Abstract
Background
Preterm birth is a worldwide health concern due to its various negative consequences. Therefore, the prevention of preterm birth is a top priority for healthcare systems in all countries.
Objective
To compare the effectiveness of vaginal versus intramuscular progesterone in the prevention of preterm delivery.
Methods
This randomized clinical trial was conducted at Shahid Sadoughi Hospital in Yazd, Iran, from November 21, 2012 to January 20, 2015. Seventy-eight pregnant women with singleton pregnancy and one risk factor of preterm delivery were included in the study. The subjects were assigned randomly to two groups, with group one receiving Cyclogest and group two receiving 17-α hydroxyprogesterone caproate. Subsequently, we analyzed drug complications during pregnancy, delivery time, neonatal outcomes, and patients’ satisfaction among the two groups. The data were analyzed using SPSS version 16. We used descriptive statistics, chi-squared, t-test, and ANOVA for the analyses of primary and secondary outcomes.
Results
Among the 39 births in group one, 33.3% occurred preterm, and, among the 39 births in group two, 30.7% occurred preterm (< 37 weeks). The mean gestational ages at delivery in groups 1 and 2 were 37.07 ± 2.23 and 36.81 ± 2.77 weeks, respectively (p = 0.765). Other variables were not significantly different between the two groups, including birth weight (p = 0.745), Apgar scores for the first and fifth minutes (p = 0.574, 0.630), length of stay in the neonatal intensive care unit (NICU) when the newborns needed hospitalization (p = 0.358), and the patients’ satisfaction with the drugs that were used (p = 0.615).
Conclusions
In this study, vaginal progesterone and intramuscular progesterone had the same levels of effectiveness, safety and acceptance by patients in the prevention of preterm delivery. Therefore, both can be used for this purpose in clinical practices, but more studies are needed.
Keywords: pregnancy, preterm delivery, progestins
1. Introduction
Preterm delivery, which is defined as delivery after 20 weeks and before 37 completed weeks, is one of the common complications of pregnancy (1). Worldwide, approximately 13 million infants are born prematurely each year (2). Reports indicate that between 5 and 25% of all pregnancies result in premature delivery. Despite improvements in perinatal care, the rate of preterm delivery in developing countries is higher than in developed countries, and, in recent years, further increases have been observed (3).
Preterm delivery has many negative consequences for newborns, their families, and society, and it is the leading cause of neonatal mortality and long-term morbidity (1, 3). Thus, in recent years, preventing preterm delivery has become a major priority for healthcare systems in most countries (2). The first step in preventing preterm delivery is the correct identification of those who are at risk (3). Several markers could be used as indicators for the prediction of preterm delivery (4), but the strongest determinants are a history of prior preterm delivery and a short cervix (2–4). After the indicators have been identified, effective interventions should be implemented to prevent preterm births. To date, treatment with drugs has been the primary method used to prevent preterm deliveries (5), and many drugs with different pharmacological formulas have been developed for this purpose, with progestin agents having the greatest efficacy in preventing preterm delivery (5, 6). Currently, progestin-based drugs are available in various forms (1). In general, according to the results of previous studies, the administration of progesterone to women with short cervixes or previous histories of preterm labor may reduce the risk of preterm births (6). However, further research is needed to establish more detailed information about the formulation, route of administration, optimum dose, potential risks, and possible side effects of progesterone on perinatal outcomes; also, the available progestin drugs should be compared to identify those that are the safest and most effective (6, 7). The purpose of this study was to compare the efficacy of vaginal progesterone (Cyclogest) and intramuscular progesterone (17-α hydroxyprogesterone caproate) for the prevention of preterm delivery.
2. Material and Methods
2.1. Trial design
This study was a randomized clinical trial that was conducted from November 21, 2012 to January 20, 2015.
2.2. Participants
This clinical trial was conducted in an Iranian governmental educational hospital (Shahid Sadoughi Hospital and its integrated obstetrics and gynecology complex, Kowsar). Shahid Sadoughi Hospital is the largest hospital in Yazd, Iran, and it is affiliated with Shahid Sadoughi University of Medical Sciences. This hospital is the referral center of the southwestern area of Iran, and it has an integrated complex of obstetrics and gynecology care (Kowsar) and an Obstetrics and Gynecology clinic. The research population was comprised of pregnant women (with gestational age of 16–20 weeks) who were being cared for by the Obstetrics and Gynecology clinic at the Hospital. The gestational age was calculated according to the date of the last menstrual period (LMP) with ultrasound confirmation. Among our 78 participants, 67 women had had a sonography in the twelfth week in which the crown-rump length (CRL) had been measured for the investigation of nuchal translucency (NT). For the others, gestational age was determined based on the biparietal diameter (BPD) at the same time the length of the cervix was measured and was adjusted with the measured gestational age based on the LMP.
2.3. Selection criteria
The inclusion criteria for participation in this study were: 1) singleton pregnancy, 2) living fetus, 3) confirmed satisfaction with participating in the study, and 4) the presence of one of the preterm delivery risk factors, i.e., a history of previous preterm delivery or a short cervix (< 25 mm) as measured with trans-vaginal ultrasonography. The exclusion criteria were women with severe liver disease, hypertension, diabetes, cardiovascular disease, thromboembolic disorder, seizures, and sensitivity to hormonal therapy.
2.4. Interventions
In this clinical trial, 362 pregnant women at the gestational age of 16–20 weeks were visited at the hospital, and 53 women were excluded because they declined to participate or they met one or more of the exclusion criteria. The researchers obtained complete histories from the other women. After informed consent was obtained, the length of each woman’s cervix was obtained by trans-vaginal ultrasonography at no cost to the participants. The women who had one of the two risk factors for preterm delivery, i.e., 1) previous preterm delivery when the length of the cervix was normal or 2) the length of the cervix was < 25 mm without previous preterm delivery, were entered into the study. Women who had both risk factors of preterm delivery were not included in the study. This decision was made because, in clinical settings, women with a history of preterm birth might be more likely to have shorter cervixes and vice versa. The result was that 78 pregnant women (33 women with a history of preterm delivery and 45 women with short cervixes) participated in the study. Randomly and separately, the 78 women were divided into two groups based on the baseline risk factor. In other words, the women with a history of preterm delivery were divided randomly into two groups (i.e., a vaginal progesterone group and an intramuscular progesterone group), and the women with short cervixes also were divided randomly into two groups (i.e., a vaginal progesterone group and an intramuscular progesterone group). We did this because some previous researchers, e.g., Abd El Hameed (8), claimed that some kinds of progesterone are preferable for cases with a specific risk factor. Then, the first group received daily administration of vaginal progesterone (Cyclogest suppository, Actavis, UK limited, England), with each suppository containing 200 mg of progesterone. The other group received weekly intramuscular injections of 250 mg of progesterone (17-α hydroxyprogesterone caproate, Bayer Schering Pharma, Germany). All cases were followed until delivery. All of the required data were carefully collected and recorded during the study in the designated form. The data that were collected during routine perinatal care included any adverse effects of the drugs (e.g., headache, nausea, depression, migraine, fever, arthralgia, and myalgia), gestational age at delivery, and the infants’ outcomes. The form used to gather the data also included one question about the overall satisfaction of the participants with the drugs they received with respect to their convenience of use. In this question, the participants were asked to rate their satisfaction with the drug they received on a 5-point Likert scale (very low to very high). The participants responded to this question after delivery, and the mean score for each group was calculated. It is notable that, due to the separate allocation of women with each of the risk factors (previous preterm delivery or short cervix) to the vaginal and intramuscular progesterone groups, we analyzed the mean gestational age at delivery across four subgroups in a complementary analysis (based on the preterm delivery risk factor and the means used to administer the progesterone). While our sample size had not been calculated for this subgrouping, we conducted this analysis and presented the results to provide additional information.
2.5. Outcomes
The primary outcomes of our analyses were the rate of preterm delivery and the mean gestational age at the time of delivery in the two groups. Also, the secondary outcomes from the analyses were the adverse effects of the drugs during the course of treatment, some infants’ outcomes [including birth weight (g), Apgar scores of the first and fifth minutes, length of stay in NICU when the newborn infants required hospitalization], and the patients’ satisfaction with the drug in terms of convenience of use.
2.6. Sample size
The sample size was calculated to be 76 subjects. This sample size was calculated based on the results of previous studies (1, 9) by assuming the test power of 80% and a confidence level of 95% and using the following formula:
Where:
n = Sample size
Z1-α/2 = 1.96 when α = 5% for two-sided test
Z1-β = 0.842 when β = 20% (test power = 80%)
P = Probability of the main outcome
2.7. Randomization and blinding
In this clinical trial, stratified randomization was used with the baseline preterm delivery risk factor of the participants. For this, all participants were divided into two groups, i.e., those with short cervixes and those with previous preterm deliveries. Then, the participants in each of the groups were assigned randomly to receive either vaginal or intramuscular progesterone on a 1:1 ratio. Randomization was done by one of researchers, who did not have a role in the treatment of the participants. The randomization sequence was computer-generated, with the randomization itself conducted through SPSS16 software (SPSS, Inc., Chicago, IL, USA) (random number generation). We used a variable block size of four and six for randomized sequence generation. Also, the allocation concealment was done by the researcher who was responsible for the randomization. For this purpose, the numbered envelopes that contained the name of the drugs (Cyclogest suppository or 17-α hydroxyprogesterone caproate) were used. After being allocated randomly to the groups, all participants were referred to Hospital’s pharmacy to get their drugs. The intramuscular progesterone was injected in the Hospital’s outpatient ward. Also, the outcomes of the study were recorded by the first-year Obstetrics & Gynecology residents and midwives of the delivery ward who made no other contribution to the study.
2.8. Statistical methods
Data analysis was conducted using SPSS16 software (SPSS, Inc., Chicago, IL, USA). We used descriptive statistics, chi-squared, t-test, and ANOVA to analyze the primary and secondary outcomes. The two-tail value of p < 0.05 was considered as statistically significant. Also, before performing the statistical analyses, the normality of the variables’ distribution was examined using the K-S test.
2.9. Research ethics
The proposal for this thesis research was presented to the Ethics Committee of Shahid Sadoughi University of Medical Sciences after its scientific approval by the Obstetrics and Gynecology Department. The Ethics Committee approved the study with the number P/17/107183/3 on August 23, 2012, and the enrollment of patients began on November 21, 2012, and the study continued until January 20, 2015. This study also was registered in the Iranian Registry of Clinical Trials (irct.ir) with the ID: IRCT2015040921670N1. It also should be noted that we registered the study in irct.ir as a retrospective registration because we were allowed to conduct the thesis after the approval of the Ethics Committee, which is affiliated with the Office of the Vice Chancellor for Research at Shahid Sadoughi University of Medical Sciences. The authors confirm that all ongoing and related trials for this drug/intervention are registered. Indeed, in this study, for ethical considerations, the participants were informed about the objective and nature of the study, and each participant provided her written consent in her native language (Persian) prior to the study. Also, we were committed to keeping all of the participants’ information confidential.
3. Results
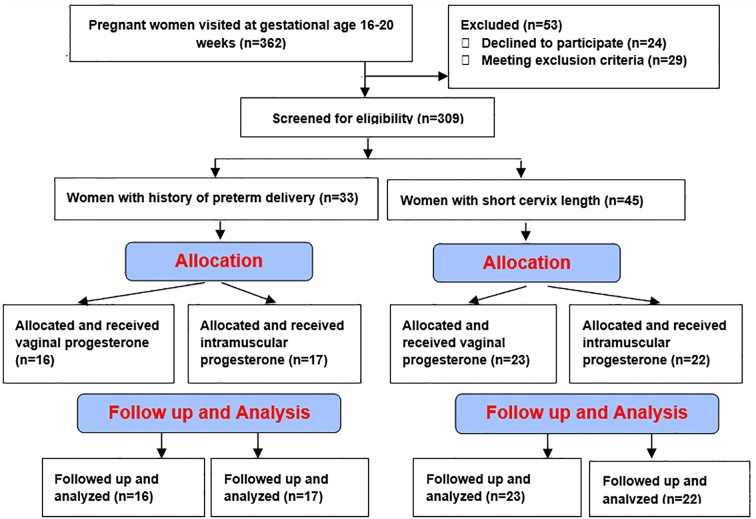
In this clinical trial, 362 pregnant women at the gestational age of 16–20 weeks were visited, 53 of whom were excluded. The remaining 309 women were screened for eligibility. After the screening, 78 participants were chosen, 33 of whom had a history of preterm delivery without short cervixes and 45 of whom had short cervixes. Then, the women separately and randomly were allocated to interventions. There were no losses of participants during the study, and all of the women were analyzed. Figure 1 shows the CONSORT flow diagram of the trial. Table 1 presents the demographic characteristics of the participants.
Figure 1.
CONSORT flow diagram of the trial
Table 1.
Demographic characteristics of participants
| Variable | Vaginal progesterone group (Mean ± SD) | Intramuscular progesterone group (Mean ± SD) | p-value |
|---|---|---|---|
| Age | 28.78 ± 6.73 | 28.76 ± 5.21 | 0.671 |
| Body Mass Index | 22.61 ± 2.22 | 23.85 ± 2.93 | 0.181 |
| Number of previous pregnancies | 1.44 ± 0.85 | 2.47 ± 2.09 | 0.064 |
| Number of previous preterm deliveries in women selected according to this risk factor | 1.28 ± 0.75 | 1.45 ± 1.16 | 0.346 |
| Length of the cervix (mm) in women selected according to this risk factor | 22.78 ± 1.36 | 22.62 ± 1.26 | 0.545 |
| Gestational age at the beginning of treatment | 16.06 ± 2.89 | 16.61 ± 1.22 | 0.101 |
As specified in Table 1, the two treatment groups (vaginal progesterone and intramuscular progesterone) showed no significant difference in demographic variables. Tables 2 and 3 present the statistics related to the outcomes. Among the 78 deliveries, 32.1% (n = 25) occurred preterm. The rates in the vaginal progesterone and intramuscular progesterone groups were 33.3% (n = 13) and 30.7% (n = 12), respectively, but this difference was not statistically significant (p = 0.088). Also, no deaths or abnormalities were observed at birth. In addition, no side effects of the drugs were observed in either of the two treatment groups. Table 3 shows that there were no significant differences in the gestational age (weeks) at delivery in the four treatment sub-groups.
Table 2.
Statistics related to the outcomes of the vaginal progesterone and intramuscular progesterone groups
| Variable | Vaginal progesterone group | Intramuscular progesterone group | p-value | ||
|---|---|---|---|---|---|
| (Mean ± SD) | n | (Mean ± SD) | n | ||
| Gestational age at delivery (weeks) | 37.07 ± 2.23 | 39 | 36.81 ± 2.77 | 39 | 0.765 |
| Birth weight (g) | 2717.20 ± 517.04 | 39 | 2785.50 ± 723.08 | 38 | 0.745 |
| Apgar score, first minute | 8.94 ± 1.39 | 39 | 9.20 ± 1.14 | 35 | 0.574 |
| Apgar score, fifth minute | 9.50 ± 1.04 | 39 | 9.66 ± 0.89 | 35 | 0.630 |
| Length of newborns’ stay in the NICU (days) in cases requiring hospitalization | 5.40 ± 3.20 | 11 | 11.70 ± 14.22* | 7 | 0.358 |
| Patients’ satisfaction with their drug | 4.11 ± 0.47 | 39 | 4.01 ± 0.79 | 39 | 0.615 |
The magnitude of this number was due to one case in the intramuscular progesterone group who increased the mean and standard deviation of this group with 38 days of hospitalization in the NICU because of sepsis. When we excluded this case from the data sheet, the length of the NICU stay for the intramuscular group decreased to 7.30 ± 2.86 days
Table 3.
Comparison of gestational age at delivery in four subgroups
| Variable | Group 1 | Group 2 | Group 3 | Group 4 | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | ||
| gestational age at delivery (weeks) | 35.57 ± 2.54 | 16 | 38.02 ± 1.43 | 23 | 35.89 ± 3.57 | 17 | 37.63 ± 1.61 | 22 | 0.088 |
Group 1: Intervention: vaginal progesterone + Risk factor: history of preterm labor; Group 2: Intervention: vaginal progesterone + Risk factor: short cervix length; Group 3: Intervention: intramuscular progesterone + Risk factor: history of preterm labor; Group 4: Intervention: intramuscular progesterone + Risk factor: short cervix length
4. Discussion
Efficacy findings showed that 67.8%, 11.6%, and 20.6% of all deliveries occurred as term, preterm (< 37 weeks), and very preterm (< 34 weeks), respectively. These results confirmed that progesterone therapy had acceptable efficacy in the prevention of preterm labor. Our other results indicated that the frequency of preterm delivery and mean gestational age at delivery were not statistically different between the vaginal progesterone and intramuscular progesterone groups. This indicated that these two modes of administering progesterone had essentially the same efficacy in preventing preterm delivery. El-gharib & El-hawary showed that vaginal progesterone and intramuscular progesterone had essentially the same effectiveness in the prevention of preterm delivery (10). Abd El Hameed (8) confirmed the results of El-gharib & El-hawary, as our study does. In contrast, Maher et al., in a randomized clinical trial to compare the efficacy of vaginal progesterone and intramuscular progesterone, found that the vaginal progesterone group had rates of delivery before 28, 32, and 34 weeks that were lower than those in the intramuscular progesterone group (11). Therefore, they concluded that vaginal progesterone was significantly more effective in the prevention of preterm delivery (11). Also, Edwards et al., in their systematic review and meta-analysis, confirmed the efficacy of all types of application of progesterone therapy in the prevention of preterm delivery, but they focused on the necessity of further research to compare directly the efficacy of different types of progesterone usage in the prevention of preterm delivery (12).
In our study, in addition to the comparison of vaginal progesterone and intramuscular progesterone, the participants were divided into four groups according to their risk factor (history of preterm delivery or short cervixes) and received progesterone (vaginally or intramuscularly) to determine their comparative effectiveness more precisely in a complementary analysis by comparing the mean gestational ages at delivery of these four groups. In this analysis, no significant difference was observed between the four groups. However, in women with short cervixes, the mean gestational age at delivery in the vaginal progesterone group was slightly higher than in the intramuscular progesterone group (p = 0.574). However, in those with previous preterm delivery, the mean gestational age at delivery was slightly higher in the intramuscular progesterone group (p = 0.846), but neither of these differences was significant. However, the comparison of the mean gestational age at delivery of each progesterone group based on the preterm delivery risk factor showed that women with short cervixes had longer pregnancies than women with preterm delivery history in both progesterone groups (p = 0.018, 0.206); these results were statistically significant in the vaginal progesterone group. Although few studies are available with the same analysis, Abd El Hameed conducted a study of women with short cervixes and found that vaginal progesterone had greater efficacy than intramuscular progesterone (i.e., preterm deliveries of 9.09% and 28.9%, respectively) (8). However, in women with histories of preterm delivery, no difference was found in the efficacies of vaginal progesterone and intramuscular progesterone (i.e., preterm deliveries of 16.6% and 14.3%, respectively) (8). Therefore, our results are in good agreement with the results of Abd El Hameed with respect to pregnancies among women with short cervixes and no previous preterm delivery.
In accordance with the side effects of selected interventions during pregnancy, we did not observe any case of adverse effects of the drugs in either of the groups, demonstrating that vaginal progesterone and intramuscular progesterone have acceptable and equal safety in the prevention of preterm delivery. In their study in 2012, Khandelwal et al. found that vaginal progesterone resulted in no complications in preventing preterm delivery (3). Also, the studies of Hassan et al. and O’Brien et al. indicated that the rate of adverse effects of vaginal progesterone was similar to that of the placebo (13, 14). Check et al., Katz et al., and Kester also confirmed the safety of hydroxyprogesterone caproate (15–17). Indeed, according to the comparison of the side effects of these two different uses of progesterone, Maher et al. showed that the rate of side effects in women treated with vaginal progesterone was lower than in the case of intramuscular progesterone, i.e., 7.5% as opposed to 14.1% (11). Therefore, this study concluded that the use of vaginal progesterone was safer than the use of intramuscular progesterone (11). Also, in the study of El-gharib & El-hawary, the rate of side effects of intramuscular progesterone was reported to be higher than that of vaginal progesterone (10). However, in our study, we did not observe any adverse effects associated with either type of usage of the drug, so we cannot offer a definitive conclusion on this matter.
In this study, in addition to comparing the efficacy and safety of vaginal progesterone and intramuscular progesterone in the prevention of preterm delivery, we assessed their effects on some infant outcomes. Based on the results, the birth weights of the two groups were not statistically different. Also, 72.2% of the infants born to women taking Cyclogest and 73.6% of the infants born to women taking hydroxyprogesterone caproate group weighed more than 2,500 g at birth. Abd El Hameed studied three groups, i.e., vaginal progesterone, intramuscular progesterone, and control groups, and confirmed the positive effect of both vaginal progesterone and intramuscular progesterone on birth weight (8). In this study, similar to our results, the birth weight of newborns of the vaginal progesterone and intramuscular progesterone groups was not significantly different (8). Also, Saleh Gargari et al., Bomba et al., Hassan et al., Dudas et al., and Dodd et al. confirmed the positive effects of progesterone on increasing infants’ weights at birth (13, 18–21). But the study of O’Brien et al. on Cyclogest and the studies of Hassan et al. and Durnwaldet et al. on hydroxyprogesterone caproate (13, 14, 22) did not find the same effects. In our study, the mean Apgar scores of the first and fifth minutes in the two study groups were not significantly different. Also, 28.20% of the infants born to the Cyclogest group and 17.90% of infants born to the hydroxyprogesterone caproate group were admitted to the NICU, but the difference of the length of stay in the NICU between the two groups was not significant. It is notable that the six-day difference between the lengths of the infants’ stays in the NICU in the two groups was due to one case in the intramuscular progesterone group. This one case increased the mean and standard deviation of this group with 38 days of hospitalization in the NICU because of sepsis. This infant was born after 31 weeks and four days of pregnancy. When we excluded this case from the data sheet, the length of the NICU stay for the intramuscular group decreased to 7.30 ± 2.86 days. In terms of Apgar scores and need for admission to the NICU, our results were similar to those of Abd El Hameed et al. (8). Also, Saleh Gargari et al., Hassan et al., and De-Franco et al. confirmed the positive effects of progesterone on reducing admissions to the NICU (13, 18, 23). We did not observe any cases of perinatal mortality and birth time abnormalities in our study. Consistent with our findings, the studies of Edwards et al. and Conde-Agudelo et al., Abd El Hameed, Hassan et al. and Berghella, et al. confirmed the positive effects of progesterone on reducing perinatal mortality and morbidity (8, 12, 13, 24, 25). But in the studies of Saleh Gargari et al., O’Brien et al. and Fonseca et al. on Cyclogest, the rate of infant mortality in the progesterone group was not significantly different from the placebo group (14, 18, 26). Also, the meta-analysis of Dodd et al. of 11 large randomized clinical trials on the effects of progesterone to prevent preterm delivery showed the same results (27). In this study, we also assessed the satisfaction of the pregnant women with the drug they received in terms of convenience of use. The findings showed that 100% of participants had a satisfaction level of moderate or better. Also, satisfaction scores were not significantly different between the two groups that were studied. In this regard, Khandelwal et al. in their study in 2012 found that vaginal progesterone was acceptable to women due to its ease of use (3).
Our study had some limitations. It was limited to singleton pregnancies, so we cannot generalize our findings to all pregnancies. Also, we had no placebo group, which could have provided more detailed information. We decided to have no placebo group because our main objective was to compare the efficacy of different progestins in the prevention of preterm delivery based on their route of administration with the presumption that progesterone is effective in preventing preterm delivery. Also, although some similar studies have been conducted with fewer samples, the potentially limited sample size in our study could be mentioned as another limitation. Studies with larger sample sizes can verify our results. In our study, the patients were not blind to the allocation and only members of the staff (first-year Ob & Gyn residents and midwives of the delivery ward) recorded the outcomes of the study, and those staff members were blind to the allocation of the medications in the study. Despite these limitations, our study also had some strong points. The first was the analysis of the effect of the use of progesterone on infant outcomes. We know that the ultimate purpose of interventions designed to prevent preterm delivery is the improvement of infant outcomes. Therefore, we analyzed the infant outcomes in our study groups up to the time of delivery in addition to the main objective of the study. Also, in the randomization process, we separately allocated our samples to two groups based on their risk factor, because, as mentioned previously, some previous research has claimed that some types of usage of progesterone are preferable for cases with a specific risk factor. Also, the fairly strict inclusion and exclusion criteria as well as the study design and interventions protocol are other strengths of this study.
5. Conclusions
Based on our results, we can conclude that vaginal progesterone and intramuscular progesterone can be equally useful in the prevention of preterm delivery, but we think that further studies are needed to compare the effectiveness of different types of usage of progesterone in preventing preterm delivery.
Acknowledgments
This paper was extracted from an Obstetrics and Gynecology residency thesis at Shahid Sadoughi University of Medical Sciences in Yazd, Iran. The authors appreciate the assistance and cooperation provided by the staff members in the Obstetrics and Gynecology Department at Shahid Sadoughi Hospital, and we sincerely appreciate all of the women who participated in the study. Also, we appreciate the statistical consultation and assistance provided by Ms. Farimah Shamsi (Ph.D. candidate in biostatistics and faculty member in the Epidemiology and Biostatistics Department at Shahid Sadoughi University of Medical Sciences).
Footnotes
iThenticate screening: August 23, 2015, English editing: September 17, 2015, Quality control: October 06, 2015
Trial Registration: The trial was registered at the Iranian Registry of Clinical Trials (http://www.irct.ir) with the Irct ID: IRCT2015040921670N1.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: There is no conflict of interest to be declared.
Authors’ contributions: All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Bernstein P, Berck D, Burgess T, Dayal A, Einstein F, Florio Ph, et al. Preventing preterm birth: The role of 17 α hydroxyprogeterone caproate. ACOG District II. 2009 [Google Scholar]
- 2.Dodd JM, Crowther CA. The role of progesterone in prevention of preterm birth. Int J Womens Health. 2009;1:73–84. doi: 10.2147/ijwh.s4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khandelwal M. Vaginal progesterone in risk reduction of preterm birth in women with short cervix in the midtrimester of pregnancy. Int J Womens Health. 2012;4:481–490. doi: 10.2147/IJWH.S28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper RL. Is there enough evidence to support the use of 17 Alpha-Hydroxyprogesterone Caproate in preventing preterm labor in healthy women who have had a prior preterm delivery? IJAPA. 2010;7(2) [Google Scholar]
- 5.Regmi MC, Rijal P, Agrawal A, Uprety D. Progesterone for Prevention of Recurrent Preterm Labor after Arrested Preterm Labor: A Randomized Controlled Trial. Gynecol Obstet. 2012;2(4) doi: 10.4172/2161-0932.1000125. [DOI] [Google Scholar]
- 6.Farine D, Mundle WR, Dodd J. The Use of Progesterone for Prevention of Preterm Birth. JOGC. 2008;202:68–72. [Google Scholar]
- 7.Nisha S, Uma S, Shikha S. Comparative Study of Nifedipine and Isoxpurine as Tocolytics for Preterm Labor. Journal Obstets & Gynecol India (September–October) 2011;61(5):512–515. doi: 10.1007/s13224-011-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abd El Hameed AA. Vaginal versus intramuscular progesterone in the prevention of preterm labor and their effect on uterine and fetal blood flow. Middle East Fertility Society Journal. 2012;17:163–169. doi: 10.1016/j.mefs.2011.12.003. [DOI] [Google Scholar]
- 9.Da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 10.El-Gharib MN, El-Hawary TM. Matched sample comparison of intramuscular versus vaginal micronized progesterone for prevention of preterm birth. J Matern Fetal Neonatal Med. 2013;26(7):716–9. doi: 10.3109/14767058.2012.755165. [DOI] [PubMed] [Google Scholar]
- 11.Maher MA, Abdelaziz A, Ellaithy M, Bazeed MF. Prevention of preterm birth: a randomized trial of vaginal compared with intramuscular progesterone. Acta Obstet Gynecol Scand. 2013;92(2):215–22. doi: 10.1111/aogs.12017. [DOI] [PubMed] [Google Scholar]
- 12.Velez Edwards DR, Likis FE, Andrews JC, Woodworth AL, Jerome RN, Fonnesbeck CJ, et al. Progestogens for preterm birth prevention: a systematic review and meta-analysis by drug route. Arch Gynecol Obstet. 2013;287(6):1059–66. doi: 10.1007/s00404-013-2789-9. [DOI] [PubMed] [Google Scholar]
- 13.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):687–96. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 15.Check JH, Rankin A, Teichman M. The risk of fetal anomalies as a result of progesterone therapy during pregnancy. Fertil Steril. 1986;45:575–7. doi: 10.1016/s0015-0282(16)49292-7. [DOI] [PubMed] [Google Scholar]
- 16.Katz Z, Lancet M, Skornik J, Chemke J, Mogilner BM, Klinberg M. Teratogenicity of progestogens given during the first trimester of pregnancy. Obstet Gynecol. 1985;65:775–80. [PubMed] [Google Scholar]
- 17.Kester PA. Effects of prenatally administered 17 alphahydroxyprogesterone caproate in adolescent males. Arch Sex Behav. 1984;13:441–55. doi: 10.1007/BF01541429. [DOI] [PubMed] [Google Scholar]
- 18.Saleh Gargari S, Habibolahi M, Zonobi Z, Khani Z, Sadat Sarfjoo F, Kazemi Robati A, et al. Outcome of Vaginal Progesterone as a Tocolytic Agent: Randomized Clinical Trial. ISRN Obstetrics and Gynecology. 2012 doi: 10.5402/2012/607906. Article ID 607906, 5 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bomba-Opon DA, Kosinska-Kaczynska K, Kosinski P, Wegrzyn P, Kaczynski B, Wielgos M. Vaginal progesterone after tocolytic therapy in threatened preterm labor. J Matern Fetal Neonatal Med. 2012;25(7):1156–9. doi: 10.3109/14767058.2011.629014. [DOI] [PubMed] [Google Scholar]
- 20.Dudas I, Gidai J, Czeizel AE. Population-based case-control teratogenic study of hydroxyprogesterone treatment during pregnancy. Congenit Anom. 2006;46(4):194–8. doi: 10.1111/j.1741-4520.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 21.Dodd JM, Crowther CA, Cincotta R, Flenady V, Robinson JS. Progesterone supplementation for preventing preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2005;84:526–533. doi: 10.1111/j.0001-6349.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- 22.Durnwald CP, Lynch CD, Walker H, et al. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. Am J Obstet Gynecol. 2009;201(4):410.e1–5. doi: 10.1016/j.ajog.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFranco EA, O’Brien JM, Adair CD, Lewis DF, Hall DR, Fusey S, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 24.Conde-Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O’Brien JM, Cetingoz E, et al. Vaginal progesterone vs cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison meta analysis. AJOG. 2013;208( 1):42.e1–42.e18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, et al. 17-alpha hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010;202(4):351.e1–6. doi: 10.1016/j.ajog.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–9. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 27.Dodd JM, Flenady VJ, Cincotta R, Crowther CA. Progesterone for the Prevention of Preterm Birth: A Systematic Review. Obstet Gynecol. 2008;112(1):127–134. doi: 10.1097/AOG.0b013e31817d0262. [DOI] [PubMed] [Google Scholar]