Abstract
Objective
To determine if there is a difference in the risk of endophthalmitis after an intravitreal steroid injection compared to an anti-vascular endothelial growth factor agent (anti-VEGF) injection
Design
Retrospective cohort study
Participants
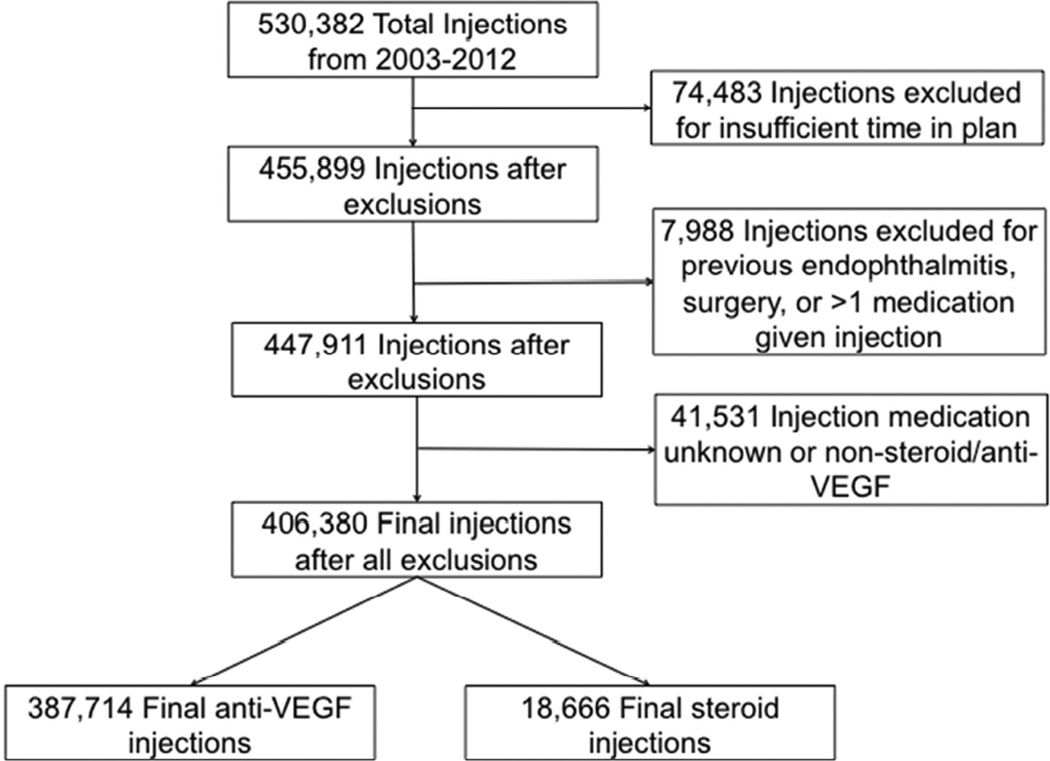
75,249 beneficiaries in a large national US medical claims database representing 406,380 intravitreal injections
Methods
Data were searched for all intravitreal injections (CPT 67028) performed between 2003 and 2012. Cohorts were created based on injections using anti-VEGF agents (bevacizumab, ranibizumab, aflibercept and pegaptanib) and intraocular steroids (triamcinolone and dexamethasone). Endophthalmitis was defined as having a new endophthalmitis diagnosis (ICD9 360.0×) and either a “tap-and-inject” procedure (CPT67015, 67025), a vitrectomy (67036) or an intravitreal antibiotic injection on the same day, between 1 and 14 days post-injection. Exclusion occurred for any history of endophthalmitis, <6 months in the plan or <1 month follow up. The main outcome measure was the odds of endophthalmitis using logistic regression while controlling for injection-associated diagnosis, age, race and gender.
Results
387,714 anti-VEGF injections and 18,666 steroid intravitreal injections were performed and were followed by 73 (rate=0.019% or 1/5283 anti-VEGF injections) and 24 (rate= 0.13% or 1/778 steroid injections) cases of endophthalmitis respectively. After controlling for diagnosis, age, race and gender, the odds ratio for endophthalmitis occurring was 6.92 (95% CI: 3.54–13.52, p<0.001) times higher post-steroid injection compared to anti-VEGF injections.
Conclusions
The rate of endophthalmitis post-intravitreal steroid injection in a national cohort was 0.13% (1/778 injections). This rate conferred a significantly increased odds ratio of 6.92 for endophthalmitis compared to anti-VEGF agents.
Keywords: endophthalmitis, steroids, anti-VEGF, intravitreal injections, pharmacoepidemiology
Introduction
Intravitreal injections have become a central component of treating numerous retinal diseases.1–3 Due to the proven efficacy of injections and patients’ acceptance of this form of treatment, the number of medications delivered in this manner continues to expand.4–6 In addition to anti-vascular endothelial growth factor (anti-VEGF) agents, older medication classes, such as steroids, have grown in number as well. The past decade has seen the FDA approve new formulations for intravitreal steroids including the dexamethasone implant (Ozurdex®, Allergan, Irvine, CA) and a preservative free triamcinolone (TRIESENCE®, Alcon, Fort Worth, TX). These are being offered as alternative therapies for various retinal diseases in place of the more commonly used off-label generic form of triamcinolone.7
Although rare, endophthalmitis remains the most visually devastating adverse event associated with intravitreal injections. Despite the increase in choices, little is known about the comparative risk of endophthalmitis after intravitreal steroids. The largest study to date combined 2 clinical trials to find 1 case of endophthalmitis out of 2009 steroid injections for a rate of 0.05% (95% confidence interval (CI) 0.001% to 0.277%).8 Other large observational series found rates that ranged from 0% (0 cases in 1135 injections) to 0.87% (8/922).9–11 These studies and others have inferred the possibility of a higher endophthalmitis risk for steroids compared to other medication classes, but due to small sample sizes of injections and sparse cases, no direct testing of this theory has been reported.7,12,13
Ongoing studies are currently evaluating the role intraocular steroids should play in macular edema secondary to both diabetes and retinal vein occlusions (RVO).14,15 An informed therapeutic recommendation; however, will not be complete without knowing all of the comparative benefits and risks, including rates of adverse events. The objective of this study is to describe the rate of endophthalmitis after an intravitreal steroid injection in a large national U.S. cohort and compare the rate of endophthalmitis post intravitreal injection to that of anti-VEGF agents.
Methods
Dataset
The Clinformatics Data Mart Database (OptumInsight, Eden Prairie, MN) was used for this study. This is an administrative medical claims database that contains the de-identified medical claims of all beneficiaries from a large insurer in the United States. It includes all outpatient medical claims (office visits, procedures and medications given), as well as demographic data for each beneficiary during their enrollment in the insurance plan. The subset of data available for this study included all patients in the database from January 1st, 2003 to December 31, 2012. Due to the de-identified nature of the database, the University of Pennsylvania’s Institutional Review Board deemed this study exempt from review.
Subjects
Two cohorts were created based on the type of medication used during the intravitreal injection, steroid or anti-VEGF agent. All forms of triamcinolone and dexamethasone available for intravitreal use were aggregated into the steroid cohort, whereas all injections of bevacizumab, ranibizumab, aflibercept, and pegaptanib were collected for the anti-VEGF cohort. Each instance of an intravitreal injection (CPT code 67028) was used as the index date and was considered an independent observation. For inclusion into the study, individuals had to have at least 6 consecutive months in the insurance plan prior to the index date and 1 month after the index date. Each patient that had a bilateral code (CPT 50) used with an intravitreal injection code was counted to have had 2 injections. (See Online only Table 1 for complete list of codes used during this study.)
Injections were excluded from analysis for any previous diagnosis of endophthalmitis. To reduce the possibility that endophthalmitis was associated with a procedure other than an intravitreal injection, all index dates that occurred less than 15 days after an intraocular surgery or had an intraocular surgery occur between the index date and a diagnosis of endophthalmitis were excluded as well. Lastly, intravitreal injections that had a drug code for both a steroid and anti-VEGF agent on the same day were also removed from analysis.
Outcome measures
The main outcome was the occurrence of endophthalmitis. This was considered to have occurred when a new diagnosis of endophthalmitis was made in conjunction with having a “tap and inject” procedure, a vitrectomy or an intravitreal injection of antibiotics. Cases had to occur from 1–14 days after index date. Those that occurred on day “0” (the same day as the index date) were excluded due to an inability to distinguish which treatment occurred first during the 24-hour period of the day.
The primary outcome measure was the odds of developing endophthalmitis after an intravitreal injection of steroids compared to an intravitreal injection of an anti-VEGF agent. Odds ratios were calculated by logistic regression for both univariate and multivariate analyses. Covariates of interest included basic demographic information; age, race and gender which were collected at the time of the index date. Additionally the injection–associated diagnosis was categorized as either age-related macular degeneration (AMD), diabetic macular edema (DME), retinal vein occlusions (RVO) or other. It has been previously suggested that the rate of endophthalmitis after intravitreal injection is decreasing with time.13 To account for this possible variation in rates with time in the model, the year the injection was performed was included. STATA® 12 (College Station, Texas) software was used for all statistical analysis. Results of the analyses were considered statistically significant for p<0.05 (two-tailed).
Results
A total of 406,380 intravitreal injections given to 75,249 patients between 2003 and 2012 met inclusion criteria for the study. Of these 387,714 injections were for Anti-VEGF agents and 18,666 were for steroids. (See Table 2 for injection demographics and types.) Generic triamcinolone accounted for the majority of steroid injections (67.7%) and bevacizumab (76.6%) was the most common anti-VEGF agent. The steroid cohort was significantly younger and had higher percentages of males and Black patients compared to the anti-VEGF cohort (p<0.001 for all comparisons). This cohort also had higher percentages of injections associated with diabetic and “other” diagnoses (p<0.001) compared to anti-VEGF agents, which were mostly associated with AMD.
Table 2.
Baseline characteristics of anti-vascular endothelial growth factors (anti-VEGF) and steroids
| Anti-VEGF | Steroid | P value | |
|---|---|---|---|
| Total injections | 387,714 | 18,666 | |
| Avg age in years (±SD) | 75.1(9.6) | 66.3(11.9) | <0.001 |
| Female | 59.3% | 51.6% | <0.001 |
| Race (%) | <0.001 | ||
| White | 79.6% | 71.0% | |
| Black | 6.4% | 12.0% | |
| Hispanic | 4.4% | 7.9% | |
| Asian | 1.3% | 1.8% | |
| Other/Unknown | 8.3% | 7.3% | |
| Diagnosis (%) | <0.001 | ||
| Age-related Macular Degeneration | 309,692 (79.9%) | 2,412 (12.9%) | |
| Diabetes Mellitus | 36,788 (9.4%) | 6,455 (34.6%) | |
| Retinal Vein Occlusion | 30,836 (8.0%) | 2,972 (15.9%) | |
| Other | 8,210 (2.1%) | 6,703 (35.9%) | |
| Multiple | 2,188 (0.6%) | 124 (0.7%) | |
| Injections/calendar year | |||
| 2003 | 0 | 444 | |
| 2004 | 0 | 1,166 | |
| 2005 | 2,097 | 1,786 | |
| 2006 | 8,468 | 1,837 | |
| 2007 | 18,347 | 1,864 | |
| 2008 | 27,806 | 1,879 | |
| 2009 | 44,819 | 2,279 | |
| 2010 | 68,043 | 2,192 | |
| 2011 | 98,703 | 2,309 | |
| 2012 | 119,431 | 2,910 | |
| Number of Injections | |||
| Bevacizumab | 296,942 | ||
| Ranibizumab | 87,305 | ||
| Pegaptanib | 3410 | ||
| Aflibercept | 57 | ||
| Triamcinolone | 12,634 | ||
| Pres. Free Triamcinolone | 4,690 | ||
| Dexamethasone | 627 | ||
| Dexamethasone Implant | 715 |
Ninety-seven cases of endophthalmitis were identified between the two cohorts (73 in the anti-VEGF cohort and 24 in the steroid cohort). On average these cases occurred 4.15 (SD±3.31) days after injection. Rates of endophthalmitis were 0.02% (1/5324) for anti-VEGF injections and 0.13% (1/778) injections in the steroid group. Univariate logistic regression analysis (Table 3) showed no significant associations between post injection endophthalmitis and gender (OR=0.90, 95% CI: 0.63–1.30; p=0.57) or race (ORs=0.69–1.10, p=0.35–0.79 for all races). Each year of older age slightly decreased the odds of endophthalmitis (OR=0.98, 95% CI: 0.97–1.00; p=0.012). Each successive year in which the injection was performed was associated with decreased odds of endophthalmitis (i.e. an injection performed in 2012 was associated with a decreased OR of endophthalmitis of 0.86 compared to 2011) (OR=0.86, 95% CI: 0.79–0.95; p<0.001). With diabetes as the comparator group, AMD had a significantly lower odds (OR=0.56, 95% CI: 0.33–0.92, p=0.023) and the “other” group of diagnoses had a significantly higher odds of endophthalmitis (OR=2.18, 95% CI: 1.14–4.20; p=0.019). RVO had no significant association. Intravitreal steroid injections had a significantly increased odds ratio for endophthalmitis of 6.85 (95% CI: 4.32–10.87, p<0.001).
Table 3.
Unadjusted odds ratios for developing endophthalmitis
| Unadjusted Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age | 0.98 | 0.97–1.00 | 0.012 |
| Gender* | 0.90 | 0.63–1.30 | 0.57 |
| Year of Injection** | 0.86 | 0.79–0.95 | 0.001 |
| Race*** | |||
| Black | 1.10 | 0.55–2.17 | 0.79 |
| Hispanic | 0.69 | 0.26–1.89 | 0.48 |
| Asian | - | - | - |
| Unknown | 0.69 | 0.32–1.49 | 0.35 |
| Diagnosis**** | |||
| AMD | 0.56 | 0.33–0.92 | 0.023 |
| Retinal Vein Occlusion | 0.63 | 0.29–1.40 | 0.26 |
| Other | 2.18 | 1.14–4.20 | 0.019 |
| Multiple | - | - | - |
| Steroids | 6.85 | 4.32–10.87 | <0.001 |
Male comparator
Compares each successive year
White is the comparator group
Diabetes as the comparator group
“-“ Too little data for calculation
After controlling for diagnosis, race, age, year of injection and gender in the multivariate analysis, the steroids odds ratio for endophthalmitis was still significantly higher at 6.92 (95% CI: 3.54–13.52, p<0.001) compared to anti-VEGF agents. No other covariates had a significant association with the occurrence of endophthalmitis once each of the other variables was adjusted for in the model.(Table 4) There was insufficient data to calculate odds ratios for individual types of steroids.
Table 4.
Multivariate odds ratios for developing endophthalmitis
| Adjusted Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age | 0.99 | 0.97–1.01 | 0.47 |
| Gender* | 1.01 | 0.67–1.52 | 0.96 |
| Year of Injection** | 0.99 | 0.89–1.10 | 0.813 |
| Race*** | |||
| Black | 0.86 | 0.40–1.89 | 0.71 |
| Hispanic | 0.54 | 0.17–1.72 | 0.30 |
| Asian | - | - | - |
| Unknown | 0.72 | 0.31–1.64 | 0.43 |
| Diagnosis**** | |||
| AMD | 1.19 | 0.58–2.43 | 0.63 |
| Retinal Vein Occlusion | 0.93 | 0.38–2.24 | 0.87 |
| Other | 1.15 | 0.51–2.59 | 0.74 |
| Multiple | - | - | - |
| Steroids | 6.92 | 3.54–13.52 | <0.001 |
Male comparator
Compares each successive year
White is the comparator group
Diabetes as the comparator group
“-“ Too little data for calculation
Discussion
Using a national database that included 18,666 intravitreal steroid injections, a post-injection endophthalmitis rate of 0.13% (1/778) was found. This rate corresponds well with earlier reports, which have ranged from 0% (0/1135 injections) to 0.87% (8/922).9–11 After adjusting for medication class, this study showed no significant association between endophthalmitis and the injection-associated disease. This contradicts the previously reported idea that injections given to patients with diabetes mellitus are at a higher risk for endophthalmitis due to relative immune suppression.10 Additionally, after controlling for medication class, no association was seen between year of injection and endophthalmitis, suggesting previous reports showing a decrease in endophthalmitis rates after injections over time where more likely related to a change in the type of injections being used than an increase in the safety of the procedure.
Recently, much of the focus of intravitreal injection related endophthalmitis research has been concentrated on the anti-VEGF agents due to their exponential increase in usage since 2006. The idea that steroids may confer a higher risk for post-injection endophthalmitis; however, is not a new one and has been postulated previously.7,12,13 Despite these concerns, a comparative study to test these ideas has never been performed, likely due to the infrequency of the outcome. To the best of the authors’ knowledge, this study represents the largest collection of intravitreal steroid-related injections and cases of endophthalmitis to date. Because of this large sample size, we were able to find that the use of steroids was associated with significantly increased odds of nearly 7 times that of anti-VEGF agents for post-injection endophthalmitis.
Several potential reasons exist to account for the difference in the elevated comparative risk found in this study. First, the gauge of the needle used for intravitreal injections is different between the medication classes (30- or 32-gauge for anti-VEGF agents; 27-, 25-gauge for triamcinolone and 22-gauge for the dexamethasone implant). This may suggest that larger wound tracts from the needles used in steroid injections allow for easier bacterial penetration into the vitreous. Additionally, the immunosuppressive nature of the steroids themselves likely contributes to these findings. Evidence for this is seen in a previous study that injected rabbit eyes with steroids and various bacterial loads. The eyes that received steroids required significantly lower bacterial counts to induce endophthalmitis compared to control eyes and also possibly reduced the time required for infection to occur.16
Every study needs to be evaluated within the context of its design. For this study, the most significant limitation is the lack of lab culture results which are not found in administrative claims data. This prevents the ability to verify endophthalmitis cases at the chart level. Not having this information could alter the study results if “sterile” endophthalmitis occurs at a considerably higher rate in steroid injections than in anti-VEGF injections.
Although this is possible, several points about this limitation need to be considered. First, despite multiple reports on sterile endophthalmitis, it is still a rare event, and as such, definitive rates of occurrence are difficult to reliably calculate, especially when making comparisons across medication classes. Additionally, limited evidence exists that “sterile” endophthalmitis is frequently observed without treatment17, meaning these cases would not be included in the study since they would not meet the study’s definition for endophthalmitis. Furthermore, the association between the increased odds of endophthalmitis and steroids is such a strong one that statistically 15 of the 23 steroid and none of the anti-VEGF associated cases would have had to be falsely diagnosed to make this association no longer significant. Lastly, recent work showing high rates of torque teno virus in culture-negative endophthalmitis has blurred the previously sharp distinction between infectious and “sterile” endophthalmitis altogether.18
Another limitation to this study is the inability to reliably study or control for pre- or post-injection antibiotic use. Administrative claims data only includes data on outpatient prescriptions that have been filled. For many years a significant number of patients were given topical antibiotic drops through in-office samples, making the tracking of this variable difficult at best in claims data. Similarly, the database does not have specific data on the setting of the injection (office-based vs. operating room); therefore differences between these settings cannot be tested. Despite this, it is reasonable to assume the vast majority of injections with this study were performed in the office as that is the predominate practice pattern throughout the United States. Next, a small possibility exists for a patient to be erroneously diagnosed with uveitis, injected with a steroid and only days later be correctly diagnosed with endophthalmitis. Although rare, this situation is unlikely to occur with an anti-VEGF gent, and therefore, would bias towards a comparatively higher endophthalmitis rate for a steroid injection. A last limitation to note is that despite no fewer than 600 of any one type of steroid, the individual sample sizes are too small to make reliable intra-class endophthalmitis risk comparisons.
Steroids have long been a proven beneficial therapeutic option in various retinal diseases. Interest into steroid research has risen lately due to the relatively recent availability of a preservative free formulation of triamcinolone and the dexamethasone implant. Some studies citing comparable visual acuity results as anti-VEGF agents and fewer injections have even begun suggesting intravitreal steroids should be first-line treatment for diabetic macular edema.19,20 Most clinical decisions are not based on just the potential benefits, but also must balance the known potential risks. This study supports the idea that the risk of endophthalmitis is not equal between treatment options and should be factored into therapeutic decision-making.
Supplementary Material
Figure 1.
Counts of injections after exclusion criteria:
Acknowledgments
Financial Support: National Institutes of Health K12 Award (Dr. Brian VanderBeek, K12-EY015398). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicting relationship exists for any author
References
- 1.Holekamp NM, Liu Y, Yeh WS, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. American journal of ophthalmology. 2014;157:825–833. doi: 10.1016/j.ajo.2013.12.018. e1. [DOI] [PubMed] [Google Scholar]
- 2.Kiss S, Liu Y, Brown J, et al. Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema. Clin Ophthalmol. 2014;8:1611–1621. doi: 10.2147/OPTH.S60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lad EM, Hammill BG, Qualls LG, et al. Anti-VEGF Treatment Patterns for Neovascular Age-Related Macular Degeneration Among Medicare Beneficiaries. American journal of ophthalmology. 2014 doi: 10.1016/j.ajo.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009;29:875–912. doi: 10.1097/IAE.0b013e3181a94f01. [DOI] [PubMed] [Google Scholar]
- 5.Francis JH, Marr BP, Brodie SE, et al. Tethered vitreous seeds following intravitreal melphalan for retinoblastoma. JAMA ophthalmology. 2014;132:1024–1025. doi: 10.1001/jamaophthalmol.2014.436. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. Journal of ophthalmic inflammation and infection. 2013;3:32. doi: 10.1186/1869-5760-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao Y, Jonas JB. Intravitreal triamcinolone. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 2011;225:1–20. doi: 10.1159/000317909. [DOI] [PubMed] [Google Scholar]
- 8.Bhavsar AR, Ip MS, Glassman AR. The risk of endophthalmitis following intravitreal triamcinolone injection in the DRCRnet and SCORE clinical trials. American journal of ophthalmology. 2007;144:454–456. doi: 10.1016/j.ajo.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westfall AC, Osborn A, Kuhl D, et al. Acute endophthalmitis incidence: intravitreal triamcinolone. Archives of ophthalmology. 2005;123:1075–1077. doi: 10.1001/archopht.123.8.1075. [DOI] [PubMed] [Google Scholar]
- 10.Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. American journal of ophthalmology. 2003;136:791–796. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 11.Jonas JB, Kreissig I, Spandau UH, et al. Infectious and non-infectious endophthalmitis after intravitreal high-dosage triamcinolone acetonide. American journal of ophthalmology. 2006;141:579–580. doi: 10.1016/j.ajo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CS, Wong AW, Lui A, et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119:1609–1614. doi: 10.1016/j.ophtha.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Nentwich MM, Yactayo-Miranda Y, Schwarzbach F, et al. Endophthalmitis after intravitreal injection: decreasing incidence and clinical outcome-8-year results from a tertiary ophthalmic referral center. Retina. 2014;34:943–950. doi: 10.1097/IAE.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed March 10th 2015];Phase II Combination Steroid and Anti-VEGF for Persistent DME. 2014 2015, at https://clinicaltrials.gov/ct2/show/NCT01945866?term=DRCR+protocol+U&rank=1.)
- 15.Ozurdex Versus Ranibizumab Versus Combination for Central Retinal Vein Occlusion (ORION) ClinicalTrials.gov. 2013 (Accessed at https://clinicaltrials.gov/ct2/show/NCT01827722.)
- 16.Bucher RS, Hall E, Reed DM, et al. Effect of intravitreal triamcinolone acetonide on susceptibility to experimental bacterial endophthalmitis and subsequent response to treatment. Archives of ophthalmology. 2005;123:649–653. doi: 10.1001/archopht.123.5.649. [DOI] [PubMed] [Google Scholar]
- 17.Nelson ML, Tennant MT, Sivalingam A, et al. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003;23:686–691. doi: 10.1097/00006982-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Lee AY, Akileswaran L, Tibbetts MD, et al. Identification of torque teno virus in culture-negative endophthalmitis by representational deep DNA sequencing. Ophthalmology. 2015;122:524–530. doi: 10.1016/j.ophtha.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121:2473–2481. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Khurana RN, Palmer JD, Porco TC, Wieland MR. Dexamethasone intravitreal implant for pseudophakic cystoid macular edema in patients with diabetes. Ophthalmic surgery, lasers & imaging retina. 2015;46:56–61. doi: 10.3928/23258160-20150101-09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.