Article first published online 6 August 2015.
Key Words: inflammatory bowel disease, venous thromboembolism, clot lysis profiles, fibrinolysis and risk assessment
Background:
The occurrence of thromboembolic events (TE) is an important extraintestinal manifestation in patients with inflammatory bowel disease (IBD). The aim of this study was to compare fibrinolysis and clot lysis parameters between (1) patients with IBD and healthy controls and (2) patients with IBD with TE (IBD + TE) and without TE (IBD − TE).
Methods:
One hundred thirteen healthy controls and 202 patients with IBD, of which 84 patients with IBD + TE and 118 patients with IBD − TE, were included in this case–control study. Three clot lysis parameters (area under the curve, 50% clot lysis time, and amplitude) were determined using a clot lysis assay. Plasminogen activator inhibitor 1 (PAI-1) and thrombin activatable fibrinolysis inhibitor concentrations were determined by enzyme-linked immunosorbent assay.
Results:
PAI-1 antigen, active PAI-1, and intact thrombin activatable fibrinolysis inhibitor concentrations, as well as 50% clot lysis time and area under the curve, were significantly associated with the presence of IBD (all P < 0.05). The median time between TE and plasma collection was 5.0 (1.8–11.0) years. Comparing IBD + TE versus IBD − TE, active to total PAI-1 ratio (0.36 [0.24–0.61] versus 0.24 [0.13–0.40]), area under the curve (31 [24–49] versus 22 [13-31]), 50% clot lysis time (110 [64–132] versus 95 [70–126] minutes), and amplitude (0.295 [0.222–0.436] versus 0.241 [0.168–0.308]) were significantly higher in IBD + TE (all P <0.05) and remained higher after adjustment for age, gender, C-reactive protein, type of disease, presence of comorbidities, and disease activity.
Conclusions:
Patients with IBD have an altered clot lysis profile compared with healthy controls. Clot lysis parameters differ significantly between patients with IBD with and without a history of TE and should be included in the risk assessment.
Crohn's disease (CD) and ulcerative colitis are the 2 major manifestations of inflammatory bowel disease (IBD), a chronic relapsing inflammatory condition characterized by local and systemic inflammation. The occurrence of thromboembolic events (TE) is a life-threatening complication in IBD. Patients are 3-fold more likely to develop TE compared with the healthy population. In active disease, this relative risk increases to 16-fold.1 In both CD and ulcerative colitis, thrombosis seems to occur to a similar extent and correlates with the extent of disease, namely pancolonic involvement in ulcerative colitis and colonic involvement in CD.2 Deep vein thrombosis (DVT) and pulmonary embolism (PE) are the most common types of TE in IBD and represent significant morbidity and mortality. Patients with IBD with TE have a 2-fold higher mortality rate than patients without IBD with TE.3,4 Data obtained from the United Kingdom primary care database showed that of 17 evaluated chronic illnesses, only cancer and heart failure had a greater risk for venous TE than IBD.5 Frequency of hereditary thrombophilia is similar in patients with IBD and without IBD.6 Nonetheless, acquired prothrombotic risk factors such as immobility, surgery, use of intravenous catheters, vitamin deficiencies, and excessive inflammation are common in patients with IBD.2
Despite research efforts investigating the occurrence of TE in patients with IBD, the etiology is still not well understood and multifactorial. Hypofibrinolysis could possibly lead to TE. Fibrinolysis is initiated when plasminogen is converted into plasmin by plasminogen activators. Both active plasminogen activator inhibitor 1 (PAI-1) and activated thrombin activatable fibrinolysis inhibitor (TAFIa) hamper plasmin formation and are considered as 2 major fibrinolysis inhibitors. High active PAI-1 concentrations and an increased extent of TAFI activation have been associated with the occurrence of TE.7–11
Because only active PAI-1 attenuates fibrinolysis through the formation of a complex with tissue-type plasminogen activator, it is very important to discriminate active from total PAI-1 antigen (comprising latent, substrate, and active PAI-1). Current available assays can measure total PAI-1 antigen and active PAI-1, which allows calculating the active to total PAI-1 ratio. On activation of intact TAFI, the activation peptide of TAFI (AP-TAFI) is released form the active moiety (active TAFI, TAFIa). Subsequently, TAFIa cleaves C-terminal lysine residues of partially degraded fibrin, thereby abolishing the cofactor function of fibrin in the conversion of plasminogen to plasmin. The extent of TAFI activation can be determined by measuring the released activation peptide (AP-TAFI).12
PAI-1 and TAFI concentrations have been studied in patients with IBD, but discrepant findings have been reported.13–15 To the best of our knowledge, no comparison was made so far between patients with IBD with and without a history of TE. In this study, using a comprehensive panel of PAI-1 and TAFI assays, we measured total PAI-1 antigen, active PAI-1, intact TAFI, and AP-TAFI in plasma of healthy controls (HC) and patients with IBD with and without a history of TE. In addition, we compared the clot lysis profiles between those groups.
METHODS
Study Design
Between February 2009 and May 2012, 124 patients with IBD in whom a TE occurred between January 1987 and March 2014 were seen at the Gastroenterology Division of the University Hospitals of Leuven. Patients with a TE before the diagnosis of IBD were excluded. TE included both venous and arterial thrombosis. Venous TE comprised DVT, PE, thrombophlebitis and other veins, and arterial TE comprised ischemic events, myocardial infarction, and other arteries. Finally, 84 patients with IBD with a history of TE (IBD + TE) met the inclusion criteria. In the same period, 118 patients with IBD without a history of TE (IBD − TE) and 113 HC were selected as control groups in this case–control study. HC and patients gave written informed consent to participate in the IRB-approved “Vlaamse Erfelijkheidsstudie Crohn and Colitis ulcerosa” (VLECC) biobank (B322201213950/S53684) and filled out a questionnaire before the collection of blood samples.
Plasma Collection
A venipuncture in the antecubital vein was performed while the patients and controls remained in a seated upright position. Blood was collected in a 2.7 mL BD Vacutainer (Becton, Dickinson and Co., Franklin Lakes, NJ) containing 3.2% buffered sodium citrate, serving as an anticoagulant. Platelet poor plasma was obtained by centrifugation at 1800g for 10 minutes at room temperature and stored at −20°C.
Clinical Characteristics
Medical records were used to collect demographic features, type of TE, disease activity, comorbidities, and IBD medication. Disease activity was assessed based on physician's global assessment including, where possible, objective markers of inflammation (e.g., C-reactive protein [CRP]) and imaging. Comorbidities such as disorders in liver, heart, kidney, prostate, joint, thyroid, pancreas or ankylosing spondylitis, cancer, gout, iron-deficiency anemia, diabetes, chronic obstructive pulmonary disease or asthma, and thrombophilia abnormalities were recorded. Medication taken for IBD included 5-amino salicylic acids (5-ASA), corticosteroids (CS), thiopurines, methotrexate, cyclosporine, and biologicals. Use of general pain killers was not recorded.
Laboratory Analysis
TAFI and PAI-1 Concentrations
TAFI and PAI-1 concentrations were determined by in-house developed enzyme-linked immunosorbent assays (ELISAs), earlier described in Reference 7. Total PAI-1 antigen (latent, active, and complexed) was measured by the MA-31C9/MA-55F4C12-HRP ELISA and active PAI-1 using MA-21F7C4/MA-51H8 ELISA.16–18 The active to total PAI-1 ratio (ratio PAI-1) was calculated. Intact TAFI antigen was measured using MA-T12D11/MA-T30E5A2-HRP ELISA and AP-TAFI using MA-T12D11/MA-T18A8-HRP ELISA.19 All measurements were performed in duplicate. A buffer solution (blank) and plasma pool were included as negative and reference control, respectively. The plasma pool was prepared from blood obtained from 29 healthy individuals (15 women and 14 men) with a mean age of 33 years (range, 23–57 years). The blood was collected, processed, and stored as described above.
Clot Lysis Assay
Clot lysis assay was performed as earlier described in Reference 20. Briefly, human plasma (final concentration of 30%) was mixed with dilution Tris-buffer in a 96-well microtiter plate. Tissue-type plasminogen activator (180 pM) was added to induce lysis and CaCl2 (10.6 mM) to initiate clot formation. The clot lysis turbidity profile of every control/patient was determined by measuring the optical density at 405 nm every 2 minutes for 240 minutes. The area under the curve (AUC), the 50% clot lysis time (50% CLT), and the amplitude were calculated. The AUC is an integrated measurement reflecting the overall coagulation/fibrinolysis profile. The 50% CLT is the time in minutes between the time point of maximum turbidity and the midpoint of the maximum turbid to clear transition, representing the fibrinolysis rate. The amplitude is the maximal clot absorbance, indicating clot formation. All measurements were performed in duplicate.
Statistics
Quantitative data were summarized by mean and standard deviation (mean ± SD) or median (interquartile ranges) for normally and nonnormally distributed continuous variables, respectively. Shapiro–Wilk normality test was used to assess the normality of continuous variables. Receiver operating characteristics (ROC) analysis, upper left cutoff level determination, and multiple logistic regression analyses were performed using the software R (version 3.0.2, http://www.r-project.org/). Corrections were made for age, gender, disease type, disease activity, the presence of comorbidities, platelet count, CRP, and WBC. Univariate analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA). Unpaired t tests and Mann–Whitney tests were used to compare 2 groups of unpaired continuous data and analysis of variance tests to compare more than 2 groups of unpaired data. Fisher's exact test was used to compare 2 groups of unpaired categorical data. To analyze correlations, the Spearman correlation test was used. P values less than 0.05 were considered statistically significant.
RESULTS
HC Versus Patients with IBD
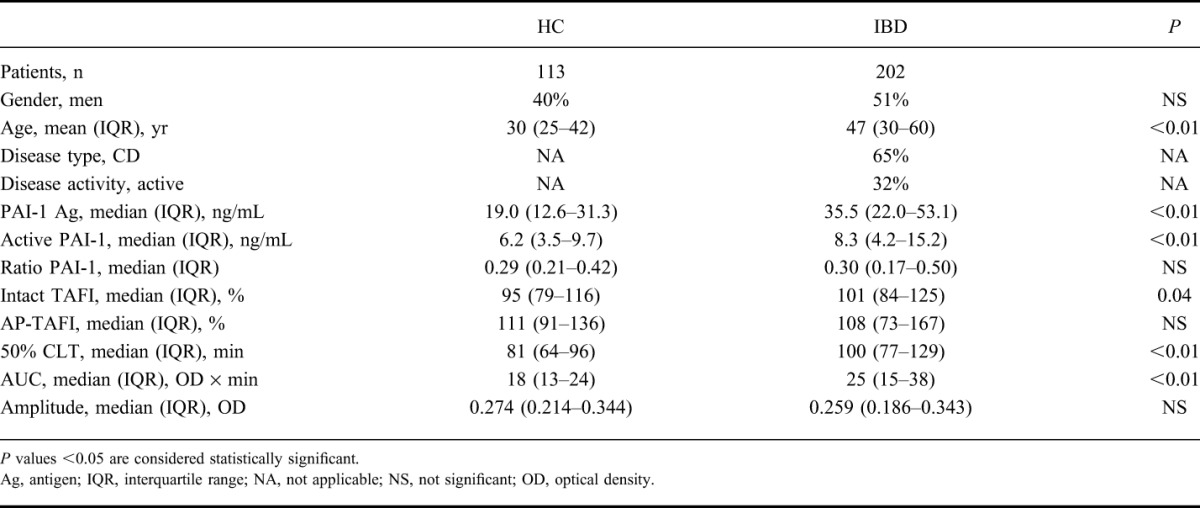
The median (interquartile range) age of 113 HC (40% males) was significantly lower than the median age of 202 patients with IBD (51% males, 65% CD) (30 [25–42] years versus 47 [30–60] years, P < 0.05). Mann–Whitney tests revealed that total PAI-1 antigen, active PAI-1, intact TAFI, AUC, and 50% CLT were significantly lower in HC compared with patients with IBD (Table 1). These parameters remained associated with IBD after corrections for age and gender (P < 0.05). Active to total PAI-1 ratio, AP-TAFI, and amplitude were not significantly different between HC and patients with IBD.
TABLE 1.
Clinical Characteristics, Fibrinolysis Proteins, and Clot Lysis Parameters in HC and Patients with IBD
Of the 202 patients with IBD, 64 were identified with active disease (55% males, 58% CD, median age of 37 [26–48] years) versus 136 patients with inactive disease (49% males, 69% CD, median age of 48 [33–59] years). Comparing parameters between both groups revealed that only the amplitude was significantly higher in patients with IBD suffering from active disease (0.298 [0.212–0.372] versus 0.244 [0.179–0.329], respectively, P < 0.05).
Patients with IBD with and Without CS Treatment
At the time of sampling, 43 patients received CS therapy (51% males, median age of 42 [29–51] years) and 159 patients did not receive CS therapy (51% males, median age of 44 [31–58] years). Comparing patients with IBD with and without CS treatment, total PAI-1 antigen (47.6 [27.8–64.8] ng/mL versus 32.2 [20.1–50.9] ng/mL, P < 0.05), active PAI-1 (14.7 [5.7–27.1] ng/mL versus 7.5 [4.1–14.4] ng/mL, P < 0.05), and intact TAFI (119% [93%–146%] versus 98% [83%–119%], P < 0.05) were significantly higher in patients receiving CS treatment. Active to total PAI-1 ratio, AP-TAFI, and the 3 studied clot lysis parameters were not different between both therapy groups. Interestingly, also after corrections for age, gender, type of disease, disease activity and presence of comorbidities, total PAI-1 antigen, active PAI-1, and intact TAFI remained associated with the use of CS therapy. Age, gender, disease type, and presence of comorbidities were not associated with CS therapy intake, whereas activity of disease was highly associated (P < 0.05).
Patients with IBD with and Without a History of Thrombosis
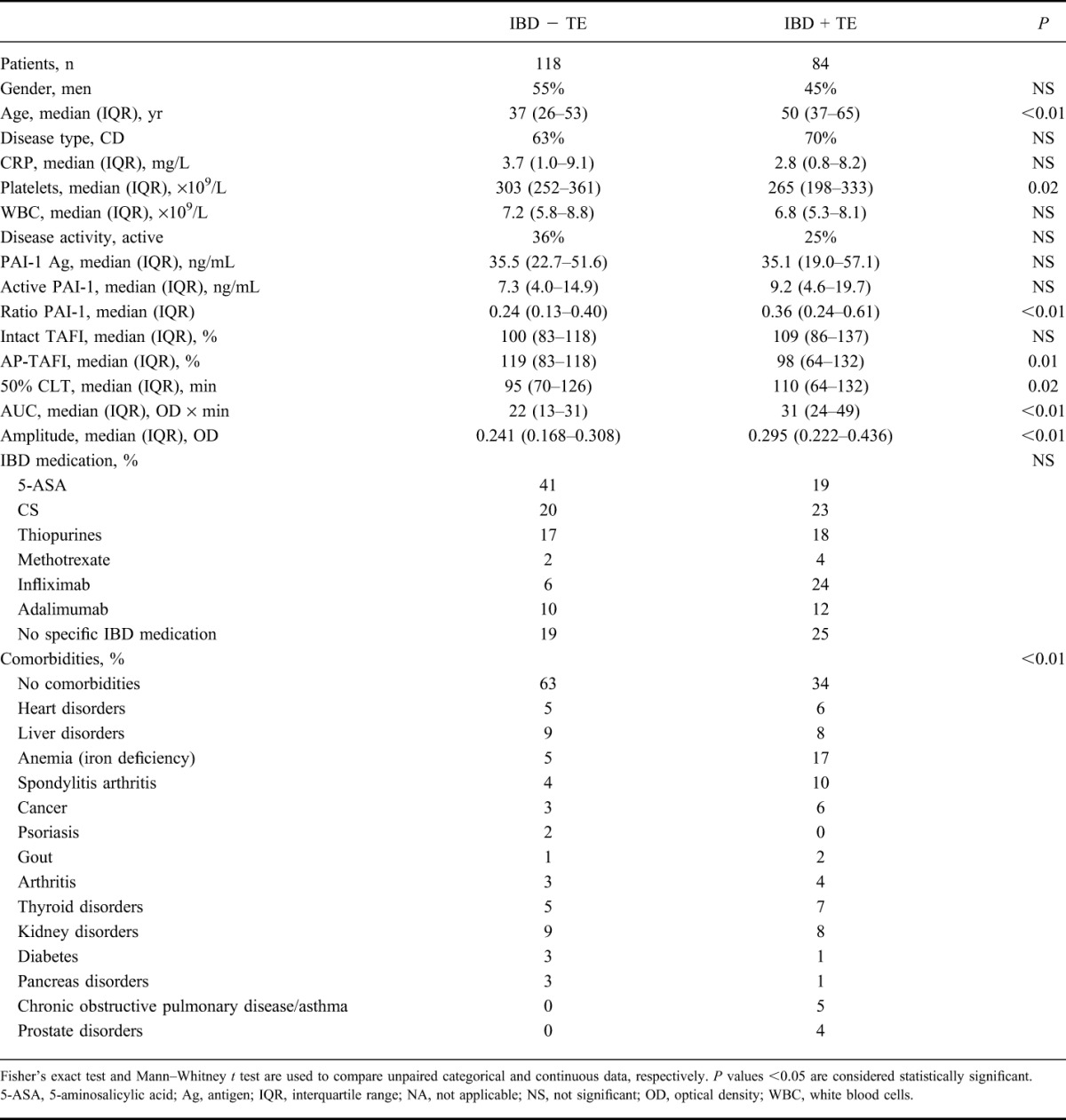
Eighty-four patients with IBD + TE (45% males, 70% CD) and 118 patients with IBD − TE (55% males, 63% CD) were included. Fisher's exact test revealed that gender and disease type were not significantly different between both groups. In the IBD + TE cohort, 70 of the 84 (83%) patients with IBD were identified with a venous TE compared with 14 of the 84 (17%) with an arterial TE. Of the 84 patients, 28 (33%) were identified with a DVT, 11 (13%) with a PE, 5 (6%) with both a DVT and a PE, 12 (14%) with a venous TE other than DVT or PE, 14 (17%) with thrombophlebitis, 12 (14%) with ischemic TE or myocardial infarction, and 2 (2%) with an arterial TE. At the time of TE, 60 of the 84 patients (71%) had active disease.
At the time of sampling, 25% of the patients were identified with active disease and 23% used CS therapy in the IBD + TE group, which was not significantly different from the IBD − TE group, 36% and 20%, respectively (P > 0.05). A significant higher percentage of patients with comorbidities was detected in the IBD + TE group compared with the IBD − TE group, 66% versus 37%, respectively (P < 0.01) (Table 2). Univariate Mann–Whitney tests (IBD + TE versus IBD − TE) showed significant differences in age, active to total PAI-1 ratio, AP-TAFI, AUC, 50% CLT, and amplitude (Table 2). No difference in intact TAFI, PAI-1 antigen, active PAI-1, CRP, or WBC was detected (Table 2). Multiple logistic regression modeling revealed that active to total PAI-1 ratio, AUC, 50% CLT, and amplitude remained associated with the presence of TE after correcting for age, gender, disease activity, presence of comorbidities, disease type, CRP, platelets, and WBC (P < 0.05).
TABLE 2.
Clinical Characteristics, Fibrinolysis Proteins, and Clot Lysis Parameters in Patients with IBD, Without Thrombosis (IBD − TE) and with Thrombosis (IBD + TE)
Disease Activity
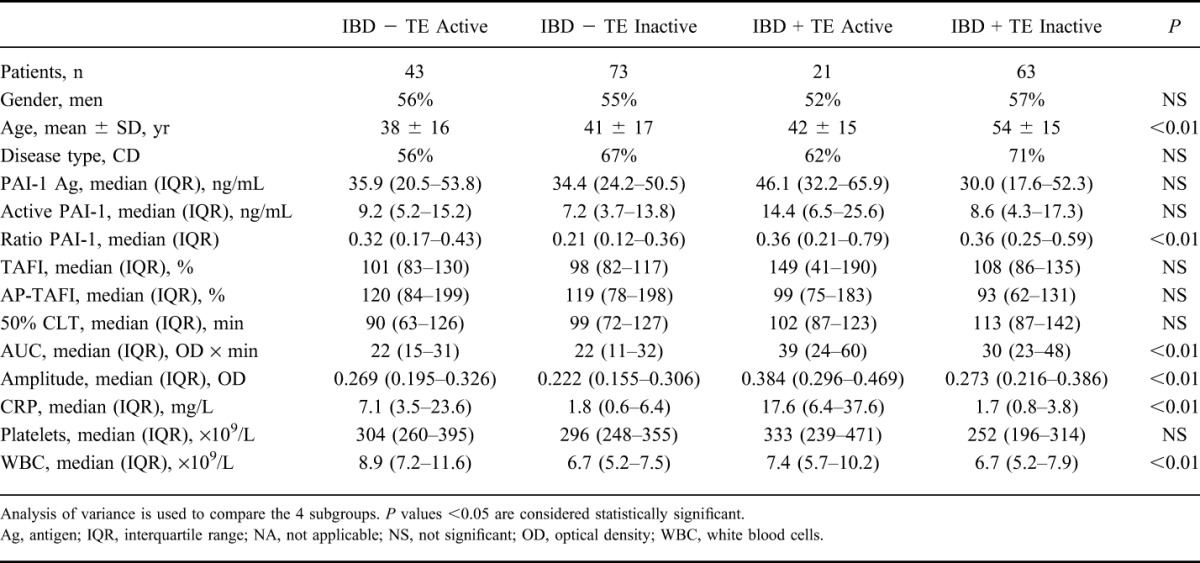
The patients with IBD were allocated into 4 subgroups based on the presence of TE and the activity of disease at the time of sampling (IBD + TE and active disease, IBD + TE and inactive disease, IBD − TE and active disease, and IBD − TE and inactive disease). Analysis of variance analysis showed that age, CRP, WBC, active to total PAI-1 ratio, AUC, and amplitude were significantly different between the 4 groups (Table 3).
TABLE 3.
Clinical Characteristics, Fibrinolysis Proteins, and Clot Lysis Parameter of Patients with IBD Divided into 4 Subgroups Based on a History of TE and Disease Activity at the Time of Plasma Collection
ROC Analysis
ROC analysis was performed to distinguish patients with IBD + TE from HC. The clot lysis parameter AUC was the best discriminator with an area under the ROC curve of 0.80 (95% confidence interval, 0.73–0.87) and an optimal cutoff level of 23 optical density × minutes (specificity of 73% and sensitivity of 77%) (Fig. 1).
FIGURE 1.
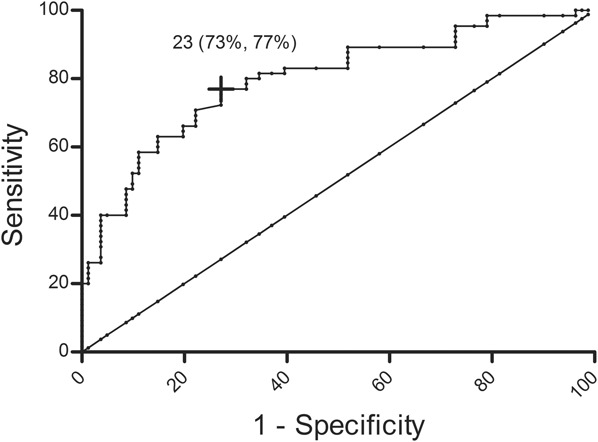
Receiver operating curve of the clot lysis parameter AUC, patients with IBD + TE compared with HC. The clot lysis parameter AUC had an area under the receiver operating curve (95% CI) of 0.80 (0.73–0.87). The cross depicted in black presents the best top-left cutoff, 23 optical density × minutes, with a specificity of 73% and a sensitivity of 77%.
C-reactive Protein
The proinflammatory acute phase reactant CRP correlated with the amplitude in both IBD + TE (r = 0.30, P = 0.01) and IBD − TE (r = 0.36, P < 0.01) but not with AUC or 50% CLT. CRP also correlated significantly with PAI-1 antigen in patients with IBD + TE (r = 0.26, P = 0.03), however not with active PAI-1 or active to total PAI-1 ratio. No correlation between CRP and any of the PAI-1 measurements was observed in patients with IBD − TE.
Time Between Occurrence of Thrombosis and Plasma Collection
The median (interquartile range) time interval from IBD diagnosis until first TE diagnosis was 9.0 (3.0–17.0) years. The median (range) time between plasma collection and TE event was 5.0 (0.04–24) years. No correlation was found between the time interval (time between TE and plasma sampling), and CRP, platelets, WBC, PAI-1 antigen, active PAI-1, active to total PAI-1 ratio, intact TAFI, AP-TAFI, 50% CLT, AUC, or amplitude.
DISCUSSION
The occurrence of thromboembolic complications in IBD is associated with a significant impact on mortality.3,4 The pathogenesis of TE in IBD has been studied intensively, and many risk factors have been described without identifying one major risk factor. The complex interplay between inflammation and coagulation plays definitely a key role. Several hemostatic abnormalities have been reported in patients with IBD, among them altered TAFI and PAI-1 concentrations. TAFI and PAI-1 are 2 major fibrinolysis inhibitors that have been associated with the occurrence of TE,7–11 although conflicting results are reported concerning their concentrations in patients with IBD.13–15
This study revealed that fibrinolysis parameters, such as PAI-1 antigen, active PAI-1 and intact TAFI, and clot lysis parameters, such as the 50% CLT and the AUC, are significantly associated with the presence of IBD disease, compared with HC. The increase in both active and total PAI-1 may explain why no difference in the ratio of active to total PAI-1 was observed. In 2008, Koutroubakis et al13 have shown that PAI-1 activity levels are higher in patients with IBD compared with HC and are higher in patients with active disease compared with patients with inactive disease, which is in agreement with the increase in active PAI-1 concentration observed in patients with IBD included in this study, although we could not correlate PAI-1 concentrations (total, active, or ratio) with disease activity. PAI-1 is an acute phase reactant, of which the expression is upregulated in response to proinflammatory cytokines such as tumor necrosis factor alpha, known to be associated with IBD severity.21 Interestingly, comparing PAI-1 concentrations across patients with IBD with and without TE revealed that in particular, the ratio of active to total PAI-1 is significantly higher in patients with TE. This is attributed to a higher concentration of active PAI-1, although the total concentration of PAI-1 antigen did not differ between both groups. It is therefore important to make a distinction between both the active and inactive forms of the fibrinolysis inhibitor.
Although CS are used in the treatment of IBD for their broad anti-inflammatory effect, it also has a well-known procoagulant effect. Next to its influence on coagulation factors, CS can enhance the synthesis of the antifibrinolytic protein PAI-1.22,23 In this study, we showed indeed that at the time of sample collection, patients with IBD on CS therapy had higher total PAI-1 and active PAI-1 concentrations compared with patients not receiving CS therapy. This association remained significant after correcting for disease activity, age, gender, disease type, and the presence of comorbidities. Because we recently showed that active PAI-1 is associated with TE7 and IBD is known to induce a hypercoagulable state, we want to emphasize that it is important to determine whether the use of CS will further enhance this process. Reducing steroid use has been proposed as one of the actions that can potentially reduce the incidence of TE in IBD.24
Saibeni et al14 showed increased intact TAFI levels in patients with IBD compared with HC, whereas Koutroubakis et al13 found lower intact TAFI levels in patients with IBD. The latter study suggested that the decreased intact TAFI levels might result from enhanced consumption owing to TAFI activation. In both studies, commercial ELISAs were used that cannot discriminate between intact TAFI and activated TAFI. Owczarek et al15 measured activated TAFI levels with a functional assay and found that TAFIa levels were higher in patients with IBD compared with HC. However, they used a reference value for HC instead of actually measuring TAFIa in HC. This study revealed that intact TAFI and AP-TAFI were both associated with the presence of IBD disease. Furthermore, we did not detect a difference for intact TAFI or for AP-TAFI between patients with IBD with and without a history of TE. Altogether, we conclude that TAFI does most likely not play a significant role in the occurrence of TE in patients with IBD, which is in line with a previous report investigating the role of TAFI in the occurrence of venous thrombosis.7
So far many prothrombotic abnormalities have been described in IBD; however, specific biomarkers to identify patients at risk for TE are still lacking. A global clot lysis assay generates a profile, which indicates the interplay between coagulation and fibrinolysis. In this study, all 3 clot lysis parameters studied (AUC, 50% CLT, and amplitude) were significantly higher in patients with IBD with a history of TE compared with patients with IBD without a history of TE. Multivariate analysis showed that after corrections, the 3 parameters remained associated with the presence of TE. Furthermore, these profiles showed that the coagulation–fibrinolysis system is altered in patients with IBD with TE, independently of the time after occurrence of TE because the time between TE and plasma collection did not correlate with any of the 3 parameters. CRP correlated significantly with the amplitude but not with the 50% CLT, suggesting that the amplitude indicating the clot formation is associated more with inflammation compared with the clot lysis. The increase in amplitude might be a reflection of high fibrinogen levels or an increased endogenous thrombin potential because an increase in both fibrinogen and thrombin has previously been reported in IBD.25–28 Furthermore, fibrinogen is known to be an acute phase reactant29–31 and has been linked with an increase in maximal clot absorbency (turbidity).32
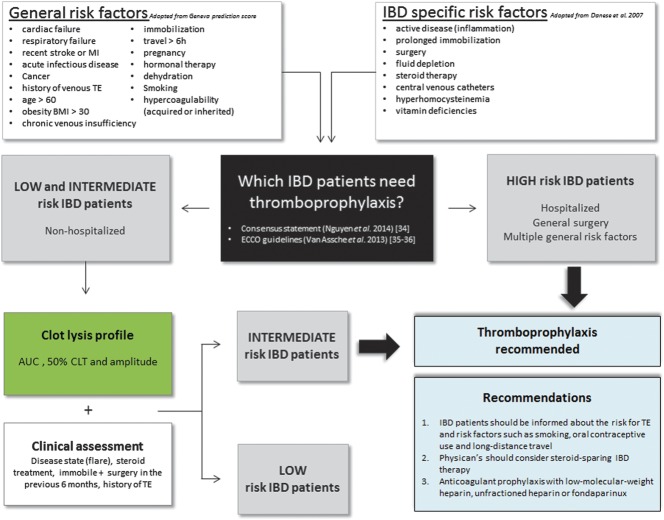
It has been estimated that risk-appropriate thromboprophylaxis may reduce the incidence of thrombosis up to 70%.33 We propose therefore a risk assessment (Fig. 2) in which clinical data and clot lysis profile parameters will be combined to identify patients with IBD who need special attention. In this study, patients with IBD with a history of TE had a significant higher percentage of comorbidities (66% versus 37%, respectively), reflecting again the need for a profound clinical assessment. A history of TE, presence of cardiovascular diseases or other comorbidities, use of oral contraceptives, smoking behavior, obesity, immobility, genetic predisposition for TE, and presence of recent surgery should be assessed (Fig. 2). In this study, a threshold AUC that distinguished patients with IBD + TE from HC was delineated. The cutoff reveals an acceptable sensitivity (77%) and specificity (73%), allowing to identify patients at risk. However, further stratification through assessing IBD-specific risk factors such as active disease, steroid therapy, and prolonged immobility will increase these sensitivity and specificity percentages, emphasizing the importance to combine clot lysis and clinical data in the risk assessment (Fig. 2). Recent surgery is a major risk factor for thrombosis, and therefore, longer thromboprophylaxis after surgery is required, especially in patients with IBD who are prone to develop TE. Besides the assessment of recent surgery, a history of TE is as well a very important parameter to implement in the risk assessment model because it has been shown that patients with IBD have a high risk for recurrence of TE.34 Furthermore, a steroid-sparing anti–tumor necrosis factor therapy may be the treatment of choice for patients with IBD with severe disease and additional risk factors for TE.20,35 We recommend that the selected patients with IBD should be advised on additional risk factors such as smoking behavior, the use of oral contraceptives, and long-distance travel. In addition, physician's should consider a steroid-sparing IBD therapy, and in case anticoagulant prophylaxis is needed, low-molecular-weight heparin, unfractionated heparin, or fondaparinux can be given (see recommendations in Fig. 2).36–38
FIGURE 2.
Proposal for a risk assessment for patients with IBD at risk to develop a thrombosis. Patients with IBD are stratified according to their general and IBD-specific risk factors for TE into high-risk patients and intermediate/low-risk patients. Hospitalized patients with IBD or patients with IBD with multiple general risk factors are considered “high risk” and thromboprophylaxis is recommended. For nonhospitalized patients with IBD, the need for thromboprophylaxis is less clear. Patients with IBD with risk factors such as surgery in the previous 6 months, a history of TE, IBD flares, prolonged immobility, or long-time steroid treatment are considered more at risk for TE. A global clot lysis assay will help, in this particular case, to determine if the patient is considered as “intermediate risk,” then thromboprophylaxis is recommended, or “low risk,” then no thromboprophylaxis is recommended.
In conclusion, to the best of our knowledge, this is the first study to compare fibrinolysis and clot lysis parameters in patients with IBD with and without TE revealing that both the clot lysis profile and the ratio of active to total PAI-1 are altered in patients with IBD with a history of TE. However, to prove a direct cause–effect relation between the fibrinolysis and clot lysis parameters and the occurrence of TE, prospective studies are warranted.
ACKNOWLEDGMENTS
The authors would like to thank Vera Ballet (Translational Research in GastroIntestinal Disorders, Department of Gastroenterology, UZ Leuven) for collecting plasma samples of IBD patients and healthy controls.
Author contributions: L. Bollen performed research, interpreted the data, and implemented statistical analysis. M. Peeters performed part of the research. N. Vande Casteele, G. Van Assche, M. Ferrante, W. Van Moerkercke, P. Declerck, and S. Vermeire designed research and contributed to manuscript review. A. Gils designed research, coordinated the experiments, and reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by the Fund for Scientific Research Flanders G.0464.10.
L. Bollen is a Research Assistant of the Research Foundation-Flanders (FWO-Vlaanderen), Belgium. N. Vande Casteele received lectures and consultancy fees from AbbVie, MSD and Janssen Biologics, UCB, and Pfizer. G. Van Assche received financial support for research from MSD and AbbVie; received lecture fees from MSD, AbbVie, Takeda, and Ferring; consultant for MSD, AbbVie, Takeda, Ferring, Genentech/Roch, and Pfizer. M. Ferrante received financial support for research from Takeda; received lecture fees from MSD, AbbVie, Janssen, Chiesi, Tillotts, and Zeira; consultant for AbbVie, Janssen, MSD, Boehringer Ingelheim, and Ferring. S. Vermeire received financial support for research from MSD and AbbVie; received lectures fees from MSD, AbbVie, Takeda, Falk, and Tillotts; is a consultant for MSD, AbbVie, Takeda, Falk, Ferring, Shire, Galapagos, Hospira, Mundipharma, Genentech/Roch, Pfizer, and Celgene. A. Gils received financial support for research from Pfizer; received lectures fees from MSD, AbbVie, Janssen Biologics, and Pfizer. The remaining authors have no conflict of interest to disclose.
A. Gils is the guarantor of the article.
REFERENCES
- 1.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–663. [DOI] [PubMed] [Google Scholar]
- 2.Danese S, Papa A, Saibeni S, et al. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. 2007;102:174–186. [DOI] [PubMed] [Google Scholar]
- 3.Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011;106:713–718. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:2272–2280. [DOI] [PubMed] [Google Scholar]
- 5.Huerta C, Johansson S, Wallander MA, et al. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–943. [DOI] [PubMed] [Google Scholar]
- 6.Thompson NP, Wakefield AJ, Pounder RE. Inherited disorders of coagulation appear to protect against inflammatory bowel disease. Gastroenterology. 1995;108:1011–1015. [DOI] [PubMed] [Google Scholar]
- 7.Bollen L, Peetermans M, Peeters M, et al. Active PAI-1 as marker for venous thromboembolism: case-control study using a comprehensive panel of PAI-1 and TAFI assays. Thromb Res. 2014;134:1097–1102. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer ME, Lisman T, de Groot PG, et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116:113–121. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Cushman M, Heckbert SR, et al. Prospective study of fibrinolytic markers and venous thromboembolism. J Clin Epidemiol. 2003;56:598–603. [DOI] [PubMed] [Google Scholar]
- 10.Crowther MA, Roberts J, Roberts R, et al. Fibrinolytic variables in patients with recurrent venous thrombosis: a prospective cohort study. Thromb Haemost. 2001;85:390–394. [PubMed] [Google Scholar]
- 11.Prins MH, Hirsh J. A critical review of the evidence supporting a relationship between impaired fibrinolytic activity and venous thromboembolism. Arch Intern Med. 1991;151:1721–1731. [PubMed] [Google Scholar]
- 12.Ladenvall C, Gils A, Jood K, et al. Thrombin activatable fibrinolysis inhibitor activation peptide shows association with all major subtypes of ischemic stroke and with TAFI gene variation. Arterioscler Thromb Vasc Biol. 2007;27:955–962. [DOI] [PubMed] [Google Scholar]
- 13.Koutroubakis IE, Sfiridaki A, Tsiolakidou G, et al. Plasma thrombin-activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1 levels in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2008;20:912–916. [DOI] [PubMed] [Google Scholar]
- 14.Saibeni S, Bottasso B, Spina L, et al. Assessment of thrombin-activatable fibrinolysis inhibitor (TAFI) plasma levels in inflammatory bowel diseases. Am J Gastroenterol. 2004;99:1966–1970. [DOI] [PubMed] [Google Scholar]
- 15.Owczarek D, Undas A, Foley JH, et al. Activated thrombin activatable fibrinolysis inhibitor (TAFIa) is associated with inflammatory markers in inflammatory bowel diseases TAFIa level in patients with IBD. J Crohns Colitis. 2012;6:13–20. [DOI] [PubMed] [Google Scholar]
- 16.Meissenheimer LM, Verbeke K, Declerck PJ, et al. Quantitation of vervet monkey (Chlorocebus aethiops) plasminogen activator inhibitor-1 in plasma and platelets. Thromb Haemost. 2006;95:902–903. [PubMed] [Google Scholar]
- 17.Declerck PJ, Alessi MC, Verstreken M, et al. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988;71:220–225. [PubMed] [Google Scholar]
- 18.Declerck PJ, Verstreken M, Collen D. An immunofunctional assay for active plasminogen activator inhibitor-1 (PAI-1). Fibrinolysis. 1988;2(suppl 2):77–78. [Google Scholar]
- 19.Ceresa E, Brouwers E, Peeters M, et al. Development of ELISAs measuring the extent of TAFI activation. Arterioscler Thromb Vasc Biol. 2006;26:423–428. [DOI] [PubMed] [Google Scholar]
- 20.Bollen L, Vande Casteele N, Peeters M, et al. Short-term effect of infliximab is reflected in the clot lysis profile of patients with inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2015;21:570–578. [DOI] [PubMed] [Google Scholar]
- 21.Gruber F, Hufnagl P, Hofer-Warbinek R, et al. Direct binding of Nur77/NAK-1 to the plasminogen activator inhibitor 1 (PAI-1) promoter regulates TNF alpha-induced PAI-1 expression. Blood. 2003;101:3042–3048. [DOI] [PubMed] [Google Scholar]
- 22.van Zaane B, Nur E, Squizzato A, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8:2483–2493. [DOI] [PubMed] [Google Scholar]
- 23.Halleux CM, Declerck PJ, Tran SL, et al. Hormonal control of plasminogen activator inhibitor-1 gene expression and production in human adipose tissue: stimulation by glucocorticoids and inhibition by catecholamines. J Clin Endocrinol Metab. 1999;84:4097–4105. [DOI] [PubMed] [Google Scholar]
- 24.Wallaert JB, De Martino RR, Marsicovetere PS, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum. 2012;55:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnin P, Coelho J, Pocard M, et al. Anti-TNF alpha therapy early improves hemodynamics in local intestinal and extraintestinal circulations in active Crohn's disease. J Crohns Colitis. 2013;7:451–459. [DOI] [PubMed] [Google Scholar]
- 26.Alkim H, Ayaz S, Alkim C, et al. Continuous active state of coagulation system in patients with nonthrombotic inflammatory bowel disease. Clin Appl Thromb Hemost. 2011;17:600–604. [DOI] [PubMed] [Google Scholar]
- 27.Owczarek D, Cibor D, Salapa K, et al. Reduced plasma fibrin clot permeability and susceptibility to lysis in patients with inflammatory bowel disease: a novel prothrombotic mechanism. Inflamm Bowel Dis. 2013;19:2616–2624. [DOI] [PubMed] [Google Scholar]
- 28.Saibeni S, Saladino V, Chantarangkul V, et al. Increased thrombin generation in inflammatory bowel diseases. Thromb Res. 2010;125:278–282. [DOI] [PubMed] [Google Scholar]
- 29.Skogen WF, Senior RM, Griffin GL, et al. Fibrinogen-derived peptide B beta 1-42 is a multidomained neutrophil chemoattractant. Blood. 1988;71:1475–1479. [PubMed] [Google Scholar]
- 30.Drake WT, Lopes NN, Fenton JW, II, et al. Thrombin enhancement of interleukin-1 and tumor necrosis factor-alpha induced polymorphonuclear leukocyte migration. Lab Invest. 1992;67:617–627. [PubMed] [Google Scholar]
- 31.Sower LE, Froelich CJ, Carney DH, et al. Thrombin induces IL-6 production in fibroblasts and epithelial cells. Evidence for the involvement of the seven-transmembrane domain (STD) receptor for alpha-thrombin. J Immunol. 1995;155:895–901. [PubMed] [Google Scholar]
- 32.Kwasny-Krochin B, Gluszko P, Undas A. Unfavorably altered fibrin clot properties in patients with active rheumatoid arthritis. Thromb Res. 2010;126:e11–e16. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e185S–e194S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novacek G, Weltermann A, Sobala A, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779–787, 787.e771. [DOI] [PubMed] [Google Scholar]
- 35.Higgins PD, Skup M, Mulani PM, et al. Increased risk of venous thromboembolic events with corticosteroid vs biologic therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;13:316–321. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–848.e836. [DOI] [PubMed] [Google Scholar]
- 37.Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: special situations. J Crohns Colitis. 2010;4:63–101. [DOI] [PubMed] [Google Scholar]
- 38.Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1–33. [DOI] [PubMed] [Google Scholar]