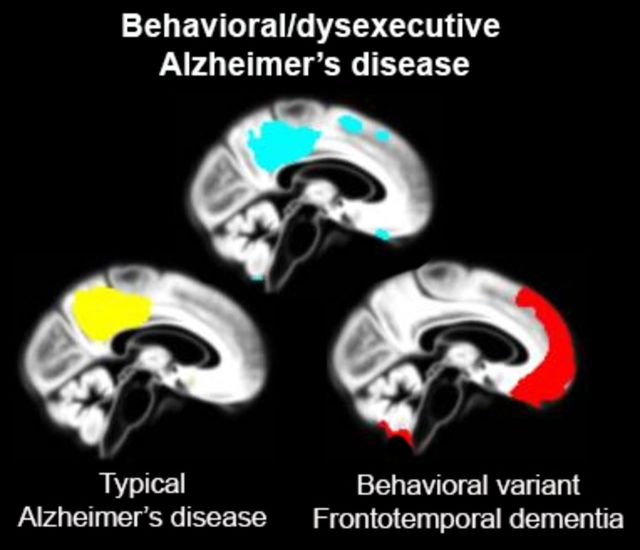
Relatively little is known about behavioural- and dysexecutive-predominant presentations of Alzheimer’s disease, collectively known as ‘frontal’ Alzheimer’s disease. Ossenkoppele et al. compare these two syndromes, revealing classical temporoparietal atrophy and relative sparing of frontal cortex in both, and propose that they are redefined as the ‘behavioural/dysexecutive variant of Alzheimer’s disease’.
Keywords: Alzheimer’s disease; frontotemporal dementia; frontal, behaviour; executive function
Relatively little is known about behavioural- and dysexecutive-predominant presentations of Alzheimer’s disease, collectively known as ‘frontal’ Alzheimer’s disease. Ossenkoppele et al. compare these two syndromes, revealing classical temporoparietal atrophy and relative sparing of frontal cortex in both, and propose that they are redefined as the ‘behavioural/dysexecutive variant of Alzheimer’s disease’.
Abstract
A ‘frontal variant of Alzheimer’s disease’ has been described in patients with predominant behavioural or dysexecutive deficits caused by Alzheimer’s disease pathology. The description of this rare Alzheimer’s disease phenotype has been limited to case reports and small series, and many clinical, neuroimaging and neuropathological characteristics are not well understood. In this retrospective study, we included 55 patients with Alzheimer’s disease with a behavioural-predominant presentation (behavioural Alzheimer’s disease) and a neuropathological diagnosis of high-likelihood Alzheimer’s disease (n = 17) and/or biomarker evidence of Alzheimer’s disease pathology (n = 44). In addition, we included 29 patients with autopsy/biomarker-defined Alzheimer’s disease with a dysexecutive-predominant syndrome (dysexecutive Alzheimer’s disease). We performed structured chart reviews to ascertain clinical features. First symptoms were more often cognitive (behavioural Alzheimer’s disease: 53%; dysexecutive Alzheimer’s disease: 83%) than behavioural (behavioural Alzheimer’s disease: 25%; dysexecutive Alzheimer’s disease: 3%). Apathy was the most common behavioural feature, while hyperorality and perseverative/compulsive behaviours were less prevalent. Fifty-two per cent of patients with behavioural Alzheimer’s disease met diagnostic criteria for possible behavioural-variant frontotemporal dementia. Overlap between behavioural and dysexecutive Alzheimer’s disease was modest (9/75 patients). Sixty per cent of patients with behavioural Alzheimer’s disease and 40% of those with the dysexecutive syndrome carried at least one APOE ε4 allele. We also compared neuropsychological test performance and brain atrophy (applying voxel-based morphometry) with matched autopsy/biomarker-defined typical (amnestic-predominant) Alzheimer’s disease (typical Alzheimer’s disease, n = 58), autopsy-confirmed/Alzheimer’s disease biomarker-negative behavioural variant frontotemporal dementia (n = 59), and controls (n = 61). Patients with behavioural Alzheimer’s disease showed worse memory scores than behavioural variant frontotemporal dementia and did not differ from typical Alzheimer’s disease, while executive function composite scores were lower compared to behavioural variant frontotemporal dementia and typical Alzheimer’s disease. Voxel-wise contrasts between behavioural and dysexecutive Alzheimer’s disease patients and controls revealed marked atrophy in bilateral temporoparietal regions and only limited atrophy in the frontal cortex. In direct comparison with behavioural and those with dysexecutive Alzheimer’s disease, patients with behavioural variant frontotemporal dementia showed more frontal atrophy and less posterior involvement, whereas patients with typical Alzheimer’s disease were slightly more affected posteriorly and showed less frontal atrophy (P < 0.001 uncorrected). Among 24 autopsied behavioural Alzheimer’s disease/dysexecutive Alzheimer’s disease patients, only two had primary co-morbid FTD-spectrum pathology (progressive supranuclear palsy). In conclusion, behavioural Alzheimer’s disease presentations are characterized by a milder and more restricted behavioural profile than in behavioural variant frontotemporal dementia, co-occurrence of memory dysfunction and high APOE ε4 prevalence. Dysexecutive Alzheimer’s disease presented as a primarily cognitive phenotype with minimal behavioural abnormalities and intermediate APOE ε4 prevalence. Both behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease presentations are distinguished by temporoparietal-predominant atrophy. Based on the relative sparing of frontal grey matter, we propose to redefine these clinical syndromes as ‘the behavioural/dysexecutive variant of Alzheimer’s disease’ rather than frontal variant Alzheimer’s disease. Further work is needed to determine whether behavioural and dysexecutive-predominant presentations of Alzheimer’s disease represent distinct phenotypes or a single continuum.
Introduction
The first symptom in the evolution of Alzheimer’s disease is usually episodic memory impairment, but visual and language predominant variants of Alzheimer’s disease have been well characterized and termed posterior cortical atrophy (Benson et al., 1988; Crutch et al., 2012) and logopenic variant primary progressive aphasia (Mesulam et al., 2008; Gorno-Tempini et al., 2011). A less prevalent phenotype is the ‘frontal variant’ of Alzheimer’s disease, referring to a clinical presentation of predominantly behavioural and/or dysexecutive deficits with Alzheimer’s disease as the primary aetiology.
The first description of a frontal variant of Alzheimer’s disease was provided by Johnson et al. (1999) in three patients with early and predominant executive dysfunction in the face of amyloid plaque and neurofibrillary tangle pathology. Several subsequent studies have reported on a dysexecutive phenotype of Alzheimer’s disease (Binetti et al., 1996; Back-Madruga et al., 2002; Snowden et al., 2007; Wolk et al., 2010; Dickerson et al., 2011; Mez et al., 2013), but only few included autopsy/biomarker-confirmed Alzheimer’s disease patients. Other autopsy (Forman et al., 2006; Balasa et al., 2011; Mendez et al., 2013, Blennerhassett et al., 2014), clinical (Larner, 2006; Woodward et al., 2010), and case (Taylor et al., 2008; Habek et al., 2010; Herrero-San Martin et al., 2013) studies have shown that the spectrum of frontal variant Alzheimer’s disease also comprises patients with early personality and behavioural changes such as disinhibition, apathy or compulsiveness. The clinical picture of frontal variant Alzheimer’s disease may mimic that of behavioural variant frontotemporal dementia (FTD), as illustrated by the 10–40% of patients clinically diagnosed with behavioural variant FTD who are found to have Alzheimer’s disease pathology on amyloid PET (Rabinovici et al., 2011; Ossenkoppele et al., 2013a) or post-mortem evaluation (Varma et al., 1999; Forman et al., 2006; Alladi et al., 2007; Beach et al., 2012).
Although frontal variant Alzheimer’s disease has been incorporated into new diagnostic criteria for Alzheimer’s disease dementia (McKhann et al., 2011; Dubois et al., 2014), little is known about the initial symptoms, risk factors, genetic predispositions, behavioural and neuropsychological profiles and co-pathologies that characterize this phenotype. There is a need for better understanding of neurodegenerative diseases that cross boundaries of distinct clinical entities, as this may improve clinicians’ ability to discern the histopathological cause of dementia. In this retrospective study, we assembled a large sample of patients with autopsy/biomarker-defined Alzheimer’s disease with behavioural and/or dysexecutive deficits. We aimed to better characterize the clinical, neuropsychological, neuroimaging and neuropathological features of these clinical syndromes, and compared these features to those found in matched behavioural variant FTD and patients with ‘typical’ (amnestic-predominant) Alzheimer’s disease. We hypothesized that patients with Alzheimer’s disease selected based on behavioural or dysexecutive-predominant presentations would show a less severe behavioural profile and more impaired cognition than patients with behavioural variant FTD, and would display brain atrophy in both anterior regions characteristic of behavioural variant FTD and posterior regions characteristic of Alzheimer’s disease.
Materials and methods
Participants
A total of 253 subjects were included in the study. We identified 75 patients with pathological or biomarker evidence of Alzheimer’s disease pathology and a behavioural or dysexecutive-predominant clinical presentation. These included 46 patients selected for a behavioural-predominant presentation (behavioural Alzheimer’s disease), 20 selected for a dysexecutive-predominant presentation (dysexecutive Alzheimer’s disease), and nine patients who met criteria for both behavioural and dysexecutive Alzheimer’s disease (described below). We further included 58 patients with typical Alzheimer’s disease, 59 with behavioural variant FTD and 61 control subjects matched with the behavioural-dysexecutive Alzheimer’s disease patients for demographical variables and (for patients) disease severity. All subjects were recruited from research cohorts at the University of California San Francisco (UCSF, n = 138) Memory and Aging Center and from the VU University Medical Center (VUMC, n = 115) Amsterdam Dementia Cohort (van der Flier et al., 2014). All patients underwent standard dementia screening that included a medical history and physical examination, a structured caregiver interview, brain MRI and neuropsychological testing. Mini-Mental State Examination (MMSE, n = 250; Folstein et al., 1975), global Clinical Dementia Rating scale (CDR, n = 207, Morris, 1993), Geriatric Depression Scale (GDS, n = 196; Yesavage et al., 1982) and Neuropsychiatric Inventory (NPI, n = 186; Kaufer et al., 2000) were administered at baseline. APOE genotyping was performed in 215 subjects. The clinical diagnosis was established by consensus by a multidisciplinary team. Controls were recruited through advertisements in newspapers and underwent the same diagnostic procedures (Ossenkoppele et al., 2012a; Lehmann et al., 2013a). Informed consent was obtained from all subjects or their assigned surrogate decision-makers, and the UCSF and VUMC institutional review boards for human research approved the study.
Clinical definitions
As there are no consensus clinical criteria for frontal variant Alzheimer’s disease, a group of behavioural neurologists (G.D.R., Y.A.L.P., P.S.) and neuropsychologists (R.O., W.vdF., J.H.K.) developed the criteria presented below to identify Alzheimer’s disease patients with prominent behavioural and/or dysexecutive presentations. Throughout the manuscript, ‘behavioural Alzheimer’s disease’ and ‘dysexecutive Alzheimer’s disease’ refer to groups of Alzheimer’s disease patients selected based on behavioural-predominant or dysexecutive-predominant presentations (specific to this study), respectively, while the generic term ‘behavioural/dysexecutive variant Alzheimer’s disease’ refers to the full spectrum of behavioural/dysexecutive presentations.
Behavioural-predominant presentations of Alzheimer’s disease
The behavioural Alzheimer’s disease group was defined as patients with in vivo evidence of amyloid pathology on PET or in CSF, and/or autopsy-confirmation (see ‘Neuropathology and Alzheimer’s disease biomarkers’ section), in addition to (i) a clinical diagnosis of possible behavioural variant FTD; or (ii) a differential diagnosis of both possible behavioural variant FTD and probable Alzheimer’s disease listed in their medical charts; or (iii) a clinical diagnosis of ‘frontal variant’ Alzheimer’s disease during life. A database search between January 1999 and January 2014 revealed 55 patients (UCSF, n = 33; VUMC, n = 22) who met these criteria. Although not a prerequisite for inclusion in the behavioural Alzheimer’s disease group, 48/55 patients had full neuropsychological testing available. The baseline clinical diagnoses were ‘frontal variant’ Alzheimer’s disease (n = 23), behavioural variant FTD (n = 15) or included both Alzheimer’s disease and behavioural variant FTD in the differential diagnosis (n = 17). The mean follow-up period was 1.5 ± 1.1 years with a mean of 3.3 ± 2.5 clinical evaluations. The last available clinical diagnosis included 37 patients with ‘frontal variant’ Alzheimer’s disease, 13 patients with behavioural variant FTD, and five patients with both Alzheimer’s disease and behavioural variant FTD as part of the differential diagnosis.
Dysexecutive-predominant presentations of Alzheimer’s disease
The dysexecutive Alzheimer’s disease group consisted of patients for whom the first clinical impression was mild cognitive impairment due to Alzheimer’s disease or Alzheimer’s disease dementia (supported by biomarkers/autopsy), subsequently showing selective impairment of executive functions on extensive neuropsychological testing. First, we searched databases for patients with a clinical diagnosis of probable Alzheimer’s disease or mild cognitive impairment due to Alzheimer’s disease with (i) positive PET or CSF amyloid biomarkers and/or autopsy-confirmation (see ‘Neuropathology and Alzheimer’s disease biomarkers’ section); (ii) a full neuropsychological test battery available; and (iii) MMSE ≥ 18 (to minimize interference of other cognitive domains with executive functions with advancing dementia). After exclusion of patients with posterior cortical atrophy or logopenic variant primary progressive aphasia, 111 patients at UCSF and 104 at VUMC were eligible. Next, z-scores were computed for memory and executive function as described in the neuropsychology section below. The composite score for executive function was subtracted from the memory composite score, and the quartile with worst executive function relative to memory performance was selected. The mean residual (executive z-score minus memory z-score) for patients with dysexecutive variant Alzheimer’s disease was −1.02 ± 0.72 (range: − 2.82–0.74, negative in 27/29 patients) and 1.64 ± 1.19 (range: −1.39–4.77, positive in 52/56) for the amnestic Alzheimer’s disease group (P < 0.001). The memory clinics of UCSF and VUMC specifically focus on early-onset dementia patients, a population more frequently demonstrating focal cortical symptoms such as aphasia, apraxia and agnosia, compared to late-onset Alzheimer’s disease (Mendez et al., 2012). To identify predominantly dysexecutive syndromes—rather than more global non-amnestic presentations of Alzheimer’s disease—only patients with executive function composite scores at least z = 0.5 lower than language and visuo-spatial function scores were selected from the quartile with worst executive function relative to memory performance. This resulted in 19 patients with dysexecutive Alzheimer’s disease from UCSF and 10 from VUMC. Nine patients met inclusion criteria for both behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease, and were included in both groups for the analyses.
Control groups
We identified 58 patients with autopsy/biomarker-confirmed typical Alzheimer’s disease and 59 patients with autopsy-confirmed/Alzheimer’s disease biomarker-negative behavioural variant FTD who were matched to the behavioural-dysexecutive Alzheimer’s disease patients for age, sex, education, MMSE and CDR. We also included 61 cognitively normal controls matched for age, sex and education. The typical Alzheimer’s disease patients fulfilled National Institute on Ageing-Alzheimer’s Association (NIA-AA) criteria for probable Alzheimer’s disease with at least intermediate-likelihood of Alzheimer’s disease pathophysiology (McKhann et al., 2011) or mild cognitive impairment due to Alzheimer’s disease (Albert et al., 2011) based on positive amyloid markers or autopsy, did not meet criteria for any non-amnestic variant of Alzheimer’s disease, and had memory-predominant presentations. Patients with behavioural variant FTD met the core clinical criteria proposed by Neary et al. (1998) or Rascovsky et al. (2011), and were either autopsy-confirmed or had negative amyloid markers.
Neuropathology and Alzheimer’s disease biomarkers
Patients were only included after autopsy and/or biomarker confirmation of Alzheimer’s disease pathology. The breakdown of the number of autopsy, PET and CSF assessments for each diagnostic group is provided in Table 1. Amyloid PET and/or amyloid-β42 and tau biomarkers in CSF were used for assessment of Alzheimer’s disease pathology during life. Amyloid PET and CSF biomarkers correlate with a neuropathological diagnosis of Alzheimer’s disease (Strozyk et al., 2003; Ikonomovic et al., 2008) and there is excellent concordance between PET and CSF (Landau et al., 2013; Zwan et al., 2014). Amyloid PET scans [using 11C-Pittsburgh compound B (PiB) (Klunk et al., 2004) in the vast majority and 18F-flutemetamol (Vandenberghe et al., 2010) in four patients] were performed using previously described procedures (Rabinovici et al., 2011; Ossenkoppele et al., 2012a). Parametric images were assessed by experienced raters and visually interpreted as amyloid-positive or amyloid-negative. CSF data were collected at VUMC only using a previously described method (van der Flier et al., 2014). A cut-off of total tau/amyloid-β42 ratio > 0.52 was used to classify the CSF as ‘Alzheimer’s disease-like’ (Duits et al., 2014).
Table 1.
Demographic and clinical characteristics according to diagnostic group
| Behavioural/dysexecutive variant Alzheimer’s disease | Behavioural presentation Alzheimer’s disease | Dysexecutive presentation Alzheimer’s disease | Typical Alzheimer’s disease | Behavioural variant FTD | Controls | |
|---|---|---|---|---|---|---|
| N | 75 | 55* | 29* | 58 | 59 | 61 |
| Agea | 65.8 ± 8.5 | 64.7 ± 8.8 | 69.2 ± 8.5 | 64.4 ± 8.6 | 63.8 ± 6.8 | 63.7 ± 8.1 |
| Sex (% male) | 68.0 | 72.7 | 60.7 | 65.5 | 71.2 | 62.3 |
| Education (years)b | 15.5 ± 3.1 | 15.7 ± 2.3 | 15.7 ± 2.7 | 15.8 ± 2.5 | 15.4 ± 3.2 | 17.3 ± 1.9 |
| MMSEc | 22.7 ± 5.6 | 22.5 ± 5.4 | 24.6 ± 3.3 | 22.5 ± 4.1 | 23.7 ± 5.4 | 29.4 ± 0.7 |
| CDRd | 0.9 ± 0.6 | 0.9 ± 0.4 | 0.8 ± 0.3 | 0.9 ± 0.5 | 1.1 ± 0.7 | 0 ± 0 |
| GDSe | 3.4 ± 2.9 | 3.2 ± 2.8 | 3.7 ± 3.2 | 2.9 ± 2.1 | 5.0 ± 3.4 | 2.0 ± 2.6 |
| NPIf | 14.3 ± 16.8 | 15.4 ± 17.6 | 12.3 ± 18.1 | 7.0 ± 11.0 | 21.9 ± 20.0 | 2.7 ± 1.2 |
| % APOE ε4 carriersg | 51.7 | 59.5 | 40.0 | 72.1 | 18.9 | 16.7 |
| APOE ε4++/+−/−−g | 6/25/29 | 6/19/17 | 2/8/15 | 14/17/12 | 0/10/43 | 3/7/50 |
| TIV (l) | 1.60 ± 0.17 | 1.60 ± 0.15 | 1.61 ± 0.19 | 1.59 ± 0.15 | 1.64 ± 0.16 | 1.57 ± 0.14 |
| Autopsy-confirmed | 24 | 17 | 12 | 8 | 21 | - |
| PET/CSF biomarkers | 41/22 | 28/18 | 15/10 | 26/29 | 23/23 | - |
Data are presented as mean ± SD unless indicated otherwise. Differences between groups were assessed using ANOVA with post hoc LSD tests [age, education, MMSE, CDR, Geriatric Depression Scale (GDS), Neurophsychiatric Inventory (NPI) and total intracranial volume (TIV)], χ2 (sex and APOE ε4 status), and Kruskal-Wallis with post hoc Mann-Whitney U-tests (number of APOE ε4 alleles).
aDysexecutive Alzheimer’s disease > other groups, P < 0.05.
bControls > patients, P < 0.01.
cPatients < controls, P < 0.001; behavioural Alzheimer’s disease + typical Alzheimer’s disease < dysexecutive Alzheimer’s disease, P < 0.05.
dPatients > controls, P < 0.001; behavioural variant FTD > dysexecutive Alzheimer’s disease, P < 0.01.
eBehavioural/dysexecutive Alzheimer’s disease+behavioural Alzheimer’s disease+dysexecutive Alzheimer’s disease+behavioural variant FTD > controls, P < 0.05; behavioural variant FTD > behavioural Alzheimer’s disease/dysexecutive Alzheimer’s disease + behavioural Alzheimer’s disease + typical Alzheimer’s disease, P < 0.01.
fBehavioural/dysexecutive Alzheimer’s disease + behavioural Alzheimer’s disease + dysexecutive Alzheimer’s disease + behavioural variant FTD > controls, P < 0.05; behavioural Alzheimer’s disease/dysexecutive Alzheimer’s disease + behavioural Alzheimer’s disease > typical Alzheimer’s disease, P < 0.05; behavioural variant FTD > dysexecutive Alzheimer’s disease + typical Alzheimer’s disease, P < 0.05.
gBehavioural/dysexecutive Alzheimer’s disease + behavioural Alzheimer’s disease + dysexecutive Alzheimer’s disease + typical Alzheimer’s disease > behavioural variant FTD + controls, P < 0.05; typical Alzheimer’s disease > dysexecutive Alzheimer’s disease, P < 0.05.
*Nine patients met criteria for both behavioural and dysexecutive Alzheimer’s disease and were included in both groups.
APOE = Apolipoprotein E; ++ = ε4 homozygous, +− = ε4 heterozygous; −− = ε4 negative.
Comprehensive neuropathological assessments were performed following previously described standard procedures for the evaluation of dementia (Lee et al., 2011; Schoonenboom et al., 2012). Patients were included if they met NIA-Reagan neuropathological criteria for intermediate or high-likelihood Alzheimer’s disease (Hyman and Trojanowski, 1997). The neuropathological diagnosis of FTD was made using standard criteria (Cairns et al., 2007; Mackenzie et al., 2010). Further microscopic investigations were performed to assess the presence of co-pathologies such as cerebral amyloid angiopathy, cerebrovascular disease and Lewy body disease (Montine et al., 2012). Argyrophilic grain disease, TAR DNA-binding protein 43 (encoded by TARDBP) and argyrophilic thorny astrocyte clusters were noted when observed during neuropathological examination but were not assessed systematically across the entire cohort. Neuropathological protocols have been modified over time (autopsies were performed between 2004 and the present) as novel protein inclusions and analytical methods were detected. Missing data are indicated as ‘unknown’ in Table 2.
Table 2.
Neuropathological findings in behavioural and dysexecutive variant Alzheimer’s disease patients
| # | Phenotype | Age at Diagnosis | Sex | MMSE | Possible bvFTD criteria meta | Baseline diagnosis | Last diagnosis | Diagnosis-to-autopsy (months) | NIA-Reagan likelihoodb | CAA | CVD | LB | AGD | TDP | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | bAD | 42 | M | 18 | A/C/D/E | bvFTD | bvFTD | 45 | High | Absent | No | Absent | Unknown | Unknown | |
| 2 | bAD | 57 | F | 11 | A | bAD | bAD | 64 | High | Absent | No | Amygdala | Unknown | Unknown | |
| 3 | bAD | 63 | M | 28 | A | bAD | bAD | 52 | High | Absent | No | Amygdala | Unknown | Unknown | |
| 4 | bAD | 60 | F | 10 | A/D/E/F | bvFTD | bvFTD | 22 | High | Mild | No | Absent | Unknown | Unknown | |
| 5 | bAD | 57 | M | 16 | A/B/E | bvFTD | bvFTD | 118 | High | Moderate | No | Limbic | Unknown | Unknown | |
| 6 | bAD | 52 | F | 30 | A/B/E | bAD/bvFTD | bAD | 82 | High | Moderate | No | Absent | Absent | Absent | |
| 7 | bAD | 71 | M | 20 | A/D | bAD | bAD | 25 | High | Moderate | No | Absent | Unknown | Unknown | |
| 8 | bAD | 60 | M | 22 | C | bAD/bvFTD | bAD/bvFTD | 85 | High | Moderate | No | Amygdala | Absent | Absent | |
| 9 | bAD | 58 | M | Unknown | B/C/D/F | bAD/bvFTD | bAD | 58 | High | Absent | No | Absent | Unknown | Unknown | |
| 10 | bAD | 58 | M | 24 | B/F | bAD | bAD | 41 | High | Mild | Yesd | Limbic | Limbic | Absent | |
| 11 | bAD | 56 | M | 11 | B/C | bAD | bAD | 53 | High | Moderate | No | Absent | Absent | Absent | |
| 12 | bAD | 56 | F | 26 | B/D/E | bAD | bAD | 50 | High | Mild | Yese | Absent | Absent | Absent | ATAC |
| 13 | bAD+deAD | 81 | M | 23 | A/C/D/F | bvFTD | bvFTD | 15 | High | Absent | No | Absent | Unknown | Unknown | PSPc |
| 14 | bAD+deAD | 76 | F | 28 | C/F | bAD/bvFTD | bAD/bvFTD | 43 | High | Absent | No | Absent | Unknown | Unknown | |
| 15 | bAD+deAD | 58 | M | 28 | B/D/F | bvFTD/PSP | bvFTD/PSP | 63 | High | Unknown | No | Absent | Unknown | Absent | PSPc |
| 16 | bAD+deAD | 73 | M | 27 | B/C | bAD | bAD | 53 | High | Unknown | No | Neocortical | Unknown | Unknown | |
| 17 | bAD+deAD | 83 | M | 19 | A/B/C/D/E/F | bvFTD | bvFTD | 82 | High | Severe | Yesf | Limbic | Limbic | Unclassifiable | ATAC |
| 18 | deAD | 65 | F | 25 | F | AD | AD | 74 | High | Absent | Yesg | Absent | Unknown | Absent | |
| 19 | deAD | 72 | M | 25 | F | AD | AD | 101 | High | Mild | No | Limbic | Limbic | Unknown | ATAC |
| 20 | deAD | 62 | M | 25 | F | AD/CBD | AD | 80 | High | Mild | Yesh | Absent | Unknown | Absent | |
| 21 | deAD | 75 | F | 28 | F | AD | AD | 62 | High | Mild | Yesi | Absent | Limbic | Absent | |
| 22 | deAD | 59 | M | 18 | B/C | AD | AD | 74 | High | Absent | No | Absent | Absent | Absent | |
| 23 | deAD | 81 | M | 19 | None | AD | AD | 72 | High | Moderate | No | Amygdala | Unknown | Absent | |
| 24 | deAD | 67 | M | 25 | F | AD | AD | 47 | High | Moderate | No | Neocortical | Unknown | Unknown |
bAD = behavioural-predominant presentation Alzheimer’s disease; deAD = dysexecutive-predominant presentation Alzheimer’s disease; M = male; F = female; PSP = progressive supranuclear palsy; ADNC = Alzheimer’s disease neuropathological change; CAA = cerebral amyloid angiopathy; CVD = cerebrovascular disease; DLB = Lewy bodies; AGD = argyrophilic grain disease; TDP = TAR DNA-binding protein; ATAC = argyrophilic thorny astrocyte clusters.
aRascovsky et al. (2011) criteria for possible behavioural variant FTD: A = disinhibition, B = apathy, C = loss of empathy or sympathy, D = perseverative, Stereotype or compulsive/ritualistic behaviour, E = hyperorality and dietary changes, F = dysexecutive neuropsychological profile.
bHyman and Trojanowski (1997): all patients had frequent neuritic plaques (CERAD) and neurofibrillary tangles stage V or VI (Braak).
cMixed primary Alzheimer’s disease/ progressive supranuclear palsy neuropathological diagnosis.
dOne lacunar infarct, scarce middle insula and angular gyrus microinfarcts, mild to moderate arteriolosclerosis.
eScarce microinfarction in putamen.
fScarce microinfarction in globus pallidus, moderate arteriolosclerosis.
gLacunar infarcts in right external globus pallidus and putamen.
hLacunar infarct left putamen/pallidum/subinsular white matter.
iModerate microinfaction thalamus and angular gyrus, moderate arteriolosclerosis.
Clinical data
Structured chart reviews in patients with behavioural Alzheimer’s disease and those with dysexecutive Alzheimer’s disease were performed by R.O., Y.A.L.P. and G.D.R. to ascertain clinical features (see the example form in the Supplementary material). Five randomly selected charts of both UCSF and VUMC patients were jointly reviewed to ensure sufficient internal consistency. Information was collected on the first symptom(s) reported by the patient and/or caregiver (i.e. cognitive, behavioural and/or motor), medical history, family history of dementia or other neurological/psychiatric disorders, whether each of the diagnostic criteria for behavioural variant FTD (Rascovsky et al., 2011) and probable Alzheimer’s disease (McKhann et al., 2011) were met, clinical diagnosis after the first visit, final clinical diagnosis, follow-up period and number of evaluations.
Neuropsychology
Cognitive functioning was assessed using neuropsychological tests completed at baseline that were either performed at both UCSF and VUMC (Rabinovici et al., 2010; Ossenkoppele et al., 2012b) or that measure equivalent neuropsychological constructs. Four major cognitive domains were covered: memory (delayed recall of the Californian Verbal Learning Test and Benson Figure Test at UCSF and delayed recall of a Dutch version of the Rey Auditory Verbal Learning Test and the Visual Association Test at VUMC), executive functioning (Digit Span backwards, Trail Making Test part B, Stroop Color-Word card subtask and Letter Fluency at both UCSF and VUMC), language (Category Fluency at both sites, Boston Naming Test at UCSF and Visual Association Test picture naming at VUMC) and visuo-spatial functioning [Number Location from the Visual Object Space and Perception battery and (modified) Rey Complex Figure copy test at both centres]. Population-based age, sex and education adjusted normalized scores were available for all memory and executive function tasks, but not for all language and visuo-spatial tests. We therefore converted raw language and visuo-spatial test scores to z-scores within the final selection (presented in Table 1) of patients and controls. For language and visuo-spatial functions, z = 0 therefore reflects the average performance of the present study population, whereas for memory and executive function z = 0 reflects the average performance of the general population. Finally, composite means were calculated for each cognitive domain by averaging z-scores for all tasks within a given cognitive domain.
Magnetic resonance imaging
Acquisition
Structural MRI scans were available for 46/55 patients with behavioural Alzheimer’s disease, 26/29 patients with dysexecutive Alzheimer’s disease, 58/58 patients with typical Alzheimer’s disease, 57/59 patients with behavioural variant FTD, and 61/61 control subjects. At UCSF, T1-weighted images were acquired on a 1.5 T (Magnetom Avanto System/Magnetom VISION system, Siemens, n = 87) or 3 T (Tim Trio, Siemens, n = 51) unit. At VUMC, MRI scans were performed on a 1 T (Magnetom Impact, Siemens, n = 21), 1.5 T (Sonata, Siemens, n = 23) or 3 T (SignaHDxt, GE Healthcare, n = 66) unit. Acquisition parameters have been published previously (Sluimer et al., 2008; Ossenkoppele et al., 2012a; Lehmann et al., 2013a; Moller et al., 2013). The proportion of subjects scanned on each scanner was balanced across groups and all imaging statistical models included scanner type and acquisition site as nuisance variables.
Voxel-based morphometry
MRI data were segmented using the New Segment toolbox implemented in the Statistical Parametric Mapping (SPM) 8 software (Wellcome Trust Centre for Neuroimaging, Institute of Neurology at University College London). Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) was used to generate a study-specific template by aligning the grey matter images non-linearly to a common space. Native grey and white matter images were spatially normalized to the DARTEL template using individual flow fields, with modulation applied to preserve the total amount of signal. Images were smoothed using an 8-mm full-width at half-maximum isotropic Gaussian kernel. Images were inspected visually after each step in the processing pipeline, and the final smoothed-modulated-warped grey matter images were checked for sample homogeneity using the VBM8 toolbox to identify potential outliers. Next, we performed voxel-wise contrasts between the four different patient groups (plus a combined behavioural/dysexecutive Alzheimer’s disease group) and the healthy control subjects. We additionally performed voxel-wise contrasts against controls for patients with autopsy-confirmed behavioural Alzheimer’s disease/dysexecutive Alzheimer’s disease (n = 24) and for patients with behavioural Alzheimer’s disease who were initially diagnosed with behavioural variant FTD (n = 13). Finally, we directly compared the patient groups (combined behavioural/dysexecutive Alzheimer’s disease, typical Alzheimer’s disease and behavioural variant FTD). The primary models included age, sex, total intracranial volume, scanner type and centre as nuisance variables. Secondary models additionally included MMSE (as a proxy of disease severity) as a nuisance variable. Results are displayed at an uncorrected threshold of P < 0.001.
Frequency maps
As voxel-based morphometry group-level analyses potentially wash out individual effects, we generated frequency maps across the brain to visualize the proportion of patients within each phenotype who showed reduced grey matter density compared to controls (Supplementary Fig. 1). These maps show the relative involvement of each brain region for each diagnostic group. First, we performed voxel-wise regressions in SPM8 on a set of nuisance factors (age, sex, total intracranial volume, scanner type and centre) to estimate their effect on smoothed-modulated-warped grey matter probability images (resulting from VBM processing) in our group of healthy controls. This resulted in beta maps for the nuisance variables as well as a residual map for each healthy control. Next, we used these values to compute a voxel-wise map of W-scores for each patient using the formula: W-score = (actual − expected) / SD, where actual is the smoothed-modulated-warped grey matter probability for a given patient at a given voxel, expected is the predicted grey matter probability for that voxel using the nuisance factors and beta weights from the regression in healthy controls, and SD is the standard deviation of the residuals for that voxel among the controls. Thus, W-scores (mean = 0, SD = 1 in the control group, similar to z-scores) show, at each voxel, where a patient’s grey matter probability would fall on the normal distribution of grey matter probabilities in healthy controls, after taking nuisance factors into account (Jack et al., 1997; La Joie et al., 2012). Our last steps were to binarize the W-score map for each patient at W < −1, and to allow for comparison between diagnostic groups we generated frequency maps to show the proportion of patients with suprathreshold W-scores for each voxel across the brain.
Statistical analyses
Differences between groups for baseline characteristics were assessed using ANOVA with post hoc Fischer’s least significant difference (LSD) tests for continuous variables and χ2 and Kruskal-Wallis with post hoc Mann-Whitney U-tests for dichotomous or categorical data. Differences in neuropsychological function across groups were assessed using ANOVA with post hoc Fischer’s LSD tests.
Results
Participants
Demographic and clinical characteristics are presented in Table 1. Clinical groups consisted of mildly impaired patients with mean MMSE scores ranging from 22 to 25 and an average CDR of ∼1. Patients with behavioural Alzheimer’s disease (mean age: 64.7 ± 8.8, median: 64.1, range: 43.1–83.5) and dysexecutive Alzheimer’s disease (mean age: 69.2 ± 8.5, median: 71.3, range: 53.7–83.5) were relatively young at time of diagnosis and more often male (73% and 61%, respectively) than female. Of the patients with behavioural Alzheimer’s disease and patients with dysexecutive Alzheimer’s disease, 59.5% and 40% carried at least one APOE ε4 allele, respectively.
Behavioural-predominant presentations of Alzheimer’s disease
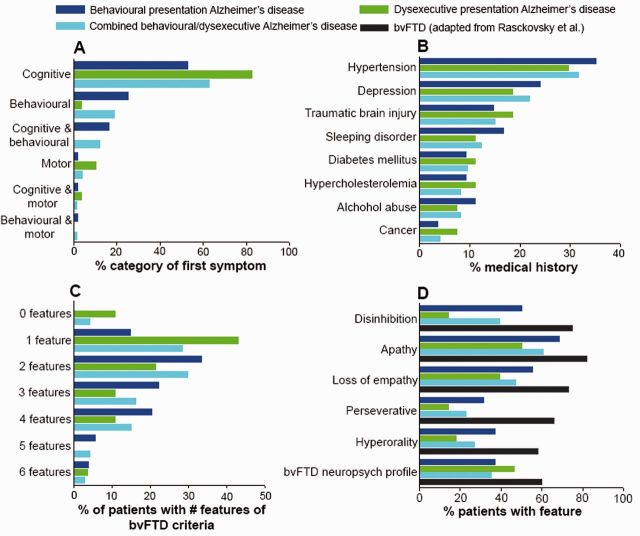
Patients presented initially with cognitive difficulties (53%) more often than with behavioural changes (25%, Fig. 1A). The breakdown of cognitive complaints at baseline consisted of memory impairment (26/55, 47%), executive dysfunction (4/55, 7%), both (21/55, 38%) or neither memory nor executive dysfunction (4/55, 7%). Hypertension, depression, sleep disorder and traumatic brain injury were the most frequently mentioned conditions in the medical history (Fig. 1B). Fifty-two per cent met international consensus criteria for possible behavioural variant FTD (Rascovsky et al., 2011). The majority of patients were close to the threshold of ≥3 of 6 core behavioural/cognitive symptoms required for a diagnosis of possible behavioural variant FTD [2/6 (33%), 3/6 (22%) or 4/6 (20%), Fig. 1C], and had fewer behavioural symptoms compared to patients with behavioural variant FTD. Apathy was more prominent than disinhibition and loss of empathy, while hyperorality and perseverative/compulsive behaviours were less common (Fig. 1D). Figure 1D and the Neuropsychiatric Inventory scores also indicate that the behavioural profile of patients with behavioural Alzheimer’s disease was less profound than that of patients with behavioural variant FTD. Neuropsychiatric Inventory domains often cited in typical Alzheimer’s disease such as anxiety, agitation, and irritability were less prevalent in patients with behavioural Alzheimer’s disease than in those with behavioural variant FTD.
Figure 1.
Clinical features. Frequency of (A) first symptoms reported by patients and caregivers, (B) self-reported medical conditions in the past history, (C) the number of core behavioural/cognitive symptoms met of diagnostic criteria for behavioural variant FTD (bvFTD; Rascovsky et al., 2011), and (D) these behavioural/cognitive features.
Dysexecutive-predominant presentations of Alzheimer’s disease
Patients with dysexecutive Alzheimer’s disease predominantly presented with cognitive deficits (83%, Fig. 1A). The breakdown of cognitive complaints at baseline consisted of memory impairment (11/29, 38%), executive dysfunction (3/29, 10%), both (11/29, 38%) or neither memory nor executive dysfunction (4/29, 14%). Hypertension, depression, and traumatic brain injury were among the most frequently mentioned conditions in the medical history (Fig. 1B). There was mild overlap between dysexecutive and behavioural Alzheimer’s disease as nine patients fulfilled inclusion criteria for both groups. Seven of the patients with dysexecutive/behavioural Alzheimer’s disease met international consensus criteria for possible behavioural variant FTD (Rascovsky et al., 2011), whereas none of the dysexecutive Alzheimer’s disease only patients met behavioural variant FTD criteria. Most patients experienced only one (43%) or two (21%) core behavioural/cognitive symptoms (Fig. 1C). Apathy was the most common behavioural feature, followed by a behavioural variant FTD neuropsychological profile and loss of empathy, while disinhibition, hyperorality and perseverative/compulsive behaviours were less prevalent (Fig. 1D).
Neuropsychology
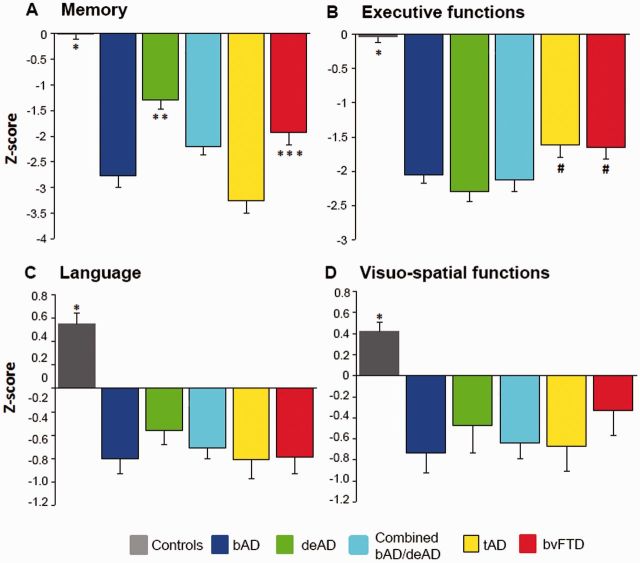
Patient groups showed worse neuropsychological functions than controls in all cognitive domains (P < 0.001). Behavioural Alzheimer’s disease patients had worse composite memory scores than dysexecutive Alzheimer’s disease (P < 0.001) and behavioural variant FTD (P < 0.01) patients, and memory scores did not significantly differ from typical Alzheimer’s disease patients (P = 0.06, Fig. 2A). Both dysexecutive Alzheimer’s disease and behavioural Alzheimer’s disease patients had lower composite executive function scores than typical Alzheimer’s disease ( < 0.05) and behavioural variant FTD patients (P < 0.05), and did not differ from each other (P = 0.29, Fig. 2B). The patient groups did not differ in composite scores for language and visuo-spatial functions (Fig. 2C and D).
Figure 2.
Neuropsychological performance. Composite z-scores for (A) memory, (B) executive functions, (C) language and (D) visuo-spatial functions. Differences between groups were assessed using ANOVA. *All patient groups < controls, P < 0.001; **behavioural Alzheimer’s disease (bAD; P < 0.001), typical Alzheimer’s disease (tAD; P < 0.001), behavioural Alzheimer’s disease/dysexecutive Alzheimer’s disease (bAD/deAD; P < 0.05) and behavioural variant FTD (bvFTD; P < 0.05) < dysexecutive Alzheimer’s disease; ***behavioural Alzheimer’s disease (P < 0.01) and typical Alzheimer’s disease (P < 0.001) < behavioural variant FTD; #dysexecutive Alzheimer’s disease < typical Alzheimer’s disease (P < 0.01) and behavioural variant FTD (P < 0.01) and behavioural Alzheimer’s disease/dysexecutive Alzheimer’s disease and behavioural Alzheimer’s disease < typical Alzheimer’s disease (P < 0.05) and behavioural variant FTD (P < 0.05).
Magnetic resonance imaging
Voxel-based morphometry: patients versus controls
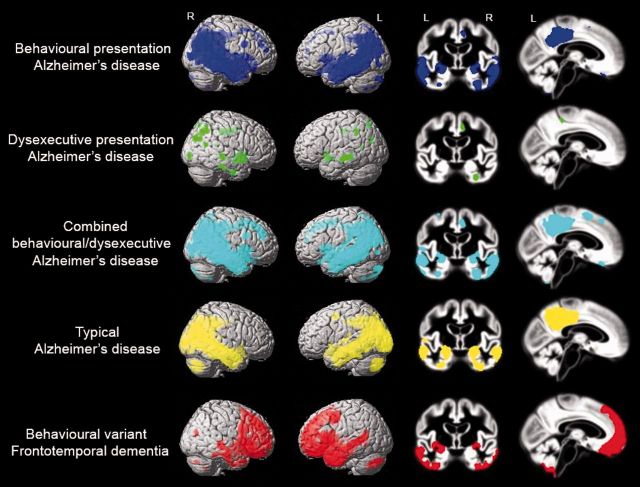
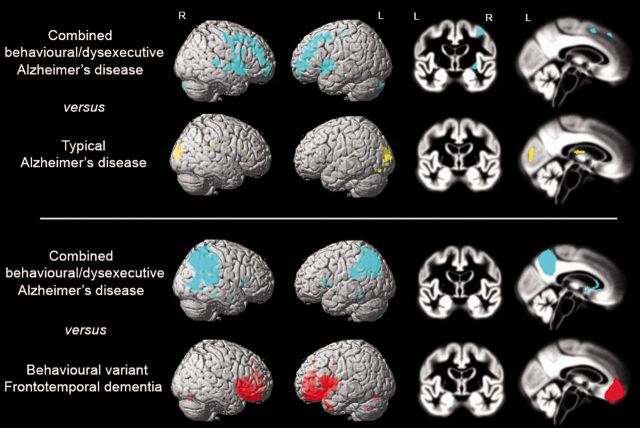
We performed voxel-wise contrasts between the different patient groups and controls (Fig. 3), adjusted for age, sex, total intracranial volume, scanner type and centre. Characteristic patterns of brain atrophy were seen in patients with typical Alzheimer’s disease (involving large areas of the temporoparietal cortex, including posterior cingulate, precuneus and the medial temporal lobes) and patients with behavioural variant FTD (in medial frontal cortex, anterior cingulate, frontal insula and bilateral anterior temporal lobes). Compared with healthy controls at P < 0.001 uncorrected, the combined behavioural/dysexecutive Alzheimer’s disease group showed a pattern of brain atrophy that was strikingly similar to that observed in the typical Alzheimer’s disease patients, with limited atrophy in the frontal cortex (Fig. 3). Patients with behavioural Alzheimer’s disease showed a predominant temporoparietal atrophy pattern, with additional involvement of small regions of the left orbitofrontal cortex, frontal poles and middle and superior frontal gyri (at P < 0.001 uncorrected). The effect in frontal regions disappeared when applying a more stringent statistical threshold [P < 0.05 family-wise error (FWE) corrected], while the classical posterior Alzheimer’s disease regions survived (Supplementary Table 1). Voxel-wise contrasts between controls and the autopsy-confirmed behavioural Alzheimer’s disease patients showed a pattern of atrophy similar to that of the total behavioural Alzheimer’s disease group (Supplementary Fig. 2A). Patients with behavioural Alzheimer’s disease initially diagnosed with behavioural variant FTD (thus excluding baseline ‘frontal Alzheimer’s disease’ and Alzheimer’s disease/behavioural variant FTD differential diagnoses) had similar lateral temporoparietal atrophy compared to the entire behavioural Alzheimer’s disease group, but showed more frontal (mainly dorsolateral) and insular atrophy and less mesioparietal involvement (Supplementary Fig. 2B) when compared against controls. At P < 0.001 uncorrected, the dysexecutive Alzheimer’s disease group showed diffusely distributed grey matter reductions in superior, middle and inferior temporal gyrus, anterior and posterior cingulate, inferior parietal lobule and parahippocampal gyrus. Apart from the anterior cingulate gyrus, no significant clusters were observed in the frontal cortex. Only one cluster in the left middle temporal cortex survived P < 0.05 FWE correction. Supplementary Table 1 includes the coordinates of local maxima and their anatomical labels, T-values, P-values and cluster sizes of all voxel-wise contrasts described above. In a secondary model including MMSE as an additional covariate the effects attenuated differences between patients and controls (particularly for typical Alzheimer’s disease and behavioural variant FTD patients, see Supplementary Fig. 3), but the observed patterns of atrophy were largely similar.
Figure 3.
Voxel-wise comparisons of grey matter volumes between healthy controls and the different diagnostic groups. Contrasts were adjusted for age, sex, total intracranial volume, scanner type and centre. Results are superimposed on the SPM8 single-subject template (left) and the study-specific DARTEL template (right), and displayed at P < 0.001 uncorrected. Supplementary Table 1 includes the coordinates of local maxima and their anatomical labels, T-values, P-values and cluster sizes.
Voxel-based morphometry: direct patient contrasts
Figure 4 shows voxel-wise contrasts between the patient groups, adjusted for age, sex, total intracranial volume, scanner type and centre. At P < 0.001 uncorrected, the combined behavioural/dysexecutive Alzheimer’s disease group showed greater involvement of various prefrontal and temporal regions whereas patients with typical Alzheimer’s disease were more affected in the occipital cortex. Apart from a small cluster in the left medial frontal gyrus in patients with behavioural/dysexecutive Alzheimer’s disease, both groups did not differ at P < 0.05 FWE corrected. Direct comparisons between behavioural/dysexecutive Alzheimer’s disease and behavioural variant FTD revealed a clearly distinct atrophy pattern with posterior involvement in behavioural/dysexecutive Alzheimer’s disease and anterior involvement in behavioural variant FTD, which largely survived P < 0.05 FWE correction. Additional adjustment for disease severity (using MMSE) essentially did not change the results (Supplementary Fig. 4). Supplementary Table 2 includes the coordinates of local maxima and their anatomical labels, T-values, P-values and cluster sizes of all voxel-wise contrasts described above.
Figure 4.
Voxel-wise comparisons of grey matter volumes between combined behavioural/dysexecutive variant Alzheimer’s disease, typical Alzheimer’s disease and behavioural variant FTD patients. Contrasts were adjusted for age, sex, total intracranial volume, scanner type and centre. Results are superimposed on the SPM8 single-subject template (left) and the study-specific DARTEL template (right), and displayed at P < 0.001 uncorrected. Supplementary Table 1 includes the coordinates of local maxima and their anatomical labels, T-values, P-values and cluster sizes.
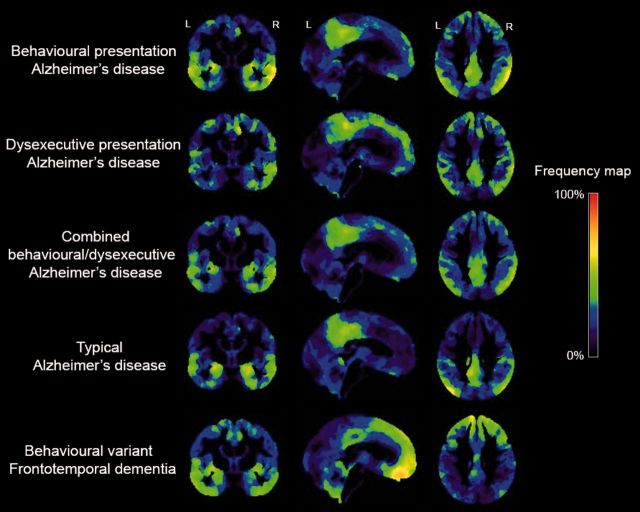
Figure 5.
Frequency of brain atrophy. Frequency maps showing the proportion of patients within each diagnostic group with suprathreshold values (W < −1) compared to a group of healthy controls. Hot colours indicate that patients with behavioural/dysexecutive variant Alzheimer’s disease (i) strikingly overlapped with patients with typical Alzheimer’s disease; and (ii) showed greater involvement of the frontal cortex than patients with typical Alzheimer’s disease although to a lesser extent than patients with behavioural variant FTD patients. The methodology for computing these frequency maps is explained in more detail in Supplementary Fig. 1.
Frequency maps of brain atrophy
To further assess the relative involvement of the frontal cortex we computed frequency maps showing for each voxel the proportion of patients with suprathreshold W-scores (W < −1). Visual inspection of the W-score maps indicate that patients in both behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease groups showed more frequent involvement in frontal cortical regions (i.e. anterior cingulate, orbitofrontal cortex, middle and superior frontal gyrus) compared to patients with typical Alzheimer’s disease, but markedly less than patients with behavioural variant FTD (Fig. 4). Also, the frequencies were highest in regions typically affected in Alzheimer’s disease such as posterior cingulate, precuneus and lateral temporoparietal regions. Patients with dysexecutive Alzheimer’s disease showed more frequent relative involvement of the frontal and parietal cortex than patients with behavioural Alzheimer’s disease, whereas patients with behavioural Alzheimer’s disease showed more (medial) temporal lobe involvement than patients with dysexecutive Alzheimer’s disease.
Neuropathology
Neuropathological data were available for 12 patients with behavioural Alzheimer’s disease, seven with dysexecutive Alzheimer’s disease and five with mixed behavioural/dysexecutive Alzheimer’s disease. The mean interval from time of diagnosis to autopsy was 60.9 ± 23.8 months. All patients with behavioural/dysexecutive Alzheimer’s disease met NIA-Reagan neuropathological criteria (Hyman and Trojanowski, 1997) for high-likelihood Alzheimer’s disease, with frequent neuritic plaque scores (Mirra et al., 1991) and Braak stage V (n = 4) or VI (n = 20) for neurofibrillary tangles (Braak and Braak, 1991; Braak et al., 2006). For eight patients, Thal amyloid-β plaque stage (Thal et al., 2002) was assessed. All had stage V and thus classified for ‘high’ Alzheimer’s disease neuropathological change according to recently proposed NIA-AA guidelines (Montine et al., 2012). Of all patients, two had mixed dementia with Alzheimer’s disease and progressive supranuclear palsy as co-primary neuropathological diagnosis. Both patients met formal criteria for possible behavioural variant FTD during life and additionally met our criteria for dysexecutive Alzheimer’s disease. One patient was clinically diagnosed with behavioural variant FTD and the other with mixed behavioural variant FTD and progressive supranuclear palsy. Several co-pathologies were observed in our neuropathological sample: cerebral amyloid angiopathy in 14/22 (64%, six mild, seven moderate and one severe), cerebrovascular disease in 6/24 (25%, see legend of Table 2 for specification), Lewy body disease in 10/24 (42%, four amygdala-predominant, four transitional limbic and two diffuse neocortical), argyrophilic grain disease in 4/9 (44%, all limbic), and TARDBP in 1/12 patients (8%, unclassifiable-limbic). Additionally, argyrophilic thorny astrocyte clusters (Munoz et al., 2007) were observed in three patients, and one patient had a large pituitary adenoma.
Discussion
In this retrospective study we assessed the clinical, neuropsychological, morphological and neuropathological features of a clinical syndrome that is known in the literature as the frontal variant of Alzheimer’s disease. We compared autopsy/biomarker-defined Alzheimer’s disease patients selected based on ‘behavioural’ or ‘dysexecutive’ predominant presentations against carefully matched and autopsy/biomarker-confirmed typical Alzheimer’s disease and behavioural variant FTD patients, along with a group of healthy controls. As a group, patients with Alzheimer’s disease selected based on behavioural-predominant presentations more often presented initially with cognitive than behavioural symptoms, both memory and executive functions were more impaired than in behavioural variant FTD, and prevalence of APOE ε4 was high. Patients with Alzheimer’s disease selected based on dysexecutive features presented as a primarily cognitive phenotype with minimal behavioural abnormalities and intermediate APOE ε4 prevalence. Both behavioural and dysexecutive Alzheimer’s disease patients were distinguished by temporoparietal-predominant atrophy. Based on the relative sparing of frontal grey matter in most patients, we propose future reference to these clinical syndromes as ‘the behavioural/dysexecutive variant of Alzheimer’s disease’ rather than frontal Alzheimer’s disease.
Clinical features and neuropsychological profiles
In line with Forman et al. (2006), patients with behavioural Alzheimer’s disease presented twice as often with cognitive as opposed to behavioural symptoms. About 80% of patients were around the threshold required to meet clinical criteria for possible behavioural variant FTD (meeting 2–4 of 6 criteria, with 3/6 criteria required for the diagnosis of ‘possible behavioural variant FTD’; Rascovsky et al., 2011), while 52% met formal criteria, illustrating the diagnostic dilemmas these patients can produce. Figure 1D and the Neuropsychiatric Inventory scores indicate that the severity of behavioural symptoms was greater in patients with behavioural variant FTD than behavioural Alzheimer’s disease. On the other hand, patients with behavioural Alzheimer’s disease had the most profound neuropsychological deficits, showing equivalent memory performance to patients with typical Alzheimer’s disease and worse executive functioning than behavioural variant FTD and typical Alzheimer’s disease patients. Altogether, these findings suggest that a combination of early primary cognitive deficits, objectively-confirmed memory deficits and an intermediate behavioural profile can help to differentiate behavioural Alzheimer’s disease from behavioural variant FTD clinically. In line with our hypothesis, dysexecutive Alzheimer’s disease presented as a primarily cognitive phenotype with minimal behavioural abnormalities (25% of patients with dysexecutive Alzheimer’s disease was also included in the behavioural Alzheimer’s disease group). Breakdown of the first cognitive symptoms revealed that patients with dysexecutive Alzheimer’s disease and/or their caregivers rarely complain about dysexecutive features (only 10%), suggesting that their symptoms are often misclassified as ‘memory-related’.
Potential risk factors
The proportion of patients with behavioural Alzheimer’s disease carrying an APOE ε4-allele (59.5%) was within the range typically observed in patients with Alzheimer’s disease (Poirier et al., 1993; Farrer et al., 1997). APOE ε4 is known to predispose for medial temporal lobe vulnerability and memory dysfunction (Wolk et al., 2010; Ossenkoppele et al., 2013b; Lehmann et al., 2014; Manning et al., 2014) and so could partially account for these features. The prevalence of APOE ε4-carriers in patients with dysexecutive Alzheimer’s disease (40%) was intermediate to that of typical Alzheimer’s disease and controls, which is in accordance with other studies in non-amnestic variants of Alzheimer’s disease (Pa et al., 2009; Wolk et al., 2010; van der Flier et al., 2011; Mez et al., 2013). Future research should assess for potential other genetic risk factors contributing to behavioural/dysexecutive variant Alzheimer’s disease. In agreement with previous reports (Duara et al., 1996; Devi et al., 2004), approximately half of the behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease groups had a positive family history (first or second degree) of dementia or a psychiatric disorder. Medical histories of behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease patients showed roughly equivalent presence of most conditions, e.g. hypertension (30–35%), diabetes mellitus (∼10%) and sleeping disorder (11–17%), compared to epidemiological studies (Wild et al., 2004; Nwankwo et al., 2013). The proportion of depression (18–24% received treatment) and traumatic brain injury (15–19% experienced at least one moderate or severe event) seemed overrepresented in behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease patients compared to the general population (Soldatos and Lugaresi, 1987; Andrade et al., 2003; Corrigan et al., 2010). However, an ascertainment bias due to the retrospective nature and clinical setting of this study should be taken into account.
Clinico-anatomical dissociation
In behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease patients, the frontal lobe, traditionally considered to be the regulatory core of behaviour and executive functioning (Damasio et al., 1994; Bruen et al., 2008), was relatively spared compared to the temporo-parietal regions in voxel-based morphometry group-level analyses (Fig. 3). Although slightly more affected than in typical Alzheimer’s disease, frontal atrophy was less prominent than we hypothesized a priori based on the behavioural and dysexecutive phenotypes. There are several explanations for this clinico-anatomical dissociation. First, we used somewhat liberal criteria to define our behavioural Alzheimer’s disease group. Due to lack of consensus clinical criteria, behavioural Alzheimer’s disease has often been operationalized as patients meeting behavioural variant FTD criteria in the face of primary Alzheimer’s disease pathology at autopsy (Forman et al., 2006; Mendez et al., 2013). To capture more broadly the clinical spectrum of these patients, we did not only include patients who met formal criteria for behavioural variant FTD, but also included patients who were clinically labelled as having ‘frontal Alzheimer’s disease’ or having a differential diagnosis consisting of both behavioural variant FTD and behavioural Alzheimer’s disease. When only looking at patients with behavioural Alzheimer’s disease who were initally diagnosed with behavioural variant FTD, frontal atrophy was more prominent than that in the total behavioural Alzheimer’s disease group, but temporoparietal atrophy remained predominant (Supplementary Fig. 2B). While previous case studies and our clinical experience demonstrate that profound frontal involvement can be observed in patients with behavioural Alzheimer’s disease, these single-subject effects may have been washed in group-level voxel-based morphometry analyses. The W-score frequency maps (Fig. 4) should be more sensitive to individual atrophy patterns, and indeed visual inspection of these maps indicated more frontal involvement compared to the voxel-based morphometry results (Fig. 3). Second, vascular damage in frontal white matter may result in fronto-parietal disconnection (Neufang et al., 2011) and has been associated with both neuropsychiatric symptoms (Kim et al., 2013) and executive dysfunction (Sjobeck et al., 2010) in Alzheimer’s disease. Similarly, lesions in basal ganglia that affect fronto-subcortical circuitries have been shown to exert behavioural or dysexecutive symptoms (Reed et al., 2004; Pa et al., 2009). Third, it is conceivable that structural MRI did not capture the full extent of neurodegeneration as a recent study did show increased frontal hypometabolism in patients with Alzheimer’s disease with the highest score on a behavioural questionnaire (Woodward et al., 2014). This suggests that 18F-fluorodeoxyglucose (FDG) PET might be able to detect neuronal injury in an earlier stage. Finally, patients with behavioural Alzheimer’s disease and those with dysexecutive Alzheimer’s disease might have reduced frontal reserve related to neurodevelopmental factors, life events (e.g. traumatic brain injury), or differences in premorbid personality traits or genes that regulate behaviour. This would make them more vulnerable to frontal dysfunction when general brain homeostasis is disturbed and could potentially trigger a behavioural/dysexecutive profile when pathogenic processes occur. Future studies applying functional MRI, 18F-FDG PET, quantification of white matter integrity, subcortical volumetrics or premorbid personality questionnaires are essential to further unravel the neurobiology underlying behavioural/dysexecutive variant Alzheimer’s disease.
Neuroimaging distinction between behavioural/dysexecutive variant Alzheimer’s disease and behavioural variant FTD
Clinicians often struggle to differentiate Alzheimer’s disease from behavioural variant FTD (Rabinovici et al., 2011), and diagnostic decision-making will likely be even more complex in patients with a behavioural/dysexecutive presentation of Alzheimer’s disease. A key finding of this study is that structural MRI clearly distinguished the two groups, as behavioural variant FTD patients showed a characteristic atrophy pattern in anterior brain regions, while the behavioural/dysexecutive variant Alzheimer’s disease group showed a classical Alzheimer’s disease pattern involving wide regions of the temporoparietal cortex. Direct statistical comparison between the groups survived P < 0.05 FWE correction, and even within a subset (n = 13) of patients with autopsy/biomarker-defined Alzheimer’s disease with an initial clinical diagnosis of behavioural variant FTD the temporoparietal cortex was the predominant locus of brain atrophy. This suggests that posterior versus anterior brain atrophy on structural MRI provides helpful information when clinicians are uncertain whether Alzheimer’s disease or frontotemporal lobar degeneration (FTLD) pathology is driving a behavioural/dysexecutive presentation.
Neuropathological findings
In the subset of patients with behavioural/dysexecutive Alzheimer’s disease who underwent autopsy (n = 24), dual Alzheimer’s disease and FTLD pathology as co-primary cause for dementia was restricted to two patients with mixed progressive supranuclear palsy and Alzheimer’s disease. Another patient harboured unclassifiable limbic/paralimbic TARDBP (also known as TDP) inclusions, which was considered a contributing, rather than the causative, pathology by the neuropathologist. FTLD-tau and FTLD-TDP pathology were thus relatively rare (<10% of all patients), and occurred at a lower rate compared to previous studies demonstrating non-Alzheimer’s disease tauopathy in 10–40% and TDP inclusions in 19–57% of more typical Alzheimer’s disease patients (Varma et al., 1999; Forman et al., 2006; Alladi et al., 2007; Amador-Ortiz et al., 2007; Beach et al., 2012; Josephs et al., 2014). Several other co-pathologies were frequently present, but in general these were less extensive compared to the classical plaque and tangle pathology and were thought to have contributed little to the patient’s clinical deficits. The proportion of Lewy body disease (42%), cerebrovascular disease (25%) and argyrophilic grain disease (44%) in our patients with behavioural/dysexecutive Alzheimer’s disease falls within the range of previous reports on sporadic Alzheimer’s disease (Hansen et al., 1990; Mirra et al., 1991; Braak and Braak, 1998; Hamilton, 2000; Togo et al., 2002), while cerebral amyloid angiopathy (64%) may be somewhat less frequent compared to ∼90% found in earlier neuropathological studies in Alzheimer’s disease (Kalaria and Ballard, 1999; Attems, 2005). Due to the young average age of patients with behavioural/dysexecutive Alzheimer’s disease, comparisons against post-mortem studies in typical Alzheimer’s disease should be interpreted with caution as it is known that the onset of a diversity of pathologies accelerates with ageing (Schneider et al., 2007). We further found argyrophilic thorny astrocyte clusters in three patients (Munoz et al., 2007). These 4-repeat tau-positive inclusions may be of interest for future studies as they preferentially localize in frontotemporal and parietal grey–white matter junctions, and may be a common neuropathological feature in atypical manifestations of Alzheimer’s disease (Munoz et al., 2007). Future studies will also need to further assess the distribution of amyloid plaques and neurofibrillary tangles in patients with behavioural/dysexecutive Alzheimer’s disease (Johnson et al., 1999; Blennerhassett et al., 2014), which was not assessed in our study.
Behavioural/dysexecutive variant Alzheimer’s disease: a continuum or distinct clinical entities?
Driven by previous studies and clinical experience, we developed two distinct sets of inclusion criteria for behavioural-predominant and dysexecutive-predominant Alzheimer’s disease patients that cover the spectrum of ‘frontal Alzheimer’s disease’. Although several potential mechanisms were identified that may differ between behavioural and dysexecutive-predominant presentations of Alzheimer’s disease (i.e. APOE ε4 status and cognitive/behavioural profiles), the presence of a single (behaviour) rather than a double dissociation (behaviour and executive dysfunction), suggests at least a certain degree of overlap between the two phenotypes. In this study, we reported results for both combined and distinct groups. Whether these patients represent a single phenotype on a continuum including behavioural features and executive dysfunction, or two separate clinical entities is subject to future studies. We can only conclude that: (i) the majority of patients selected based on behavioural-predominant presentations also had memory deficits proportionate to or greater than executive impairment; (ii) there were also patients selected based on dysexecutive-predominant presentations showing no significant behavioural changes; and (iii) applying our inclusion criteria resulted in modest overlap between the groups (9/75, 12%).
Strengths and limitations
The strengths of this study are the relatively large sample of patients with behavioural/dysexecutive Alzheimer’s disease, the availability of both antemortem clinical, neuropsychological and neuroimaging data and post-mortem data, and the direct comparison against well-characterized patients with typical Alzheimer’s disease and behavioural variant FTD. There are also some limitations. First, the low prevalence of behavioural Alzheimer’s disease and dysexecutive Alzheimer’s disease compelled us to include patients seen over the course of 15 years. This fact, in addition to data acquisition at two different sites, resulted in some heterogeneity in diagnostic work-up, as dementia screening and neuropathological protocols evolved over time, higher field-strength MRI scanners were implemented, and there are differences in the timing of clinical assessment and amyloid status determination (i.e. PET/CSF versus autopsy). Second, this is a retrospective study, and clinical data were obtained following standardized chart reviews. In cases of incomplete or ambiguous descriptions, some degree of subjective judgement on the part of the investigators is unavoidable. Also, an ascertainment bias in both directions (i.e. information may be absent in the charts or, in contrast, may be mentioned more frequently as prompted by clinicians) should be taken into account. Third, patients with memory-predominant Alzheimer’s disease were relatively young (64.4 ± 8.6 years) as they were age-matched to patients with behavioural Alzheimer’s disease, and may differ in neuropsychological profile and atrophy pattern from late-onset amnestic Alzheimer’s disease patients. Fourth, as opposed to autopsy-confirmed patients only, we applied in vivo CSF and PET biomarkers as supporting evidence for underlying Alzheimer’s disease pathology. Although this does not necessarily exclude the presence of frontotemporal lobar degeneration pathology, our autopsy data show that the likelihood of mixed primary pathologies was low (<10%). Finally, in contrast to previous autopsy studies (Johnson et al., 1999; Blennerhassett et al., 2014) we were not able to investigate the distribution of amyloid plaque and neurofibrillary tangle pathology across the brain.
Clinical relevance
Clinical, imaging and neuropathological features of patients with behavioural/dysexecutive Alzheimer’s disease are summarized in Table 3 and may serve as a roadmap to identify these patients in a clinical setting. This is important for studying the effects of disease-modifying agents and for appropriate symptomatic treatment (e.g. patients with behavioural/dysexecutive variant Alzheimer’s disease may show clinical benefit from acetylcholinesterase inhibitors). The key diagnostic features to distinguish behavioural Alzheimer’s disease from behavioural variant FTD are the magnitude of memory impairment and brain atrophy affecting predominantly classical Alzheimer’s disease regions in the temporoparietal cortex such as the medial temporal lobes, posterior cingulate and precuneus. Additional clues are provided by a cognitive onset of the disease, presence of an APOE ε4 allele and a behavioural profile that is generally less severe than that observed in patients with behavioural variant FTD. Conversely, patients with a dysexecutive presentation without prominent behavioural changes are more likely to have underlying Alzheimer’s disease than FTLD pathology. In conclusion, the present study underscores that clinicians should look beyond the prominent and often intrusive behavioural changes, and take full advantage of all information being provided by current diagnostic tools. We propose to start the diagnostic process with extensive history-taking and neuropsychological testing, followed by a brain MRI scan. Voxel-based morphometry yielded a classical Alzheimer’s disease pattern in patients with behavioural/dysexecutive Alzheimer’s disease, thus the NIA-AA criteria (McKhann et al., 2011; incorporating neurodegenerative biomarkers, could be more specific than criteria proposed by (Dubois et al., 2014). Finally, if available, amyloid PET or CSF biomarkers could be used to exclude Alzheimer’s disease as the causative aetiology in patients with a behavioural or a dysexecutive clinical presentation.
Table 3.
Key clinical and neuroimaging features of behavioural/dysexecutive variant Alzheimer’s disease
| Clinical features | Typical Alzheimer’s disease | Behavioural/dysexecutive variant Alzheimer’s disease | Behavioural variant FTD | |
|---|---|---|---|---|
| Phenotype | Memory-predominant | Behavioural-predominant | Dysexecutive-predominant | Behavioural |
| Genetic predisposition | ||||
| APOE ε4 positive | Frequent (∼60%)a | Frequent (59.5%) | Intermediate (40.0%) | Rare (<20%)b |
| Clinical | ||||
| Age-at-onset | Late-onset | Early-onset | Early-onset | Early-onset |
| First symptom | Cognitive (memory) | Cognitive > behavioural | Cognitive (executive) | Behavioural |
| Cognition | ||||
| Episodic memory | Impaired | Impaired | Relatively spared | Typically spared |
| Executive function | Relatively spared | Impaired | Impaired | Impaired |
| Behaviour | Relatively spared | Impaired | Mostly spared | Impaired |
| MRI | ||||
| Atrophy pattern | Medial temporal and temporoparietal lobes | Temporoparietal cortex | Temporoparietal cortex | Frontal and anterior temporal lobes |
APOE prevalence estimates derived from aMichaelson (2014) bVerpillat et al. (2002).
Supplementary Material
Acknowledgements
The authors would like to thank Daniel Schonhaut for editing and scientific discussion, Sander Verfaillie for data support, Gaël Chételat and Brigitte Landeau for the inspiration to create W-score frequency maps, Robin Ketelle, Matthew Growdon, Jung Jang, Baber Khan, Teresa Wu for administrative support, and the patients and families for their dedication to research.
Glossary
Abbreviations
- CDR
Clinical Dementia Rating
- FTD
frontotemporal dementia
- MMSE
Mini-Mental State Examination
- UCSF
University of California San Francisco
- VUMC
VU University Medical Center
Funding
This research was funded by Marie Curie FP7 International Outgoing Fellowship [628812] (to R.O.); The donors of [Alzheimer’s Disease Research], a program of BrightFocus Foundation (to R.O.); National Institute on Aging grants [R01-AG045611] (to G.D.R.), [P01-AG1792403] (to B.L.M. and W.W.S.), [P50-AG023501] (to G.D.R., W.W.S. and B.L.M.), [R01-AG027859] (to W.J.J.), Tau Consortium (to G.D.R., W.W.S. and W.J.J); Consortium for Frontotemporal Dementia Research (to W.W.S. and B.L.M.); John Douglas French Alzheimer’s Foundation (to G.D.R. and B.L.M.); State of California Department of Health Services Alzheimer’s Disease Research Centre of California grant [04-33516] (to B.L.M). Research of the VUMC Alzheimer center is part of the neurodegeneration research program of the Neuroscience Campus Amsterdam. The VUMC Alzheimer center is supported by Alzheimer Nederland and Stichting VUMC funds. The clinical database structure was developed with funding from Stichting Dioraphte. Aegon NV sponsored a three-month visiting professorship of Dr. Rabinovici to VUMC Alzheimer center, which was partly dedicated to work on the present project.
Supplementary material
Supplementary material is available at Brain online.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer Dement 2011; 7: 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, et al. Focal cortical presentations of Alzheimer's disease. Brain 2007; 130: 2636–45. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol 2007; 61: 435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res 2003; 12: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J. Sporadic cerebral amyloid angiopathy: pathology, clinical implications, and possible pathomechanisms. Acta Neuropathol 2005; 110: 345–59. [DOI] [PubMed] [Google Scholar]
- Back-Madruga C, Boone KB, Briere J, Cummings J, McPherson S, Fairbanks L, et al. Functional ability in executive variant Alzheimer's disease and typical Alzheimer's disease. Clin Neuropsych 2002; 16: 331–40. [DOI] [PubMed] [Google Scholar]
- Balasa M, Gelpi E, Antonell A, Rey MJ, Sanchez-Valle R, Molinuevo JL, et al. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology 2011; 76: 1720–5. [DOI] [PubMed] [Google Scholar]
- Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropath Exp Neurol 2012; 71: 266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol 1988; 45: 789–93. [DOI] [PubMed] [Google Scholar]
- Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, Trabucchi M. Executive dysfunction in early Alzheimer's disease. J Neurol Neurosurg Psychiatry 1996; 60: 91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ. Distribution of pathology in frontal variant Alzheimer's disease. J Alzheimers Dis 2014; 39: 63–70. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006; 112: 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm 1998; 105: 801–19. [DOI] [PubMed] [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain 2008; 131: 2455–63. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007; 114: 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil 2010; 25: 72–80. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol 2012; 11: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 1994; 264: 1102–5. [DOI] [PubMed] [Google Scholar]
- Devi G, Williamson J, Massoud F, Anderson K, Stern Y, Devanand DP, et al. A comparison of family history of psychiatric disorders among patients with early- and late-onset Alzheimer's disease. J Neuropsychiatry Clin Neurosci 2004; 16: 57–62. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA.Alzheimer's Disease Neuroimaging I. Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry 2011; 82: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duara R, Barker WW, Lopez-Alberola R, Loewenstein DA, Grau LB, Gilchrist D, et al. Alzheimer's disease: interaction of apolipoprotein E genotype, family history of dementia, gender, education, ethnicity, and age of onset. Neurology 1996; 46: 1575–9. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–29. [DOI] [PubMed] [Google Scholar]
- Duits FH, Teunissen CE, Bouwman FH, Visser PJ, Mattsson N, Zetterberg H, et al. The cerebrospinal fluid “Alzheimer profile”: Easily said, but what does it mean? Alzheimer Dement 2014; 10: 713–23. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278: 1349–56. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006; 59: 952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habek M, Hajnsek S, Zarkovic K, Chudy D, Mubrin Z. Frontal variant of Alzheimer's disease: clinico-CSF-pathological correlation. Can J Neurol Sci 2010; 37: 118–20. [PubMed] [Google Scholar]
- Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 2000; 10: 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology 1990; 40: 1–8. [DOI] [PubMed] [Google Scholar]
- Herrero-San Martin A, Villarejo-Galende A, Rabano-Gutierrez A, Guerrero-Marquez C, Porta-Etessam J, Bermejo-Pareja F. Frontal variant of Alzheimer's disease. Two pathologically confirmed cases and a literature review [in Spanish]. Rev Neurol 2013; 57: 542–8. [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropath Exp Neurol 1997; 56: 1095–7. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 2008; 131: 1630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 1997; 49: 786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol 1999; 56: 1233–9. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol 2014; 127: 811–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord 1999; 13 (Suppl 3): S115–23. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000; 12: 233–9. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kang SJ, Kim C, Kim GH, Jeon S, Lee JM, et al. The effects of small vessel disease and amyloid burden on neuropsychiatric symptoms: a study among patients with subcortical vascular cognitive impairments. Neurobiol Aging 2013; 34: 1913–20. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–19. [DOI] [PubMed] [Google Scholar]
- La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, et al. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer's disease dementia. J Neurosci 2012; 32: 16265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 2013; 74: 826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner AJ. Frontal variant Alzheimer's disease: a reappraisal. Clin Neurol Neurosurg 2006; 108: 705–8. [DOI] [PubMed] [Google Scholar]
- Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol 2011; 70: 327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Karydas A, Coppola G, O'Neil JP, et al. Greater medial temporal hypometabolism and lower cortical amyloid burden in ApoE4-positive AD patients. J Neurol Neurosurg Psychiatry 2014; 85: 266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Jr, Corbetta-Rastelli C, Weiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain 2013a; 136: 844–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Madison CM, Ghosh PM, Seeley WW, Mormino E, Greicius MD, et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer's disease. Proc Nat Acad Sci 2013b; 110: 11606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010; 119: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EN, Barnes J, Cash DM, Bartlett JW, Leung KK, Ourselin S, et al. APOE epsilon4 is associated with disproportionate progressive hippocampal atrophy in AD. PloS One 2014; 9: e97608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic presentations of early-onset Alzheimer's disease. Am J Alzheimers Dis Other Dement 2012; 27: 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MF, Joshi A, Tassniyom K, Teng E, Shapira JS. Clinicopathologic differences among patients with behavioural variant frontotemporal dementia. Neurology 2013; 80: 561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008; 63: 709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez J, Cosentino S, Brickman AM, Huey ED, Manly JJ, Mayeux R. Dysexecutive versus amnestic Alzheimer disease subgroups: analysis of demographic, genetic, and vascular factors. Alzheimer Dis Assoc Disord 2013; 27: 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson DM. ApoE4: The most prevalent yet understudied risk factor for Alzheimer's disease. Alzheimer's Dement 2014; 10: 861–8. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991; 41: 479–86. [DOI] [PubMed] [Google Scholar]
- Moller C, Vrenken H, Jiskoot L, Versteeg A, Barkhof F, Scheltens P, et al. Different patterns of gray matter atrophy in early- and late-onset Alzheimer's disease. Neurobiol Aging 2013; 34: 2014–22. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012; 123: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–4. [DOI] [PubMed] [Google Scholar]
- Munoz DG, Woulfe J, Kertesz A. Argyrophilic thorny astrocyte clusters in association with Alzheimer's disease pathology in possible primary progressive aphasia. Acta Neuropathol 2007; 114: 347–57. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–54. [DOI] [PubMed] [Google Scholar]
- Neufang S, Akhrif A, Riedl V, Forstl H, Kurz A, Zimmer C, et al. Disconnection of frontal and parietal areas contributes to impaired attention in very early Alzheimer's disease. J Alzheimer Dis 2011; 25: 309–21. [DOI] [PubMed] [Google Scholar]
- Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief 2013; 133: 1–8. [PubMed] [Google Scholar]
- Ossenkoppele R, Prins ND, Pijnenburg YA, Lemstra AW, van der Flier WM, Adriaanse SF, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement 2013a; 9: 414–21. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Tolboom N, Foster-Dingley JC, Adriaanse SF, Boellaard R, Yaqub M, et al. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. Eur J Nuc Med Mol Imaging 2012a; 39: 990–1000. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, van der Flier WM, Zwan MD, Adriaanse SF, Boellaard R, Windhorst AD, et al. Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 2013b; 80: 359–65. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Zwan MD, Tolboom N, van Assema DM, Adriaanse SF, Kloet RW, et al. Amyloid burden and metabolic function in early-onset Alzheimer's disease: parietal lobe involvement. Brain 2012b; 135: 2115–25. [DOI] [PubMed] [Google Scholar]
- Pa J, Boxer A, Chao LL, Gazzaley A, Freeman K, Kramer J, et al. Clinical-neuroimaging characteristics of dysexecutive mild cognitive impairment. Ann Neurol 2009; 65: 414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 1993; 342: 697–9. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, Alkalay A, Racine CA, O'Neil JP, Janabi M, et al. Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden. Brain 2010; 133: 512–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011; 77: 2034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Eberling JL, Mungas D, Weiner M, Kramer JH, Jagust WJ. Effects of white matter lesions and lacunes on cortical function. Arc Neurol 2004; 61: 1545–50. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197–204. [DOI] [PubMed] [Google Scholar]
- Schoonenboom NS, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 2012; 78: 47–54. [DOI] [PubMed] [Google Scholar]
- Sjobeck M, Elfgren C, Larsson EM, Brockstedt S, Latt J, Englund E, et al. Alzheimer's disease (AD) and executive dysfunction. A case-control study on the significance of frontal white matter changes detected by diffusion tensor imaging (DTI). Arch Gerontol Geriatr 2010; 50: 260–6. [DOI] [PubMed] [Google Scholar]
- Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, et al. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology 2008; 248: 590–8. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Stopford CL, Julien CL, Thompson JC, Davidson Y, Gibbons L, et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex 2007; 43: 835–45. [DOI] [PubMed] [Google Scholar]
- Soldatos CR, Lugaresi E. Nosology and prevalence of sleep disorders. Sem Neurol 1987; 7: 236–42. [DOI] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 2003; 60: 652–6. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Probst A, Miserez AR, Monsch AU, Tolnay M. Clinical course of neuropathologically confirmed frontal-variant Alzheimer's disease. Nat Clin Pract Neurol 2008; 4: 226–32. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002; 58: 1791–800. [DOI] [PubMed] [Google Scholar]
- Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, et al. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropath Exp Neurol 2002; 61: 547–56. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer's disease: the case of the missing APOE varepsilon4 allele. Lancet Neurol 2011; 10: 280–8. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Pijnenburg YA, Prins N, Lemstra AW, Bouwman FH, Teunissen CE, et al. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimer Dis 2014; 41: 313–27. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010; 68: 319–29. [DOI] [PubMed] [Google Scholar]
- Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DM, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry 1999; 66: 184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpillat P, Camuzat A, Hannequin D, Thomas-Anterion C, Puel M, Belliard S, et al. Apolipoprotein E gene in frontotemporal dementia: an association study and meta-analysis. Eur J Hum Genet 2002; 10: 399–405. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–53. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC.Alzheimer's Disease Neuroimaging I. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Nat Acad Sci 2010; 107: 10256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M, Jacova C, Black SE, Kertesz A, Mackenzie IR, Feldman H, et al. Differentiating the frontal variant of Alzheimer's disease. Int J Ger Psychiatry 2010; 25: 732–8. [DOI] [PubMed] [Google Scholar]
- Woodward MC, Rowe CC, Jones G, Villemagne VL, Varos TA. Differentiating the frontal presentation of Alzheimer's disease with FDG-PET. J Alzheimer Dis 2014; 44: 233–42. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17: 37–49. [DOI] [PubMed] [Google Scholar]
- Zwan M, van Harten A, Ossenkoppele R, Bouwman F, Teunissen C, Adriaanse S, et al. Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort. J Alzheimer Dis 2014; 41: 801–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.