Abstract
Cells must know the local levels of available oxygen and either alter their activities or relocate to more favorable environments. Prolyl 4-hydroxylases are emerging as universal cellular oxygen sensors. In animals, these oxygen sensors respond to decreased oxygen availability by up-regulating hypoxia-inducible transcription factors. In protists, the prolyl 4-hydroxylases appear to activate E3-SCF ubiquitin ligase complexes potentially to turn over their proteomes. Intracellular parasites respond to decreased oxygen by utilizing both types of oxygen-sensing pathways. Since parasites are exposed to diverse oxygen tensions during their life cycle, oxygen sensing is likely a critical process and this review will discuss how these oxygen-sensing mechanisms contribute to the behavior of these unicellular eukaryotes.
O2 sensing is important for cellular life in an aerobic atmosphere
As an atmospheric gas that dissolves in aqueous biological solvents, O2 is a thermodynamically favorable acceptor for electrons and protons in mitochondrial respiration and other metabolic pathways. Its consumption by aerobic metabolism, combined with its limited solubility and slow diffusion in water, leads to concentration gradients of O2 within cells, tissues, and the local environments [1–4]. Cells have evolved multiple autonomous mechanisms to rapidly reorganize their metabolism to cope with O2 depletion [5,6]. These range from the rapid formation of short-lived reactive oxygen species (ROS) to longer-term changes in a cell's transcript and protein repertoire. Together these alterations impact the cell's metabolism and other activities such as motility to enable a cell to migrate to areas of higher O2 availability. As an example, when the soil amoeba Dictyostelium is exposed to low O2 it migrates toward higher O2 levels where it can continue its developmental program and differentiate into a fruiting body [7].
Cells express several types of O2 sensors. One class directly binds O2 effecting a conformational change that modifies the protein's function [8]. A second class includes proteins such as oxidases and non-heme dioxygenases that utilize O2 as a substrate for the biochemical reactions that they catalyze. The non-heme dioxygenases have an Fe center that coordinates and splits O2, distributing one of the O-atoms to the target substrate [9], and the other to α-ketoglutarate (αKG or 2-OG), which decomposes into succinate and CO2 (Fig. 1A). These dioxygenases include a variety of enzymes such as demethylases and hydroxylases that can modify several types of amino acids and nucleic acids. This review will focus primarily on non-heme dioxygenases and in particular prolyl 4-hydroxylases (P4Hs) since they are the best characterized O2 sensors in protozoans and animals and have potential roles in other eukaryotes and bacteria [10].
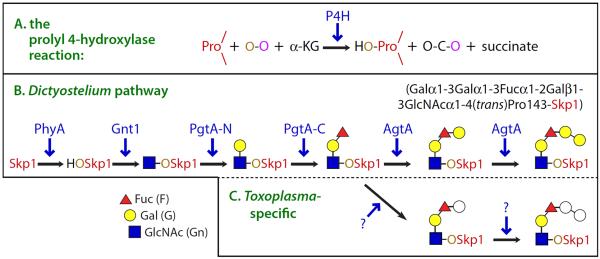
Fig. 1.
The Skp1 modification pathway in Dictyostelium and Toxoplasma. (A) Summary of the reaction catalyzed by the P4H class of non-heme dioxygenases, in which a protein-linked Pro residues is subject to hydroxylation at its 4-position by an O-atom derived from O2 (O-O). The other O-atom is transferred to α-KG, which decomposes into CO2 and succinate. (B) The hydroxyl group generated by the action of the P4H PhyA on Skp1 provides the site for glycosylation by five glycosyltransferase activities encoded by 3 genes in Dictyostelium. (C) Toxoplasma also assembles a pentasaccharide with the same Hex-Hex-dHex-Hex-HexNAc– composition (unpublished data). Tg-PhyA mediates Skp1 hydroxylation and is required for glycosylation, which has been confirmed by mass spectrometry (in preparation). gnt1 and pgtA are conserved in Toxoplasma, and likely modify Toxoplasma Skp1 as they do in Dictyostelium. However, the physical order of the N- and C-terminal catalytic domains of Dd-PgtA, which mediate the addition of β-Gal and α-Fuc, respectively, is reversed in Toxoplasma.
Transcription-dependent O2-sensing in animals
Transcriptome rewiring is a key mechanism by which animals (and many fungi) respond to decreased O2-availability and PHDs (prolyl hydroxylase domain proteins) are the O2 sensing P4Hs most important in regulating these O2-regulated transcription factors. They function by hydroxylating two conserved prolines in the α subunit of the heterodimeric hypoxia inducible factor (HIF) transcription factor, resulting in the formation of (4S,2R)-hydroxyproline, (Hyp) [6,9,11]. Prolyl hydroxylated HIFα is recognized by and targeted for proteasome-dependent degradation by the VHL ubiquitin ligase complex. Thus, HIFα is rapidly degraded under O2 replete conditions. However, the PHDs have low affinity for O2 and when O2 becomes limiting, as can occur in tissue environments, HIFα accumulates, translocates to the nucleus, binds HIFβ (also called ARNT), and activates target gene expression. Many of these genes, such as glycolytic enzymes and vascular endothelial growth factor, are important to coordinate a cell's response to decreased O2 availability. HIFα is regulated by another non-heme dioxygenase, Factor-inhibiting-HIF (FIH), which hydroxylates an asparagine in the protein's C-terminal transcriptional coactivator domain [12,13]. This O2-dependent process diminishes transactivation activity, which reinforces the action of PHDs in regulating HIF1α activity. Besides HIF1α, PHDs regulate other proteins including PKM2 [14] and the TRPA1 [15] ion channels. Thus, hydroxylation of prolines and other amino acid is emerging as a widespread post-translational modification and we expect that these lists will continue to grow.
O2-sensing in amoebae
While HIFα is restricted to animals, P4Hs are evolutionarily conserved and found not only in all animals [16] but fungi, amoebazoa, and, evidently, prokaryotes [10]. Thus P4Hs appear to predate the emergence of HIFα in animals, raising the question of what their targets are in other organisms and whether they are involved in O2-sensing. The discovery of a Dictyostelium P4H (DdPhyA), which hydroxylates Pro143 in the Skp1 subunit of the SCF class of E3-Ub ligases, raised the possibility that this P4H enzyme is an O2 sensor [17] (Fig. 1B).
Dictyostelium resides in the soil where O2 availability is governed by the rate of infusion from the atmosphere, and depletion by microbial and root metabolism. The amoebae use bacteria as a nutrient source and under starvation the amoeba differentiate into dormant spores. This involves a developmental program resulting in cell aggregation to form a polarized multicellular slug that migrates to the soil surface and transforms into a fruiting body consisting of dormant spores that survive until activated by a nutrient rich environment. O2 is one sensory modality used by Dictyostelium to assess whether they are above ground at a site appropriate for fruiting body formation. Culmination therefore occurs in an O2 dependent manner and the O2 set point for culmination, which is well above that required for metabolism, can be raised or lowered by forced under- or over-expression of the DdPhyA [18*]. Since purified DdPhyA has a Km for O2 above 21%, this suggests that O2 control of culmination is mediated via PhyA activity. This mechanism is supported by pulse-labeling studies with 35S-Met, that showed that Skp1 hydroxylation lags behind synthesis at low, but not normal, O2 levels [19*]. When Dictyostelium is cultured under standard submerged conditions, development requires high atmospheric levels of O2 (70–100%) by a mechanism that depends on PhyA and Skp1 hydroxylation [20]. The data suggest that O2 is limiting for hydroxylation of newly synthesized Skp1, which might have a privileged role in forming regulatory SCF complexes composed of nascent FBPs.
Not only is Skp1 a substrate for PhyA it is a functional mediator of PhyA activity in cells. Skp1 under-expression has the same effect on the O2-setpoint for culmination as PhyA over-expression, suggesting that hydroxylation reduces Skp1 activity [21*]. Conversely, Skp1 overexpression raises the O2-setpoint similar to disrupting phyA. As expected, the activity of Skp1 is suppressed by forced overexpression of PhyA, and is not additive with genetic depletion of phyA [21*]. Paradoxically, Skp1 with a point mutation at the position of Pro143 exhibits less activity, though this might reflect separate effects on protein folding and function. Over-expression of Skp1 also inhibits sporulation, a subsequent developmental step that occurs as prespore cells rise to the top of the fruiting body. In contrast to culmination, inhibition depends on its Skp1 hydroxylation. Thus in addition to being the major if not only functional mediator of PhyA action, Skp1 contributes multiple functions in development that vary in their dependence on its hydroxylation.
Skp1 prolyl hydroxylation renders it a substrate for Gnt1 [22], the first of three glycosyltransferases (GTs) that assemble a linear pentasaccharide on Hyp143 (Fig. 1B). This raises the question of whether hydroxylation alone controls Skp1 function, or simply enables subsequent glycosylation. Both answers are correct. Genetic disruption of gnt1 results in an O2 requirement for culmination that lies between that of phyA-KO and w/t cells, and also yields morphologically defective fruiting bodies at any O2 level [22]. A KO of the second GT (pgtA), which allows for only a single sugar (GlcNAc) to be added, has an O2 requirement that is very similar to that of gnt1-KO cells, but rescues the defective fruiting body morphology. Remarkably, KO of the third GT (AgtA), allowing for the trisaccharide to be assembled, reverts the O2-dependence almost back to that of phyA-KO cells and has unexpectedly delayed early development [23]. Accumulating evidence suggests that AgtA has a second function as a Skp1 binding protein, via its WD40 repeat domain, that dampens its function regardless of its glycosylation status [24]. Consistent with this, the high O2-requirement of agtA-KO cells is ameliorated by genetic reduction of Skp1 levels [21*]. Thus glycosylation and the three GTs modulate the signal generated by prolyl hydroxylation of Skp1.
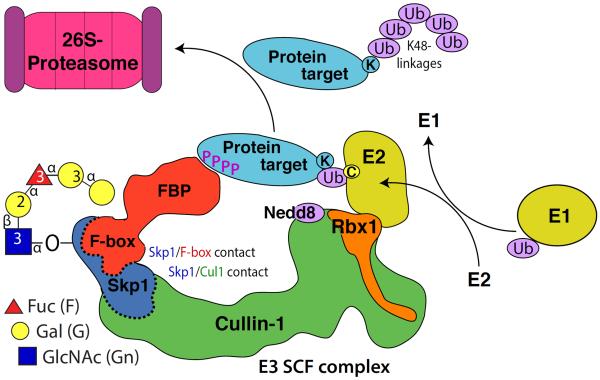
The primary function of Skp1 as part of the E3-SCF ubiquitin complex is to bind the F-box domain of F-box proteins (FBP) (Fig. 2). FBPs are heterogeneous multidomain proteins that serve as receptors for substrates to be poly-ubiquitinated and subsequently degraded [25]. Dictyostelium is predicted to have 55 FBPs and the mechanism(s) controlling binding specificity to Skp1 is complex but important since E3SCFUb-ligases likely control the fates of hundreds if not thousands of cellular proteins. Co-immunoprecipitation studies in Dictyostelium suggest that two distinct FBPs (FbxD and FbxA) preferentially associate with fully glycosylated Skp1 compared to unmodified Skp1 [19*]. In addition, FbxD binds better to the trisaccharide form of Skp1 (in agtA KO strains) than unmodified Skp1 although not as well as it does to fully modified Skp1. While, structural data indicate that addition of GlcNAc promotes increased α-helical content in Skp1 [26], further glycosylation has little on Skp1 structure suggesting that the peripheral sugars of the pentasaccharide regulate FBP binding through different mechanisms. Taken together these data indicate that O2 regulated Skp1 hydroxylation/glycosylation has various novel effects on Skp1 and serves to complement other SCF regulatory mechanisms that include the neddylation of cullin-1 which controls the exchange activity of Cand1 at the Cullin-1/Skp1 interaction site [27,28]. Further studies are needed to identify target substrates of Dictyostelium FBPs to determine how changes to the cell's proteome mediate O2-dependent culmination and development. In addition, we have identified putative Skp1-modification genes in other disease-causing amoebazoa, such as Acanthamoeba, suggesting that O2-sensing may contribute to virulence in ocular keratitis.
Fig. 2.
Skp1 hydroxylation and glycosylation likely regulate the SCF complex, an E3 ubiquitin ligase that marks target proteins with K48-linked polyubiquitin chains that are recognized by the proteasome for degradation. Polyubiquitination involves successive transfers of Ub from Ub-E2 donors that cycle on, off, and on again after recharging from Ub-E1. The process is controlled by, in some cases, substrate priming such as phosphorylation (P=PO4) as indicated. Ubiquitination is also regulated by neddylation of Cul1, which is under the control of the COP9 signalosome and other factors and promotes flexible tethering of Rbx1 and its associated Ub-E2 to allow access to the Ub target site (K=Lys) on the substrate. Neddylation also excludes Cand1 (not shown), which allows docking of FBP/Skp1 complexes to Cul1 (dashed interface). Skp1 modifications, which occur near its C-terminus, are proposed to promote interactions of FBPs with Skp1 (dashed interface), providing novel SCF-specific regulation. Modified from [42].
O2-sensing in Toxoplasma
Toxoplasma gondii is an unrelated protozoan that can infect almost all known mammals. It is passed between hosts by digestion of either infectious oocysts shed in feline (the parasite's definitive host) fecal material or tissue cysts found in undercooked meat from other infected mammals [29]. The acidic environment of the stomach releases parasites from oocysts or tissue cysts and once these parasites reach the anaerobic environment of the gut they infect intestinal epithelial cells, transform into replicative tachyzoites, and use recruited inflammatory monocytes and dendritic cells to disseminate via a Trojan Horse mechanism to diverse tissues throughout the body. In tissues, tachyzoites can develop into tissue cysts that are impervious to either anti-parasitic drugs or the resulting immune response.
Thus, like Dictyostelium, Toxoplasma is exposed to diverse O2 tensions during its life cycle and how the parasite senses and responds to changing O2 levels has been an open area of investigation. A clue about how Toxoplasma senses O2 came from the discovery that homologues of the Dictyostelium Skp1 modification pathway genes are conserved in Toxoplasma [30] (Fig. 1C). Toxoplasma PhyA (TgPhyA) is 40% similar in sequence to DdPhyA and can complement DdphyA-KO mutants in O2-dependent culmination [31*]. Furthermore, TgSkp1, which contains the equivalent of Pro143, is glycosylated in a TgPhyA-dependent fashion, and purified TgPhyA is capable of hydroxylating TgSkp1 in vitro [31*]. Genetic disruption of the TgphyA gene reveals that while TgPhyA is not essential its loss does reduce parasite growth at atmospheric O2 and this growth defect is intensified at 0.5% O2, a low but physiologically relevant O2 tension. Slow growth suggests that, in the absence of an O2 sensor, the parasite is unable to accommodate to the stress of low O2. Based on studies in Dictyostelium, these results suggest that Skp1 prolyl hydroxylation and/or glycosylation allows Toxoplasma to adapt to decreased O2 availability by altering the parasite's proteome.
Biochemical analysis of TgPhyA activity revealed an unexpectedly high affinity for O2 [31*], which was in contrast to DdPhyA and the PHDs that have apparent low affinities for O2. Thus, only low O2 levels, like those encountered in the gut, would be expected to impact TgPhyA activity. Nevertheless, TgPhyA can function in place of DdPhyA in O2-dependent culmination [31*] suggesting that TgPhyA is regulated by both O2 (at very low levels) and αKG, as its Km for αKG is similar to that of DdPhyA [31*]. Since αKG is generated by the O2-dependent TCA cycle, it is possible that TgPhyA senses O2 indirectly via changes in αKG. Whether DdPhyA (and the metazoan PHDs) are similarly regulated awaits metabolomic profiling of Toxoplasma and Dictyostelium grown at various O2 tensions.
Other protists and pathogens may employ the Skp1 mechanism to sense O2
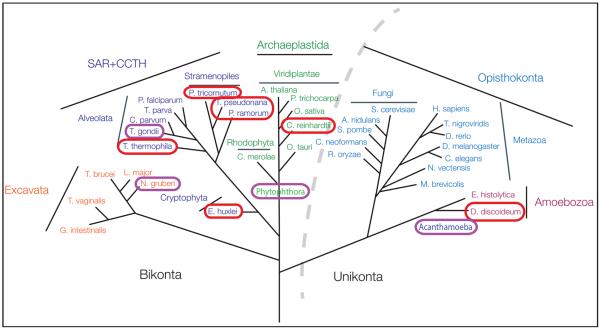
Genome analyses indicate that PhyA, Gnt1, and the target Pro residue in Skp1 co-exist in representatives of all 6 major protist phyla, but are evidently absent from fungi, animals, and higher plants (Fig. 3). Their broad but sporadic presence in diverse protozoans suggest that this is an ancient pathway that was retained during evolution via secondary gene loss. A striking example of this conservation is that the Skp1/PhyA/Gnt1 pathway, which was originally discovered in Dictyostelium and is present in other amoebazoae (e.g., Acanthamoeba), is conserved in Toxoplasma and closely related coccidians (e.g. Neospora) as well as in distantly related oomycetes (e.g. Phytophthora). But, the pathway is absent in other amoebazoae (e.g., Entamoeba spp.) and apicomplexans including other coccidia (Eimeria) and hemosporidia (Plasmodium). This is significant because many of the organisms with the Skp1 modification pathway are human and agricultural pathogens and this pathway represents a novel drug target since their hosts do not express it.
Fig. 3.
Phylogenetic distribution of Skp1 modification genes. Organisms predicted to modify Skp1, based on possession of phyA-like and gnt1-like genes, and a Skp1 with the equivalent of Pro143 in Dictyostelium Skp1, are encircled. Those in violet can cause human or plant disease. Phylogenetic tree of eukaryotic evolution is from [43].
Other O2-sensing mechanisms in fungal and protist pathogens
The secondary loss of Skp1 modification genes may have occurred in organisms that live anaerobically, only experience constant O2 levels, or acquired other O2 sensing mechanisms. We identified in apicomplexan, including those lacking PhyA such as Plasmodium, a second related P4H we have named PhyB (unpublished data). Further studies are needed to identify PhyB target(s) and determine how PhyB senses O2.
Trypanosomatids, such as the important human parasites Trypanosoma cruzi, T. brucei, and Leishmania spp., and other diversely related protists (e.g., euglenoids) lack genetic evidence for PhyA-related P4Hs. Thus, other O2-sensing proteins are likely to be utilized by these organisms and O2 and α-ketoglutarate utilizing dioxygenases are likely candidates. These organisms have a novel nuclear DNA modification called base J that affects transcription initiation and termination [32,33]. Base J is formed by the action of a DNA-thymine hydroxylase (JBP1/2), which is a non-heme containing O2 and α-ketoglutarate utilizing dioxygenase, and the resulting 5-hydroxymethyluracil (hmU) is capped with a β-glucose. Thus, the base J pathway parallels the Skp1 modification pathway in which a hydroxylated target serves as a glycosyltransferase substrate. Base J synthesis is reduced in Trypanosoma cruzi cells exposed to hypoxia suggesting that the JBP1/2 are O2 sensors in trypanosomes [34*].
Protozoans may utilize other types of O2 sensors. For example, non-heme O2- and αKG-dependent dioxygenases that may function as O2 sensors include asparagine, histidine and arginine hydroxylases [35] and Jumonji-domain containing proteins that epigenetically regulate gene expression by promoting Lys methylation and histone hydroxylation [9,36,37]. Finally other proteins may sense changes in O2 availability due to O2's ability to directly induce structural changes to those proteins that it binds. As an example, O2 binding to a Fe-heme prosthetic group in an adenylate cyclase of L. major stimulates cAMP formation [38*].
Intracellular parasites utilize both parasite and host O2 sensing
As a parasite traverses through the body, oxygen sensors such as PhyA and JBP1/2 are likely important for parasite survival and virulence. An infection is, however, a complex environment consisting of multiple types of cells (e.g. parasites, resident tissue cells, and recruited leukocytes) and each must properly function in order for the host and/or parasite to survive and thrive. Toxoplasma and other obligate intracellular parasites must ensure that both it and its host cell are in a metabolic state permissive for parasite growth. The first clue that host oxygen sensing plays a role in establishing this state was the finding that Toxoplasma activates host HIF1α [39]. Surprisingly, this was not simply a consequence of establishing a hypoxic microenvironment due to parasite oxygen consumption. Rather, Toxoplasma specifically activates HIF-1 by a mechanism that involves activin receptor signaling and inactivation of host PHD2 expression and activity [40]. Most significantly, parasite activation of HIF-1 is required for parasite growth under physiological O2 tensions and does so, in part, by increasing host hexokinase-2 expression (unpublished results).
Other intracellular parasites such as Leishmania and Theileria activate HIF-1 in the infected host cell although it isn't clear whether this is needed for parasite growth or supports a host defense pathway. In macrophages, Leishmania activates HIF-1 through a distinct mechanism involving transcriptional upregulation of HIF-1α and depletion of intracellular Fe to cause reduced PHD activity [41]. This mechanism is reminiscent to how HIF-1 is activated to promote resistance to pathogenic bacteria [42] suggesting that HIF-1 acts to promote resistance to Leishmania.
Summary and implications for the future
O2-sensing is important for protists to grow in the varied environments that they encounter. We are beginning to see that multiple mechanisms have evolved to sense and respond to changes in O2-availability, and even to perhaps manipulate the O2-consumption of host cells for their benefit. We highlighted here recent progress in the elucidation of biochemical mechanisms involving a homolog of the animal PHD O2-sensor whose function is not to regulate the stability of a single transcriptional factor but rather the entire proteome itself. The restriction of this pathway to unicellular parasites and other protists implies that rapid protein turnover in response to altered O2-levels may be more adaptive for single cells than the slower-acting transcriptional mechanism utilized by animals. Clearly much remains to be done to confirm this speculative model, to determine if it is the evolutionary precursor of the animal O2-sensing mechanism, and to assess its utility as a drug target.
Acknowledgments
Research from the authors' labs summarized in this article was supported by grants from the NIH (R01AI069986, R01GM037539, R01GM084383), the Oklahoma Center for the Advancement of Science and Technology, and the Mizutani Foundation for Glycoscience.
References
- 1.Fenchel T, Finlay B. Oxygen and the spatial structure of microbial communities. Biol Rev Camb Philos Soc. 2008;83:553–569. doi: 10.1111/j.1469-185X.2008.00054.x. [DOI] [PubMed] [Google Scholar]
- 2.Fenchel T. Respiration in heterotrophic unicellular eukaryotic organisms. Protist. 2014;165:485–492. doi: 10.1016/j.protis.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Jones DP. Intracellular diffusion gradients of O2 and ATP. Am J Physiol. 1986;250:C663–675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi E, Sato M. Imaging of oxygen gradients in monolayer cultured cells using green fluorescent protein. Am J Physiol Cell Physiol. 2010;299:C1318–1323. doi: 10.1152/ajpcell.00254.2010. [DOI] [PubMed] [Google Scholar]
- 5.Ward JP. Oxygen sensors in context. Biochim Biophys Acta. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonner JT, Lamont DS. Behavior of cellular slime molds in the soil. Mycologia. 2005;97:178–184. doi: 10.3852/mycologia.97.1.178. [DOI] [PubMed] [Google Scholar]
- 8.Girvan HM, Munro AW. Heme sensor proteins. J Biol Chem. 2013;288:13194–13203. doi: 10.1074/jbc.R112.422642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loenarz C, Schofield CJ. Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem Sci. 2011;36:7–18. doi: 10.1016/j.tibs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Scotti JS, Leung IK, Ge W, Bentley MA, Paps J, Kramer HB, Lee J, Aik W, Choi H, Paulsen SM, Bowman LA, Loik ND, Horita S, Ho CH, Kershaw NJ, Tang CM, Claridge TD, Preston GM, McDonough MA, Schofield CJ. Human oxygen sensing may have origins in prokaryotic elongation factor Tu prolyl-hydroxylation. Proc Natl Acad Sci U S A. 2014;111:13331–13336. doi: 10.1073/pnas.1409916111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaelin WG., Jr Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335–45. doi: 10.1101/sqb.2011.76.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RW, Elkins JM, Oldham NJ, Bhattacharya S, Gleadle JM, Ratcliffe PJ, Pugh CW, Schofield CJ. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 13.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- 16.Loenarz C, Coleman ML, Boleininger A, Schierwater B, Holland PW, Ratcliffe PJ, Schofield CJ. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 2011;12:63–70. doi: 10.1038/embor.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Wel H, Ercan A, West CM. The Skp1 prolyl hydroxylase from Dictyostelium is related to the hypoxia-inducible factor-alpha class of animal prolyl 4-hydroxylases. J Biol Chem. 2005;280:14645–14655. doi: 10.1074/jbc.M500600200. [DOI] [PubMed] [Google Scholar]
- 18*.West CM, van der Wel H, Wang ZA. Prolyl 4-hydroxylase-1 mediates O2 signaling during development of Dictyostelium. Development. 2007;134:3349–3358. doi: 10.1242/dev.000893. [DOI] [PubMed] [Google Scholar]; This paper demonstrates that Dictyostelium PhyA is an O2 sensor.
- 19*.Sheikh MO, Xu Y, van der Wel H, Walden P, Hartson SD, West CM. Glycosylation of Skp1 promotes formation of Skp1-Cullin-1-F-box protein complexes in Dictyostelium. Mol Cell Proteomics. 2015;14:66–80. doi: 10.1074/mcp.M114.044560. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that low O2 is rate limiting for the hydroxylation of nascent Skp1 in cells, and demonstrates that a function of Skp1 hydroxylation/glycosylation is to alter the repertoire of the F-box proteins with which it associates.
- 20.Xu Y, Wang ZA, Green RS, West CM. Role of the Skp1 prolyl-hydroxylation/glycosylation pathway in oxygen dependent submerged development of Dictyostelium. BMC Dev Biol. 2012;12:31. doi: 10.1186/1471-213X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Wang ZA, Singh D, van der Wel H, West CM. Prolyl hydroxylation- and glycosylation-dependent functions of Skp1 in O2-regulated development of Dictyostelium. Dev Biol. 2011;349:283–295. doi: 10.1016/j.ydbio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reveals that Skp1 is a major mediator of PhyA-dependent O2-signaling in Dictyostelium.
- 22.Zhang D, van der Wel H, Johnson JM, West CM. Skp1 prolyl 4-hydroxylase of Dictyostelium mediates glycosylation-independent and -dependent responses to O2 without affecting Skp1 stability. J Biol Chem. 2012;287:2006–2016. doi: 10.1074/jbc.M111.314021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZA, van der Wel H, Vohra Y, Buskas T, Boons GJ, West CM. Role of a cytoplasmic dual-function glycosyltransferase in O2 regulation of development in Dictyostelium. J Biol Chem. 2009;284:28896–28904. doi: 10.1074/jbc.M109.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer CM, Sheikh MO, Zhang D, West CM. Novel regulation of Skp1 by the Dictyostelium AgtA α-galactosyltransferase involves the Skp1-binding activity of its WD40 repeat domain. J Biol Chem. 2014;289:9076–9088. doi: 10.1074/jbc.M113.528679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skaar JR, Pagan JK, Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol. 2013;14:369–381. doi: 10.1038/nrm3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheikh MO, Schafer CM, Powell JT, Rodgers KK, Mooers BH, West CM. Glycosylation of Skp1 affects its conformation and promotes binding to a model F-box protein. Biochemistry. 2014;53:1657–1669. doi: 10.1021/bi401707y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshaies RJ, Emberley ED, Saha A. Control of cullin-ring ubiquitin ligase activity by nedd8. Subcell Biochem. 2010;54:41–56. doi: 10.1007/978-1-4419-6676-6_4. [DOI] [PubMed] [Google Scholar]
- 28.Pierce NW, Lee JE, Liu X, Sweredoski MJ, Graham RL, Larimore EA, Rome M, Zheng N, Clurman BE, Hess S, Shan SO, Deshaies RJ. Cand1 promotes assembly of new SCF complexes through dynamic exchange of F box proteins. Cell. 2013;153:206–215. doi: 10.1016/j.cell.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blader IJ, Koshy AA. Toxoplasma gondii development of its replicative niche: in its host cell and beyond. Eukaryot Cell. 2014;13:965–976. doi: 10.1128/EC.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West CM, Wang ZA, van der Wel H. A cytoplasmic prolyl hydroxylation and glycosylation pathway modifies Skp1 and regulates O2-dependent development in Dictyostelium. Biochim Biophys Acta. 2010;1800:160–171. doi: 10.1016/j.bbagen.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Xu Y, Brown KM, Wang ZA, van der Wel H, Teygong C, Zhang D, Blader IJ, West CM. The Skp1 protein from Toxoplasma is modified by a cytoplasmic prolyl 4-hydroxylase associated with oxygen sensing in the social amoeba Dictyostelium. J Biol Chem. 2012;287:25098–25110. doi: 10.1074/jbc.M112.355446. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated that the Skp1/PhyA pathway is conserved in protists and that PhyA is important for Toxoplasma growth at low O2 levels.
- 32.Reynolds D, Cliffe L, Förstner KU, Hon CC, Siegel TN, Sabatini R. Regulation of transcription termination by glucosylated hydroxymethyluracil, base J, in Leishmania major and Trypanosoma brucei. Nucleic Acids Res. 2014;42:9717–9729. doi: 10.1093/nar/gku714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Luenen HG, Farris C, Jan S, Genest PA, Tripathi P, Velds A, Kerkhoven RM, Nieuwland M, Haydock A, Ramasamy G, Vainio S, Heidebrecht T, Perrakis A, Pagie L, van Steensel B, Myler PJ, Borst P. Glucosylated hydroxymethyluracil, DNA base J, prevents transcriptional readthrough in Leishmania. Cell. 2012;150:909–921. doi: 10.1016/j.cell.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Cliffe LJ, Hirsch G, Wang J, Ekanayake D, Bullard W, Hu M, Wang Y, Sabatini R. JBP1 and JBP2 proteins are Fe2+/2-oxoglutarate-dependent dioxygenases regulating hydroxylation of thymidine residues in Trypanosome DNA. Mol Cell Biol. 2011;31:1690–1700. doi: 10.1074/jbc.M112.341974. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrates that Base J synthesis is regulated by Fe2+/2-oxoglutarate-dependent dioxygenases and that trypanosome virulence gene expression is regulated by O2.
- 35.Chowdhury R, Sekirnik R, Brissett NC, Krojer T, Ho CH, Ng SS, Clifton IJ, Ge W, Kershaw NJ, Fox GC, Muniz JR, Vollmar M, Phillips C, Pilka ES, Kavanagh KL, von Delft F, Oppermann U, McDonough MA, Doherty AJ, Schofield CJ. Ribosomal oxygenases are structurally conserved from prokaryotes to humans. Nature. 2014;510:422–426. doi: 10.1038/nature13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-Fernández EM, Tarhonskaya H, Al-Qahtani K, Hopkinson RJ, McCullagh JS, Schofield CJ, Flashman E. Investigations on the oxygen dependence of a 2-oxoglutarate histone demethylase. Biochem J. 2013;449:491–496. doi: 10.1042/BJ20121155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webby CJ, Wolf A, Gromak N, Dreger M, Kramer H, Kessler B, Nielsen ML, Schmitz C, Butler DS, Yates JR, 3rd, Delahunty CM, Hahn P, Lengeling A, Mann M, Proudfoot NJ, Schofield CJ, Böttger A. Jmjd6 catalyses lysyl-hydroxylation of U2AF65, a protein associated with RNA splicing. Science. 2009;325:90–93. doi: 10.1126/science.1175865. [DOI] [PubMed] [Google Scholar]
- 38*.Sen Santara S, Roy J, Mukherjee S, Bose M, Saha R, Adak S. Globin-coupled heme containing oxygen sensor soluble adenylate cyclase in Leishmania prevents cell death during hypoxia. Proc Natl Acad Sci U S A. 2013;110:16790–16795. doi: 10.1073/pnas.1304145110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work identified an O2 regulated adenylate cyclase as a novel O2 sensing pathway in Leishmania that is required for parasite survival at low O2.
- 39.Spear W, Chan D, Coppens I, Johnson RS, Giaccia A, Blader IJ. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–352. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 40.Wiley M, Sweeney KR, Chan DA, Brown KM, McMurtrey C, Howard EW, Giaccia AJ, Blader IJ. Toxoplasma gondii activates hypoxia-inducible factor (HIF) by stabilizing the HIF-1alpha subunit via type I activin-like receptor kinase receptor signaling. J Biol Chem. 2010;285:26852–26860. doi: 10.1074/jbc.M110.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh AK, Mukhopadhyay C, Biswas S, Singh VK, Mukhopadhyay CK. Intracellular pathogen Leishmania donovani activates Hypoxia inducible factor-1 by dual mechanism for survival advantage within macrophage. PLoS ONE. 2012;7:e38489. doi: 10.1371/journal.pone.0038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werth N, Beerlage C, Rosenberger C, Yazdi AS, Edelmann M, Amr A, Bernhardt W, von Eiff C, Becker K, Schäfer A, Peschel A, Kempf VA. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS One. 2010;5:e11576. doi: 10.1371/journal.pone.0011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizushima T, Yoshida Y, Kumanomidou T, Hasegawa Y, Suzuki A, Yamane T, Tanaka K. Structural basis for the selection of glycosylated substrates by SCF(Fbs1) ubiquitin ligase. Proc Natl Acad Sci USA. 2007;104:5777–5781. doi: 10.1073/pnas.0610312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koumandou VL, Wickstead B, Ginger ML, van der Giezen M, Dacks JB, Field MC. Molecular paleontology and complexity in the last eukaryotic common ancestor. Crit Rev Biochem Mol Biol. 2013;48:373–396. doi: 10.3109/10409238.2013.821444. [DOI] [PMC free article] [PubMed] [Google Scholar]