Abstract
Objective
To determine the relationship between left ventricular cardiac output (LVCO), superior vena cava (SVC) flow, and brain injury during whole-body therapeutic hypothermia.
Study design
Sixteen newborns with moderate or severe hypoxic-ischemic encephalopathy were studied using echocardiography during and immediately after therapeutic hypothermia. Measures were also compared with 12 healthy newborns of similar postnatal age. Newborns undergoing therapeutic hypothermia also had a cerebral magnetic resonance imaging as part of routine clinical care on postnatal day 3–4.
Results
LVCO was markedly reduced (mean+/−SD: 126+/−38 mL/kg/min) during therapeutic hypothermia, whereas SVC flow was maintained within expected normal values (88+/− 27 mL/kg/min) such that it represented 70% of the LVCO. The reduction in LVCO during therapeutic hypothermia was mainly accounted by a reduction in heart rate (99 +/− 13 BPM versus 123 +/− 17 BPM; p<0.001) compared to immediately post-warming, in the context of myocardial dysfunction. Neonates with documented brain injury on MRI showed higher SVC flow pre-rewarming, compared to newborns without brain injury (p=0.013).
Conclusion
Newborns with perinatal hypoxic-ischemic encephalopathy showed a preferential systemic-to cerebral redistribution of cardiac blood flow during whole-body therapeutic hypothermia, which may reflect a lack of cerebral vascular adaptation in newborns with more severe brain injury.
Keywords: Hypoxic Ischemic Encephalopathy (HIE), Magnetic resonance imaging (MRI), Superior vena cava flow, Newborn, Perinatal asphyxia, Echocardiography
INTRODUCTION
Neonatal hypoxic-ischemic encephalopathy (HIE) is an important cause of neurodevelopment morbidity and of mortality in term and preterm infants with an estimated 1.2 million deaths every year worldwide (1–3). Newborns with asphyxia and HIE often suffer multiple organ failure as a result of the hypoxic-ischemic insult, including myocardial dysfunction (4). In the last years, therapeutic hypothermia (TH) has become standard of care for newborns with moderate-to-severe hypoxic-ischemic encephalopathy (HIE), as this treatment has been shown to improve survival and long-term neurodevelopment (5, 6). Based on animal experimental models, the clinical benefit of TH in HIE presumably occurs through a reduction in secondary neuronal damage following reperfusion of the primary insulted brain (7). Studies conducted before the use of TH became standard have demonstrated that cerebral auto-regulation is impaired in newborns who develop more severe brain injury (8). Since the introduction of TH as a treatment, studies have showed a reduction in cerebral blood flow in newborns undergoing TH, suggesting that this may play an important role in preventing secondary neuronal damage (9). However, the extent of systemic-to-cerebral blood flow redistribution that occurs during the period of TH has not been documented.
Newborns are generally able to maintain adequate blood pressure at low temperature achieved during TH (10, 11). In a recent study examining the cardiac hemodynamic effect of whole-body TH in newborns with HIE, using echocardiography, authors concluded that left ventricular cardiac output (LVCO) is reduced by about 67% during TH when compared to measures obtained after rewarming. This suggests that TH may limit the extent of systemic blood flow available to vital organs, including the brain (10). However, a limitation of this latter study by Gebauer et al is the absence of measures of cerebral blood flow. As commented by the authors, it is unclear what degree of systemic-to-cerebral blood flow redistribution occurs in newborns with HIE treated with TH (10). It is also unclear how such mechanisms of blood flow redistribution may influence the subsequent risk of cerebral injury due to secondary neuronal damage (12). Echocardiography offers a valid, non-invasive method of measuring superior vena cava (SVC) flow, which is largely a reflection of cerebral blood flow in newborns (13). To address changes in the hemodynamic systemic and cerebral circulation in newborns with moderate or severe HIE undergoing TH, we prospectively determined LVCO and SVC blood flow immediately before and after rewarming, and validated our findings using a reference group of healthy newborns.
METHODS
Study population
Newborns admitted to the Neonatal Intensive Care Unit of the Children’s & Women’s Health Centre of British Columbia (Canada) and treated with whole-body TH for moderate or severe HIE [based on the Sarnat staging system (14)] were prospectively enrolled between January 2009 and June 2010, following parental informed consent. Newborns were treated with TH within 6 hours of life according to our institutional standards if they presented moderate or severe HIE [based on the Sarnat staging system], were of gestational age ≥35 weeks and if they met at least two of the following criteria: Apgar score ≤5 at 10 minutes, mechanical ventilation or resuscitation at 10 minutes and cord or early arterial/venous blood gas pH <7.00 or base deficit ≥12 within 60 minutes of birth. TH was administered according to the published Infant Cooling Evaluation (ICE) trial method of cooling, at ambient environmental temperature, by applying refrigerated gel packs, as necessary to reach a target core body temperature of 33 and 34°C measured using a rectal temperature probe, for 72 hours (15). In all infants, rewarming was initiated exactly at 72 hours +/− one hour and proceeded at a rate not exceeding 0.5°C every 2 hours. After rewarming, core temperature was strictly maintained between 36.0 and 36.5°C until 96 hours since the initiation of TH. In comparison, a control group of healthy term-born newborns with no clinical evidence of HIE or cardiovascular dysfunction (usually admitted for transient feeding difficulties or for investigation of unrelated causes) were also assessed using echocardiography. The study was approved by the University of British Columbia Clinical Research Ethics Board.
Echocardiography
In newborns undergoing TH (cases), two echocardiography assessments were performed: the first study was performed before initiation of rewarming and the second study was performed within 6–12 hours after the end of the progressive rewarming process. Healthy control newborns were assessed at a similar post-natal age between 72 and 96 hours after birth. Echocardiography assessments were performed by two neonatologists (OH or PML) experienced in echocardiography, using a 7S-RS phased array transducer on a Vivid i BT09 Ultra-Portable High-End echocardiography instrument (GE Healthcare, AB Canada). The following measures were obtained: LVCO was calculated by multiplying the stroke volume (SV) using the aortic valve diameter in a mid-parasternal long-axis 2D view, the velocity time interval measured by pulse Doppler in an apical 5-chamber view and the heart rate; superior vena cava (SVC) flow was calculated by multiplying the averaged minimal and maximal excursion of the SVC diameter in a high parasternal view 2D view, the velocity time interval measured by pulse Doppler over at least three representative heart beat in low subcostal view, and heart rate. Fractional shortening was calculated from a parasternal long-axis view using M-mode. All flow measures were indexed on weight. The left ventricular myocardial performance (Tei) index was calculated from an M-mode view as described (16, 17). The patency of, and flow direction across the ductus arteriosus and atrial septum were also assessed, as described (18).
Brain imaging
Newborns undergoing TH also had a cerebral magnetic resonance imaging (MRI) as part of clinical care on post-natal day 3 to 4, after rewarming. MRI findings that were considered positive for brain injury were restricted water diffusion on diffusion-weighted images, with or without accompanying T1-weighted imaging signal intensity changes, in the basal nuclei, watershed area or a total pattern of injury (both basal nuclei and watershed area involvement), as we previously described (19). All MRI studies were scored by an experienced clinical pediatric neuroradiologist.
Statistical analyses
Based on measures obtained in healthy term neonates, we estimated that between 10 and 19 newborns would provide 80% power (p<0.05) to detect a 15 to 20% difference in SVC or LVCO in non-paired analyses (calculated with: http://www.stat.ubc.ca/~rollin/stats/ssize/n2.html). Differences in echocardiography measurements: LVCO, HR, stroke volume (SV), before and after rewarming, or between newborns with or without MRI evidence of brain injury were assessed using a Wilcoxon matched-pairs signed ranks test. Differences in echocardiography measurements between newborns undergoing TH and healthy control newborns were assessed using a student t-test. A p value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics version 20 (IBM, Chicago, Illinois).
RESULTS
Newborns treated with TH were comparable with the healthy term-born control newborns with regard to their birth weight (mean±SD: 3.49 ± 0.52 versus 3.54 ± 0.43 kg; p=0.78). The clinical markers of severity of the HIE in newborns treated with HT are detailed in table 1. Moderate (n = 15) or severe HIE (n = 1) was diagnosed in all 16 newborns, of whom 13 had seizures. None of the newborns died before the rewarming was completed. The median duration of endotracheal ventilator support in newborns with perinatal HIE was 3 days (range 0 to 6 days). Nine (56%) of newborns were ventilated at the time of the pre-rewarming echocardiography assessment and of these, 8 remained ventilated during the post-rewarming echocardiography study. Five (31%) of the infants required inotropic support during TH which was also continued after rewarming. The doses of dopamine used during TH and throughout the rewarming were generally low (<7 μg/kg/min). None of the infants required volume expansion during the rewarming phase. One infant received inhaled nitric oxide due to pulmonary hypertension, which was also continued throughout the rewarming phase. All infants undergoing TH received an infusion of morphine (dose range: 10 to 30 μg/kg/hour) during the TH, and 13 (81%) continues to receive it in the early period post rewarming. Three of the newborns with HIE demonstrated a left-to-right shunting across a patent ductus arteriosus (diameter >0.2 cm in two newborns) and 6 demonstrated left-to-right atrial shunting. Residual ductus arteriosus and atrial shunts were present during the postrewarming echocardiographic assessment in 2 and 5 newborns, respectively. None of the healthy comparison newborns had significant patent ductus arteriosus or atrial shunts at the time of echocardiographic assessment.
Table 1.
Clinical characteristics of newborns with hypoxic ischemic encephalopathy treated with Therapeutic Hypothermia
| Clinical characteristic | N=16 newborns | |
|---|---|---|
| Birth weight, mean+/−SD (g) | 3.49 +/− 0.52 | |
| Arterial cord pH<7.0 (%)§ | 53 | |
| Apgar score 5 min<5 (%) | 69 | |
| HIE Staging n(%)* | Moderate | 15(94) |
| Severe | 1 (6) | |
| Organ dysfunction n(%)† | 5(31) | |
| Seizure n(%) | 13(81) | |
| Use of >1 anticonvulsant n(%) | 1(6) | |
Cord gas was missing in one newborn;
Either Kidney failure defined as a rise in serum creatinine >90 μM (1 mg/dL), or increase in alanine aminotransferase over twice the upper normal limit (40 IU/L);
Based on Sarnat staging.
Echocardiographic measurements
In newborns with HIE, the first and second echocardiography assessments were done at a mean±SD of 62 ± 16 hours of post-natal life during TH and at 96 ±8 hours of life post- the first study was performed 6–12 hours before initiation of rewarming and the second study was performed within 6–12 hours after the end of the progressive rewarming process. In comparison, the echocardiography assessments were done at a mean±SD of 90 ± 15 hours of life in control newborns.
A comparison of measures for newborns with perinatal HIE before and after rewarming, and in healthy newborns is presented in table 2 and figure 1. LVCO was significantly reduced during TH in newborns with HIE compared to measures obtained post-rewarming (p<0.001), although left ventricular contractility did not change, as assessed by fractional shortening (table 2). When compared to control newborns, LVCO during TH was 57% of values observed in healthy newborns of the same post-natal age. In contrast, measures of cephalic (SVC) blood flow in newborns with HIE remained comparable pre- and post- rewarming, and were also comparable to measures obtained in healthy controls (table 2). This reduction in LVCO was accounted by a reduction in heart rate as well as a reduction in stroke volume (table 2). The left ventricular myocardial performance (Tei) index was significantly increased in infants with HIE indicative of myocardial dysfunction (table 2). Because of the preserved SVC flow in the context of a reduction in LVCO, 70% of the systemic blood flow (LCVO) was distributed cephalically (SVC flow) during TH. This proportion remained higher post-rewarming compared to healthy controls (table 2).
Table 2.
Hemodynamic changes in asphyxiated newborns treated with TH compared to healthy control newborns.
| Newborns with hypoxic-ischemic encephalopathy (n=16) | Healthy newborns (n=12) | P value* | |||
|---|---|---|---|---|---|
| Parameter | Pre-rewarming | Post-rewarming | P value | ||
| HR, mean±SD (BPM) | 99±13 | 123±17 | <0.001 | 136±12 | 0.036 |
| Systolic BP, mean±SD (mmHg) | 61±11 | 63±9 | 0.56 | ND | |
| Diastolic BP, mean±SD (mmHg) | 39±6 | 41± 6 | 0.42 | ND | |
| Core temperature, mean±SD (°C) | 34.0±1.3 | 36.8±0.4 | <0.001 | ND | |
| LVCO, mean±SD (mL/kg/min) | 126±38 | 172±27 | <0.001 | 222±33 | <0.001 |
| SV, mean±SD (mL/kg/min) | 1.26±0.27 | 1.41±0.26 | 0.083 | 1.63±0.16 | 0.016 |
| Fractional Shortening, mean±SD (%) | 34.8±7.6 | 36.9±8.7 | 0.477 | 35±9 | 0.921 |
| LVMPI, mean±SD (circumference/sec) | 0.39 ± 0.07 | 0.35±0.06 | 0.101 | 0.30±0.04 | 0.033 |
| SVC, mean±SD (mL/kg/min) | 88±27 | 92±21 | 0.423 | 91±14 | 0.920 |
| SVC/LVCO, mean±SD (%) | 70±9.4 | 54±12 | <0.001 | 42±9 | 0.005 |
LVCO: left ventricular cardiac output; HR: Heart rate; SV: Stroke volume; FS: fractional shortening; SVC: Superior vena cava blood flow; LVMPI: Left ventricular myocardial performance index; BP: Blood pressure; ND: Not measured.
Comparing healthy newborns with newborns with asphyxia post-rewarming.
Figure 1.
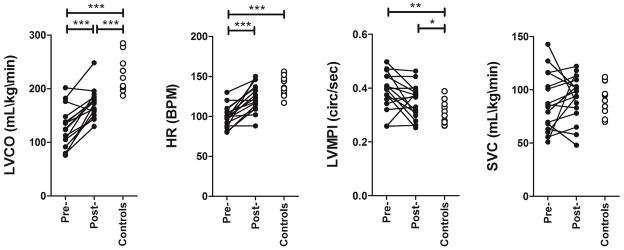
Comparisons of echocardiography measurements pre- and post-rewarming in infants with HIE (dark circles) and in healthy infants (clear circles). LVCO: left ventricular cardiac output; HR: heart rate; LVMPI: Left ventricular myocardial performance index = Tei index; SVC: superior vena cava flow.
Neurological outcomes
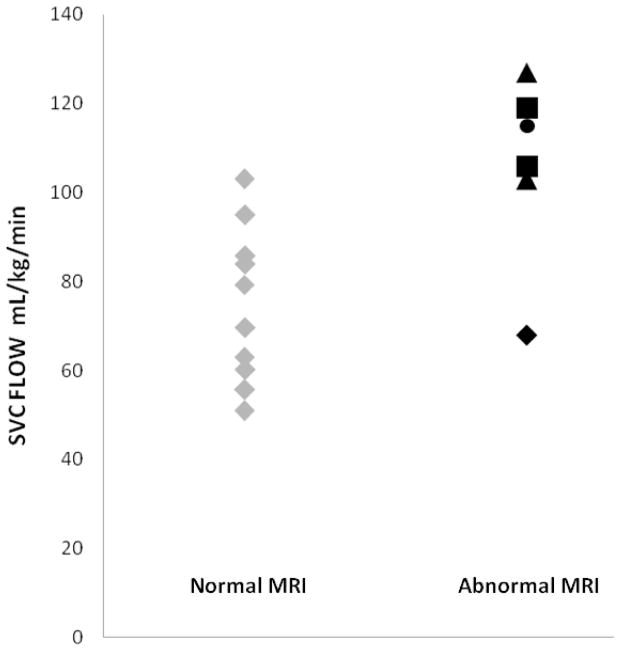
Six newborns had brain injury on MRI: 1 basal nuclei, 2 watershed, 2 total, and 1 with stroke. A majority of newborns with perinatal HIE showed elevated lactates. The newborns with HIE, with or without brain injury did not differ in Apgar scores, peak lactate levels and arterial cord gases (table 3). SVC flows were higher in newborns with brain injury on MRI in the pre-rewarming phase, compared to newborns without brain injury (mean 105 (range 68–127 mL/kg/min) versus 75 (range 51–103 mL/kg/min) P value = 0.013) (table 3). Points plot of SVC measurements during TH according to MRI findings are shown in Figure 2. Other measurements of LVCO, heart rate, stroke volume (SV), as well as of post-rewarming SVC flow did not differ between infants with or without brain injury (table 3).
Table 3.
Initial and pre-rewarming characteristics in asphyxiated newborns with and without abnormal MRI findings
| Parameter | Normal MRI (n=10) | Abnormal MRI (n=6) | P value |
|---|---|---|---|
| Clinical characteristics | |||
| 5 minutes Apgar Score, mean±SD | 4±1.4 | 4.2±3.2 | 0.9 |
| 10 minutes Apgar Score, mean±SD | 5.6±2 | 4.8±3 | 0.67 |
| Cord PH, mean±SD | 7±0.18 | 6.97±0.16 | 0.96 |
| Peak Lactate level, median (IQ range) mmol/L | 5.9 (6.3) | 4.7 (9.3) | 0.85 |
| Pre-rewarming echocardiographic measures | |||
| Heart rate, mean±SD (beats per min.) | 100±13.7 | 98±14 | 0.81 |
| Systolic blood pressure, mean±SD (mmHg) | 64±12.8 | 58±8.3 | 0.29 |
| Diastolic blood pressure, mean±SD (mmHg) | 39.2±6.1 | 39.8±7 | 0.86 |
| Core temperature, mean±SD (°C) | 33.4±0.32 | 33.5±0.5 | 0.75 |
| LVMPI, mean±SD (circumference/sec) | 0.38±0.08 | 0.4±0.045 | 0.32 |
| LVCO, mean±SD (mL/kg/min) | 130±39 | 120±37 | 0.6 |
| SVC, mean±SD (mL/kg/min) | 75±27 | 105±29 | 0.013* |
| Ventilated, n (%) | 4 (40%) | 5 (83%) | 0.09 |
SVC: Superior vena cava blood flow; LVMPI: Left ventricular myocardial performance index; LVCO: left ventricular cardiac output; SD: standard deviation
p value < 0.05
Figure 2.
Points plot of SVC Flow measurements in the newborns with hypoxic ischemic encephalopathy during hypothermia according to MRI results. (triangles): Anterior or posterior watershed, cortex and white matter pattern of injury; (diamonds): Watershed area single focal infarction; (circles): Basal ganglia involvement; (squares): Total injury: basal ganglia extensive injury with anterior and posterior watershed and cortical involvement. SVC = Superior Vena Cava flow.
DISCUSSION
Using echocardiography, we document the hemodynamic changes occurring in the systemic and cerebral circulation in newborns with HIE undergoing whole-body TH. For comparison, we performed the same measures in a reference group of healthy term newborns without signs of HIE. Our data indicate a significant reduction in LVCO during TH, to almost 60% of values observed in healthy newborns. When comparing measures of systemic (LVCO) and cephalic (SVC) blood flow, we observed a preferential 70% redistribution of LVCO to the brain. To our knowledge, this is the first study comparing systemic and cephalic blood flow in newborns with HIE in the era of clinical TH.
Our study enhances our understanding of the hemodynamic adaptation occurring during whole-body TH. First, it provides an important validation of previous data demonstrating a marked reduction in LVCO during TH (10). In our study population, the reduction in LVCO during TH was mainly accounted by a reduction in heart rate when compared to measures post-rewarming, whereas contractility was decreased to a lesser extent as assessed using fractional shortening. However, this reduction in heart rate occurred in the context of an important degree of myocardial dysfunction likely due to the asphyxia, as evidenced by the persistence of significant dysfunction immediately post-rewarming. One could question whether TH was accompanied by a worsening of pulmonary hemodynamics leading to decreased left heart filling or perhaps increased left-to-right shunting across the patent foramen ovale, although the marginal changes in stroke volume during and after rewarming argues against this as a major possibility. In absence of significant cardiac shunts in this series of newborns, we can assume that the LVCO is an accurate reflection of pre-ductal systemic blood flow. Also, the lower LVCO was also likely not related to changes in afterload as blood pressure was maintained in all these infants (data not shown). Bradycardia is a well reported consequence of TH (11), due to prolonged QT and PR intervals (20), but can also be observed in newborns with more severe myocardial hypoxic-ischemic insult (4).
By combining measures of LVCO and SVC in a group of newborns undergoing whole-body TH, we demonstrate a relative increase in systemic-to-cerebral blood flow during TH despite a reduction in LVCO. The preferential redistribution of LVCO to the cerebral circulation implies more limited systemic blood flow to other vital organs, including the kidneys and gastrointestinal system during TH in the context of HIE. Few studies have documented the cerebral hemodynamic changes occurring in newborns with HIE in the era of TH. In healthy neonates, cerebral blood flow normally accounts for a high proportion of systemic blood flow (13). The pattern of brain injury on MRI is recognized as a robust predictor of neurodevelopmental outcome in infants with HIE, including those treated with TH (21, 22). In our study, newborns with documented brain injury showed higher cerebral blood flow compared to newborns without brain injury. Changes in LVCO to the brain may be influenced by regional changes in vascular resistance or perfusion pressure related to a decreased core temperature.
Although our sample size is small, our data are consistent with two other studies reporting an increased cerebral blood flow beyond 24 hours after the initial insult in TH-treated newborns with HIE who develop more severe brain injury (23, 24). Two explanations may account for this cephalic redistribution of LVCO in TH-treated newborns with brain injury. Such process may represent a physiological adaptation, or “sparing effect”. Alternatively, it may reflect a greater loss of cerebrovascular auto-regulation in more severe cases (25). In experimental models of hypoxic-ischemic injury, a decrease in cerebral oxygenation and/or cerebral perfusion alleviates the extent of reperfusion-driven cerebral injury, suggesting that the relative increase in cerebral blood flow during TH is maladaptive (26, 27). In clinical trials in newborns with HIE, TH resulted in only partial improvements in death and neurological outcomes (15, 28, 29). The reasons why some infants appear to not benefit from TH remain unclear. In light of our data, the preferential redistribution of LVCO to the cerebral circulation may be a useful marker to identify a lack of therapeutic response to TH. In order to confirm these findings, a prospective study including a larger number of infants is required, with the collection of detailed clinical and echocardiography hemodynamic outcomes.
Our study has limitations. A majority of newborns in our study met criteria for moderate HIE, with only one newborn having severe HIE. Therefore, our data more closely reflect the majority of infants who may benefit from TH according to clinical trials (15, 28, 29). Future studies are required to understand how outcomes can be improved in infants with severe HIE. Due to its observational nature, we cannot exclude that at least part of the changes in hemodynamic measures observed before and after rewarming may have been influenced by other aspects of neonatal intensive care, including a recovery of myocardial function following the hypoxic-ischemic insult. It is also possible that these newborns may differ in other aspects of their physiology, therefore limiting more detailed comparisons between the newborns with HIE and control newborns. However, the relatively short interval between the two echocardiography studies suggests that the greater extent of the hemodynamic changes observed are due to TH. With respect to changes in the administration of morphine, the small dosages used in our study are unlikely to have results in significant hemodynamic effects. Altogether, our results mandate a more attentive use of echocardiography in the assessment and management of newborns with myocardial failure undergoing TH.
In conclusion, we demonstrate a markedly limited LVCO in newborns with HIE undergoing TH, together with a marked systemic-to-cerebral redistribution of blood flow. The increase cerebral blood flow in the context of a limited LVCO in newborns with documented brain injury on MRI is consistent with an impaired cerebral auto-regulation in a subgroup of TH-treated newborns. Future studies are required to determine whether a failure of cephalic redistribution of systemic blood flow during TH is predictive of a neurological recovery.
Acknowledgments
We are grateful to Dr. Kenneth Poskitt from the Department of Pediatrics, Division of Radiology, University of British Columbia, Vancouver Canada, for the help in the reading, interpretation and scoring of the MRI studies of the newborns with HIE enrolled to this study. We are also grateful to Drs. Patrick Pladys and Alain Beuchee, from the University of Rennes (France), for the extreme generosity of time they demonstrated training neonatologists in targeted-neonatal echocardiography. We thank the families who participated in this study.
PML is support by a Clinician-Scientist Award from the Child and Family Research Institute and a Career Investigator Award from the Michael Smith Foundation for Health Research. SPM is supported by the Bloorview Children’s Hospital Chair in Paediatric Neuroscience, with previous support from a Canada Research Chair (Tier 2) and Michael Smith Foundation for Health Research (MSFHR) Scholar award.
List of abbreviations
- HIE
hypoxic-ischemic encephalopathy
- LVCO
Left ventricular cardiac output
- MRI
Magnetic resonance imaging
- SD
Standard deviation
- SV
Stroke Volume
- SVC
Superior vena cava
- TH
Therapeutic hypothermia
- LVMPI
Left ventricular myocardial performance index (=Tei index)
Footnotes
Disclosure: There is no conflict of interests. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Reference List
- 1.Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ. 2005 Jun;83(6):409–17. [PMC free article] [PubMed] [Google Scholar]
- 2.van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. 2007 Jul;166(7):645–54. doi: 10.1007/s00431-007-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez FF, Miller SP. Does perinatal asphyxia impair cognitive function without cerebral palsy? Arch Dis Child Fetal Neonatal Ed. 2006 Nov;91(6):F454–F459. doi: 10.1136/adc.2005.092445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong K, Franklin O, Sweetman D, Molloy EJ. Cardiovascular dysfunction in infants with neonatal encephalopathy. Arch Dis Child. 2012 Apr;97(4):372–5. doi: 10.1136/adc.2011.214205. [DOI] [PubMed] [Google Scholar]
- 5.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;(4):CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Edwards AD, Azzopardi DV. Therapeutic hypothermia following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006 Mar;91(2):F127–F131. doi: 10.1136/adc.2005.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990 Jul;117(1 Pt 1):119–25. doi: 10.1016/s0022-3476(05)72459-8. [DOI] [PubMed] [Google Scholar]
- 9.Ancora G, Maranella E, Locatelli C, Pierantoni L, Faldella G. Changes in cerebral hemodynamics and amplitude integrated EEG in an asphyxiated newborn during and after cool cap treatment. Brain Dev. 2009 Jun;31(6):442–4. doi: 10.1016/j.braindev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Gebauer CM, Knuepfer M, Robel-Tillig E, Pulzer F, Vogtmann C. Hemodynamics among neonates with hypoxic-ischemic encephalopathy during whole-body hypothermia and passive rewarming. Pediatrics. 2006 Mar;117(3):843–50. doi: 10.1542/peds.2004-1587. [DOI] [PubMed] [Google Scholar]
- 11.Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics. 2000 Jul;106(1 Pt 1):92–9. doi: 10.1542/peds.106.1.92. [DOI] [PubMed] [Google Scholar]
- 12.Kluckow M. Functional echocardiography in assessment of the cardiovascular system in asphyxiated neonates. J Pediatr. 2011 Feb;158(2 Suppl):e13–e18. doi: 10.1016/j.jpeds.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed. 2000 May;82(3):F182–F187. doi: 10.1136/fn.82.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976 Oct;33(10):696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011 Aug;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 16.Cui W, Roberson DA. Left ventricular Tei index in children: comparison of tissue Doppler imaging, pulsed wave Doppler, and M-mode echocardiography normal values. J Am Soc Echocardiogr. 2006 Dec;19(12):1438–45. doi: 10.1016/j.echo.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995 Aug;26(2):135–6. [PubMed] [Google Scholar]
- 18.Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. Eur J Echocardiogr. 2011 Oct;12(10):715–36. doi: 10.1093/ejechocard/jer181. [DOI] [PubMed] [Google Scholar]
- 19.Chau V, Poskitt KJ, Sargent MA, Lupton BA, Hill A, Roland E, et al. Comparison of computer tomography and magnetic resonance imaging scans on the third day of life in term newborns with neonatal encephalopathy. Pediatrics. 2009 Jan;123(1):319–26. doi: 10.1542/peds.2008-0283. [DOI] [PubMed] [Google Scholar]
- 20.Vassallo SU, Delaney KA, Hoffman RS, Slater W, Goldfrank LR. A prospective evaluation of the electrocardiographic manifestations of hypothermia. Acad Emerg Med. 1999 Nov;6(11):1121–6. doi: 10.1111/j.1553-2712.1999.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010 Jan;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005 Nov;147(5):609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai T, Higuchi R, Higa A, Tsuno Y, Hiramatsu C, Sugimoto T, et al. Correlation between echocardiographic superior vena cava flow and short-term outcome in infants with asphyxia. Early Hum Dev. 2013 Jan 14; doi: 10.1016/j.earlhumdev.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Wintermark P, Hansen A, Gregas MC, Soul J, Labrecque M, Robertson RL, et al. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am J Neuroradiol. 2011 Dec;32(11):2023–9. doi: 10.3174/ajnr.A2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum JL, Almli CR, Yundt KD, Altman DI, Powers WJ. Higher neonatal cerebral blood flow correlates with worse childhood neurologic outcome. Neurology. 1997 Oct;49(4):1035–41. doi: 10.1212/wnl.49.4.1035. [DOI] [PubMed] [Google Scholar]
- 26.Niatsetskaya ZV, Charlagorla P, Matsukevich DA, Sosunov SA, Mayurasakorn K, Ratner VI, et al. Mild hypoxemia during initial reperfusion alleviates the severity of secondary energy failure and protects brain in neonatal mice with hypoxic-ischemic injury. J Cereb Blood Flow Metab. 2012 Feb;32(2):232–41. doi: 10.1038/jcbfm.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura S, Kusaka T, Yasuda S, Ueno M, Miki T, Koyano K, et al. Cerebral blood volume combined with amplitude-integrated EEG can be a suitable guide to control hypoxic/ischemic insult in a piglet model. Brain Dev. 2013 Aug;35(7):614–25. doi: 10.1016/j.braindev.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 29.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005 Feb 19;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]