Supplemental Digital Content is Available in the Text.
Key Words: HIV care engagement, retention, antiretroviral use, viral load suppression
Background:
Ensuring that people living with HIV are accessing and staying in care is vital to achieving optimal health outcomes including antiretroviral therapy (ART) success. We sought to characterize engagement in HIV care among participants of a large clinical cohort in Ontario, Canada, from 2001 to 2011.
Methods:
The Ontario HIV Treatment Network Cohort Study (OCS) is a multisite HIV clinical cohort, which conducts record linkage with the provincial public health laboratory for viral load tests. We estimated the annual proportion meeting criteria for being in care (≥1 viral load per year), in continuous care (≥2 viral load per year ≥90 days apart), on ART, and with suppressed viral load <200 copies per milliliter. Ratios of proportions according to socio-demographic and clinical characteristics were examined using multivariable generalized estimating equations with a log-link.
Results:
A total of 5380 participants were followed over 44,680 person-years. From 2001 to 2011, we observed high and constant proportions of patients in HIV care (86.3%–88.8%) and in continuous care (76.4%–79.5%). There were statistically significant rises over time in the proportions on ART and with suppressed viral load; by 2011, a majority of patients were on ART (77.3%) and had viral suppression (76.2%). There was minimal variation in HIV engagement indicators by socio-demographic and HIV risk characteristics.
Conclusions:
In a setting with universal health care, we observed high proportions of HIV care engagement over time and an increased proportion of patients attaining successful virologic suppression, likely due to improvements in ART regimens and changing guidelines.
INTRODUCTION
Ensuring that people living with HIV are accessing and staying in care is vital to achieving optimal health outcomes including antiretroviral therapy (ART) adherence and prevention of secondary transmission.1–4 A number of jurisdictions are quantifying patterns of care engagement among persons diagnosed with HIV with an eye to identifying potential barriers against the full realization of these individual and population benefits.5–9 The continuum of care engagement or “HIV care cascade” is a framework that depicts the degree to which people infected with HIV are diagnosed in a timely fashion, become engaged in HIV care, and are successfully treated with ART such that they achieve virologic suppression.5 Findings in 1 setting may not be generalizable to another, given differences in health care systems and populations affected by local HIV epidemics. International comparisons indicate variation in the percent loss from 1 cascade step to the next that may vary considerably,10 suggesting that locally informed strategies may be required to improve retention and ultimately the proportion with suppressed viral load.
Estimation of HIV care engagement indicators commonly uses administrative health data from clinics, laboratory systems, or health insurance programs.5,8,9,11–13 Although such data sources typically benefit from being population-based, they may lack patient-level characteristics (eg, HIV risk factors, race/ethnicity, immigration status) or, for those measures that are collected, may have extensive missing data. Data from consented participants in ongoing cohort studies typically have far better ascertainment of such characteristics but may be prone to selection biases because of differential enrollment and attrition. Ideally, one would triangulate using both types of data sources for robust inferences.
Our overall objective was to characterize HIV care engagement among participants of a large clinical cohort in Ontario, Canada, that conducts record linkage with population-based administrative health databases. All cohort participants have been diagnosed with HIV and were successfully linked to HIV care; nevertheless, this population can inform patterns of subsequent HIV care engagement after linkage to care. Specifically, we sought to estimate the annual proportions in care, in continuous care, on ART, and with suppressed viral load from 2001 to 2011 and to examine whether patterns in these outcomes varied temporally or according to socio-demographic characteristics.
METHODS
Data Sources
The province of Ontario, Canada, has the largest population of people living with HIV nationally, with an estimated total of 32,542 diagnosed as of 2011 (PHAC 2012). Its health care system provides publicly funded access to physician services. The Ontario HIV Treatment Network Cohort Study (OCS) is a multisite clinical cohort of people attending HIV care. Its design has been described previously.14 Briefly, its source population consists of people aged 16 and older diagnosed with HIV infection who entered medical care at specialty HIV clinics. The 10 participating clinics include hospital-based outpatient clinics and community-based practices, which collectively serve over 3 quarters of the approximately 13,000 patients undergoing viral load testing annually in the province.15 Overall, 43% of clinic patients were active participants (range, 20%–79% across clinics). Clinical data recorded during routine health care are abstracted from clinic records. From 1995 to 2007, participants self-completed a questionnaire at enrollment. Since 2008, participants have been interviewed annually. The OCS conducts record linkage with the HIV viral load database at the Public Health Ontario Laboratories (PHOL), which is the sole provider of such testing provincially. The study protocol, consent forms, and research instruments received ethical approval from the University of Toronto Human Subjects Review Committee and from the individual study sites.
Study Population and HIV Indicators
We used cohort data available as of December 31, 2012 to define our study population (Fig. 1). Denominators for annual estimates were dynamic and reflected entries and exits from observation. Entries occurred on January 1, 2001, or entry to HIV care, whichever was later. Participants with a record of death (as reported by clinics) were excluded in the year of death and all subsequent years. Otherwise, data were truncated on December 31, 2011, to maximize availability of clinical data as manual chart reviews were incomplete for 2012. We distinguished loss-to-follow-up from a participating OCS clinic versus entirely from provincial HIV care. Participants were considered “lost-to-clinic-follow-up,” if no CD4 cell count, viral load, or other medical chart-based data were available for from the OCS clinic 18 months or longer as of December 31, 2011. However, we continued to passively follow up such individuals in provincial HIV care through record linkage with viral loads ordered by other health care providers in non-OCS clinics available in the HIV viral load database at the PHOL.
FIGURE 1.
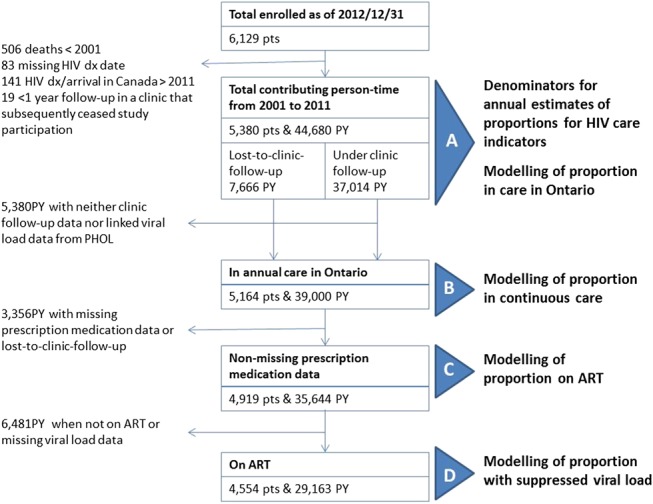
Inclusion criteria for analysis of HIV care engagement indicators, the OHTN cohort study, 2001–2011. Dx, diagnosis; PTs, participants; ART, antiretroviral treatment; PHOL, Public Health Ontario Laboratories.
Annual indicators of HIV care engagement were being in care, in continuous care, on ART, and having suppressed viral load. Clinical encounter dates were unavailable. Therefore, we used viral load or CD4 cell count testing as a proxy measure of clinical encounters, as has been done elsewhere.9,13,16 Both laboratory measures were available from clinical chart abstractions for persons remaining under follow-up at participating OCS clinics. When participants were lost-to-clinic-follow-up, linkage with the PHOL viral load database allowed us to observe viral load testing ordered by any health care provider in Ontario; however, for such years, CD4 cell counts, ART status, and regimens were unknown.
For each year, we defined being “in care” as having at least 1 viral load or CD4 cell count17 and “continuous care” as having at least 2 viral loads or CD4 cell counts at least 90 days apart.18 Being “on ART” was classified based on prescription medication recorded in medical charts; it was defined as a record of ART initiation in that year or any preceding year, with no record of having stopped ART in this year. “Suppressed viral load” was defined as a value <200 copies per milliliter at the last measurement in the year.18,19
Participants who were not in care for a given year (as defined above using laboratory tests) were classified as not being in continuous care, on ART, or having suppressed viral load for that year. For years when participants were lost-to-clinic-follow-up but were still in care according to linked viral loads from the PHOL, we carried forward the last known ART status but explored the influence of this versus alternative assumptions in sensitivity analysis.
Data Analysis
We conducted all statistical analyses using SAS version 9.3 (SAS Institute, Inc., Cary, NC). Participant characteristics at HIV diagnosis and how these varied over time were explored using descriptive statistics. We then estimated the annual proportion who met the criteria for each HIV care indicator among the denominator of all enrolled participants and who had no record of death as of that year; results are reported with 95% confidence intervals (CIs). We conducted further descriptive analyses to characterize combinations of CD4 cell count and viral load.
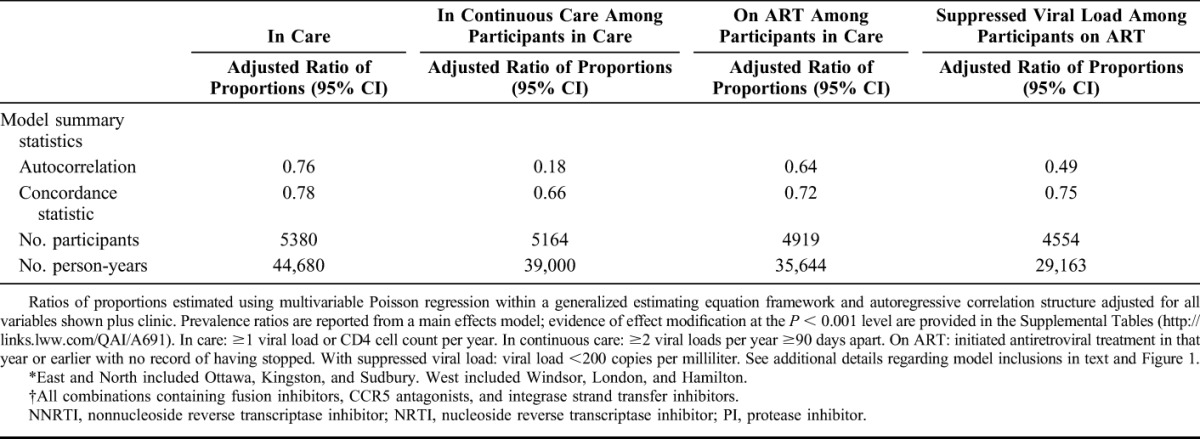
Next, we identified differences in annual HIV care indicators. We determined a priori that statistically significant proportional differences (at the P < 0.05 level) and with a magnitude of 5% or greater would be deemed to have public health and clinical significance. We examined associations for the time-invariant measures of sex, HIV risk factors, and race/ethnicity, as well as time-varying measures (updated annually): calendar year, age, time since HIV diagnosis, and region of Ontario where receiving HIV care. We estimated the ratio of prevalence proportions rather than odds ratios because the latter overestimates the ratio of proportions for common outcomes. We used Poisson regression with generalized estimating equations and an autoregressive correlation structure to account for repeated measures as recommended for ratios of proportions.20
Model inclusions were conditional (Fig. 1). We modeled the outcome “in care” for each person-year among all enrolled participants and who had no record of death as of that year, including person-time for participants lost-to-clinic-follow-up, as HIV care from other providers in Ontario remained observable through record linkage with the viral load database at PHOL. Models for “continuous care” for each year were restricted to person-years for participants in care (ie, at least 1 viral load or CD4 cell count abstracted from clinic charts or linkage with at least 1 viral load from the PHOL). We restricted models for being “on ART” for each year to person-years for participants in care and also for years with nonmissing prescription medication data (requiring exclusion of person-years lost-to-clinic-follow-up). Inclusion of person-years in models for suppressed viral load was conditional on being in care, having nonmissing prescription medication data, being on ART in that year, and nonmissing viral load result(s) (excludes person-years lost-to-clinic-follow-up).
We adjusted multivariable models for the clinic site at which the participant enrolled to account for unmeasured differences in patient populations. When data were missing for a covariate, we either classified the observation using an “unknown” category or we reclassified the observation within another category where appropriate. All covariates were retained in multivariable models unless removal was necessary to address collinearity. We checked for effect modification by time or by sex using interactions terms with all factors (except clinic site) and reported those statistically significant at the P < 0.001 level.
RESULTS
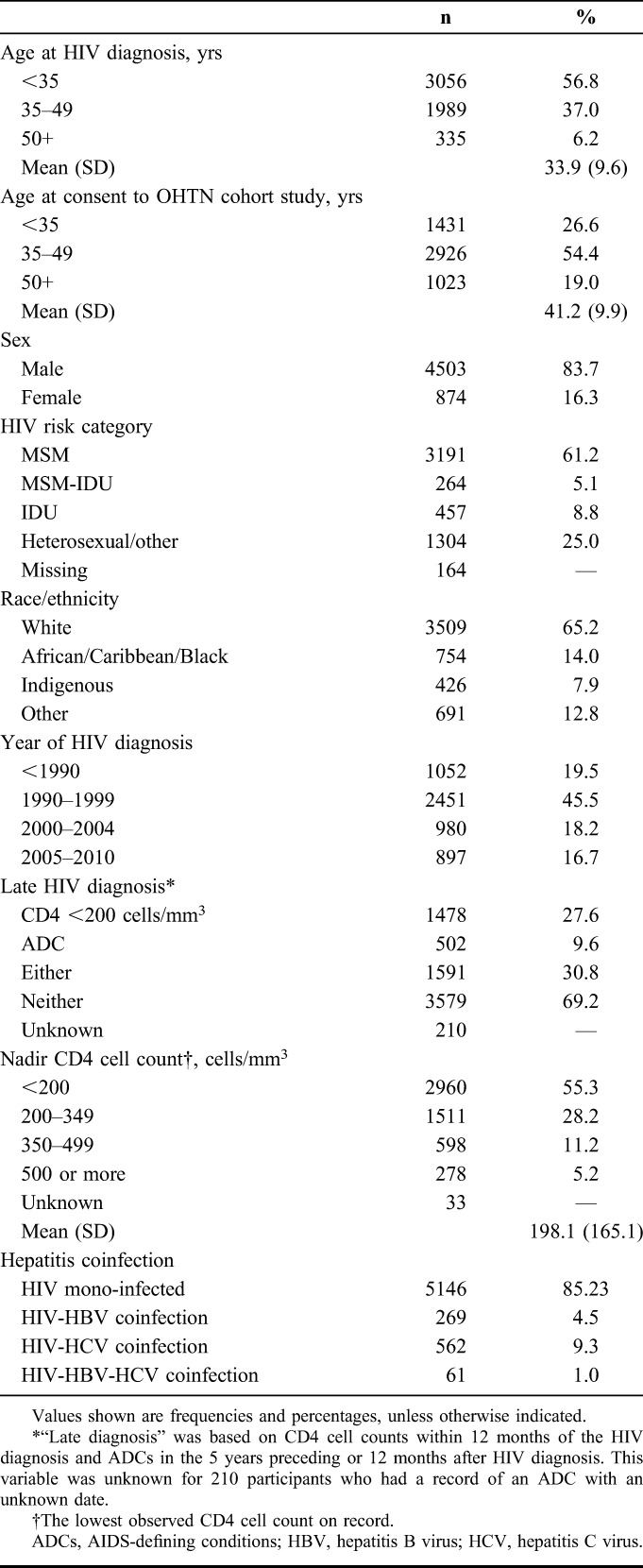
A total of 5380 participants were included in the analysis (Fig. 1). A majority were male, reported sex with men as an HIV risk factor, lived in Toronto, and were white (Table 1). At diagnosis, most were younger than 35 years. A total of 30.8% had evidence of late diagnosis according to CD4 cell count <200 cells per cubic millimeter or presence of an AIDS-defining condition. From 2001 to 2011, participants were under observation for a median of 10 person-years (interquartile range: 6–11), summing to 44,680 person-years in total. Deaths were reported for 7.3% (392/5380) of participants during follow-up. Participant characteristics varied over the study period as the cohort aged, some were lost to care, and new participants enrolled (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A691). By 2011, the average age was 47 years, most were diagnosed >10 years ago, and greater proportions were female, reported heterosexual HIV risk factor, were of nonwhite race, and were born outside Canada.
TABLE 1.
Characteristics of 5380 Participants in the OHTN Cohort Study Included in at Least 1 Year of Analysis for HIV Care Engagement Indicators, 2001–2011
Patterns of HIV Care Engagement
One quarter (24.9%, 1337/5380) had at least 1 year for which they were not in care, but, for the majority of person-years, participants were in care (87.3%, 39,000/46,680). For persons lost-to-clinic-follow-up as of 2011, the final year of analysis, 42.0% (of 958) had a viral load submitted to the provincial laboratory indicating that they were still in HIV care elsewhere; it is unknown what remaining proportion moved out-of-province or were deceased.
Time trends for annual indicators were examined among all enrolled participants including persons lost-to-clinic-follow-up in the denominator but censoring those who died in the year of death (Fig. 2). The annual proportions in care and in continuous care were generally stable and ranged from 86.3% to 88.8% and 76.4%–79.5%, respectively.
FIGURE 2.
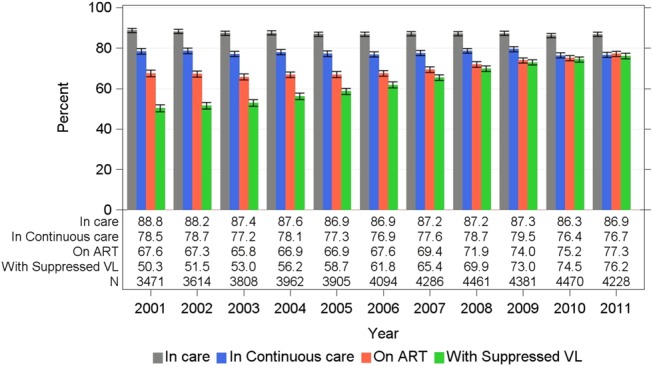
Proportion meeting HIV care engagement indicators among enrolled participants of the OHTN cohort study, 2001–2011. Proportions shown with 95% CIs. For each year and all indicators shown, the denominator included all participants ever enrolled and who had no record of death as of that year. In care: ≥1 viral load or CD4 cell count per year. In continuous care: ≥2 viral loads per year ≥90 days apart. On ART: initiated antiretroviral treatment in that year or earlier with no record of having stopped. With suppressed viral load: viral load <200 copies per milliliter. Figure 1 and text provide details.
Conversely, the proportion on ART rose from 67.6% (95% CI: 66.0% to 69.2%) in 2001 to 77.3% (95% CI: 76.0% to 78.5%) in 2011. In a sensitivity analysis, we excluded treatment-naive participants with CD4 cell counts ≥350 cells per cubic millimeter; as expected, the proportions on ART were higher and rose from 74.0% (95% CI: 72.4 to 75.5) in 2001 to 82.3% (95% CI: 81.1 to 83.5) in 2011. Moreover, the estimated proportion on ART varied according to assumptions about status for persons not in care for a given year or who were lost-to-clinic-follow-up (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A691).
Concomitantly, the proportion of enrolled participants with suppressed viral load increased from 50.3% (95% CI: 48.7% to 52.0%) in 2001 to 76.2% (95% CI: 74.8% to 77.4%) in 2011. Reclassification of viral load “blips” (detectable viral load <500 copies per milliliter for the last measure in the year when a suppressed value was observed for the measures immediately preceding and following it) produced little (<0.5%) difference in the estimated proportion suppressed.
Considering CD4 cell count and viral load for those under active clinic follow-up, the proportion with optimal status (suppressed viral load <200 copies per milliliter and CD4 cell count ≥350 cells per cubic millimeter) increased from 39.0% (95% CI: 37.0% to 41.0%) in 2001 to 68.8% (95% CI: 67.2% to 70.4%) in 2011 (P < 0.0001), with lesser proportions with suboptimal combinations of viral load and CD4 over the period (see Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/A691).
Factors Associated With HIV Care Indicators
There were minor differences in the proportion meeting the various HIV care indicator criteria in unadjusted (Table 2) and adjusted (Table 3) multivariable models, according to socio-demographic and clinical characteristics. Care engagement was higher among older adults for all indicators except being in annual care. Similarly, people with a history of injection drug use (IDU) were approximately 5% less likely to meet indicator criteria. However, men who have sex with men (MSM) with a history of IDU were equally as likely to be in annual care as MSM without IDU history (adjusted ratio of proportions: 1.00, 95% CI: 0.98 to 1.03; see Table S3, Supplemental Digital Content, http://links.lww.com/QAI/A691). People on ART were more likely to be in continuous care (Tables 2 and 3), although by 2011 we observed that the proportion in continuous care declined with years on ART (see Table S4, Supplemental Digital Content, http://links.lww.com/QAI/A691).
TABLE 2.
Unadjusted Analysis of Factors Associated With Annual HIV Care Engagement Among Participants Enrolled in the OHTN Cohort Study, 2001–2011
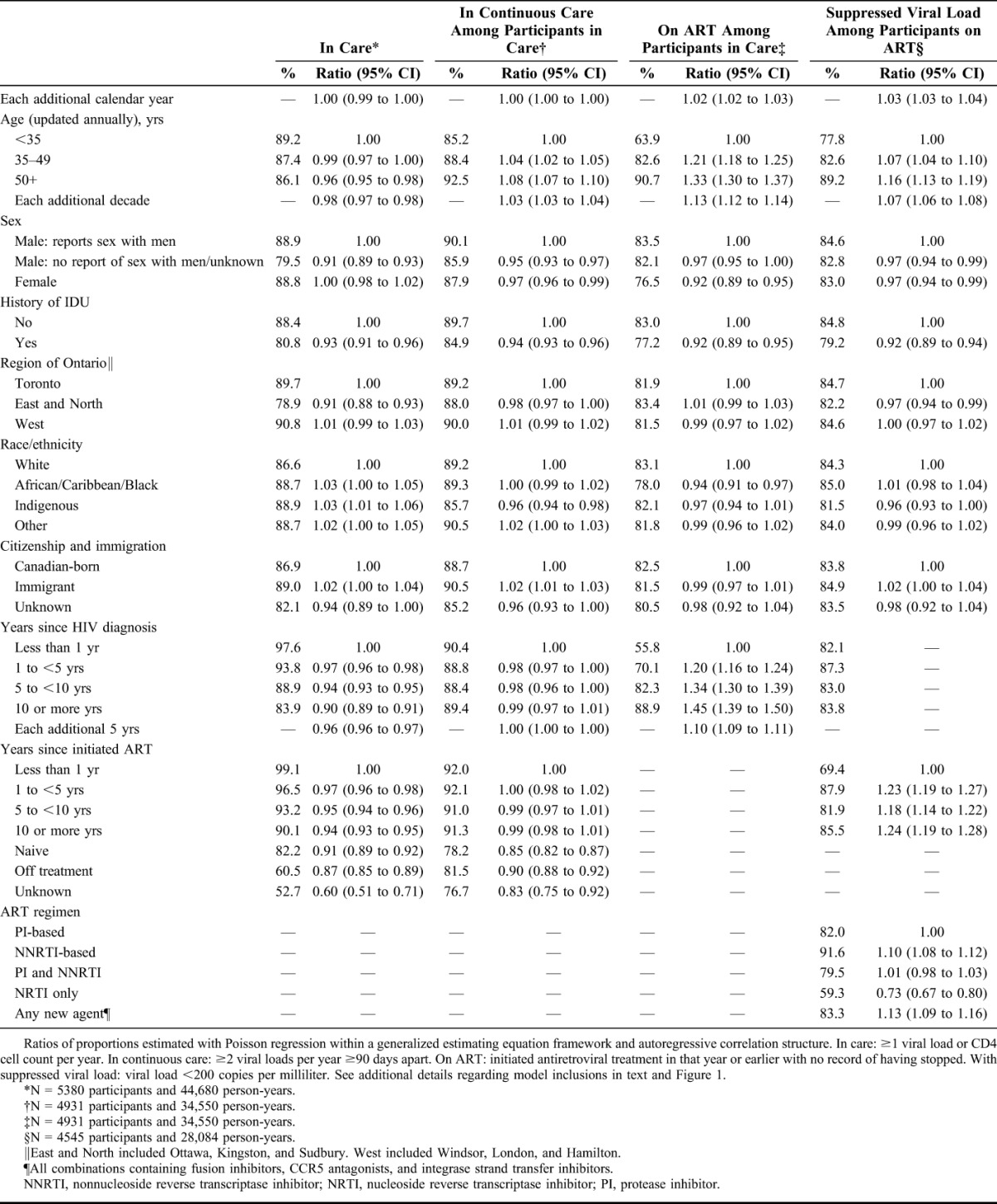
TABLE 3.
Multivariable Analysis of Factors Associated With Annual HIV Care Engagement Among Participants Enrolled in the OHTN Cohort Study, 2001–2011
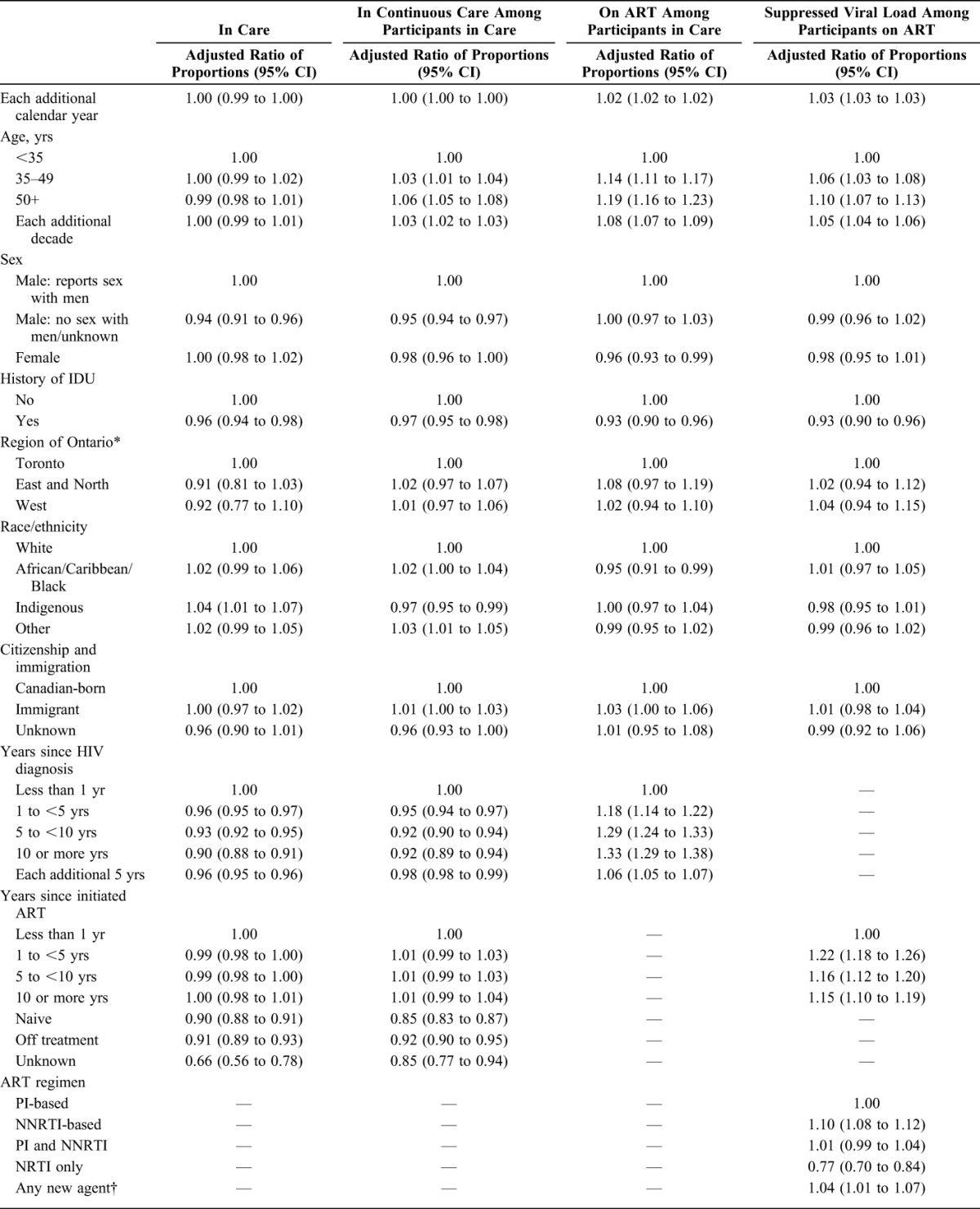
Statistically significant sex differences were noted. Men who did not report sex with men were about 5% less likely to be in annual care compared with MSM (Tables 2 and 3); this difference was especially pronounced by 2011 (adjusted ratio of proportions: 0.93, 95% CI: 0.89 to 0.96; see Table S3a, Supplemental Digital Content, http://links.lww.com/QAI/A691). Fewer women were classified as being on ART (76.5% per year, on average) compared with MSM (83.5%, on average); the magnitude of this difference diminished on adjustment for other characteristics (Table 3). For those on ART, however, there were no sex differences in the proportions with suppressed viral load.
A higher proportion of indigenous individuals were in annual care compared with white individuals, but a lower proportion of them were in continuous care. Although these differences reached statistical significance, the absolute differences in proportions were less than 5%. People of African/Caribbean/Black race/ethnicity were 5% less likely to be on ART than white individuals (adjusted ratio of proportions: 0.95, 95% CI: 0.91 to 0.99); among naive participants, we detected no difference in CD4 cell count in this ethnic group compared with others (data not shown). There was no independent effect on HIV indicators according to country of birth. However, there was a statistically significant sex interaction such that female immigrants were more likely to be on ART compared with Canadian-born women (adjusted ratio of proportions: 1.16, 95% CI: 1.09 to 1.24; see Table S5, Supplemental Digital Content, http://links.lww.com/QAI/A691).
Viral suppression was most likely among older adults, people without a history of IDU, and among participants on nonnucleoside reverse transcriptase inhibitor–based regimens (Tables 2 and 3). Those on nucleoside reverse transcriptase inhibitor–only regimens were least likely to be suppressed. As expected, participants on ART for more than 1 year were more likely to be suppressed than those in their first year of treatment. Furthermore, there was a statistically significant time interaction. Earlier in time, the proportion suppressed declined beyond 5 years postinitiation, whereas by 2011 one was equally likely to be suppressed (relative to the first year of treatment) no matter how long one had been on treatment (see Tables S6 and S7, Supplemental Digital Content, http://links.lww.com/QAI/A691). Prognostic factors were qualitatively indistinguishable whether we defined suppressed viral load as <200 copies per milliliter or below the limit of detection (data not shown).
DISCUSSION
From 2001 to 2011 among participants who enrolled in an HIV clinical cohort in Ontario, Canada, we observed high and consistent annual proportions of patients in HIV care (86%–89%) and in continuous care (76%–80%). By 2011, a majority of patients were on ART (77%) and had successful viral suppression (76% with a viral load <200 copies per milliliter). The rise in the proportion with suppressed viral load over time is consistent with previous observations in our cohort,21 as well as in British Columbia, Canada,9 and the United States.17 We attribute these gains primarily to improvements in ART regimens, specifically their tolerability and simplified dosing,22 as well as changes to clinical guidelines recommending earlier treatment initiation and advising against treatment interruption.23–25
Strengths of our analysis include use of data from a large and generally representative cohort of people in HIV care compared with cumulative HIV diagnoses in Ontario.26 As of 2011, cohort participants under active follow-up at OCS clinics constituted 28% of the approximately 13,000 people undergoing viral load testing in the province.15 Nevertheless, when compared with nonvolunteer patients at OCS clinics, OCS participants tend to be older, were diagnosed in the more distant past, and are generally healthier as measured by CD4 cell count and viral load.27 Our linkage with the PHOL viral load database allowed us to continue to passively follow those lost-to-clinic-follow-up and determine whether they had evidence of ongoing HIV care elsewhere in the province. This approach mitigated attrition bias.
Potential limitations include selection and information biases. Estimates of HIV care engagement from clinical cohorts such as the OCS are likely more optimistic than population-based estimates. Indeed, comparisons of OCS participants undergoing viral load testing in 2011 with the entire population of patients with at least 1 viral load in Ontario that year suggests more successful engagement within the cohort: 86% of participants had 2 or more viral loads versus 81% of all testers, and 89% of participants had a viral load <500 copies per milliliter compared with 77% of all testers.15 We propose that OCS data produce estimates of the upper limits of the distributions of care engagement for all people with HIV in the province. Our use of a proxy measure of HIV care encounters, namely viral load and CD4 cell count orders, may have underestimated the proportion in care or in continuous care through misclassification. In the North American AIDS Cohort Collaboration on Research and Design, laboratory tests had a high degree of agreement (85%) with recorded clinic visit encounter dates; however, use of laboratory tests dates tends to underestimate retention by 3%–9% compared with encounter dates.28 Conversely, we may have overestimated the proportion on ART as medication stop dates are not as well recorded in medical charts as start dates. Moreover, among participants with annual gaps in care or who were lost-to-clinic-follow-up, classification of ART status was sensitive to assumptions although we were conservative in our primary analysis.
Most differences in HIV care engagement across subpopulations in the cohort were minor (<5%), suggesting generally uniform engagement among people who have successfully linked to care. Specialty HIV clinics in Ontario are government-funded and are generally located in teaching hospitals or are affiliated with academic institutions. They are typically staffed by infectious diseases specialty clinicians supported by interdisciplinary teams, which may include primary care physicians, nurses, social workers, psychiatrists, pharmacists, hepatologists, nephrologists, and endocrinologists. Many have close connections with local AIDS service organizations. Patients attending these specialty clinics may receive more comprehensive care for HIV and other comorbidities. In particular, we observed that older adults and MSM in our cohort had excellent care engagement profiles. Nevertheless, even among this cohort of patients attending specialty HIV clinics in a jurisdiction with universal health care, there were signals of potential barriers to optimal care engagement.
Younger adults were consistently less likely than their older counterparts to be in care, in continuous care, on ART, and experiencing viral suppression. This is in keeping with findings in other settings8,13,16,29,30 and may reflect ongoing challenges, including drug use, having been diagnosed with HIV more recently, stigma, concerns about confidentiality, lack of transportation options, feelings of invincibility, and an inferior connection with health care, community-based AIDS service organizations, and other community services in general.31–34
Persons with a history of IDU were at risk for less HIV care engagement across all indicators, as observed elsewhere.13,35,36 To effect positive change in health policy and practice, ongoing research in this domain must further explore the nuances of drug use history to determine to what extent differences are driven by ongoing substance use versus underlying causes, such as low socioeconomic status, housing instability, and unmet mental health needs. That the proportion in care among MSM did not vary with IDU history is suggestive of the heterogeneity in this exposure and its effects.
Our finding that continuous HIV care was less common among heterosexual men despite adjustment for IDU history has also been observed in the United States and sub-Saharan Africa.37,38 In Ontario, qualitative research suggests that heterosexual men may avoid seeking health care because of the potentially negative social consequences of being seen engaged with organizations that provide HIV-related care.39 Consequently, heterosexual men's health may be compromised because of delays in seeking help and remaining in care.
We observed few differences in HIV care engagement according to race/ethnicity. Our findings of minor differences between indigenous peoples and other ethnicities were unexpected, given well-documented health care access challenges for this population.40–43 Indigenous cohort participants were predominantly living in urban areas, which may not reflect care engagement in rural and remote settings. People who reported African, Caribbean, or Black race/ethnicity were less likely than white individuals to have initiated ART, even after accounting for sex, HIV risk factors, and immigration status. Nevertheless, they were equally likely to attend HIV care and have viral suppression when on treatment. This is different from the United States, where retention is markedly lower among non-Hispanic Blacks,13,16,44–46 or in the United Kingdom and Europe, where rates of clinic nonattendance for 1 or more years was the highest among people of sub-Saharan African origin.29,47 In the OCS, we did not observe suboptimal care engagement according to immigration status. Indeed, compared with women who were Canadian-born, immigrant women were more likely to be on ART. Potential explanations include the integration of HIV and social services for immigrant communities in Ontario and possibly greater familiarity with advanced HIV disease in home countries with less HIV care and treatment access.48
Being on ART was prognostic for being in continuous care; however, we noted that by 2011 the proportion in continuous care declined with years on ART. This may be due to changing practice with less frequent monitoring for HIV patients who are healthy and successfully suppressed. Ongoing surveillance of “continuous” HIV care will require reassessment of retention definitions, particularly those based on frequency of viral load monitoring.
Our findings have implications for efforts to promote optimal engagement in HIV care. They suggest that places such as Ontario, with universal health coverage and a long history of investing in HIV services, can engage most demographic groups consistently in care. Where we do experience gaps in care engagement, Ontario should take strategic action. Younger people and people with a history of drug use should be a priority for engagement interventions, whereas programs to increase attendance at clinic visits should explore targeted approaches to reach heterosexual men and people who have not yet initiated or who have stopped ART. As part of our strategy to improve consistent engagement in HIV care, our team will explore the impact of social determinants of health (including socioeconomic status). We encourage other jurisdictions to do the same where data permit.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all of the people living with HIV who volunteered to participate in the OHTN Cohort Study and the work and support of the past and present members of the OCS Governance Committee: Adrian Betts, Anita C. Benoit, Les Bowman, Tracey Conway, Patrick Cupido (Chair), Tony Di Pede, Brian Finch, Michael J. Hamilton, Brian Huskins, Rick Kennedy, Ken King, Nathan Lachowsky, Joanne Lindsay, Shari Margolese, John McTavish, Colleen Price, Lori Stoltz, Darien Taylor, Rosie Thein, and Drs. Ahmed Bayoumi, Evan Collins, Curtis Cooper, Clemon George, Troy Grennan, Claire Kendall, and Greg Robinson. The authors thank all the interviewers, data collectors, research associates and coordinators, nurses and physicians who provided support for data collection and extraction. The authors also thank OHTN staff data management and IT support (Robert Hudder, Nahid Qureshi, and Kevin Challacombe). The authors also thank the Public Health Ontario Laboratories for supporting record linkage with the HIV viral load test database.
APPENDIX 1. Members of the OHTN Cohort Study Team
The Ontario HIV Treatment Network (OHTN) Cohort Study Team consists of Dr. Sean B. Rourke (principal investigator), University of Toronto, St. Michael's Hospital, and OHTN.; Dr. Ann N. Burchell (co-principal investigator), OHTN; Dr. Sandra Gardner, OHTN; Dr. Sergio Rueda, OHTN; Dr. Ahmed Bayoumi, Dr. Kevin Gough and Dr. Darrell Tan, St. Michael's Hospital; Dr. Jeffrey Cohen, Windsor Regional Hospital; Dr. Curtis Cooper, Ottawa General Hospital; Dr. Don Kilby, University of Ottawa Health Services; Dr. Mona Loutfy and Dr. Fred Crouzat, Maple Leaf Medical Clinic; Dr. Anita Rachlis and Dr. Nicole Mittmann, Sunnybrook Health Sciences Centre; Dr. Janet Raboud and Dr. Irving Salit, Toronto General Hospital; Dr. Michael Silverman, St. Joseph's Health Care; Dr. Roger Sandre, Sudbury Regional Hospital; and Dr. Gerald Evans and Dr. Wendy Wobeser, Hotel Dieu Hospital.
Footnotes
The Ontario HIV Treatment Network (OHTN) Cohort Study is funded by the AIDS Bureau, Ontario Ministry of Health and Long-Term Care. Other supports include a Canadian Institutes of Health Research (CIHR) New Investigator award to A.N.B. and T.A.; a CIHR Fellowship Award to C.K.; an OHTN Applied HIV Research Chair to C.C.; an OHTN Chair and the Toronto and Western Hospital Foundation Skate Dream Fund award to J.R.
Preliminary findings from this work were presented at the Canadian Association for HIV Research Conference, May 1–4, 2014, St John's, Newfoundland.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
The opinions, results, and conclusions are those of the authors and no endorsement by the Ontario HIV Treatment Network or Public Health Ontario is intended or should be inferred.
Members of the OHTN Cohort Study Team are listed in Appendix 1.
REFERENCES
- 1.World Health Organization (WHO). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: WHO; 2013. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. Accessed June 4, 2015. [PubMed] [Google Scholar]
- 2.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–833; W-284 W-285 W-286 W-287 W-288 W-289 W-290 W-291 W-292 W-293 W-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2013. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed June 4, 2015. [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks G, Gardner LI, Craw J, et al. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010;24:2665–2678. [DOI] [PubMed] [Google Scholar]
- 7.Coleman S, Boehmer U, Kanaya F, et al. Retention challenges for a community-based HIV primary care clinic and implications for intervention. AIDS Patient Care STDS. 2007;21:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleishman JA, Yehia BR, Moore RD, et al. ; HIV Research Network. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosyk B, Montaner JSG, Colley G, et al. The cascade of HIV care in British Columbia, Canada, 1996-2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raymond A, Hill A, Pozniak A. Large disparities in HIV treatment cascades between eight European and high-income countries-analysis of break points. J Int AIDS Soc. 2014;17(4 suppl 3):19507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heath K, Samji H, Nosyk B, et al. Cohort profile: seek and treat for the optimal prevention of HIV/AIDS in British Columbia (STOP HIV/AIDS BC). Int J Epidemiol. 2014;43:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Center for Disease Control and Prevention (CDC). Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep. 2011;60:1618–1623. [PubMed] [Google Scholar]
- 13.Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2013;62:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rourke SB, Gardner S, Burchell AN, et al. Cohort profile: the Ontario HIV Treatment Network Cohort Study (OCS). Int J Epidemiol. 2012;42:402–411. [DOI] [PubMed] [Google Scholar]
- 15.Remis RS, Sullivan A, Wu K, et al. CAHR abstracts. Trends in HIV viral load testing in Ontario, 1996–2011 [P103]. Can J Infect Dis Med Microbiol. 2013;24(suppl SA):5–130. [Google Scholar]
- 16.Hall HI, Gray KM, Tang T, et al. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr. 2012;60:77–82. [DOI] [PubMed] [Google Scholar]
- 17.Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Monitoring HIV Care in the United States: Indicators and Data Systems. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 19.Department of Health and Human Services, blog.aids.gov. Secretary Sebelius Approves Indicators for Monitoring HHS-Funded HIV Services. 2012. Available at: http://blog.aids.gov/2012/08/secretary-sebelius-approves-indicators-for-monitoring-hhs-funded-hiv-services.html. Accessed June 3, 2014. [Google Scholar]
- 20.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raboud J, Blitz S, Walmsley S, et al. Effect of gender and calendar year on time to and duration of virologic suppression among antiretroviral-naïve HIV-infected individuals initiating combination antiretroviral therapy. HIV Clin Trials. 2010;11:340–350. [DOI] [PubMed] [Google Scholar]
- 22.Raboud J, Li M, Walmsley S, et al. Once daily dosing improves adherence to antiretroviral therapy. AIDS Behav. 2011;15:1397–1409. [DOI] [PubMed] [Google Scholar]
- 23.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. United States: Department of Health and Human Services; 2006. [Google Scholar]
- 24.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. United States: Department of Health and Human Services; 2008. [Google Scholar]
- 25.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. United States: Department of Health and Human Services; 2009. [Google Scholar]
- 26.Remis RS, Swantee C, Liu J. Report on HIV/AIDS in Ontario 2009. Toronto, Canada: University of Toronto; 2012. Available at: http://www.ohemu.utoronto.ca/doc/PHERO2009_report_final.pdf. Accessed April 9, 2013. [Google Scholar]
- 27.Raboud J, DeSheng S, Burchell AN, et al. Representativeness of an HIV cohort of the sites from which it is recruiting: results from the Ontario HIV Treatment Network (OHTN) cohort study. BMC Med Res Methodol. 2013;13 Available at: http://www.biomedcentral.com/content/pdf/1471-2288-13-31.pdf. Accessed June 4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebeiro PF, Horberg MA, Gange SJ, et al. Strong agreement of nationally recommended retention measures from the Institute of Medicine and Department of Health and Human Services. PLoS One. 2014;9:e111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndiaye B, Ould-Kaci K, Salleron J, et al. Incidence rate and risk factors for loss to follow-up in HIV-infected patients from five French clinical centres in Northern France—January 1997 to December 2006. Antivir Ther. 2009;14:567–575. [PubMed] [Google Scholar]
- 30.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Public Health Agency of Canada. Population-Specific HIV/AIDS Status Report: HIV/AIDS and Other Sexually Transmitted and Blood Borne Infections Among Youth in Canada. Ottawa, Canada: Public Health Agency of Canada; 2014. [Google Scholar]
- 32.Barclay TR, Hinkin CH, Castellon SA, et al. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychol. 2007;26:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee L, Rand CS, Ellen JM, et al. Factors informing HIV providers' decisions to start antiretroviral therapy for young people living with behaviorally acquired HIV. J Adolesc Health. 2014;55:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yehia BR, Schranz AJ, Momplaisir F, et al. Outcomes of HIV-infected patients receiving care at multiple clinics. AIDS Behav. 2014;18:1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mocroft A, Kirk O, Aldins P, et al. Loss to follow-up in an international, multicentre observational study. HIV Med. 2008;9:261–269. [DOI] [PubMed] [Google Scholar]
- 36.Milloy MJ, Kerr T, Bangsberg DR, et al. Homelessness as a structural barrier to effective antiretroviral therapy among HIV-seropositive illicit drug users in a Canadian setting. AIDS Patient Care STDS. 2012;26:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaller ND, Fu JJ, Nunn A, et al. Linkage to care for HIV-infected heterosexual men in the United States. Clin Infect Dis. 2011;52(suppl 2):S223–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills EJ, Bakanda C, Birungi J, et al. Male gender predicts mortality in a large cohort of patients receiving antiretroviral therapy in Uganda. J Int AIDS Soc. 2011;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antoniou T, Loutfy MR, Glazier RH, et al. “Waiting at the dinner table for scraps”: a qualitative study of the help-seeking experiences of heterosexual men living with HIV infection. BMJ Open. 2012;2:e000697. 10.1136/bmjopen-2011-000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monette L, Rourke SB, Tucker R, et al. Housing status and health outcomes in Aboriginal people living with HIV/AIDS in Ontario: the positive spaces, healthy places study. Canadian Journal of Aboriginal Community-based HIV/AIDS Research. 2009;2:41–60. [Google Scholar]
- 41.Shah BR, Gunraj N, Hux JE. Markers of access to and quality of primary care for aboriginal people in Ontario, Canada. Am J Public Health. 2003;93:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasser KE, Himmelstein DU, Woolhandler S. Access to care, health status, and health disparities in the United States and Canada: results of a cross-national population-based survey. Am J Public Health. 2006;96:1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao S, Manns BJ, Culleton BF, et al. Access to health care among status aboriginal people with chronic kidney disease. CMAJ. 2008;179:1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dombrowski JC, Kent JB, Buskin SE, et al. Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. AIDS. 2012;26:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naar-King S, Bradford J, Coleman S, et al. Retention in care of persons newly diagnosed with HIV: outcomes of the outreach initiative. AIDS Patient Care STDS. 2007;21(suppl 1):S40–S48. [DOI] [PubMed] [Google Scholar]
- 46.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerver SM, Easterbrook PJ, Anderson M, et al. Sexual risk behaviours and sexual health outcomes among heterosexual black caribbeans: comparing sexually transmitted infection clinic attendees and national probability survey respondents. Int J STD AIDS. 2011;22:85–90. [DOI] [PubMed] [Google Scholar]
- 48.Worthington C, Este D, Strain KL, et al. African immigrant views of HIV service needs: gendered perspectives. AIDS Care. 2013;25:103–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


