Abstract
Introduction
Ovarian cancer is the second most common malignancy in women, the most common cause of gynecologic cancer deaths, and most patients have advanced stage disease at the time of diagnosis. The purpose of this study was to estimate the 5-year survival of patients with epithelial ovarian cancer based on age, tumor histology, stage of disease, and type of treatment.
Methods
This study was conducted on 120 patients with epithelial ovarian cancer referred to Shahid Sadoughi hospital and Shah Vali oncology clinic of Yazd from 2006 to 2012. Demographic data and patient records were studied to evaluate the treatment outcome, pathology of the tumor, and stage of disease. Finally, the overall survival rate and tumor-free survival of patients was assessed.
Results
The mean patient age was 53.87± 14.11 years. Most participants had stage I (36.7%) or stage II (35%) disease. Serous adenocarcinoma (57.6%) was the most common pathology found in patients with epithelial ovarian cancer. The overall survival of patients in this study was significantly associated with the histological tumor type (p = 0.000) and disease stage (p = 0.0377). Stage I (84.18%) and serous adenocarcinoma (72.81%) demonstrated the best survival. The tumor-free survival rates were not associated with histology types (p = 0.079), surgical procedure (p = 0.18), or chemotherapy (p = 0.18).
Conclusion
The survival of patients with epithelial ovarian cancer was significantly associated with disease stage. Serous adenocarcinoma also had the best prognosis among the pathologies studied. Therefore, early detection of ovarian cancer can substantially increase the survival rate.
Keywords: Epithelial ovarian cancer, epidemiologic points, survival rate, tumor pathology
1. Introduction
Ovarian cancer is the second most common malignancy in women and the most common cause of gynecologic cancer deaths (1). Approximately 85 to 90% of malignant ovarian tumors are epithelial cancer types (2), which are typically diagnosed in advanced stages of disease (3). The incidence of ovarian cancer has been steadily increasing over the past 10 years in many countries, reaching the overall lifetime risk of 1.8% (4). The survival rate of women widespread metastatic disease is a dismal 10–20% for 5 years survival (1). This poor overall prognosis is due to the lack of screening tools for early stage disease, the nonspecific nature of symptoms, and drug resistance in advanced disease (5). Age-related incidence of ovarian cancer increases with a steep gradient from 20 to 80 years and then decreases (6). Less than 20% of epithelial cancer are diagnosed before the onset of menopause (7). The mean age at diagnosis for invasive epithelial ovarian cancer is 51 years (8). Several studies have shown that patient survival is dependent on several factors such as disease stage, tumor histology and treatment (9). 5-year survival for other forms of cancer range from 30 to 50% (10, 11). If diagnosed and treated while the disease is confined to the ovary, the 5-year survival rate is 95%; however, only about 29% of all cases are detected at this early stage (12). Cytoreductive surgery and chemotherapy are the mainstay for the treatment of advanced epithelial ovarian cancer (4) and primary optimal surgery (residual tumor less than one centimeter), the most important factor affecting the survival of patients. Stage of the disease and the amount of residual tumor tissue in tumor reduction surgery is also effective on survival (9). Due to the epidemiologic changes and increased incidence in younger women, a reexamination of patient survival in the Shahid Sadoughi hospital in Yazd province is necessary, as it serves as a referral center for patients with ovarian cancer in women in the southeastern region of the country.
2. Material and Methods
This was a descriptive-analytic, cross-sectional study, was studied population included all patients with epithelial ovarian cancer referred to Shahid Sadoughi hospital and Shah Vali oncology clinic Yazd between May 2006 and September 2012. Shahid Sadoughi Hospital serves as a referral center for the Southeast. Any patient with a suspicious pelvic mass is characterized by ultrasonography and a CT scan. All patients with radiographic evidence of malignancy are referred to the department of gynecologic oncology for review by an oncologist. Data were analyzed for 120 women with epithelial ovarian cancer. All variables discussed in this study were collected through a questionnaire and was completed by telephone and file review of patients. Inclusion criteria included pathologic confirmation of epitheliod ovarian histology, those who underwent treatment with surgery and/or chemotherapy, and patients who had died due to epithelial ovarian cancer or its complications. Exclusion criteria included patients with incomplete medical records. The variables studied included histological type, surgery method, chemotherapy, disease stage and age. Data was analyzed by SPSS version 17 (SPSS Inc. Chicago Illinois, USA), using Log Rank (Mentel-Cox) and Kaplan Mayer method for survival analysis. Significant level of this study was p-value<0.05.
3. Results
The mean age of women at diagnosis was 53. 87 ± 14.1 years. The average age for the onset of menses was 18.12 ± 5.7 years. The average number of conceived pregnancies 4.76 ± 3.14, with 4.08 ± 2.65 of these pregnancies coming to term. The average duration of infertility was 12.12 ± 8.04 years. After disease diagnosis and treatment, the average time to recurrence was 21.53 ± 15.12 months and mean time to death was 29.5 ± 18.54 months. 9 patients (6.7%) had a history of suffering from other cancers as well. CA-125 tumor marker was elevated (reference level < 35U/ml) in the most of the patients (55%) (Table 1).
Table 1.
Demographic characteristics of patients with epithelial ovarian cancer
| Characteristics | n | Mean ± SD | |
|---|---|---|---|
| Age of diagnosis(years) | 120 | 53.87 ± 14.116 | |
| Age of menarche (years) | 118 | 12.85 ± 1.792 | |
| Age of marriage (years) | 114 | 18.12 ± 5.742 | |
| BMI (kg/m2) | 120 | 24.89 ± 4.145 | |
| Number of pregnancies | 114 | 4.76 ± 3.143 | |
| Duration of infertility (years) | 8 | 12.12 ± 8.043 | |
| Number of Children | 111 | 4.08 ± 2.656 | |
| Time to recurrence (months) | 120 | 21.53 ± 15.126 | |
| Time to death (months) | 120 | 29.50 ± 18.54 | |
| History of other cancers in patients | Yes | 9 | 7.6% |
| No | 111 | 92.4% | |
| Tumor Marker | Increased CA- 125 | 66 | 55% |
| Increased CA-19-9 | 26 | 21.7% | |
| Increased CEA | 11 | 9.2% | |
The most common histologic subtype observed was serous adenocarcinoma with 68 patients (57.6%), while the rarest tumor pathologies observed were endometroid (13%) and poorly differentiated adenocarcinoma (7.6%). The longest average duration of tumor-free survival was seen with mucinous adenocarcinoma (47.79 months), while the shortest was seen with endometrial adenocarcinoma. An examination was undertaken to assess whether different histologic subtypes recurred differentially, but log rank comparison was not statistically signification (p = 0.0795). The rate of recurrence had been observed in a variety of pathologies were very similar (Table 2).
Table 2.
Relationship between survival and prognostic factors in epithelial ovarian cancer
| Variable | Overall survival (Months) | Recurrence-free survival (Months) | |
|---|---|---|---|
| Histological type | Serous Adenocarcinoma | 72.81 | 32.92 |
| Mucinous Adenocarcinoma | 58.1 | 47.79 | |
| Endometroid Adenocarcinoma | 7.6 | 6 | |
| Clear Cell Adenocarcinoma | 43.46 | 43.17 | |
| Poorly Differentiated Adenocarcinoma | 13 | 8 | |
| Metastatic carcinoma | 52.65 | 38.78 | |
| p-value | 0.000 | 0.0795 | |
| Surgical procedures | Cytoreductive Surgery | 48.83 | 23.89 |
| Removal of both ovaries and the uterus | 72.2 | 43.50 | |
| Complete tumor resection | 43.50 | ||
| Removal of fallopian tubes and ovaries | 84.71 | 37.21 | |
| p-value | 0.2339 | 0.1803 | |
| Chemotherapy | Received | 37.95 | 72.31 |
| Not Given | 37.66 | 77.28 | |
| p-value | 0.4012 | 0.11 | |
| Stage of disease | Stage I | 84.18 | |
| Stage II | 67.08 | ||
| Stages III | 48.73 | ||
| Stage IV | 10 | ||
| p-value | 0.0377 | ||
| Age | 18–49 | 60.07 | |
| 50–59 | 83.34 | ||
| 60–89 | 57.14 | ||
| p-value | 0.613 | ||
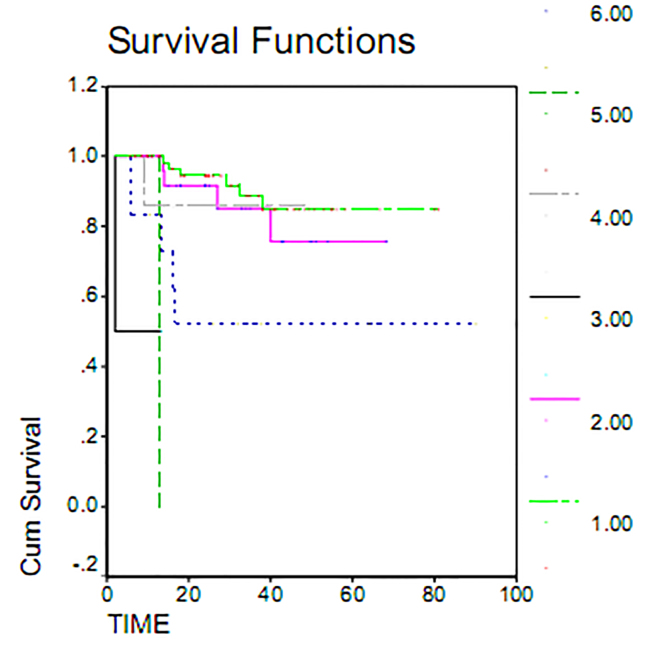
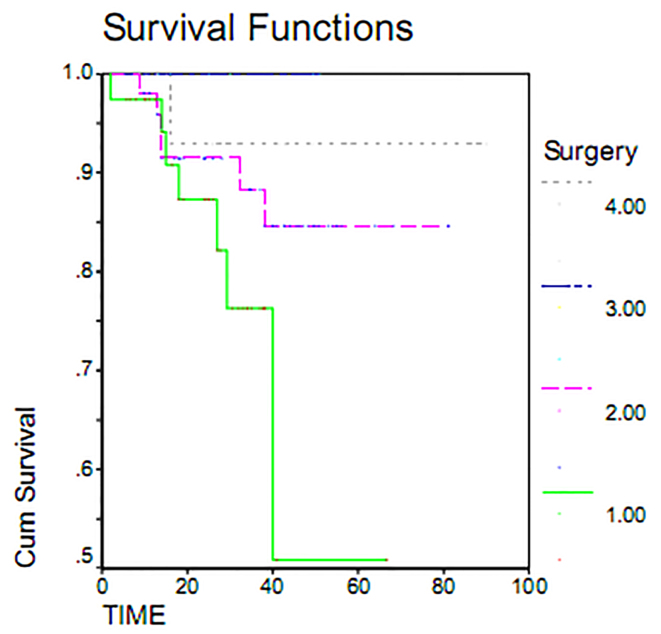
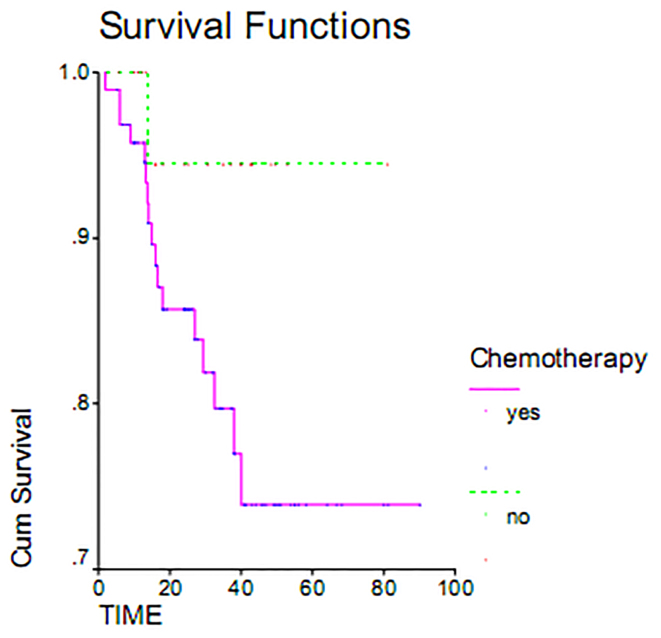
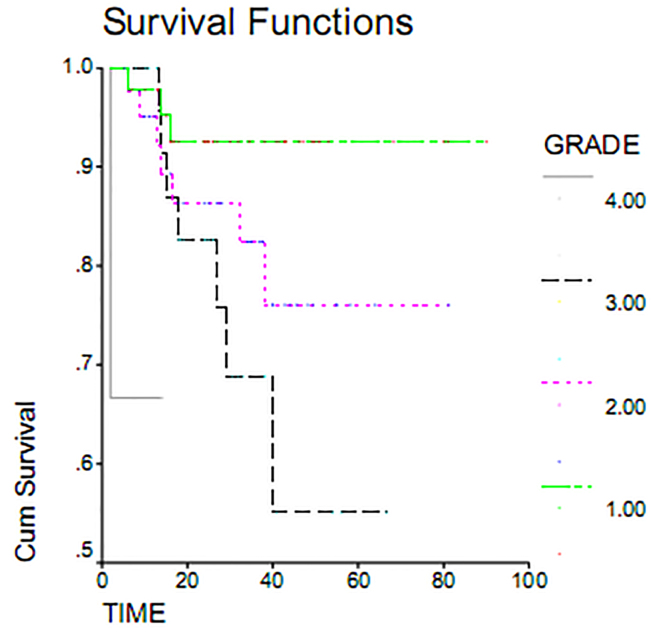
Serous adenocarcinoma, with a mean overall survival of 72.81 months, had the highest overall survival rate. Endometrial adenocarcinoma, with mean overall survival 7.6 months, had the lowest survival rate. This difference was examined using the Log Rank Test and found to be statistically significant (p = 0.000). Indeed, the overall survival varied greatly by histology (Figure 1). Maximum duration of tumor-free survival is achieved with gross total resection of the tumor, while a minimum duration of tumor-free survival was seen in staging or sub-total resection surgeries. Associated recurrence rate with surgical method was measured using the Log Rank test (p = 0.1803) and was not found to be significant. Consequently, the rates of recurrence observed using surgical methods were the same. This result was also seen in overall survival (p = 0.233) (Table 2). The overall survival of patients who underwent gross total resection was 100%, while patients undergoing surgery sub-total resection had a less than 55% survival within 60 months (Figure 2). As shown in Table 2, the average duration of tumor-free survival in patients who received chemotherapy was 37.95 months and was similar in patients who had not received chemotherapy, specifically 37.66 months. This relationship was assessed using the Log Rank test (p = 0.1803), and the treatment with chemotherapy was found not to be significant. While the mean overall survival of those who received chemotherapy was 72.31 months and was 77.28 months in women who had not undergone chemotherapy, this association was also found not to be statistically significant (p = 0.11). The 5 year survival of patients who had received chemotherapy was 73%, and patients who did not receive chemotherapy had a 95% survival at 60 months (Table 2, Figure 3). The majority of patients (71.7%) had stage I and II disease, and 28.8% and 2.5% of the patients were in stages III and IV disease, respectively, at the time of diagnosis. For patients with early stage disease, the mean overall survival was 84.18 months, while the overall survival rate most first, patients who had an average overall survival (18.84 months) had an overall survival rate for stage IV was 10 months. In second and third stages, the median duration of survival was 67.08 and 48.73 months, respectively. Therefore, the survival rate varies at different stages of disease (p = 0.037). 60-month survival in patients with stage I of disease 93%, 75% at stage II and in stage III of disease 54 percent (Table 2, Figure 4).
Figure 1.
Overall survival in patients with epithelial ovarian cancer by histology.
Figure 2.
Overall survival in patients with epithelial ovarian cancer by surgical methods.
Figure 3.
Overall survival rate in patients with epithelial ovarian cancer comparing treated by chemotherapy and without chemotherapy.
Figure 4.
Overall survival rate in patients with epithelial ovarian by histologic grade.
4. Discussion
The present study on 120 patients with epithelial ovarian cancer shows that histologic subtype and stage of disease most greatly impacted a patient’s survival, but chemotherapy and age of diagnosis had no effect. Serous adenocarcinoma is the most common pathology in this study with 68 patients (57.6%). Pill et al. (13) in a study conducted in 2002 in India with 282 ovarian cancer patients showed serous adenocarcinoma to be the most commonly seen histologic subtype. Fotopoulou et al. (14) also obtained a similar result. The results of these studies are compatible with the present study. Our results show that patients who have been diagnosed with serous adenocarcinoma have the best mean life expectancy (72.81 months) while those diagnosed with endometrial adenocarcinoma have the worst life expectancy (7.6 months). Karabuk et al. (15) conducted a study on 138 patients in Turkey, and concluded that patients who had diagnosed with serous pathology had the higher overall survival compared with patients who had a mucinous pathology (p = 0.001). But, there was no significant difference in survival free of tumor (p = 0.06); their results are consistent with the present study. The results of this study showed there was no significant difference in overall survival with type of surgery. In a sponsored study conducted by Fomitak et al. in 2006 in the Japan, progress in the treatment of epithelial ovarian cancer was reviewed and it was observed that cytoreductive or aggressive subtotal resection surgery and adjuvant chemotherapy is effective in improving survival in advanced stage. Bristow et al. (17) and Karabuk et al. (15) conducted research that also showed that surgical cytoreductive was significantly associated with increased overall survival (p < 0.001, p = 0.002). The results of these studies are inconsistent with our study. Different patient selection, especially that advanced stages were not included in those studies, could explain this discordance.
The mean survival rate was not significantly different in people who received chemotherapy compared with those who did not receive chemotherapy (p = 0.11). Tangjitgamol et al. (18) conducted a study on 781 patients with advanced ovarian cancer and concluded that there was no significant association between the overall survival and tumor-free survival between those who had undergone cytoreductive surgery with chemotherapy compared with chemotherapy alone. Psyrri et al. (12), a study on 150 patients with epithelial ovarian cancer in Athens, showed that there was no significant correlation between clinical response to chemotherapy and disease-free survival rate (p = 0.15) and overall survival (p = 0.176). Glasgow et al. (19) also showed that chemotherapy improved tumor-free survival rates, but ultimately there was no significant difference in overall survival and tumor-free survival rates in these patients. Morrison et al. (20) obtained similar results. These results are consistent with the present study. In studies by Altena et al. (21) in 2012 in the Netherlands with 23,000 epithelial ovarian cancer patients, survival of patients who received chemotherapy and surgery was significantly increased compared to patients who did not receive any treatment. A 5-year survival of 28% of those undergoing combined treatment versus 18% is inconsistent with the results of this study. In our study, the high survival rate of those who did not receive chemotherapy is suggestive that in an early tumor stage, there is no need for adjuvant chemotherapy and the effects are related to the stage of disease. Our study showed that in patients with high grade early stage there is limited benefit for chemotherapy. The results showed that the majority of patients were in stage I and II disease and overall survival were also higher than in the third and fourth stages (84.18 months, 73.08 months against 48.73 and 10 months). This highlights that patients with a suspected pelvic mass should immediately be referred for evaluation by an oncologist and undergo rapid and complete surgical excision. Psyrri et al. study (12) also found that patients in higher tumor stage, tumor-free survival is 25% and overall survival 33% compared to 8.34% and 71% for patients with low-stage tumors. Baldwin et al (22) in 2012 in Sweden determined that the 5-year survival of patients with stage 1 89% and stage 2 and 3 respectively 70 and 36%. Kirwan et al. (24) also found similar results. All the results of this study are consistent, therefore earlier detection of disease can be effective in increasing survival. In the present study, there was no significant association between age and survival time (p = 0.613). Grann et al. study (25), in Denmark showed that, with increasing age, 5-year survival rate is only slightly altered and statistically not significant. The Bristow et al. (17) study found no significant relationship between age and the mean survival (p = 0.371). As was seen for each year increase in age of only 9.0% reduction in the rate of survival, which is not statistically significant. Najafi et al. (26) concluded in their study conducted in the Fars Province that there was no significant relationship between age and increased risk of death; our data collaborates these findings.
5. Conclusions
According to this study, the survival of patients with epithelial ovarian cancer correlates inversely with disease stage. Serous adenocarcinoma had the best prognosis among other histologic subtypes studied. However, in this study, survival was not significantly associated with the type of treatment. Considering that the majority of patients were referred from other centers, lack of a comprehensive medical history and may have limited our studies results.
Acknowledgments
The authors thanks the Shahid Sadoughi hospital and Shah Vali oncology clinic Yazd for their help in this study.
Footnotes
iThenticate screening: September 26, 2015, English editing: September 30, 2015, Quality control: October 11, 2015
Conflict of Interest: There is no conflict of interest to be declared.
Authors’ contributions: All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Monk BJ, Choi DC, Pugmire G, Burger RA. Activity of bevacizumab (rhuMAB VEGF) in advanced refractory epithelial ovarian cancer. Gynecol Oncol. 2005;96(3):902–5. doi: 10.1016/j.ygyno.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Berek JS, Adashi EY, Hillard PA, Novak ER. Novak’s gynecology. Williams & Wilkins; 1996. [Google Scholar]
- 4.Ghaemmaghami F, Karimi-Zarchi M, Modares-Gilani M, Mousavi A, Behtash N. Clinical outcome of Iranian patients with advanced ovarian cancer with neoadjuvant chemotherapy versus primary debulking surgery. Asian Pac J Cancer Prev. 2008;9(4):719–24. [PubMed] [Google Scholar]
- 5.Buehler M, Tse B, Leboucq A, Jacob F, Caduff R, Fink D, et al. Meta-analysis of microarray data identifies GAS6 expression as an independent predictor of poor survival in ovarian cancer. Biomed Res Int. 2013;2013:238284. doi: 10.1155/2013/238284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sardi J, Anchezar P, Bermudez A. Favorable clinical behavior in young ovarian carcinoma patients: a rationale for conservative surgery? Int J Gynecol Cancer. 2005;15(5):762–9. doi: 10.1111/j.1525-1438.2005.00133.x. [DOI] [PubMed] [Google Scholar]
- 7.Behtash N, Zarchi MK, Gilani MM, Ghaemmaghami F, Mousavi A, Ghotbizadeh F. Ovarian carcinoma associated with pregnancy: a clinicopathologic analysis of 23 cases and review of the literature. BMC Pregnancy Childbirth. 2008;8(1):3. doi: 10.1186/1471-2393-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. International Journal of Gynecology & Obstetrics. 2009;105(2):107–8. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Ghaemmaghami F, Hassanzadeh M, Karimi-Zarchi M, Modari-Gilani M, Behtash N, Mousavi A. Centralization of ovarian cancer surgery: do patients benefit. Eur J Gynaecol Oncol. 2010;31(4):429. [PubMed] [Google Scholar]
- 10.Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108(3, Part 1):521–8. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 11.Diane M, Provencher MD. Epithelial Ovarian Cancer: Not So Asymptomatic. The Canadian Journal of Diagnosis (Women’s Health Care) Apr, 2001. pp. 75–85. Available from: http://www.stacommunications.com/journals/diagnosis/images/diagnosispdf/april01/whc.pdf.
- 12.Tanaka Y, Terai Y, Tanabe A, Sasaki H, Sekijima T, Fujiwara S, et al. Prognostic effect of epidermal growth factor receptor gene mutations and the aberrant phosphorylation of Akt and ERK in ovarian cancer. Cancer Biol Ther. 2011;11(1):50–7. doi: 10.4161/cbt.11.1.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilli GS, Suneeta K, Dhaded A, Yenni V. Ovarian tumours: a study of 282 cases. J Indian Med Assoc. 2002;100(7):420, 3–4, 47. [PubMed] [Google Scholar]
- 14.Fotopoulou C, Savvatis K, Schumacher G, Lichtenegger W, Sehouli J. Surgical outcome and survival analysis of young patients with primary epithelial ovarian cancer. Anticancer research. 2009;29(7):2809–15. [PubMed] [Google Scholar]
- 15.Karabuk E, Kose MF, Hizli D, Taşkin S, Karadağ B, Turan T, et al. Comparison of advanced stage mucinous epithelial ovarian cancer and serous epithelial ovarian cancer with regard to chemosensitivity and survival outcome: a matched case-control study. J Gynecol Oncol. 2013;24(2):160–6. doi: 10.3802/jgo.2013.24.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikkawa F, Nawa A, Ino K, Sibata K, Kajiyama H, Nomura S, et al. Advances in treatment of epithelial ovarian cancer. Nagoya journal of medical science. 2006;68(1–2):19–26. [PubMed] [Google Scholar]
- 17.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz F. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–59. doi: 10.1200/JCO.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 18.Tangjitgamol S, Manusirivithaya S, Laopaiboon M, Lumbiganon P. Interval debulking surgery for advanced epithelial ovarian cancer: a Cochrane systematic review. Gynecol Oncol. 2009;112(1):257–64. doi: 10.1016/j.ygyno.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Glasgow MA, Yu H, Rutherford TJ, Azodi M, Silasi DA, Santin AD, et al. Neoadjuvant chemotherapy (NACT) is an effective way of managing elderly women with advanced stage ovarian cancer (FIGO Stage IIIC and IV) J Surg Oncol. 2013;107(2):195–200. doi: 10.1002/jso.23171. [DOI] [PubMed] [Google Scholar]
- 20.Morrison J, Swanton A, Collins S, Kehoe S. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. The Cochrane Library. 2007 doi: 10.1002/14651858.CD005343.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Van Altena AM, Karim-Kos HE, de Vries E, Kruitwagen RF, Massuger LF, Kiemeney LA. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in the Netherlands. Gynecol Oncol. 2012;125(3):649–54. doi: 10.1016/j.ygyno.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120(3):612–8. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 23.Bamias A, Karadimou A, Soupos N, Sotiropoulou M, Zagouri F, Haidopoulos D, et al. Prognostic factors for early-stage epithelial ovarian cancer, treated with adjuvant carboplatin/paclitaxel chemotherapy: a single institution experience. Gynecol Oncol. 2011;123(1):37–42. doi: 10.1016/j.ygyno.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 24.Kirwan JM, Tincello DG, Herod JJ, Frost O, Kingston RE. Effect of delays in primary care referral on survival of women with epithelial ovarian cancer: retrospective audit. Bmj. 2002;324(7330):148–51. doi: 10.1136/bmj.324.7330.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grann AF, Thomsen RW, Jacobsen JB, Nørgaard M, Blaakær J, Søgaard M. Comorbidity and survival of Danish ovarian cancer patients from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5(Suppl 1):57. doi: 10.2147/CLEP.S47205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Najafi Z, Rivaz M, Shokrollahi P, Shamsnia M. Survival rate of women with ovarian cancer in Fars Province, Iran. Bimonthly Journal of Hormozgan University of Medical Sciences. 2013;16(6):459–65. [Google Scholar]