Abstract
Blood-oxygenation-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) studies often report inconsistent findings, probably due to brain properties such as balanced excitation and inhibition and functional heterogeneity. These properties indicate that different neurons in the same voxels may show variable activities including concurrent activation and deactivation, that the relationships between BOLD signal and neural activity (i.e., neurovascular coupling) are complex, and that increased BOLD signal may reflect reduced deactivation, increased activation, or both. The traditional general-linear-model-based-analysis (GLM-BA) is a univariate approach, cannot separate different components of BOLD signal mixtures from the same voxels, and may contribute to inconsistent findings of fMRI. Spatial independent component analysis (sICA) is a multivariate approach, can separate the BOLD signal mixture from each voxel into different source signals and measure each separately, and thus may reconcile previous conflicting findings generated by GLM-BA. We propose that methods capable of separating mixed signals such as sICA should be regularly used for more accurately and completely extracting information embedded in fMRI datasets.
Keywords: independent component analysis, neuroimaging, general linear model, neurovascular coupling, hemodynamic response function, magnetic resonance imaging
1. Introduction
Blood-oxygenation-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) has been used to investigate human brain functional organization for more than 20 years. However, findings from different fMRI studies are not always consistent (Mayberg, 2014; Parens and Johnston, 2014). For example, some studies find that BOLD signals from the so-called default mode network (DMN) or task-negative network at the medial portion of the brain negatively correlate with the BOLD signals from the so-called task-positive network at the lateral part of the brain (Carbonell et al., 2011; Fox et al., 2005). However, some other studies report that such anti-correlations are in part artificially introduced during preprocessing of fMRI datasets (Anderson et al., 2011; Chai et al., 2012; Murphy et al., 2009). Still others find that these anti-correlations are dependent on brain states or task conditions (Piccoli et al., 2015; Spreng et al., 2014). In this paper, we propose that some of these controversial findings in fMRI are probably due to several closely related brain properties, including balanced excitation and inhibition (E/I) and functional heterogeneity (Isaacson and Scanziani, 2011; Okun and Lampl, 2008), the limited spatial and temporal resolutions of fMRI, and the limited sensitivity and specificity of general-linear-model based analysis (GLM-BA) commonly used in fMRI studies.
To our knowledge, several brain properties such as balanced E/I are usually not explicitly considered in published fMRI studies. The aims of this paper are to attract the attention of fMRI investigators to these fundamental brain properties, initiate a discussion on their implications in fMRI, and consider optimal approaches for addressing them during analyzing and interpreting BOLD signals in order to improve the sensitivity and specificity of fMRI and reduce conflicting findings. We will first briefly introduce three closely related brain properties, i.e., balanced E/I, functional heterogeneity, and sparseness of neuronal activity, then discuss their potential implications in BOLD signal changes, and finally discuss a potential approach for addressing these properties in the analysis and interpretation of BOLD data.
2. Three closely related brain properties
2.1. Balanced E/I
Balanced E/I refers to the electrophysiological findings that excitatory and inhibitory synaptic activities always associate with each other at the levels of individual neurons and microcircuits in the cortex during both the resting condition and task performance (Isaacson and Scanziani, 2011; Okun and Lampl, 2008). Within any cortical region, about 20% of all neurons are GABAergic inhibitory interneurons and 80% excitatory pyramidal neurons (Druga, 2009). The interneurons and pyramidal neurons form feedforward and feedback inhibitory circuits, the building blocks of the cortex (Haider et al., 2013; Isaacson and Scanziani, 2011). Feedforward inhibition from broadly tuned inhibitory interneurons (e.g., driven by the thalamus) restricts receptive fields and enhances representation of contrast in pyramidal neurons (Li et al., 2014). Feedback inhibition mediates inhibitory responses among adjacent pyramidal neurons (Ren et al., 2007; Silberberg and Markram, 2007). Furthermore, the inhibitory interneurons form extensive synapses onto adjacent pyramidal neurons, as if to exert a ‘blanket of inhibition’ (Fino et al., 2013; Fino and Yuste, 2011; Inan et al., 2013; Karnani et al., 2014; Markram et al., 2004; Packer and Yuste, 2011). Therefore, even though inhibitory neurons are fewer than excitatory neurons in the cortex, they are powerful minorities that refine the response tuning of excitatory neurons, dominate microcircuit activities, and control information processing in the cortex (Haider et al., 2013; Li et al., 2014; Mateo et al., 2011; Miller, 2003; Okun and Lampl, 2008; Ren et al., 2007). It should be noted that balanced E/I does not mean the cortex maintains a constant E/I ratio; the E/I ratio in fact varies dynamically in different cortical regions and in the same region at different times, either during the resting condition or during different tasks (Isaacson and Scanziani, 2011).
2.2 Functional heterogeneity
Functional heterogeneity is another cortical property. The property of balanced E/I inevitably leads to different functional activities (i.e., activation vs. deactivation) among adjacent neurons. However, functionally heterogeneous neurons at adjacent locations may not always show mutual inhibition. Therefore, functional heterogeneity and balanced E/I are closely related but different properties in the brain. Electrophysiological studies have found that intermixed neurons in the same regions, including both the primary sensory and higher order association cortex, may have different functional activities (Horton and Adams, 2005; Isaacson and Scanziani, 2011; Ohki et al., 2005; Perin et al., 2011; Popivanov et al., 2014; Rothschild et al., 2010; Stettler and Axel, 2009; Swindale, 1998; Xu et al., 2013a). For example, the primary visual cortex contains a so-called “polymap”, i.e., several overlapping maps, each responsive to a unique visual property, such as edge orientation, direction of motion, and spatial location and frequency. The primary auditory and olfactory cortices show a “salt and pepper” like organization (Bandyopadhyay and Hablitz, 2007; Isaacson and Scanziani, 2011; Poo and Isaacson, 2009; Rothschild et al., 2010; Stettler and Axel, 2009). In the prefrontal cortex (PFC), neurons responsive to any specific stimulus property constitute a minority in any region, with a relatively higher density at some locations, but distributed in most or the entire PFC, without any abrupt edges (Fuster, 2009). For example, both reward- and working memory-related neurons exist in the dorsolateral PFC, medial orbitofrontal cortex (OFC), and other PFC regions, although they are not evenly distributed in these regions. More reward-related neurons concentrate in the OFC, while more working memory-related neurons reside in the lateral PFC (Ichihara-Takeda and Funahashi, 2007, 2008; Wallis and Miller, 2003). Therefore, intermixed neurons with heterogeneous functional activities exist in each PFC region, while different neuron types may be concentrated in different regions (Chafee and Goldman-Rakic, 1998; Donovan et al., 2013; Funahashi, 2013; Verduzco-Flores et al., 2009). Thus, different PFC regions show more or less common functional activities, i.e., overlap of functional networks, but each region may show a greater activity related to some central processes relative to others (Fuster, 2009).
2.3. Sparseness of neuronal activity
Sparseness of neuronal activity is another cortical property related to balanced E/I (Barth and Poulet, 2012; Wolfe et al., 2010). Electrophysiological studies find that any cortical neuron is only responsive to a few stimuli among all inputs from the environment (i.e., lifetime sparseness) (Tolhurst et al., 2009), and that only a small portion of the whole neuronal population in any cortical region exhibits activity at any instant (i.e., population sparseness), while most neurons remain silent, regardless of resting condition or task performance (Histed et al., 2009). This sparseness of neural activities is probably mediated by the inhibitory interneurons in the cortex. It has been found that the inhibitory interneurons contribute to the so-called “iceberg effect”, i.e., pyramidal neurons having narrower spiking receptive fields than subthreshold receptive fields (Isaacson and Scanziani, 2011; Priebe and Ferster, 2008). For example, in the primary somatosensory barrel cortex of behaving mice, all recorded layer 2/3 pyramidal neurons show rapid depolarization in response to whisker stimulation, but only 10% of them show spikes (Crochet et al., 2011). In the primary auditory cortex of awake rats, acoustic stimuli typically elicit increased firing rates in less than 5% of all neurons at any instant (Hromadka et al., 2008).
3. Potential implications of these brain properties for fMRI
3.1. Heterogeneous neural activity in each voxel
A typical voxel in the cortex extending 3-5 mm in each dimension contains hundreds of thousands of neurons (Druga, 2009; Logothetis, 2008; Roth and Dicke, 2012). The three cortical properties discussed above indicate that neural activities in the same voxels or brain regions are highly heterogeneous, as suggested by findings from numerous studies (Chafee and Goldman-Rakic, 1998; Donovan et al., 2013; Funahashi, 2013; Fuster, 2009; Verduzco-Flores et al., 2009). For example, Bell et al. assessed the relationships between neural activity and BOLD signal in the inferior temporal cortex in two monkeys. They first used fMRI to delineate brain regions showing significant BOLD responses to different categories of visual stimulus including face, body parts, objects, and environment scenes, then used microelectrodes to record single unit activity in each of these regions (Bell et al., 2011). They found that neurons activated and deactivated by each stimulus category were distributed throughout the entire recorded inferior temporal region, instead of being restricted within a specific area. However, within a region defined by fMRI with a specific category of visual stimulus, more neurons were activated then deactivated by stimuli in the same category, whereas more neurons were deactivated than activated by stimuli in other categories. For example, in the fMRI face region, more neurons were activated than deactivated by face, whereas fewer neurons were activated than deactivated by objects, other body parts, and scenes. Another study recorded single units from the posterior cingulate cortex (CGp) and lateral intraparietal area (LIP) in two monkeys during two attention-demanding tasks (Hayden et al., 2009). The CGp and LIP are among regions of the DMN and task-positive network, respectively. They often show reduced and increased, respectively, BOLD signal during attention-demanding tasks. Microelectrode recordings found that 35% of recorded neurons in the CGp were deactivated but only 9.4% activated during attention-demanding tasks. On the other hand, 20% of LIP neurons were activated and 7.4% deactivated. Therefore, both CGp and LIP showed heterogeneous task-related neuronal activities. Furthermore, more than 50% of recorded neurons in the CGp and LIP did not show significant changes in activity, consistent with the cortical property of sparseness of neural activity.
The three brain properties allow us to make five conclusions regarding neural activities in each voxel: 1) Some neurons may increase firing while others decrease firing simultaneously (i.e., balanced E/I) in each voxel at any instant of a resting condition or task performance. 2) Intermixed neurons in each voxel may show different timecourses of firing (i.e., functional heterogeneity). 3) Most neurons in each voxel do not increase firing at any instant (i.e., sparseness of neuronal activity). 4) Different voxels may show different ratios of activated (i.e., increased firing) and deactivated (i.e., reduced firing) neurons, because of variable E/I ratios in different voxels. 5) The ratio of activated and deactivated neurons (i.e., E/I ratio) in the same voxel may change dynamically during resting or different task conditions. These balanced and heterogeneous features of neural activities in each voxel, as determined by the three brain properties, have significant implications in relationships between neural activity and BOLD signal (i.e., neurovascular coupling) and hemodynamic response function (HRF).
3.2. Neurovascular coupling and HRF
Neurovascular coupling is one of the most important issues for properly interpreting fMRI data (Logothetis, 2008). It refers to the relationship between BOLD signal and neuronal activity, including both spiking and synaptic activity. For assessing neurovascular coupling, investigators have performed electrophysiological recording and fMRI of animal brains simultaneously, and then analyzed correlations between changes in BOLD signal and single and/or multi-unit firing rates and local field potentials (LFPs) (Hillman, 2014; Logothetis et al., 2001). Multiple studies found that neuronal activation and deactivation increased and decreased BOLD signal, respectively (Devor et al., 2007; Ekstrom, 2010; Goense et al., 2012; Logothetis, 2008; Shmuel et al., 2006), although non-neural factors such as astrocytes and local vascular features might also affect BOLD signal (Ekstrom, 2010; Hillman, 2014; Howarth, 2014). However, findings on the exact relationships between BOLD signal and other measures of neural activity were not fully consistent among different studies (Boynton, 2011; Nir et al., 2008; Renvall et al., 2014). Some studies found that neuron firing rates correlated with BOLD signal (Boynton, 2011; Nir et al., 2008), while others found that LFP but not firing rates correlated with BOLD signal and therefore proposed that BOLD signal reflected synaptic activities instead of neuron spiking (Logothetis, 2002; Logothetis et al., 2001; Viswanathan and Freeman, 2007).
There are 25,000 to 30,000 neurons/mm3 in the cortex (Roth and Dicke, 2012), and each voxel may have more than ten thousand neurons, even for high-resolution fMRI of an animal brain (e.g., ∼500 μm in each dimension). The three above-mentioned brain properties allow us to make two predictions regarding neurovascular coupling, if, as reported in multiple studies (Devor et al., 2007; Ekstrom, 2010; Goense et al., 2012; Logothetis, 2008; Shmuel et al., 2006), neuronal activation and deactivation indeed cause increases and decreases, respectively, in BOLD signal. First, different neurons in the same voxel will show different correlations between their firing rates and BOLD signal and thus different neurovascular coupling, e.g., some may show positive while others show negative correlations due to balanced E/I and functional heterogeneity. Second, neurovascular coupling may vary across voxels due to different E/I ratios in different voxels, and in the same voxels at different instants due to different E/I ratios during different tasks. These two predictions are supported by findings of variable neurovascular coupling from several animal studies (Amit and Romani, 2007; Bartolo et al., 2011; Boorman et al., 2010; Boynton, 2011; Ekstrom, 2010; Maier et al., 2008).
In one study, awake monkeys showed consistent changes in BOLD signal and neural activity (e.g., increases in both) in the V1 cortex during regular visual stimulation, but different changes in the two measures (i.e., reduced BOLD signal but not neural firing) during perception suppression (Maier et al., 2008). In another study, relative to visual single-grating stimulation, plaid pattern stimulation increased BOLD signal and the firing rates of some V1 neurons in awake monkeys but simultaneously decreased firing of other neurons, probably due to visual cross-orientation inhibition (Bartolo et al., 2011). In a third study, Boorman et al (2010) recorded neuron and BOLD responses in the rat barrel cortex to electrical stimulation of the contralateral whisker pad. Their findings indicate that more neurons are activated than deactivated in regions showing increases in BOLD signal and more neurons deactivated than activated in regions showing decreases in BOLD signal (Boorman et al., 2010). Therefore, balanced E/I and functional heterogeneity in the brain probably contribute to the different findings in neurovascular coupling from different studies. Based on these two brain properties, we propose that it is probably not a valid approach to assess neurovascular coupling by correlating changes in BOLD signal and single or multi-unit firing rates from the same voxel without considering the E/I ratio across the entire neuron population in the voxel, and that the previous conclusion that spiking does not contribute to BOLD signal is probably not valid (Logothetis, 2002; Logothetis et al., 2001), because these studies have not assessed the E/I ratio among different neurons.
Since the delay, shape, and amplitude of the HRF are dependent on neurovascular coupling, the above-discussed location and task dependent neurovascular coupling predicts that the HRF is probably variable among different voxels and in the same voxel during different tasks. This variability has been reported and discussed by several studies (Buxton, 2012; Handwerker et al., 2012; Handwerker et al., 2004). Therefore, we propose that balanced E/I and functional heterogeneity in the brain contribute to the location and task dependent variability of the HRF, in addition to other factors, such as local vascular features, discussed in previous studies (Handwerker et al., 2012). The variable neurovascular coupling and the HRF complicate the interpretation of BOLD signal changes.
3.3. Task-related changes in BOLD signal
As discussed above, both activation and deactivation are highly likely to exist concurrently in each voxel during either resting or task conditions, and this concurrent co-localized activation and deactivation (CCAD) complicates neurovascular coupling and the HRF. BOLD signals related to opposite changes in neural activities in the same voxel may cancel each other, and therefore BOLD signal changes from each voxel probably reflect changes in difference (or ratio) between CCAD, not in activation or deactivation alone (Fig. 1). A voxel may not show any BOLD signal change if its difference in CCAD does not change, even though individual neurons may change activity significantly. It may express a greater BOLD signal if its neuron population reduces total deactivation without an increase in total activation. Fig. 1 shows predicted potential relationships between BOLD signal and CCAD from a voxel in four (i.e., S1-4) of many possible scenarios. To support our point, we purposely selected scenarios of smaller activation and deactivation in task condition B (i.e., TB) relative to A (i.e., TA). However, TB may exhibit a smaller, greater, or equal positive BOLD signal as depicted in S1, S2, and S3, respectively, and a smaller negative BOLD signal in S4, relative to TA. Fig. 1 clearly demonstrates that BOLD signal changes depend not only on activation but also on deactivation, and that a greater BOLD signal is not always due to a greater activation, but may be due to a smaller deactivation (e.g., Fig.1 S2). Therefore, increases or decreases in BOLD signal at one task condition relative to another condition do not reliably indicate a greater activation or smaller deactivation at either task condition.
Fig. 1.
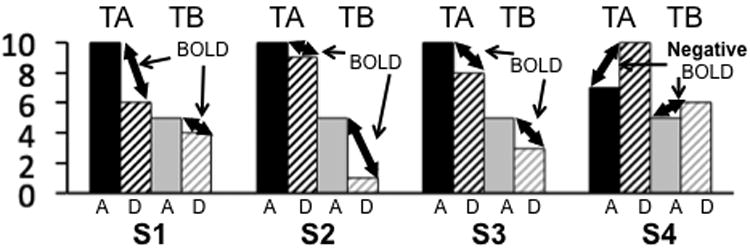
Potential relationships between BOLD signal and neuronal activity in a voxel. Solid and dashed columns represent total activation (A) and deactivation (D), respectively, in a voxel in task condition A (TA, black) and B (TB, gray), in arbitrary units. BOLD signal sizes depend on differences between A and D, not A or D alone. S1-4 shows four possible scenarios. While TB shows a smaller activation and deactivation than TA in each scenario, it does not always show a smaller BOLD signal.
4. An approach for addressing these brain properties
4.1. General-linear-model-based-analysis (GLM-BA)
FMRI studies traditionally employ a univariate analysis, i.e., general-linear-model-based-analysis (GLM-BA). It treats the BOLD signal mixture related to different neuronal activities (e.g., CCAD) from each voxel as an independent measure, and does not separate this BOLD signal mixture into different components (Logothetis, 2008; Serences and Saproo, 2012; Xu et al., 2013a). Therefore, GLM-BA is blind to balanced E/I and functional heterogeneity within a voxel. It may incorrectly report a greater brain activation in TB relative to TA of Fig. 1, when it detects a greater BOLD signal in TB, even though TB may actually have a smaller activation and deactivation, as depicted in Fig.1 S2. Therefore, GLM-BA has a limited specificity, which may lead to misinterpretation of reduced deactivation as increased activation. Furthermore, GLM-BA may show no difference in BOLD signal changes between TA and TB even though TB may actually have a smaller activation and deactivation than TA, as depicted in Fig. 1 S3. Therefore, GLM-BA has a limited sensitivity in detecting task-related changes in neuronal activity, and fMRI studies using GLM-BA may underestimate task-related changes in neural activity between task conditions and generate false negative results (Logothetis, 2008; Serences and Saproo, 2012; Xu et al., 2013a). The limited specificity and sensitivity of GLM-BA may contribute to the inconsistent data mentioned earlier. Therefore, analytical methods capable of separating a BOLD signal mixture related to CCAD in a voxel are desirable because they may have a greater sensitivity and specificity than GLM-BA in analyzing a BOLD time series. In this regard, several recent studies used at least three different methods for assessing concurrent co-localized BOLD signals during either a resting condition or task performance (Braga et al., 2013; Xu et al., 2013b; Yan et al., 2011; Yeo et al., 2013). One study used Connected Iterative Scan (CIS), a graph-theoretic clustering algorithm originally developed for analyzing community overlaps in inter-personal social networks, and reported an overlap of DMN and task-positive network during a resting condition (Yan et al., 2011). Another study used Latent Dirichlet Allocation (LDA), originally developed for text mining, and reported overlaps of multiple functional networks (FNs) during a resting condition (Yeo et al., 2013). Several other studies used a method called spatial independent component analysis (sICA). We will focus on sICA in the following text, because it is a more popular approach for assessing brain FNs in fMRI studies relative to the first two methods, and multiple recent fMRI studies have used sICA to separate BOLD signal mixtures from the same voxels and consistently reported extensive overlaps of FNs.
4.2. Spatial independent component analysis (sICA)
Independent component analysis (ICA) was originally developed for extracting hidden, unknown source signals from observed signal mixtures using higher-order statistics (Beckmann, 2012; Calhoun and Adali, 2012; Calhoun et al., 2009; Comon, 1994; McKeown et al., 1998b). In fMRI, sICA assumes that the BOLD signal from each voxel represents a linear mixture of source signals and separates it into spatially independent components (ICs) (Calhoun and Adali, 2012; Calhoun et al., 2002; Calhoun et al., 2009; McKeown et al., 1998a; McKeown et al., 1998b; McKeown and Sejnowski, 1998). In one of its earliest applications in fMRI, sICA separated the BOLD signal from one voxel into as many as six ICs (McKeown et al., 1998b). This unique capacity has been used to separate artifacts from signals in order to de-noise fMRI data (Aron and Poldrack, 2006; Beckmann, 2012; Brooks et al., 2013; Griffanti et al., 2014; Tohka et al., 2008; Yakunina et al., 2013). Over the last 15 years, sICA has arguably become one of the two most popular methods (along with seed-based approaches) for studying FNs, because it groups all voxels with synchronized source signals into one FN (Beckmann, 2012; Calhoun and Adali, 2012; Calhoun et al., 2009; Comon, 1994; McKeown et al., 1998b). Some studies describe spatial overlap of two or more FNs, indicating that sICA splits the BOLD signal from each voxel within overlapping regions into two or more FNs (Calhoun et al., 2008; Domagalik et al., 2012; Kim et al., 2009a; Kim et al., 2009b; Menz et al., 2009; St Jacques et al., 2011; van Wageningen et al., 2009; Wu et al., 2009; Zhang and Li, 2012). Very recently, we and three other groups have used sICA to systematically assess FN overlaps (Beldzik et al., 2013; Braga et al., 2013; Geranmayeh et al., 2014; Leech et al., 2012; Xu et al., 2014a; Xu et al., 2013a; Xu et al., 2013b; Yeo et al., 2013), and have consistently demonstrated: 1) that FNs often overlap with each other extensively, 2) that overlapping FNs may show concurrent but opposite task-related modulation (e.g., activation vs. deactivation), and 3) that the overlapping opposite modulations revealed by sICA may cancel each other and not show any modulation in GLM-BA. We have proposed that overlapping FNs with opposite time courses probably reflect CCAD existing in each voxel as stipulated by balanced E/I and functional heterogeneity in the brain.
4.3. Novel features of brain functional organization revealed by sICA
SICA may have a better specificity and sensitivity than GLM-BA and show novel feature of brain functional organization not revealed by GLM-BA such as extensive overlaps of FNs with opposite timecourses of task-related activities (Beldzik et al., 2013; Costumero et al., 2014; Domagalik et al., 2012; Xu et al., 2014b; Xu et al., 2015), due to its unique capacity of separating intermixed signals. In one of our recent studies, a GLM-BA revealed task-related increases, decreases, and no-changes in BOLD signal in separate brain regions. These findings are consistent with data from most fMRI studies that used GLM-BA of separated brain regions showing task-positive, negative, or absence of changes in activity. However, sICA revealed extensive overlaps of FNs showing task-related increases (i.e., task-positive FNs), decreases (i.e., task-negative FNs), and absence of changes (i.e., task-neutral FNs) in activity. Although the task-positive FNs covered most or all brain regions during highly demanding or complex cognitive tasks, so did the task-negative FNs and task-neutral FNs (Xu et al., 2015). Therefore, these FNs showed different task-related concurrent modulations, overlapped with each other, and covered almost the same brain regions, instead of being segregated among brain regions as revealed by the GLM-BA. These findings of sICA are consistent with the evidence that neurons showing task-related activation, deactivation, or no change in activation are intermixed with each other in the same brain regions (Bell et al., 2011; Borra et al., 2010; Fuster, 2009). They are also consistent with the three brain properties discussed above: balanced E/I, functional heterogeneity, and sparseness of neuronal activity. Therefore, findings from sICA and GLM-BA present a different framework of brain functional organization, i.e., overlapped and extensively distributed vs. segregated and restricted functional activities with different timecourses. The novel framework revealed by sICA should be further investigated.
The potentially greater sensitivity and specificity of sICA relative to GLM-BA may provide a novel opportunity for reconciliation of inconsistent findings of previous fMRI studies using GLM-BA, including the example presented at the beginning of this review. The property of balanced E/I in the brain predicts that anti-correlated FNs should always exist in the same and/or different brain regions. This notion is supported by the sICA finding of overlapping FNs with opposite timecourses. However, BOLD signals from different voxels do not have to show anti-correlations because they represent mixtures of different source signals including anti-correlated signals. Therefore some studies using GLM-BA may show anti-correlations between BOLD signals while some other studies may not. The novel findings from sICA along with the balanced E/I in the brain indicate to us that the opposite modulations of task-related timecourses of FNs in the same and/or different brain regions (e.g., the medial vs. lateral prefrontal cortex) may reflect balanced E/I among different FNs as discussed in a recent publication (Leech et al., 2014). Therefore, balanced E/I exists not only at the level of neurons and microcircuits, as revealed by electrophysiological studies, but also at the level of large-scale networks of the whole brain.
Conclusion
Balanced E/I, functional heterogeneity, and sparseness of neuronal activity are basic brain properties. They complicate neurovascular coupling and the HRF and the interpretation of task-related changes in BOLD signal. Thus, they probably contribute to inconsistent findings in fMRI studies using GLM-BA. Therefore, to understand brain functional organization, it is probably not adequate to use GLM-BA alone in fMRI studies. SICA has been used in fMRI studies for more than 15 years mainly due to its data-driven and model-free features. These studies usually do not describe FN overlaps and do not explicitly assess concurrent co-localized source signals from the same voxels. The recent sICA findings of overlapping FNs with different timecourses demonstrated the potential capacity of sICA for separating mixed signals in the same voxels, for reconciling extant conflicting fMRI findings, and for revealing novel features of brain functional organization. We strongly recommend that sICA or any other methods capable of separating signal mixtures should be regularly employed in addition to GLM-BA for more completely extracting information embedded in fMRI data.
Highlights.
The cortex is heterogeneous in function and maintains a balance of excitation and inhibition.
These properties indicate concurrent neuron activation and deactivation in the same voxels.
Either increased neuron activation or reduced deactivation may increase BOLD signal.
Traditional fMRI analysis may misinterpret reduced deactivation as increased activation.
Independent component analysis may reconcile conflicting findings of traditional analysis.
Acknowledgments
This work is supported by National Institutes of Health grant K01 DA027750. The author claims no conflict of interest, and acknowledges the significant contributions from Drs. Marc Potenza and Vince D. Calhoun during the original research leading to this paper. I thank Dr. Donald A. Godfrey for editing the writing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amit DJ, Romani S. Search for fMRI BOLD signals in networks of spiking neurons. Eur J Neurosci. 2007;25:1882–1892. doi: 10.1111/j.1460-9568.2007.05408.x. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Hablitz JJ. Dopaminergic modulation of local network activity in rat prefrontal cortex. J Neurophysiol. 2007;97:4120–4128. doi: 10.1152/jn.00898.2006. [DOI] [PubMed] [Google Scholar]
- Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012;35:345–355. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Bartolo MJ, Gieselmann MA, Vuksanovic V, Hunter D, Sun L, Chen X, Delicato LS, Thiele A. Stimulus-induced dissociation of neuronal firing rates and local field potential gamma power and its relationship to the resonance blood oxygen level-dependent signal in macaque primary visual cortex. Eur J Neurosci. 2011;34:1857–1870. doi: 10.1111/j.1460-9568.2011.07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF. Modelling with independent components. Neuroimage. 2012;62:891–901. doi: 10.1016/j.neuroimage.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Beldzik E, Domagalik A, Daselaar S, Fafrowicz M, Froncisz W, Oginska H, Marek T. Contributive sources analysis: a measure of neural networks' contribution to brain activations. Neuroimage. 2013;76:304–312. doi: 10.1016/j.neuroimage.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Bell AH, Malecek NJ, Morin EL, Hadj-Bouziane F, Tootell RB, Ungerleider LG. Relationship between functional magnetic resonance imaging-identified regions and neuronal category selectivity. J Neurosci. 2011;31:12229–12240. doi: 10.1523/JNEUROSCI.5865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman L, Kennerley AJ, Johnston D, Jones M, Zheng Y, Redgrave P, Berwick J. Negative blood oxygen level dependence in the rat: a model for investigating the role of suppression in neurovascular coupling. J Neurosci. 2010;30:4285–4294. doi: 10.1523/JNEUROSCI.6063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra E, Ichinohe N, Sato T, Tanifuji M, Rockland KS. Cortical connections to area TE in monkey: hybrid modular and distributed organization. Cereb Cortex. 2010;20:257–270. doi: 10.1093/cercor/bhp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM. Spikes, BOLD, attention, and awareness: a comparison of electrophysiological and fMRI signals in V1. Journal of vision. 2011;11:12. doi: 10.1167/11.5.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Sharp DJ, Leeson C, Wise RJ, Leech R. Echoes of the Brain within Default Mode, Association, and Heteromodal Cortices. J Neurosci. 2013;33:14031–14039. doi: 10.1523/JNEUROSCI.0570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JC, Faull OK, Pattinson KT, Jenkinson M. Physiological noise in brainstem FMRI. Front Hum Neurosci. 2013;7:623. doi: 10.3389/fnhum.2013.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB. Dynamic models of BOLD contrast. Neuroimage. 2012;62:953–961. doi: 10.1016/j.neuroimage.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, van Zijl PC, Pekar JJ. Independent component analysis of fMRI data in the complex domain. Magn Reson Med. 2002;48:180–192. doi: 10.1002/mrm.10202. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell F, Bellec P, Shmuel A. Global and system-specific resting-state fMRI fluctuations are uncorrelated: principal component analysis reveals anti-correlated networks. Brain Connect. 2011;1:496–510. doi: 10.1089/brain.2011.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comon P. Independent component analysis — a new concept? Signal Process. 1994;36:287–314. [Google Scholar]
- Costumero V, Barros-Loscertales A, Bustamante JC, Fuentes P, Rosell-Negre P, Ventura-Campos N, Avila C. A new window to understanding individual differences in reward sensitivity from attentional networks. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0760-6. [DOI] [PubMed] [Google Scholar]
- Crochet S, Poulet JF, Kremer Y, Petersen CC. Synaptic mechanisms underlying sparse coding of active touch. Neuron. 2011;69:1160–1175. doi: 10.1016/j.neuron.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalik A, Beldzik E, Fafrowicz M, Oginska H, Marek T. Neural networks related to pro-saccades and anti-saccades revealed by independent component analysis. Neuroimage. 2012;62:1325–1333. doi: 10.1016/j.neuroimage.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Knox PC, Skytta JA, Blayney JA, DiCenzo J. Buprenorphine from detox and beyond: preliminary evaluation of a pilot program to increase heroin dependent individuals' engagement in a full continuum of care. J Subst Abuse Treat. 2013;44:426–432. doi: 10.1016/j.jsat.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Druga R. Neocortical inhibitory system. Folia biologica. 2009;55:201–217. doi: 10.14712/fb2009055060201. [DOI] [PubMed] [Google Scholar]
- Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev. 2010;62:233–244. doi: 10.1016/j.brainresrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist. 2013;19:228–237. doi: 10.1177/1073858412456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Space representation in the prefrontal cortex. Prog Neurobiol. 2013;103:131–155. doi: 10.1016/j.pneurobio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci. 2009;21:2047–2072. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Wise RJ, Mehta A, Leech R. Overlapping Networks Engaged during Spoken Language Production and Its Cognitive Control. J Neurosci. 2014;34:8728–8740. doi: 10.1523/JNEUROSCI.0428-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense J, Merkle H, Logothetis NK. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron. 2012;76:629–639. doi: 10.1016/j.neuron.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Gonzalez-Castillo J, D'Esposito M, Bandettini PA. The continuing challenge of understanding and modeling hemodynamic variation in fMRI. Neuroimage. 2012;62:1017–1023. doi: 10.1016/j.neuroimage.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci U S A. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Adams DL. The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci. 2005;360:837–862. doi: 10.1098/rstb.2005.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Frontiers in neuroscience. 2014;8:103. doi: 10.3389/fnins.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara-Takeda S, Funahashi S. Activity of primate orbitofrontal and dorsolateral prefrontal neurons: task-related activity during an oculomotor delayed-response task. Exp Brain Res. 2007;181:409–425. doi: 10.1007/s00221-007-0941-0. [DOI] [PubMed] [Google Scholar]
- Ichihara-Takeda S, Funahashi S. Activity of primate orbitofrontal and dorsolateral prefrontal neurons: effect of reward schedule on task-related activity. J Cogn Neurosci. 2008;20:563–579. doi: 10.1162/jocn.2008.20047. [DOI] [PubMed] [Google Scholar]
- Inan M, Blazquez-Llorca L, Merchan-Perez A, Anderson SA, DeFelipe J, Yuste R. Dense and overlapping innervation of pyramidal neurons by chandelier cells. J Neurosci. 2013;33:1907–1914. doi: 10.1523/JNEUROSCI.4049-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Agetsuma M, Yuste R. A blanket of inhibition: functional inferences from dense inhibitory connectivity. Curr Opin Neurobiol. 2014;26:96–102. doi: 10.1016/j.conb.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub RL, White T, Wible C, Belger A, Bockholt HJ, Clark VP, Lauriello J, O'Leary D, Mueller BA, Lim KO, Andreasen N, Potkin SG, Calhoun VD. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009a;30:3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Mathalon DH, Ford JM, Mannell M, Turner JA, Brown GG, Belger A, Gollub R, Lauriello J, Wible C, O'Leary D, Lim K, Toga A, Potkin SG, Birn F, Calhoun VD. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull. 2009b;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Scott G, Carhart-Harris R, Turkheimer F, Taylor-Robinson SD, Sharp DJ. Spatial dependencies between large-scale brain networks. PLoS One. 2014;9:e98500. doi: 10.1371/journal.pone.0098500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Ji XY, Liang F, Li YT, Xiao Z, Tao HW, Zhang LI. A feedforward inhibitory circuit mediates lateral refinement of sensory representation in upper layer 2/3 of mouse primary auditory cortex. J Neurosci. 2014;34:13670–13683. doi: 10.1523/JNEUROSCI.1516-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mateo C, Avermann M, Gentet LJ, Zhang F, Deisseroth K, Petersen CC. In vivo optogenetic stimulation of neocortical excitatory neurons drives brain-state-dependent inhibition. Curr Biol. 2011;21:1593–1602. doi: 10.1016/j.cub.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Neuroimaging and psychiatry: the long road from bench to bedside. The Hastings Center report Spec No. 2014:S31–36. doi: 10.1002/hast.296. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Jung TP, Makeig S, Brown G, Kindermann SS, Lee TW, Sejnowski TJ. Spatially independent activity patterns in functional MRI data during the Stroop color-naming task. Proceedings of the National Academy of Sciences of the United States of America. 1998a;95:803. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998b;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Sejnowski TJ. Independent component analysis of fMRI data: examining the assumptions. Hum Brain Mapp. 1998;6:368–372. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<368::AID-HBM7>3.0.CO;2-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz MM, McNamara A, Klemen J, Binkofski F. Dissociating networks of imitation. Hum Brain Mapp. 2009;30:3339–3350. doi: 10.1002/hbm.20756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD. Understanding layer 4 of the cortical circuit: a model based on cat V1. Cereb Cortex. 2003;13:73–82. doi: 10.1093/cercor/13.1.73. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Dinstein I, Malach R, Heeger DJ. BOLD and spiking activity. Nat Neurosci. 2008;11:523–524. doi: 10.1038/nn0508-523. author reply 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci. 2008;11:535–537. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parens E, Johnston J. Neuroimaging: beginning to appreciate its complexities. The Hastings Center report Spec No. 2014:S2–7. doi: 10.1002/hast.293. [DOI] [PubMed] [Google Scholar]
- Perin R, Berger TK, Markram H. A synaptic organizing principle for cortical neuronal groups. Proc Natl Acad Sci U S A. 2011;108:5419–5424. doi: 10.1073/pnas.1016051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli T, Valente G, Linden DE, Re M, Esposito F, Sack AT, Salle FD. The Default Mode Network and the Working Memory Network Are Not Anti-Correlated during All Phases of a Working Memory Task. PLoS One. 2015;10:e0123354. doi: 10.1371/journal.pone.0123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popivanov ID, Jastorff J, Vanduffel W, Vogels R. Heterogeneous single-unit selectivity in an fMRI-defined body-selective patch. J Neurosci. 2014;34:95–111. doi: 10.1523/JNEUROSCI.2748-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57:482–497. doi: 10.1016/j.neuron.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Ren M, Yoshimura Y, Takada N, Horibe S, Komatsu Y. Specialized inhibitory synaptic actions between nearby neocortical pyramidal neurons. Science. 2007;316:758–761. doi: 10.1126/science.1135468. [DOI] [PubMed] [Google Scholar]
- Renvall V, Nangini C, Hari R. All that glitters is not BOLD: inconsistencies in functional MRI. Scientific reports. 2014;4:3920. doi: 10.1038/srep03920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G, Dicke U. Evolution of the brain and intelligence in primates. Prog Brain Res. 2012;195:413–430. doi: 10.1016/B978-0-444-53860-4.00020-9. [DOI] [PubMed] [Google Scholar]
- Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat Neurosci. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- Serences JT, Saproo S. Computational advances towards linking BOLD and behavior. Neuropsychologia. 2012;50:435–446. doi: 10.1016/j.neuropsychologia.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh WM, Turner GR. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 2014;34:14108–14114. doi: 10.1523/JNEUROSCI.2815-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. Neuroimage. 2011;57:608–616. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Swindale NV. Cortical organization: modules, polymaps and mosaics. Curr Biol. 1998;8:R270–273. doi: 10.1016/s0960-9822(98)70170-8. [DOI] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39:1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst DJ, Smyth D, Thompson ID. The sparseness of neuronal responses in ferret primary visual cortex. J Neurosci. 2009;29:2355–2370. doi: 10.1523/JNEUROSCI.3869-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wageningen H, Jorgensen HA, Specht K, Eichele T, Hugdahl K. The effects of the glutamate antagonist memantine on brain activation to an auditory perception task. Hum Brain Mapp. 2009;30:3616–3624. doi: 10.1002/hbm.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduzco-Flores S, Bodner M, Ermentrout B, Fuster JM, Zhou Y. Working memory cells' behavior may be explained by cross-regional networks with synaptic facilitation. PLoS One. 2009;4:e6399. doi: 10.1371/journal.pone.0006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. 2007;10:1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Houweling AR, Brecht M. Sparse and powerful cortical spikes. Curr Opin Neurobiol. 2010;20:306–312. doi: 10.1016/j.conb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Wu X, Lu J, Chen K, Long Z, Wang X, Shu H, Li K, Liu Y, Yao L. Multiple neural networks supporting a semantic task: an fMRI study using independent component analysis. Neuroimage. 2009;45:1347–1358. doi: 10.1016/j.neuroimage.2008.12.050. [DOI] [PubMed] [Google Scholar]
- Xu J, Calhoun VD, Pearlson GD, Potenza MN. Opposite Modulation of Brain Functional Networks Implicated at Low vs. High Demand of Attention and Working Memory. PLoS One. 2014a;9:e87078. doi: 10.1371/journal.pone.0087078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Calhoun VD, Potenza MN. The absence of task-related increases in BOLD signal does not equate to absence of task-related brain activation. J Neurosci Methods. 2014b doi: 10.1016/j.jneumeth.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Calhoun VD, Worhunsky PD, Xiang H, Li J, Wall JT, Pearlson GD, Potenza MN. Functional Network Overlap as Revealed by fMRI Using sICA and Its Potential Relationships with Functional Heterogeneity, Balanced Excitation and Inhibition, and Sparseness of Neuron Activity. PLoS One. 2015;10:e0117029. doi: 10.1371/journal.pone.0117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN, Calhoun VD. Spatial ICA reveals functional activity hidden from traditional fMRI GLM-based analyses. Frontiers in neuroscience. 2013a;7:154. doi: 10.3389/fnins.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang S, Calhoun VD, Monterosso J, Li CS, Worhunsky PD, Stevens M, Pearlson GD, Potenza MN. Task-related concurrent but opposite modulations of overlapping functional networks as revealed by spatial ICA. Neuroimage. 2013b;79:62–71. doi: 10.1016/j.neuroimage.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N, Tae WS, Lee KU, Kim SS, Nam EC. Spatiotemporal Segregation of Neural Response to Auditory Stimulation: An fMRI Study Using Independent Component Analysis and Frequency-Domain Analysis. PLoS One. 2013;8:e66424. doi: 10.1371/journal.pone.0066424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Kelley S, Goldberg M, Biswal BB. Detecting overlapped functional clusters in resting state fMRI with Connected Iterative Scan: a graph theory based clustering algorithm. J Neurosci Methods. 2011;199:108–118. doi: 10.1016/j.jneumeth.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Chee MW, Buckner RL. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 2013;88C:212–227. doi: 10.1016/j.neuroimage.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp. 2012;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]


