Abstract
The new mammalian growth factor peptide family consists of three peptides, TFF1, TFF2, and TFF3, which are secreted mainly from mucous epithelia with mucus gel. The predominant secretion of trefoil factor (TFF) occurs from gastric mucosal lining, small and large intestine, oral mucosal cells, and salivary glands. Research regarding trefoil factors is an immerging aspect in the dental field. The mucosal healing and restitution function describes about its novel role in case of chronic inflammatory conditions, but its expression from different tissue at different pathological condition shows its importance in immune response. At present, TFF expression has been detected from the severe periodontal diseased tissue samples. Future research from mild to moderate chronic periodontal diseased condition should be carried out to assess the protective response of TFF in gingival tissues. In future, assessment of TFF levels and its expression in oral mucosal tissues and oral secretions, such as saliva and gingival crevicular fluid, will provide a negative biomarker for chronic periodontal diseases and a novel therapeutic agent in oral mucosal healing.
Keywords: Peptide, Mucosa, Restitution, Chronic periodontitis
1. Introduction
The trefoil factors (TFF) are soluble proteins, comprising of small (12–22 kD) peptides, which have a common three leaved/looped structure formed by inter-chain disulphide bond. The first member of this family was discovered around thirty years back in 1980s, and was named as PSP (pancreatic spasmolytic peptide).1 The mammalian trefoil factor family consists of three peptides, TFF1, TFF2 and TFF3, which are secreted mainly from mucous epithelia with mucus gel.2 The predominant secretion of TFF occurs from gastric mucosal lining, small and large intestine, oral mucosal cells, and salivary glands.3 They are also expressed within the brain.4 The trefoil structures form homo- or hetero-dimers, either with the same peptide or with other trefoil peptide,5 and this dimerization is responsible for proteolysis and acid resistance against harsh internal environment.6 Different members of the family are expressed by different cells, such as TFF1 is expressed mainly from gastric mucosal lining,3 while TFF2 is expressed mainly from Brunner's gland of the duodenum.2 TFF3 is expressed mainly by intestinal goblet cells.3 In 1982, TFF2 was the first member of this family discovered during purification of insulin from the porcine pancreatic cells. Initially, it was named as PSP, as it showed the inhibitory effect on gastric secretion.2 The second member discovered of this family was TFF1 and was initially named as human breast cancer associated peptide 2, as it was discovered unintentionally with the gene regulated by estrogen in breast cancer cells (MCF-7).7 TFF3 is the last known member of this family, which was cloned from rat intestinal epithelial cells and was named as intestinal trefoil factor.8 The human homologue was then cloned and named hP1B.9
2. Molecular structure of TFFs
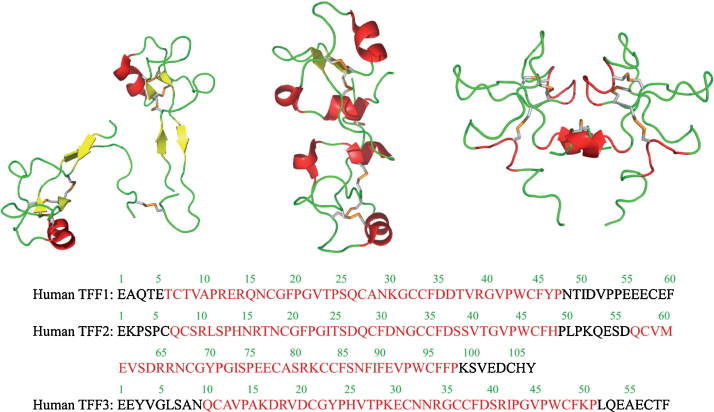
Thim L in 1989 described a new family of growth factor like peptides, which he named as “Trefoil”. He found that disulphide loop structure was a common feature in breast cancer cell associated peptide (pS2), PSP, and frog skin peptides (spasmolysines).10 Cysteine residual parts of the trefoil domain form the disulphide bond in 1–5, 2–4, 3–6 configuration.8, 10 TFF2 molecule has basically two trefoil domains attached by an interface, while TFF1 and TFF3 contain single trefoil domain. There is a free cysteine residue in position 57 and it is found to be essential for dimer configuration. The naturally occurring TFF1 and TFF3 consist of both monomers and dimers.11 The compact structure of TFF2 makes it highly resistant against the acidic environment of upper gastrointestinal tract and trypsin- and chymotrypsin-like protease enzymes.12 There is a cleft between second and third loop of the domain, which serves as the prime binding site for the oligosaccharides. This site may be used to bind the mucins and may help to maintain the mucus layer. This binding of mucins requires two domains of trefoil, and hence supports the natural monomeric existence of TFF1 and TFF3 (Fig. 1).11
Fig. 1.
3. Physiological expression of TFFs
In normal physiological condition, TFFs are mainly secreted from gastrointestinal epithelial cells and are packaged with mucus granules in the Golgi bodies to form the protective mucus layer.13
TFF1 – Stomach and upper gastrointestinal ducts are the major sites of TFF1 expression.13, 14 Small amount of TFF1 is also found to be expressed from respiratory epithelium,15 upper part of Brunner's gland, and some goblet cells. Only some few patchy cells of pancreas, gall bladder, and the duct of luminal breast cells are also found to secret TFF1.16
TFF2 – It is secreted from mucus gland of gastric mucosa, Brunner's gland acini, and distal ducts of duodenum. Some patchy and focal expression of TFF2 is observed from pancreas and gall bladder duct epithelia.13, 16
TFF3 – It is mainly secreted by intestinal epithelial and goblet cells. TFF3 is also found to be expressed from human uterus, human breast, some parts of hypothalamus, and pituitary glands in brain.4, 16 Along with TFF1, it is also found to be secreted from respiratory epithelium.15
Other than gastrointestinal tract, varying degrees of TFFs have also been seen to be expressed from respiratory, ocular mucus secreting cells,17 as well as from salivary glands,18, 19 male and female reproductive organs,20, 21 and human breast milk producing cells.22 TFF3 serum levels have been seen fluctuating during pregnancy.23 Heart, muscles, and lymphoid tissue have also been found to express TFFs.24 The diverse expression of TFFs throughout the body suggests that these peptides could have multiple and vibrant functions in the different body parts, but all the physiological aspects are still not known.
4. Pathological expression of TFFs
Wright et al. in 1990,25 described the ectopic expression of TFF1 and TFF2 in chronic gastrointestinal ulceration and it was termed as “ulcer associated cell lineage (UACL)”. UACL is also seen in inflammatory bowel disease, ulcerative colitis, peptic gastric and duodenal ulcers, chronic diverticulitis, and cholecystic patients.25, 26
TFFs are also expressed aberrantly in about 50% of gastric tumors27 and the TFF genes were found suppressed in so many cancers, such as gastric and intestinal cancers, prostate cancer,20 breast cancer,28 pancreatic, liver and gall bladder cancers,29 and oral cancers.30 In these cancerous conditions, TFFs have been used as negative prognostic markers. The levels of salivary TFF1 and TFF3 were seen to be reduced in chronic periodontitis patients. Gingival tissues of chronic periodontitis patients also showed decreased TFF3 expression. Salivary TFF3 expression is decreased in periodontal pathogenesis and is negatively related with the prominent periodontopathogens, such as Porphyromonas gingivalis and Tannerella forsythia.19
5. Functions of TFFs
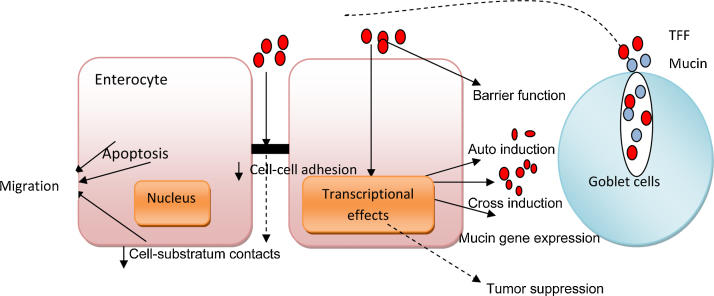
TFFs perform various functions, which include proliferation, antiapoptosis, wound repair, regeneration, neovascularisation, mucin interaction, etc. (Fig. 2).
Fig. 2.
Functions of trefoil factors.31
5.1. Restitution and regeneration
Restitution is the process that promotes epithelial-cell migration to reseal superficial wounds after injury. This involves the rapid disassembly of cell–cell and cell–substratum adhesion, de-differentiation, and spreading of surface cells over basement membrane. This is independent of cell proliferation. The process of restitution helps to prevent the loss of electrolytes and plasma from the injured tissue to outside the environment and also prevents the bacterial or foreign material invasion. It is a step in mucosal repair, which occurs just before regeneration, to restore the discontinuous mucosal lining.31
TFFs have been found to promote epithelial-cell restitution as their prime and fundamental physiological function. The recombinants of TFF1 and TFF3 have been seen to produce both monomeric and dimeric forms, which induce cell migration, but the bioactivity of monomeric TFF1 is one-eighth times less than dimeric form. Whereas, a mutation in TFF3 (Cys 57Ser – Cysteine 57 Serine) was unable to produce dimeric form and could not induce cell migration. It shows that dimerization is the important step to induce cell migration and restitution. Monomeric TFF2 contains two trefoil domains, which can induce cell migration. Epithelial repair and regeneration includes proliferation and differentiation of epithelium as two main steps. It follows the remodeling phase. TFFs help the injured mucosa to form a single layer of cells, which acts as a physical barrier, and it promotes mucosal healing.31
5.2. Migration of epithelial cells
In epithelial restitution, migration is the important step in which epithelial cells get elongated and migrate on the surface to restore the denudation. The dimerization of TFF1 and TFF3 has been found to initiate cell migration and further restitution, whereas the monomeric form could not produce cell migration and dependent restitution. The monomeric TFF2 with two trefoil domains is found to be efficient for cell migration process. TFFs have been shown to downregulate E-cadherin/b catenin receptors in cell-to-cell adhesion junctions and promote the pro-migratory effects.32 TFF3 showed beneficial effects on methotrexate-induced enteritis in an in vivo study, by enhancing the epithelial restitution on the gastrointestinal epithelial cell layers, which was damaged by methotrexate.33
5.3. Antiapoptotic property
Antiapoptotic property is very important for epithelial restitution, where epithelial cell anchorage is detached and the epithelial surface area becomes denuded. TFF3 deficiency in rat increases apoptotic cell numbers in the colonic crypts.34 TFF3 has shown antianoikic effects on the intestinal epithelial cells via NFκB signal activation. Anoikis is a form of apoptosis, in which there is disruption of cell–substratum adhesion and loss of anchorage. Antiapoptotic and antianoikic effects of TFF3 is EGF-R (epidermal growth factor-receptor) dependent and it requires TFF3 dimer.31
5.4. Angiogenesis
TFF3 has been found to induce neovascularization in human gastric carcinoma.35 TFF3 has also been found to induce hypoxia inducing factor 1 angiogenesis.36 The pro-angiogenic property of all TFFs has been compared to that of the vascular endothelial growth factor. The neovascularization property of TFFs is found to be dependent on cycloxygenase 2 (COX2) and EGF-R signal transduction.37
5.5. Immunomodulation
TFFs have been found to decrease the pro-inflammatory mediators, such as COX2 and inducible nitric oxide synthase (iNOS).38 Human TFF2 (hTFF2) has been found to decrease bacterial lipopolysaccharide (LPS)-induced iNOS and NO synthesis from monocytes.38 TFF3 acts as anti-inflammatory agent as it downregulates the NFκB signal transduction by upregulating a transcription factor TWIST.39
Tebbutt et al.40 described in their study that TFFs are increased in amount when the pro-inflammatory interleukin-6 and IL-11 get activated via either theSHP2/Erk or the JAK/STAT signal transduction pathway. The gp-130 protein gets activated by these pathways, which have direct and reciprocal effect on the transcription of TFF1 and TFF3. Other authors found that IL-6, IL-1β, and tumor necrosis factor-α decrease the transcription of TFF1 and TFF3 via C/EBPb signal transduction pathway.41 Studies suggest that TFFs can be regulated by pro-inflammatory cytokines and transcription factors, such as NFκB.39, 41
Thim et al. described about a pTFF2 binding protein (the human homologue is the gp-340 protein encoded by the DMBT-1 gene) from the porcine gastric mucosal cell membrane lining.12 It was identified as 224 kDa CRP-ductin. This protein has been found to be expressed from intestinal walls, salivary glandular cells, respiratory epithelial lining, pancreas, liver, uterus, etc.42 CRP-ductin has shown to play a role in innate immune system by interfering macrophage actions and by activating complement cascade.42
6. TFFs expression in different oral tissues
6.1. In oral keratinocytes
TFF3 promotes wound healing and regulates cell survival, growth, differentiation, proliferation, and cell migration of oral keratinocytes. Microarray real-time qPCR of mRNA analysis of TFF3 showed that this peptide improved mechanical and chemical resistance of mucin. It also stimulates oral keratinocytes during wound healing. Human recombinant of TFF3 shows their beneficial effect on stratified epithelial cells of oral keratinocytes and oral epithelial carcinogenic cells. It acts as motogen in oral keratinocytes, which help in cell mobility toward surface, which ultimately promotes restitution and wound healing.43
6.2. In saliva
TFF3 peptide was found to be expressed from all the salivary glands. It is the only known member of TFF family found in whole saliva. TFF3 is secreted along with MUC7 mucin from serous salivary glands, such as submandibular gland. It has also been found to be secreted from mucus cells of sublingual gland. Parotid gland has been found as the major source of TFF3 expression as it is the major mucus secreting salivary gland detected by western blotting.43 TFF3 was also detectable from minor salivary glands. Research should be carried out whether these peptides are to be expressed from gingival crevicular fluids (CGF).
6.3. In periodontal tissues
Studies showed there is decreased expression of TFF3 in salivary secretion as well as in periodontal tissues in chronic periodontitis patients. When the periodontal pathogens like P. gingivalis and T. forsythia are increased in the periodontal tissues, the salivary TFF3 level decreases. Infections caused by periodontal pathogens ultimately inhibit the TFF3 gene expression. Expression of TFF in human gingival tissues has been assessed by immunohistochemical study. TFF expression is independent of gender in case of chronic periodontitis patients.19 The dimers of TFF3 play important role in formation of mucin cross-link, which results in the formation of protective layers. In chronic periodontitis, the TFF3 secretion is found to be decreased, which supports the negative correlation of TFF3 and chronic inflammatory conditions.19 Research is being done in different oral diseases, such as chronic periodontitis, stomatitis, mucositis, oral cancers, and other sources, such as oral mucosal glands, GCF, pro-inflammatory cells present in oral cavity, oral keratinocytes, etc., to investigate the TFF expression and mechanism of action.
6.4. In oral cancer
In oral squamous cell carcinoma conditions, TFF2 and TFF3 expressions are found to be decreased, when compared with normal oral mucosal cells. Tissue samples of healthy and OSCC patients were compared to investigate the TFF expression by immunohistochemistry and ELISA has been done for salivary samples. The results indicated that TFF is negatively correlated with OSCC. As the tumor increased in severity, the TFF expression was downregulated.30 The patients developing oral mucositis, as a side effect of chemotherapy for cancers, are being treated with TFF3 and these trials are in clinical phase II. The oral application of TFF3 has also been found to be effective against the development and recurrence of mucositis in cancerous patients.44
7. Conclusion
Research regarding trefoil factors is an emerging aspect in the dental field. The mucosal healing and restitution function describes about its novel role in case of chronic inflammatory conditions, but its expression from different tissue at different pathological condition shows its importance in immune response. At present, TFF expression has been detected from the severe periodontal diseased tissue samples. Future research from mild to moderate chronic periodontal diseased condition should be carried out to assess the protective response of TFF in gingival tissues. In future, assessment of TFF levels and its expression in oral mucosal tissues and oral secretions, such as saliva and GCF, will provide a negative biomarker for chronic periodontal diseases and a novel therapeutic agent in oral mucosal healing.
Conflicts of interest
The authors have none to declare.
References
- 1.Kjellev S. The trefoil factor family – small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66:1350–1369. doi: 10.1007/s00018-008-8646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen K.H., Thim L., Jacobsen H.E. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul Pept. 1982;3:207–219. doi: 10.1016/0167-0115(82)90126-4. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann W., Jagla W., Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol. 2001;16:319–334. doi: 10.14670/HH-16.319. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann W., Jagla W. Cell type specific expression of secretory TFF peptides: co localization with mucins and synthesis in the brain. Int Rev Cytol. 2002;213:147–181. doi: 10.1016/s0074-7696(02)13014-2. [DOI] [PubMed] [Google Scholar]
- 5.Chadwick M.P., Westley B.R., May F.E. Homodimerization and hetero-oligomerization of the single domain trefoil protein pNR-2/pS2 through cysteine 58. Biochem J. 1997;327(Pt 1):117–123. doi: 10.1042/bj3270117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westley B.R., Griffin S.M., May F.E.B. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C. Biochemistry. 2005;44:7967–7975. doi: 10.1021/bi047287n. [DOI] [PubMed] [Google Scholar]
- 7.Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10:7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suemori S., Lynch-Devaney K., Podolsky D.K. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci USA. 1991;88:11017–11021. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser F., Poulsom R., Chinery R. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci USA. 1993;90:6961–6965. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thim L. A new family of growth factor-like peptides. Trefoil disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins) FEBS Lett. 1989;250:85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- 11.Chinery R., Bates P.A., De A., Freemont P.S. Characterisation of the single copy trefoil peptides intestinal trefoil factor and pS2 and their ability to form covalent dimers. FEBS Lett. 1995;357:50–54. doi: 10.1016/0014-5793(94)01297-e. [DOI] [PubMed] [Google Scholar]
- 12.Thim L., May F.E. Structure of mammalian trefoil factors and functional insights. Cell Mol Life Sci. 2005;62:2956–2973. doi: 10.1007/s00018-005-5484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanby A.M., Poulsom R., Singh S., Elia G., Jeffery R.E., Wright N.A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- 14.Rio M.C., Bellocq J.P., Daniel J.Y., Tomasetto C., Lathe R., Chenard M.P. Breast cancer associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988;241:705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- 15.Santos S.E., Ulrich M., Doring G., Botzenhart K., Gott P. Trefoil factor family domain peptides in the human respiratory tract. J Pathol. 2000;190:133–142. doi: 10.1002/(SICI)1096-9896(200002)190:2<133::AID-PATH518>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 16.Piggott N.H., Henry J.A., May F.E., Westley B.R. Antipeptide antibodies against the pNR-2 oestrogen regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991;163:95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- 17.Langer G., Jagla W., Behrens-Baumann W., Walter S., Hoffmann W. Ocular TFF-peptides: new mucus-associated secretory products of conjunctival goblet cells. Adv Exp Med Biol. 2002;506:313–316. doi: 10.1007/978-1-4615-0717-8_44. [DOI] [PubMed] [Google Scholar]
- 18.Jagla W. Secretion of TFF-peptides by human salivary glands. Cell Tissue Res. 1999;298:161–166. doi: 10.1007/s004419900087. [DOI] [PubMed] [Google Scholar]
- 19.Chaiyarit P., Chayasadom A., Wara-Aswapati N., Hormdee D., Sittisomwong S., Nakaresisoon S. Trefoil factors in saliva and gingival tissues of patients with chronic periodontitis. J Periodontol. 2012;83:9. doi: 10.1902/jop.2011.110431. [DOI] [PubMed] [Google Scholar]
- 20.Garraway I.P., Seligson D., Said J., Horvath S., Reiter R.E. Trefoil factor 3 is overexpressed in human prostate cancer. Prostate. 2004;61:209–214. doi: 10.1002/pros.20096. [DOI] [PubMed] [Google Scholar]
- 21.Wiede A., Hinz M., Canzler E., Franke K., Quednow C., Hoffmann W. Synthesis and localization of the mucin-associated TFF-peptides in the human uterus. Cell Tissue Res. 2001;303:109–115. doi: 10.1007/s004410000297. [DOI] [PubMed] [Google Scholar]
- 22.Vestergaard E.M., Nexo E., Wendt A., Guthmann F. Trefoil factors in human milk. Early Hum Dev. 2008;10:631–635. doi: 10.1016/j.earlhumdev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Samson M.H., Vestergaard E.M., Milman N., Poulsen S.S., Nexo E. Circulating serum trefoil factors increase dramatically during pregnancy. Scand J Clin Lab Invest. 2008;68:369–374. doi: 10.1080/00365510701767862. [DOI] [PubMed] [Google Scholar]
- 24.Cook G.A., Familari M., Thim L., Giraud A.S. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett. 1999;456:155–159. doi: 10.1016/s0014-5793(99)00940-0. [DOI] [PubMed] [Google Scholar]
- 25.Wright N.A., Poulsom R., Stamp G.W. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990;162:279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- 26.Wright N.A., Poulsom R., Stamp G. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 27.Park W.S., Oh R.R., Park J.Y. Somatic mutations of the trefoil factor family 1 gene in gastric cancer. Gastroenterology. 2000;119:691–698. doi: 10.1053/gast.2000.16483. [DOI] [PubMed] [Google Scholar]
- 28.Rio M.C., Chambon P. The pS2 gene, mRNA, and protein: a potential marker for human breast cancer. Cancer Cells. 1990;2:269–274. [PubMed] [Google Scholar]
- 29.Ohshio G., Suwa H., Kawaguchi Y. Differential expression of human spasmolytic polypeptide (trefoil factor family-2) in pancreatic carcinomas, ampullary carcinomas, and mucin-producing tumors of the pancreas. Dig Dis Sci. 2000;45:659–664. doi: 10.1023/a:1005471005289. [DOI] [PubMed] [Google Scholar]
- 30.Chaiyarit P., Utrawichian A., Leelayuwat C. Investigation of trefoil factor expression in saliva and oral mucosal tissues of patients with oral squamous cell carcinoma. Clin Oral Investig. 2012;16:1549–1556. doi: 10.1007/s00784-011-0667-z. [DOI] [PubMed] [Google Scholar]
- 31.Taupin D., Podolsky D.K. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 32.Chinery R., Jawhari A., Hattori T., Wright N.A., Bodmer W.F., Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xian C.J., Howarth G.S., Mardell C.E. Temporal changes in TFF3 expression and jejunal morphology during methotrexate-induced damage and repair. Am J Physiol. 1999;277:G785–G795. doi: 10.1152/ajpgi.1999.277.4.G785. [DOI] [PubMed] [Google Scholar]
- 34.Taupin D.R., Kinoshita K., Podolsky D.K. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci USA. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhar D.K., Wang T.C., Tabara H. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res. 2005;11:6472–6478. doi: 10.1158/1078-0432.CCR-05-0671. [DOI] [PubMed] [Google Scholar]
- 36.Furuta G.T., Turner J.R., Taylor C.T. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues S., Van Aken E., Van Bocxlaer S. Trefoil peptides as proangiogenic factors in vivo and in vitro: implication of cyclooxygenase-2 and EGF receptor signaling. FASEB J. 2003;17:7–16. doi: 10.1096/fj.02-0201com. [DOI] [PubMed] [Google Scholar]
- 38.Giraud A.S., Pereira P.M., Thim L., Parker L.M., Judd L.M. TFF-2 inhibits iNOS/NO in monocytes, and nitrated protein in healing colon after colitis. Peptides. 2004;25:803–809. doi: 10.1016/j.peptides.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y.Q., Tan X.D. TFF3 modulates NF-κB and a novel negative regulatory molecule of NF-κB in intestinal epithelial cells via a mechanism distinct from TNF-α. Am J Physiol Cell Physiol. 2005;289:C1085–C1093. doi: 10.1152/ajpcell.00185.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tebbutt N.C., Giraud A.S., Inglese M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 41.Graness A., Chwieralski C.E., Reinhold D., Thim L., Hoffmann W. Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (IL-6) and IL-8 secretion. J Biol Chem. 2002;277:18440–18446. doi: 10.1074/jbc.M200468200. [DOI] [PubMed] [Google Scholar]
- 42.Madsen J., Tornoe I., Nielsen O. CRP-ductin, the mouse homologue of gp-340/deleted in malignant brain tumors 1 (DMBT1), binds gram-positive and gram-negative bacteria and interacts with lung surfactant protein D. Eur J Immunol. 2003;33:2327–2336. doi: 10.1002/eji.200323972. [DOI] [PubMed] [Google Scholar]
- 43.Storesund T., Schreurs O., Messelt E.B., Kolltveit K.M., Schenck K. Trefoil factor family 3 expression in the oral cavity. Eur J Oral Sci. 2009;117:636–643. doi: 10.1111/j.1600-0722.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 44.Barker N.P., Peterson D.E., Akhmadullina L.I. Prophylaxis of recurrent chemotherapy-induced oral mucositis: a phase II multicenter, randomized, placebo-controlled trial of recombinant human intestinal trefoil factor (rhITF) J Clin Oncol. 2008;26:9514. doi: 10.1200/JCO.2008.21.2381. [DOI] [PubMed] [Google Scholar]