Abstract
The aim of this study is to report a case of a nonvital, discolored, maxillary central incisor bleached by 35% hydrogen peroxide gel with the use of glass ionomer cement as a mechanical barrier in an attempt to minimize the undesirable side effects of intracoronal bleaching. The patient was a 13-year-old boy complaining of a discolored nonvital upper-right central incisor and was selected for this study from the pedodontic clinic in the Shibin Elkom teaching hospital in June 2013. After successful endodontic treatment, the tooth was bleached by 35% hydrogen peroxide gel (Opalescence Xtra), activated by a standard curing light unit, and evaluated for any periapical changes by a periapical radiograph for a nine-months follow-up period. Radiographically, there was no evidence of cervical or apical resorption during the study period.
Keywords: Bleaching, discoloration, walking bleaching method, nonvital teeth, mechanical barrier
1. Introduction
With increased interest in esthetic dentistry, bleaching of discolored teeth, either vital or nonvital, has become popular. Nonvital bleaching has several advantages over other treatment options such as full veneer crowns. Difficulties in color matching and achieving the natural appearance are possible drawbacks of full-coverage restorations. In contrast, nonvital bleaching is a noninvasive procedure; it is less time consuming and economical (1, 2). Tooth discoloration varies in etiology, appearance, location, and severity (3). It could be classified as intrinsic, extrinsic, or both according to its location and etiology (4). Extrinsic discoloration is caused by chromogens derived from habitual intake of dietary sources such as wine, coffee, tea, carrots, oranges, chocolate, tobacco, mouth rinses, or plaque on the tooth surface (5), while intrinsic discoloration typically results from systemic or local causes. Systemic causes include drug-related (tetracycline), metabolic, fluorosis, and genetic (hyperbilirubinemia, amelogenesis imperfecta, and dentinogenesis imperfecta). Local causes include pulp necrosis, intrapulpal hemorrhage, pulp tissue remnants after endodontic therapy, endodontic materials, coronal filling materials, root resorption, and aging (6). Over the years, many bleaching agents have been used with varying results. Among them are oxalic acid, calcium hypochlorite, hydrogen peroxide, carbamide peroxide, and sodium perborate (2). The most commonly used agents for bleaching endodontically treated teeth are 30%–35% hydrogen peroxide and sodium perborate either in combination or separately (7). Two basic techniques have been used to bleach discolored nonvital teeth: thermocatalytic and walking bleach. Both methods have lost favor due to the potential risk, which includes cervical resorption, under- or over-lightening, the possibility of color regression, and external root resorption. Recently, a variety of products and techniques have been developed to resolve or minimize the side effects of the bleaching process. This development has been paralleled with rising interest among patients in correcting esthetic problems with their dentition (8, 9). Therefore, in the current study, many modifications have been done for bleaching techniques in an attempt to minimize the risk and side effects of the bleaching process.
2. Case presentation
A 13-year-old boy, who complained of discolored and unaesthetic appearance of his upper central incisor, was selected from the pedodontic clinic in the Shibin Elkom teaching hospital (Egypt) in June 2013. The child was free from systemic disorders, and he was not under any medications that cause darkening or staining of the teeth. Clinical and radiographic examinations were carried out. A diagnosis of nonvital maxillary right central incisor was made, based on the vitality test, which was performed by using an electric pulp tester; thus, the shade guide of the discolored tooth was assessed under normal daylight with a Vita porcelain shade guide (Vita Zahafabrik); also, a pre- and post-bleaching photograph was taken for the patient. Conventional endodontic treatment was done for the patient, and, after successful endodontic treatment, the bleaching process was undertaken using 35% hydrogen peroxide gel. The gingiva was protected by water-soluble cream (Vaseline) applied to soft tissues, and rubber dam isolation was achieved; then, 1–2 mm of the gutta-percha was removed in an apical direction beyond the cemento-enamel junction. The tooth was then washed with 3% hydrogen peroxide solution, rinsed and dried. To assure a barrier between the sealed root canal and the bleaching gel (mechanical seal), a 1–2 mm of glass ionomer cement base was placed over the gutta percha. The opalescence extra (Ultra Dent. Products, Inc. USA, Union Broach Co., Long Island City, NY) was then expressed into the opened pulp chamber and on the labial surface, and curing light was applied to activate the bleaching gel from the labial and palatal sides (10). We changed the gel and repeated the bleaching until desired results were obtained. Then, the pulp chamber was rinsed and dried and obdurated with calcium hydroxide to be left in the pulp chamber for 1 week before the final or permanent filling material (light cure composite resin) (11, 12). Clinical evaluation was recorded by comparing the tooth shade with its original one before treatment using the Vita porcelain shade guide and photographs; also, radiographic evaluation was done at 1, 3, 6, and 9 month intervals by taking periodical radiographs for the patient using the paralleling technique. The automatically developed radiographs were digitized using a full color flatbed Umax scanner (Umax Data System, Inc. USA) with a transparency adapter connected to a standard IBM compatible personal computer having a Pentium III Intel processor and a 15 in. ViewSonic monitor (ViewSonic, Business Parkway, Walnut St., USA). The scanned images were saved in a 28 gigabyte hard disk in tagged image format files (TIFF) with Adobe Photoshop Version 5.5 (Adobe System Incorporated, Seattle, WA) (13). The tooth had lightened to a suitable degree with accepted clinical success. As shown in Figure 1, periapical radiographs were taken prior to bleaching of teeth, immediately after bleaching, and at 1, 3, 6, and 9 months after bleaching. The pre-bleaching assessment for the tooth was diagnosed as having periapical pathosis; during the follow-up period, there were signs of healing since the start of root canal treatment. The presence of resorption was also assessed radiographically using the digital subtraction technique (14), and there was no evidence of cervical or progressive apical resorption (Figure 2).
Figure 1.
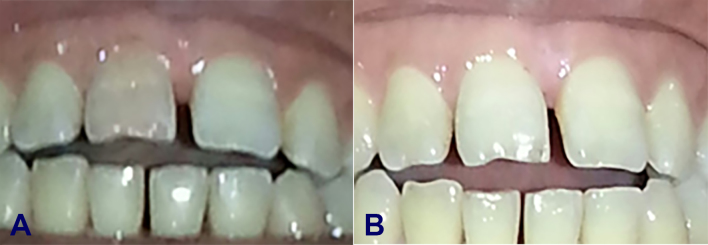
Tooth before (A) and after (B) bleaching.
Figure 2.
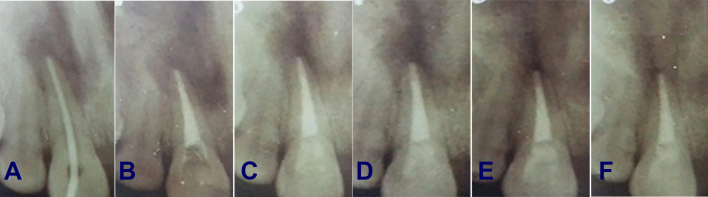
Periapical x-ray film: (A) before treatment shows periapical resorption; (B) obturation and bleaching; (C) after 1 month; (D) after 3 months; (E) after 6 months; (F) after 9 months shows no evidence of cervical resorption; also, there is a sign of improvement in the periapical region.
3. Discussion
The present study was undertaken to evaluate the trials made to minimize the side effects of bleaching agents; hence, teeth bleaching is a harmless process, but some conditions have to be respected: correct and complete soft tissue isolation (gums, lips, cheeks), in order to protect them from eventual burns caused by the peroxide. Some modifications have been done in an attempt to minimize the risk of cervical or apical resorption; thus, a base of 1–2 mm glass ionomer cement was placed over the root filling material to assure a mechanical barrier between the sealed root canal and the bleaching gel, which is in agreement with other studies (8, 9) but is in disagreement with Friedman et al. (15), as they did not use an intermediate lining prior to the bleaching material. Another modification added to the bleaching technique was that on reaching the desired shade guide; thus, the pulp chamber was obturated by calcium hydroxide for seven days before the final filling material was placed. This was necessary to allow for elimination of residual oxygen, which interferes with the polymerization of the filling material and to neutralize and render the medium alkaline that reduces the risk of cervical resorption (16). Radiographic follow-up using digital subtraction technique showed no evidence of any cervical resorption or any progressive alteration in the periapical area around the tooth, which may be attributed to the placement of the mechanical seal that prevents the leakage of hydrogen peroxide; hence, a reduced potential risk of invasive resorption was noted (17).
4. Conclusions
This case report demonstrates the successful management of a discolored nonvital tooth using 35% hydrogen peroxide gel (Opalescence Xtra) as bleaching material, effectively and safely, by following modifications and precautions to eliminate the side effects of peroxides. No evidence of cervical resorption of the tooth was observed during the follow-up period; furthermore, there were signs of improvement related to the periapical region. More advanced studies still needed to gather more information about the stability of results and to detect any adverse effect that could appear.
Acknowledgments
The author thanks the Shibin Elkom teaching hospital and Al-Farabi Colleges for supporting this case study.
Footnotes
iThenticate screening: October 05, 2015, English editing: October 14, 2015, Quality control: October 18, 2015
Conflict of Interest:
There is no conflict of interest to be declared.
References
- 1.Isaac AM, Hoen MM. Intracoronal bleaching: Concerns and considerations. J Can Dent Assoc. 1994;60:57–64. [PubMed] [Google Scholar]
- 2.Rostein I. Tooth discoloration and bleaching. In: Ingle JI, Bakland LK, editors. Endodontics. 5th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2002. pp. 845–60. [Google Scholar]
- 3.Dahl JE, Pallesen U. Tooth bleaching-a critical review of the biological aspects. Crit Rev Oral Biol Med. 2003;14:292–304. doi: 10.1177/154411130301400406. [DOI] [PubMed] [Google Scholar]
- 4.Hattab FN, Qudeimat MA, al-Rimawi HS. Dental discoloration: an overview. J Esthet Dent. 1999;11:291–310. doi: 10.1111/j.1708-8240.1999.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 5.Watts A, Addy M. Tooth discoloration and staining: a review of the literature. Br Dent J. 2001;190:309–16. doi: 10.1038/sj.bdj.4800959. [DOI] [PubMed] [Google Scholar]
- 6.Nathoo SA. The chemistry and mechanism of extrinsic and intrinsic discoloration. J Am Dent Assoc. 1997;128:6S–10S. doi: 10.14219/jada.archive.1997.0428. [DOI] [PubMed] [Google Scholar]
- 7.Attin T, Paque F, Ajam F, Lennon AM. Review of the current status of tooth whitening with the walking bleach technique. Int Endod J. 2003;36:313–29. doi: 10.1046/j.1365-2591.2003.00667.x. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzo G, Cabanes C, Cervera SL, Forner N. Clinical study of a halogen light-activated bleaching agent in non-vital teeth: Case report. Quin Int. 1996;27:383–8. [PubMed] [Google Scholar]
- 9.Amato M, Scaravilli MS, Farella M, Riccitiello F. Bleaching teeth treated endodontically:long-term evaluation of a case series. J Endod. 2006;32:376–8. doi: 10.1016/j.joen.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Walton RE, Rotstein L. Principles of Endodontics. Second edition. Chapter 23. W.B Saunders; 1996. Bleaching Discoloured Teeth: Internal and External; pp. 385–400. [Google Scholar]
- 11.Rotstein I, Zyskind D, Lewinstein I, Bamberger N. Effect of different protective base materials on hydrogen peroxide leakage during intracoronal bleaching in vitro. J Endod. 1992;18:114–7. doi: 10.1016/S0099-2399(06)81310-5. [DOI] [PubMed] [Google Scholar]
- 12.Steiner DR, West JD. A method to determine the location and shape of an intracoronal bleach barrier. J Endod. 1994;20:304–6. doi: 10.1016/S0099-2399(06)80822-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Hallender L. Digitizing of radiographs with flat bed scanner. Dent J. 1995;23:205–9. doi: 10.1016/0300-5712(95)91183-N. [DOI] [PubMed] [Google Scholar]
- 14.Jeffcoat MK. Radiographic method for the detection of progressive alveolar bone loss. J Period. 1992;63:367–72. doi: 10.1902/jop.1992.63.4s.367. [DOI] [PubMed] [Google Scholar]
- 15.Friedman S, Rotstein I, Lidfeld H, Stabholz A, Heling I. Incidence of external root resorption and esthetic results in 58 bleached pulpless teeth. Endod Dent Traumatol. 1988;4:23–6. doi: 10.1111/j.1600-9657.1988.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 16.Titley KC, Tomeck CD, Ruse ND, Knnec D. Adhesion of a composite resin to bleached and unbleached human enamel. J Endodon. 1993;19:112–5. doi: 10.1016/S0099-2399(06)80504-2. [DOI] [PubMed] [Google Scholar]
- 17.Heller D, Skriber J, Lin LM. Effects of intracoronal bleaching on external cervical root resorption. J Endo don. 1992;18:145–8. doi: 10.1016/S0099-2399(06)81407-X. [DOI] [PubMed] [Google Scholar]


