal formulation in the human colorectal cancer HCT116 model. Anticancer Res. 2012;32:4395–4404. [PubMed] [Google Scholar]
- 25.Tippayamontri T, Kotb R. Efficacy of Cisplatin and Lipoplatin™ in Combined Treatment with Radiation of a Colorectal Tumor in Nude Mouse. Anticancer Res. 2013;33:3005–3014. [PubMed] [Google Scholar]
- 26.Barth RF, Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009;94:299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathieu D, Lecomte R, Tsanaclis AM, et al. Standardization and detailed characterization of the syngeneic Fischer/F98 glioma model. Can J Neurol Sci. 2007;34:296–306. doi: 10.1017/s0317167100006715. [DOI] [PubMed] [Google Scholar]
- 28.Charest G, Mathieu D, Lepage M, et al. Polymer gel in rat skull to assess the accuracy of a new rat stereotactic device for use with the Gamma Knife. Acta Neurochir (Wien) 2009;151:677–684. doi: 10.1007/s00701-009-0298-1. [DOI] [PubMed] [Google Scholar]
- 29.Charest G, Sanche L, Fortin D, et al. Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J Neurooncol. 2013;115:365–373. doi: 10.1007/s11060-013-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charest G, Sanche L, Fortin D, et al. Glioblastoma Treatment3: Bypassing the Toxicity of Platinum Compounds by Using Liposomal Formulation and Increasing Treatment Efficiency with Concomitant Radiotherapy. Int J Oncol Biol Phys. 2011;84:244–249. doi: 10.1016/j.ijrobp.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn EL, Midthune D, Chen TT, et al. A comparison of two phase I trial designs. Stat Med. 1994;13:1799–1806. doi: 10.1002/sim.4780131802. [DOI] [PubMed] [Google Scholar]
- 33.Ausubel FM, Brent R, Kingston RE, et al. Current Protocols in Molecular Biology. Wiley; New York: 1994. [Google Scholar]
- 34.Nist. NIST/SEMANTECH e-Handbook of Statistical Methods. 2012 Retrieved January 1, 2014. [Google Scholar]
- 35.Hryniuk WM. More is better. J Clin Oncol. 1988;6:1365–1367. doi: 10.1200/JCO.1988.6.9.1365. [DOI] [PubMed] [Google Scholar]
- 36.Tippayamontri T, Kotb R, Paquette B, Sanche L. New therapeutic possibilities of combined treatment of radiotherapy with oxaliplatin and its liposomal formulations (LipoxalTM) in colorectal cancer using nude mouse xenograft. Anticancer. 2014;5312:5303–5312. [PubMed] [Google Scholar]
- 37.Boulikas T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol Rep. 2004;12:3–12. [PubMed] [Google Scholar]
- 38.Stathopoulos GP, Boulikas T, Vougiouka M, et al. Pharmacokinetics and adverse reactions of a new liposomal cisplatin (Lipoplatin): phase I study. Oncol Rep. 2005;13:589–95. [PubMed] [Google Scholar]
- 39.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 40.Rezaee M, Sanche L, Hunting DJ. Cisplatin enhances the formation of DNA single-and double-strand breaks by hydrated electrons and hydroxyl radicals. Radiat Res. 2013;179:323–331. doi: 10.1667/RR3185.1. [DOI] [PubMed] [Google Scholar]
- 41.White E, Bienemann A, Pugh J, et al. An evaluation of the safety and feasibility of convection-enhanced delivery of carboplatin into the white matter as a potential treatment for high-grade glioma. J Neurooncol. 2012;108:77–88. doi: 10.1007/s11060-012-0833-4. [DOI] [PubMed] [Google Scholar]
- 42.Dörner L, Nabavi A, Mehdorn HM. Entry points for catheter placement in convection-enhanced delivery--a retrospective anatomic analysis. Minim Invasive Neurosurg. 2009;52:229–32. doi: 10.1055/s-0029-1243259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


