Abstract
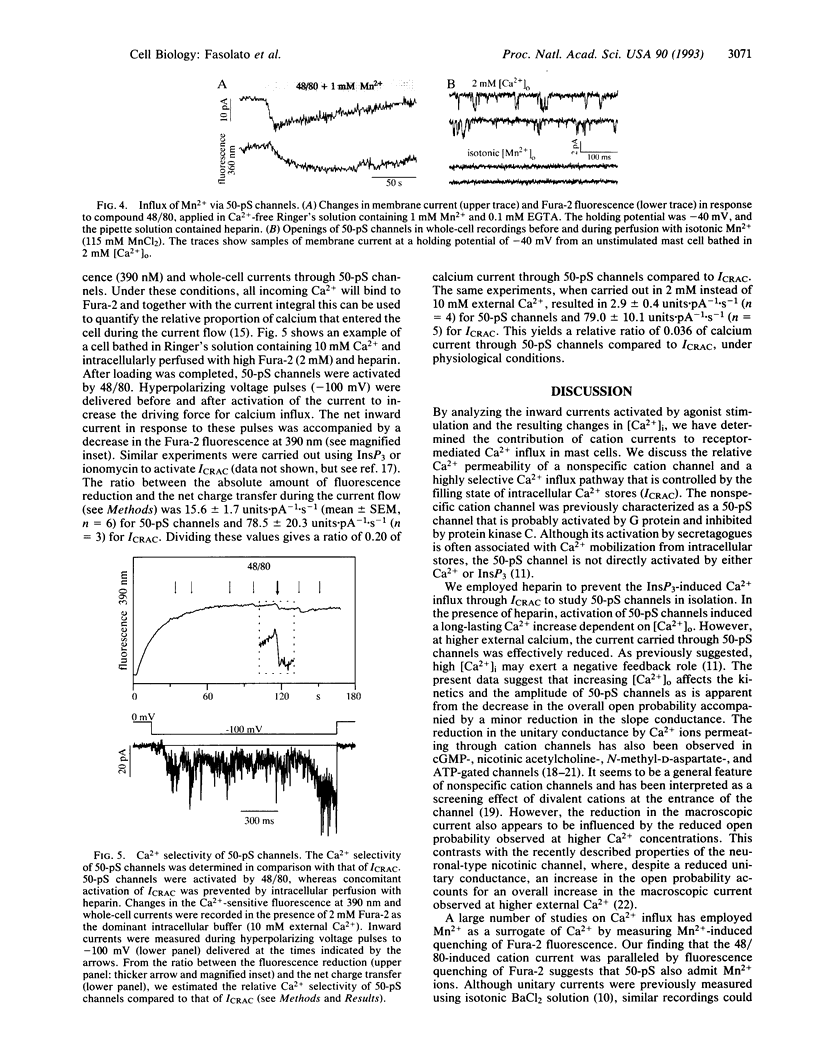
Whole-cell patch-clamp recordings of membrane currents and Fura-2 measurements of free intracellular calcium concentration ([Ca2+]i) were used to study calcium influx through receptor-activated cation channels in rat peritoneal mast cells. Cation channels were activated by the secretagogue compound 48/80, whereas a possible concomitant Ca2+ entry through pathways activated by depletion of calcium stores was blocked by dialyzing cells with heparin. Heparin effectively suppressed the transient Ca2+ release induced by 48/80 and abrogated inositol 1,4,5-trisphosphate-induced calcium influx without affecting activation of 50-pS cation channels. There was a clear correlation between changes in [Ca2+]i and the activity of 50-pS channels. The changes in [Ca2+]i increased with elevation of extracellular Ca2+. At the same time, inward currents through 50-pS channels were diminished as more Ca2+ permeated. This effect was due to a decrease in slope conductance and a reduction in the open probability of the cation channels. In physiological solutions, 3.6% of the total current was carried by Ca2+. The cation channels were not only permeable to Ca2+ but also to Mn2+, as evidenced by the quench of Fura-2 fluorescence. Mn2+ current through 50-pS channels could not be resolved at the single-channel level. Our results suggest that 50-pS cation channels partially contribute to sustained increases of [Ca2+]i in mast cells following receptor activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Comerford J. G., Dawson A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-induced Ca2+ release from rat liver microsomes. FEBS Lett. 1988 Feb 8;228(1):57–59. doi: 10.1016/0014-5793(88)80584-2. [DOI] [PubMed] [Google Scholar]
- Decker E. R., Dani J. A. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990 Oct;10(10):3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990 Oct 15;265(29):17486–17492. [PubMed] [Google Scholar]
- Kuno M., Kimura M. Noise of secretagogue-induced inward currents dependent on extracellular calcium in rat mast cells. J Membr Biol. 1992 May;128(1):53–61. doi: 10.1007/BF00231870. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Matthews G. Ion channels that are directly activated by cyclic nucleotides. Trends Pharmacol Sci. 1991 Jul;12(7):245–247. doi: 10.1016/0165-6147(91)90563-8. [DOI] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989 Nov;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Mulle C., Léna C., Changeux J. P. Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron. 1992 May;8(5):937–945. doi: 10.1016/0896-6273(92)90208-u. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Yang J., Mathie A., Hille B. 5-HT3 receptor channels in dissociated rat superior cervical ganglion neurons. J Physiol. 1992 Mar;448:237–256. doi: 10.1113/jphysiol.1992.sp019039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von zur Mühlen F., Eckstein F., Penner R. Guanosine 5'-[beta-thio]triphosphate selectively activates calcium signaling in mast cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):926–930. doi: 10.1073/pnas.88.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]




