Abstract
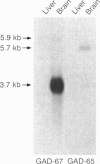
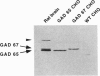
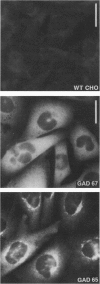
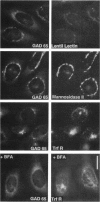
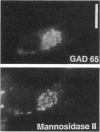
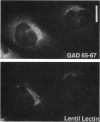
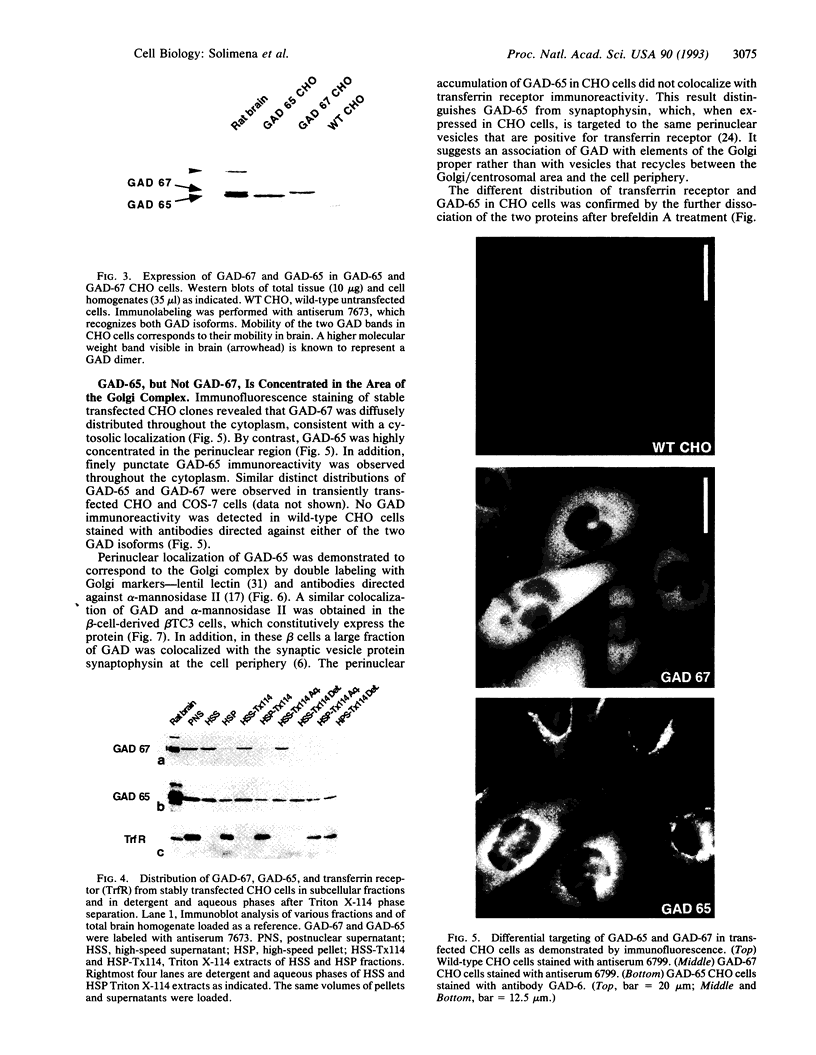
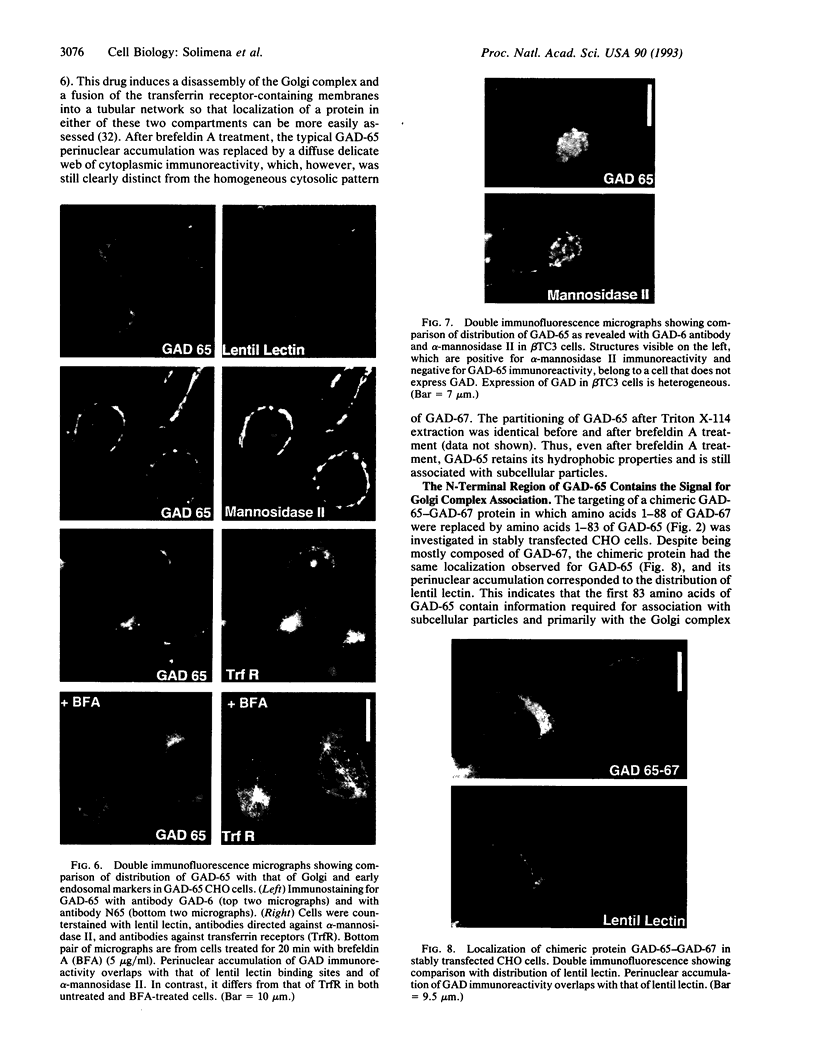
Glutamic acid decarboxylase (GAD) is the enzyme responsible for synthesis of the neurotransmitter gamma-aminobutyric acid in neurons and pancreatic beta cells. It is represented by two isoforms, GAD-65 and GAD-67, which are the products of two different genes and differ substantially only at their N-terminal regions. GAD-65 is a dominant autoantigen in stiff-man syndrome and insulin-dependent diabetes mellitus. In neurons and beta cells, GAD is concentrated around synaptic vesicles and synaptic-like microvesicles, respectively, as well as in the area of the Golgi complex. The mechanisms responsible for specific targeting of GAD to these organelles are not yet understood. The elucidation of the mechanism of subcellular targeting of GAD may be relevant to understanding its role as an autoantigen. In this study, the cloned genes for GAD-65 and GAD-67 were expressed separately in Chinese hamster ovary (CHO) cells and COS cells. While GAD-67 had a diffuse cytoplasmic localization, GAD-65 had a punctate distribution, with most of the immunoreactivity being concentrated in the area of the Golgi complex. A chimeric protein in which the 88 N-terminal amino acids of GAD-67 were replaced by the 83 N-terminal amino acids of GAD-65 was targeted to the Golgi complex, indicating that the N-terminal region of GAD-65 contains a targeting signal sufficient for directing the remaining portion of the molecule, highly similar in GAD-65 and GAD-67, to the Golgi complex-associated structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Cameron P. L., Südhof T. C., Jahn R., De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991 Oct;115(1):151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Gottlieb D. I. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988 Jun;8(6):2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christgau S., Aanstoot H. J., Schierbeck H., Begley K., Tullin S., Hejnaes K., Baekkeskov S. Membrane anchoring of the autoantigen GAD65 to microvesicles in pancreatic beta-cells by palmitoylation in the NH2-terminal domain. J Cell Biol. 1992 Jul;118(2):309–320. doi: 10.1083/jcb.118.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdö S. L., Joo F., Wolff J. R. Immunohistochemical localization of glutamate decarboxylase in the rat oviduct and ovary: further evidence for non-neural GABA systems. Cell Tissue Res. 1989 Feb;255(2):431–434. doi: 10.1007/BF00224128. [DOI] [PubMed] [Google Scholar]
- Erlander M. G., Tillakaratne N. J., Feldblum S., Patel N., Tobin A. J. Two genes encode distinct glutamate decarboxylases. Neuron. 1991 Jul;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Erlander M. G., Tobin A. J. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991 Mar;16(3):215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Katarova Z., Szabo G., Mugnaini E., Greenspan R. J. Molecular Identification of the 62 kd Form of Glutamic Acid Decarboxylase from the Mouse. Eur J Neurosci. 1990;2(3):190–202. doi: 10.1111/j.1460-9568.1990.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., Houser C. R., Tobin A. J. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991 Feb;56(2):720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Kaufman D. L., Tobin A. J. Glutamic acid decarboxylase cDNA: nucleotide sequence encoding an enzymatically active fusion protein. J Neurosci. 1987 Sep;7(9):2768–2772. doi: 10.1523/JNEUROSCI.07-09-02768.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legay F., Henry S., Tappaz M. Evidence for two distinct forms of native glutamic acid decarboxylase in rat brain soluble extract: an immunoblotting study. J Neurochem. 1987 Apr;48(4):1022–1026. doi: 10.1111/j.1471-4159.1987.tb05620.x. [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Chapman E. R., Storm D. R. Targeting of neuromodulin (GAP-43) fusion proteins to growth cones in cultured rat embryonic neurons. Neuron. 1991 Mar;6(3):411–420. doi: 10.1016/0896-6273(91)90249-y. [DOI] [PubMed] [Google Scholar]
- Moremen K. W., Touster O. Biosynthesis and modification of Golgi mannosidase II in HeLa and 3T3 cells. J Biol Chem. 1985 Jun 10;260(11):6654–6662. [PubMed] [Google Scholar]
- Persson H., Pelto-Huikko M., Metsis M., Söder O., Brene S., Skog S., Hökfelt T., Ritzén E. M. Expression of the neurotransmitter-synthesizing enzyme glutamic acid decarboxylase in male germ cells. Mol Cell Biol. 1990 Sep;10(9):4701–4711. doi: 10.1128/mcb.10.9.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991 May;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway N. D., Dawson P. A., Ho Y. K., Brown M. S., Goldstein J. L. Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J Cell Biol. 1992 Jan;116(2):307–319. doi: 10.1083/jcb.116.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimena M., De Camilli P. Autoimmunity to glutamic acid decarboxylase (GAD) in Stiff-Man syndrome and insulin-dependent diabetes mellitus. Trends Neurosci. 1991 Oct;14(10):452–457. doi: 10.1016/0166-2236(91)90044-u. [DOI] [PubMed] [Google Scholar]
- Solimena M., Folli F., Aparisi R., Pozza G., De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990 May 31;322(22):1555–1560. doi: 10.1056/NEJM199005313222202. [DOI] [PubMed] [Google Scholar]
- Solimena M., Folli F., Denis-Donini S., Comi G. C., Pozza G., De Camilli P., Vicari A. M. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N Engl J Med. 1988 Apr 21;318(16):1012–1020. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- Sorenson R. L., Garry D. G., Brelje T. C. Structural and functional considerations of GABA in islets of Langerhans. Beta-cells and nerves. Diabetes. 1991 Nov;40(11):1365–1374. doi: 10.2337/diab.40.11.1365. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Glynn S., Levi E., Mjolsness S., Hayday A. Use of a chemically modified T7 DNA polymerase for manual and automated sequencing of supercoiled DNA. Biotechniques. 1988 May;6(5):460–469. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Shackelford D. A. Structure and function of transferrin receptors and their relationship to cell growth. Biochem Soc Symp. 1986;51:117–129. [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y., Elde R. P., Morgan L. M., Kimmel J. R. Immunohistochemical studies of the GABA system in the pancreas. Neuroendocrinology. 1983;36(3):197–204. doi: 10.1159/000123456. [DOI] [PubMed] [Google Scholar]
- Wyborski R. J., Bond R. W., Gottlieb D. I. Characterization of a cDNA coding for rat glutamic acid decarboxylase. Brain Res Mol Brain Res. 1990 Aug;8(3):193–198. doi: 10.1016/0169-328x(90)90016-7. [DOI] [PubMed] [Google Scholar]