Abstract
Objectives
The treatment goal in congenital adrenal hyperplasia (CAH) is to replace glucocorticoids while avoiding androgen excess and iatrogenic Cushing’s syndrome. However, there is no consensus on how to monitor disease control. Our main objectives were to evaluate hormonal circadian rhythms and use these profiles to identify optimal monitoring times and novel disease biomarkers in CAH adults on intermediate- and long-acting glucocorticoids.
Design
This was an observational, cross-sectional study at the National Institutes of Health Clinical Center in 16 patients with classic CAH.
Methods
Twenty-four hour serum sampling for corticotropin (ACTH), 17-hydroxyprogesterone (17OHP), androstenedione (A4), androsterone, dehydroepiandrosterone (DHEA), testosterone, progesterone and 24-hour urinary pdiol and 5β-pdiol was carried out. Bayesian spectral analysis and cosinor analysis were performed to detect circadian rhythmicity. The number of hours to minimal (TminAC) and maximal (TmaxAC) adrenocortical hormone levels after dose administration were calculated.
Results
A significant rhythm was confirmed for ACTH (r2 0.95;P<0.001), 17OHP (r2 0.70; P=0.003), androstenedione (r2 0.47;P=0.043), androsterone (r2 0.80;P<0.001), testosterone (r2 0.47;P=0.042) and progesterone (r2 0.64;P=0.006). The mean (SD) TminAC and TmaxAC for 17OHP and A4 were: morning prednisone [4.3(2.3) and 9.7(3.5) hours], evening prednisone [4.5(2.0) and 10.3(2.4) hours], and daily dexamethasone [9.2(3.5) and 16.4(7.2) hours]. AUC0-24hr progesterone, androsterone and 24-hour urine pdiol were significantly related to 17OHP.
Conclusion
In CAH patients adrenal androgens exhibit circadian rhythms influenced by glucocorticoid replacement. Measurement of adrenocortical hormones and interpretation of results should take into account the type of glucocorticoid and time of dose administration. Progesterone and backdoor metabolites may provide alternative disease biomarkers.
Keywords: circadian rhythms, CAH, 17-hydroxyprogesterone, androstenedione, corticotropin, progesterone, androsterone
Introduction
Congenital adrenal hyperplasia is an autosomal recessive condition which in 95% of patients is caused by 21-hydroxylase deficiency, an enzyme of the cytochrome P450 family essential for cortisol and aldosterone biosynthesis 1. This enzyme defect results in cortisol deficiency, which by impaired negative feedback on the hypothalamic-pituitary-adrenal axis, stimulates ACTH synthesis. ACTH secretion is regulated by circadian input from the central clock in the suprachiasmatic nucleus and follows a circadian pattern with an early morning rise, reaching a peak in the morning on waking, and slowly declining towards an evening nadir 2. In untreated or most treated CAH patients this rhythm is maintained and adrenal androgens, which are also under the influence of ACTH, are released in a circadian pattern resulting in hyperandrogenism and its clinical consequences 3–5.
Patients with CAH suffer from multiple morbidities related to excess androgens or glucocorticoid over-replacement. Thus, the challenge for the endocrinologist is to achieve a balance between these two undesirable states. Endocrine Society guidelines have recommended the use of hydrocortisone in children whereas up to two-thirds of adults are commonly receiving intermediate and long-acting glucocorticoids 1, 6. A number of strategies have been suggested to help guide dose titration, such as measuring 17-hydroxyprogesterone (17OHP) and androstenedione before the early morning hydrocortisone dose in an attempt to measure peak hormone levels 7, 8. No definite criteria exist as to what time in relation to dose administration this should be performed in patients on intermediate- and long-acting glucocorticoids. Synthetic glucocorticoids have diverse pharmacokinetic characteristics with wide inter-individual variability and unpredictable effects on rhythm parameters and hormone concentrations 9, 10, making it problematic for physicians to decide on dose changes when measuring random hormone levels.
We measured hormonal circadian rhythms of CAH patients on intermediate- and long-acting glucocorticoids. In addition to routinely measured hormones we analyzed profiles of progesterone, dehydroepiandrosterone (DHEA), the precursor to pregnanetriol, 5β-pregnane-3α, 17α-diol-20-one (5β-pdiol), and the backdoor pathway metabolites androsterone (also a classic pathway product) and 5α-pregnane-3α, 17α-diol-20-one (pdiol) 11 (Figure 1). The use of multiple hormone levels at different time points provided us with a robust measurement of 24-hour hormone exposure as opposed to a single random hormone concentration as usually performed in clinical care. Our goal was to evaluate hormonal diurnal rhythms in patients with CAH, which then allowed us to determine optimal monitoring time ranges for identifying hormonal suppression and escape from hormonal control, and explore novel disease biomarkers.
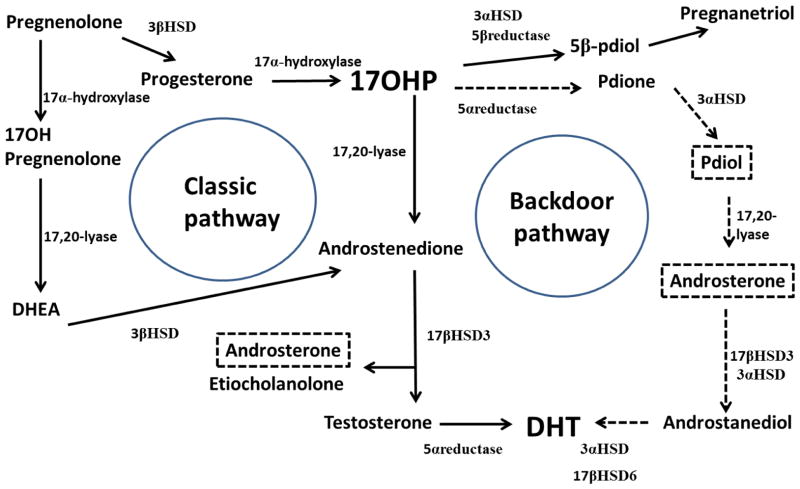
Figure 1. Pathway of adrenal steroidogenesis.
Normally, the principal route for adrenocortical androgen production is via the classic pathway (solid line arrows). In 21-hydroxylase deficiency, large amounts of 17OHP are converted to androstenedione via this classic pathway and these are the common biomarkers used in CAH to estimate disease control. Accumulating 17OHP can also be converted to pdione via an alternative route termed the backdoor pathway (dotted arrows). Androsterone is produced by both the classic and backdoor pathways. The end product for both pathways is ultimately dihydrotestosterone (DHT), the most potent androgen receptor agonist.
Patients and Methods
Study Patients
An observational, cross sectional study was carried out at the National Institutes of Health Clinical Center, Bethesda, MD. Sixteen patients with classic 21-hydroxylase deficiency (5 simple virilizing; 11 salt wasting) participated. This 24-hour serial sampling study was performed as part of the baseline visit of two clinical trials investigating the use of circadian hydrocortisone replacement (www.clinicaltrials.gov identifier no. NCT01735617 and NCT01859312) and patients were receiving their usual at home medication regimen. The diagnosis of CAH was ascertained using medical records and hormonal tests and confirmed by genotype 12. Five patients were on a daily dexamethasone dose (dose range: 0.25mg – 0.5mg, in hydrocortisone equivalent (x 80)7 9.6 – 21.3mg/m2/day). Four of these patients were taking dexamethasone in the evening whereas one patient was taking dexamethasone in the morning. Eleven patients were on twice daily prednisone (dose range: 2mg to 7.5mg (am), 2mg to 5mg (pm) in hydrocortisone equivalent (x 5)7: 12.0mg/m2/day – 33.7mg/m2/day. All patients were on a stable glucocorticoid regimen for a three month minimum. Other inclusion criteria included age 18 years and over, plasma renin activity less than 1.5 times the upper normal range and a negative pregnancy test. Patients who were taking medications that induce hepatic enzymes or interfere with glucocorticoid metabolism, spironolactone, inhaled, oral or nasal glucocorticoids apart from treatment for CAH, or had taken estrogen-containing oral contraceptive pill within 6 weeks of recruitment were excluded. Patients with clinical or biochemical evidence of hepatic, renal or psychiatric disease, were also excluded. The study was approved by the Eunice Kennedy Shriver National Institutes of Child Health and Development (NICHD) Institutional Review Board and all patients gave their written informed consent.
Study Design
All participants were admitted overnight and blood was sampled from an intravenous cannula every two hours (23:00h until 23:00h). Patients continued to take their usual medications during sampling. The following hormones were measured: ACTH, androstenedione, 17OHP, androsterone, DHEA, testosterone and progesterone. Glucocorticoid levels (dexamethasone, prednisolone) were also measured. In addition, a 24-hour urine sample, timed from 23:00h on Day 1 to 23:00h on Day 2, was collected to measure the backdoor metabolite pdiol and the precursor to pregnanetriol, 5β-pdiol (Figure 1).
Assays
Hormones were analyzed at the NIH Clinical Center (Bethesda, MD, USA) unless otherwise noted. Androstenedione, 17OHP, androsterone, DHEA, testosterone and progesterone were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) 13, 14. Within-day CVs ranged from 2.4–9.5% and between-day CVs from 3.0–9.9%. An Agilent 6490 triple-quadrupole mass spectrometer coupled with an Atmospheric Pressure Photoionization (APPI) source and Agilent 1200 Infinity series HPLC were used employing isotope dilution with deuterium labeled internal standard for each analyte. Instrument parameters were as follows: gas temperature 325°C, vaporizer 400°C, gas flow of 11 L/min, nebulizer 60psi, and capillary 4000V.
Urinary pdiol and 5β-pdiol were determined by LC-ESI-MS/MS (NICHD Biomedical Mass Spectrometry Facility, Bethesda, MD). Briefly, 1 mL aliquots of urine were treated with β-glucuronidase (Type VII-A, Sigma), followed by addition of an internal standard (13C(3) 17-hydroxyprogesterone). Steroids were then isolated by solid-phase weak anion exchange using DPX-WAX tips (DPX-Labs), eluted with isopropanol/5% ammonium hydroxide, and dried by vacuum centrifugation. The dried samples were dissolved in 0.2 mL isopropanol:water (1:1) (v/v) and analyzed by LC/MS/MS, using an Agilent 6460 QQQ mass spectrometer. Steroids were separated by reversed phase chromatography using an Acquity UPLC BEH C18 (2.1 I.D. × 150 mm) column, and eluted with a linear gradient from 100% A (water/0.1% formic acid) to 30% B (isopropanol/0.1% formic acid). Pdiol and 5β-pdiol concentrations were determined by an MRM assay. The precursor ion [MH – 2(H2O)]+ (m/z 299) was selected and product ions 161, 147, 135 were followed. Concentrations were determined from peak ratios of the pdiol or 5β-pdiol to the internal standard and standard curves generated by the addition of steroid standards to a stripped urine matrix. A linear response was obtained for levels up to 1500 pmol/mL (correlation coefficient > 0.997). Sensitivity was approximately 1 pmol/mL for either pdiol or 5β-pdiol and between-day CVs were from 4–12%. The minimum concentration of pdiol and 5β–pdiol for CAH pati ents was 3 and 20 pmol/mL respectively (median pdiol = 230 pmol/mL and 5β–pdiol = 1,600 pmol/mL). Total urinary excretion of pdiol and 5β-pdiol per 24 hr was calculated from steroid concentration and the 24 hr urine volume for each patient.
Plasma ACTH was measured using a chemiluminescence immunoassay on Siemens Immulite 2000 XPi analyzer (NIH Clinical Center) with sensitivity of 1.1 pmol/L, and intra- and inter-assay CVs 2.5% and 3.6% respectively. Dexamethasone and prednisolone were analyzed by LC-MS/MS (Mayo Medical Laboratories, Rochester, MN) with a sensitivity of 2.5nmol/L and 2.8nmol/L, respectively. For prednisolone and dexamethasone intra-assay CV were 4.9% and 6.1% at 33 and 31nmol/l, respectively whereas inter-assay CV were 8.6% and 9.3% at 324nmol/l and 8nmol/l. The following conversion values for normal ranges were used: ACTH (1.1 – 10.1pmol/L; pg/mL *0.22); 17OHP (0.4 – 5.3nmol/L; ng/dL*0.0303 ); Androstenedione (0.6 – 6.1nmol/L; ng/dL*0.0349); Androsterone (0.7 – 2.8nmol/L; ng/dL*0.0349); DHEA (0.8 – 20.8nmol/L; ng/dL*0.0347); Testosterone male (3.5 – 25.7nmol/L; ng/dL*0.0347); Testosterone female (0.3 – 2.5nmol/L; ng/dL*0.0347 ); Progesterone male (0.03 – 0.2nmol/L; ng/mL*3.18 ); Progesterone female (0.03 – 64nmol/L; ng/mL*3.18).
Statistical Analyses
Descriptive analyses were performed using SPSS Version 19 (Chicago, IL) and Microsoft Excel 2010 (Redmond, WA). Baseline variables are presented as median and interquartile (IQR) ranges as in the majority a normal distribution was not present. Correlation analysis was carried out using Spearman’s rho and differences between groups were assessed using the non-parametric Mann Whitney test. Linear regression analysis was performed to assess the relationship between classic and backdoor pathway metabolites.
Hormonal profiles were analyzed to detect the presence of circadian rhythms using Matlab Version 8.2 (Mathworks, Natick, MA). We initially took an exploratory approach, using Bayesian spectral analysis 15, 16 and, by assuming a harmonic relationship between frequency components, examined the data for evidence of a circadian fundamental frequency and the presence of individual components; this allowed us to detect a circadian rhythm, and estimate the period and number of harmonics. Hormonal data was log-transformed due to the lack of normality. A cosine curve (cosinor model), a mathematical-statistical regression technique used in chronobiological analyses of circadian rhythms 17, was then fitted to the averaged log-converted (ln) 24-hour data for each hormone found to have a circadian rhythm. By fitting in a cosinor model we were able to both confirm statistical significance of the rhythm and derive r2, the percentage of the variance that can be explained by the model17, 18. By using a p-value of statistical significance one rejects or accepts the hypothesis that the amplitude is zero and a significant p-value ascertains a significant rhythm is present.
To demonstrate circadian changes we also designed concentration-time profiles for each hormone for patients on either dexamethasone or prednisone using geometric mean (SEM) at each time point. We then measured 8-hour and 24-hour hormonal exposure which we defined by a PK approach ( WinNonlin Professional V5.2.1 software Certara, Princeton, NJ, USA). The parameters included geometric mean (10th – 90th percentiles) 8-hourly/24-hourly AUC (23:00h – 07:00h; 07:00h – 15:00h; 15:00h – 23:00h, 23:00h – 23:00h). To reduce heterogeneity, one patient receiving morning dexamethasone was evaluated separately. Two-tailed tests were performed and P-value < 0.05 was considered statistically significant.
Defining hormonal monitoring times
Circadian hormonal profiles provided robust measurements to help define optimal monitoring time ranges dependent on type and time of glucocorticoid administration. Two time parameters were used: 1) TminAC, the number of hours from dose administration to the lowest adrenocortical (AC) hormone level; 2) TmaxAC, the number of hours from dose administration to the maximal AC hormone level reached after the hormone escaped control. In some patients in whom hormone levels were above target ranges prior to dose administration and remained above these defined ranges after dosing, that is control was not achieved, TmaxAC was taken as the maximal concentration subsequent to a drop in hormone levels post dose administration. For individual variables, hormonal control was defined as: ACTH 1.1–15.2pmol/L(1.5 the upper limit of normal range), 17OHP 1.5–36nmol/L 7, 19 and normal range for androstenedione 0.6–6.1nmol/L, androsterone 0.7–2.8nmol/L, DHEA 0.8–20.8nmol/L, testosterone (males 3.5–25.7nmol/L, females 0.3–2.5nmol/L) and progesterone (males 0.03–0.2nmol/L, females 0.03–64nmol/L). Overall disease control was based on androstenedione being within the normal range. Hormonal concentrations for routinely measured hormones at TminAC and TmaxAC were recorded.
Results
Sixteen adult patients (6 women) with classic CAH participated, with median (IQR) age 23 (20 – 42) years, weight 70.9 (57.5 – 95.4) kg and BMI 25.9 (22.5 – 32.5) kg/m2. Based on early morning 07:00h androstenedione, 8 (50%) patients were in poor disease control 29 (SD 28)nmol/L. In males n=3, the androstenedione concentration was 42 (SD 37)nmol/L whilst in females n=5, this was 21 (SD 21)nmol/L. Eight patients had acceptable adrenal hormone levels 4 (SD 2)nmol/LIn males n=7, the androstenedione concentration was 4 (SD 2)nmol/L whilst in females n=1, this was 4nmol/L. Based on 17:00h levels 10 (62.5%) patients had elevated androstenedione [17 (SD 12)nmol/L; males n=4, 19 (SD 9)nmol/L; females n=6, 16 (SD 14)nmol/L] (Supplementary Table 1). Eleven patients were on prednisone twice daily at median (10th – 90th percentiles) 08:00h (07:00h – 10:00h) and 21:00h (19:00h – 23:00h) whereas four patients were taking dexamethasone in the evening at a median (10th – 90th percentiles) time of 22:00h (21:18h – 22:42h). One patient taking dexamethasone 0.375mg in the morning at 08:00h had normal hormonal control throughout most of the 24 hours.
Circadian rhythm analysis
All hormones except DHEA displayed evidence of circadian rhythm with remarkable consistency given the small sample size and inter-individual variation (Supplementary Table 1). A cosinor model, fitted to the averaged 24-hour data of all patients, resulted in a significant sinusoidal curve for ACTH (r2 0.95; P<0.001) (2 harmonics) and 17OHP (r2 0.70; P=0.003), androstenedione (r2 0.47; P=0.043), androsterone (r2 0.80; P<0.001), testosterone (r2 0.47; P=0.042) and progesterone (r2 0.64; P=0.006) (all one harmonic). The presence of a rhythm was not related to gender or type of glucocorticoid. To support these findings 8-hourly hormonal AUCs indicate higher hormonal exposure in the morning and early afternoon with decreasing levels thereafter (Table 1). Androsterone showed higher 17:00h than 07:00h levels (IQR) (Supplementary Table 1) suggesting an inverted rhythm but this was not confirmed when estimating 8 hourly AUCs (Table 1). Concentration-time profiles for all hormones revealed variable hormonal responses to drug levels (Figure 2).
Table 1.
24-Hour and 8-Hourly Hormonal Exposure in patients with Classic CAH on treatment
| Geometric mean (10th to 90th percentiles) | AUC (24 hours) [23:00 – 23:00] | AUC (23:00 – 07:00) | AUC (07:00 – 15:00) | AUC (15:00 – 23:00) |
|---|---|---|---|---|
| ACTH (pmol/L.hr) | 257 (38 – 1227) | 45 (8 – 383) | 106 (11 – 631) | 82 (15 – 417) |
| 17OHP (nmol/L.hr) | 633 (19 – 7113) | 122 (5 – 2066) | 265 (8 – 3784) | 199 (5 – 1700) |
| Androstenedione (nmol/L.hr) | 179 (30 – 1081) | 51 (11 – 325) | 65 (10 – 453) | 59 (9 – 303) |
| Androsterone (nmol/L.hr) | 121 (44 – 478) | 33 (11 – 130) | 43 (13 – 192) | 42 (14 – 157) |
| DHEA (nmol/L.hr) | 42 (10 – 730) | 13 (3 – 115) | 16 (3 – 344) | 12 (3 – 301) |
| Testosterone (males) (nmol/L.hr) | 321 (127 – 2037) | 109 (23 – 1536) | 105 (52 – 248) | 90 (36 – 252) |
| Testosterone (females) (nmol/L.hr) | 40 (22 – 131) | 12 (6 – 41) | 14 (7 – 42) | 14 (8 – 47) |
| Progesterone (males) (nmol/L.hr) | 17.0 (1.2 – 543.8) | 4.4 (0.3 – 197.2) | 6.5 (0.2 – 286.2) | 4.3 (0.2 – 184.1) |
| Progesterone (females) (nmol/L.hr) | 58.8 (13.4 – 1329.2) | 6.6 (0.2 – 321.2) | 23.2 (2.3 – 537.4) | 22.2 (6.4 – 470.6) |
AUC, area under the curve
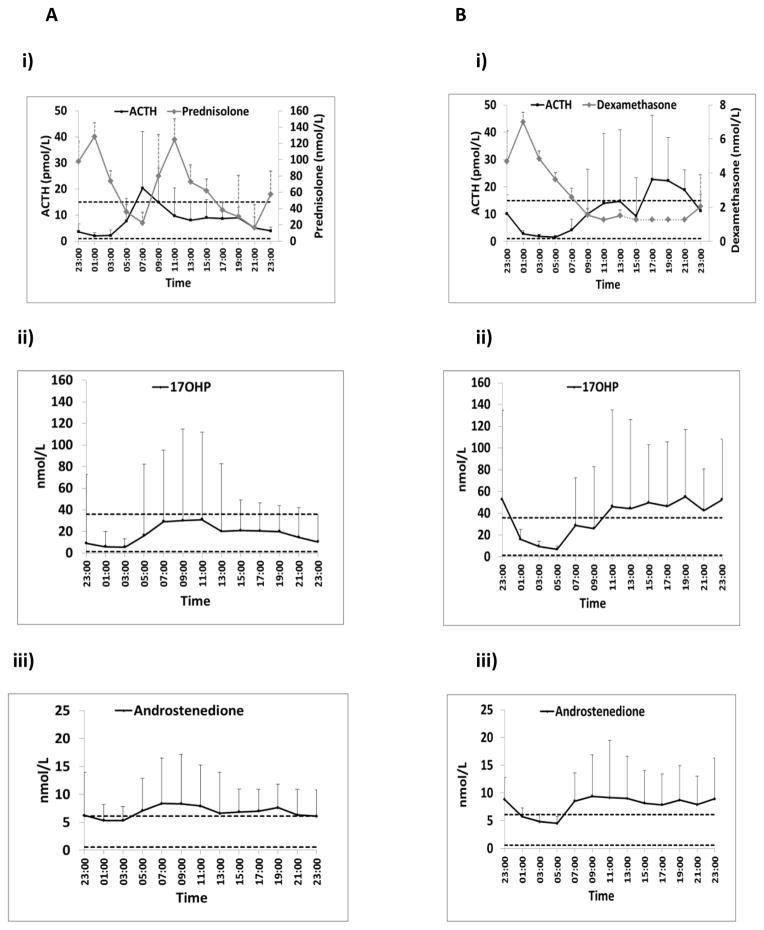
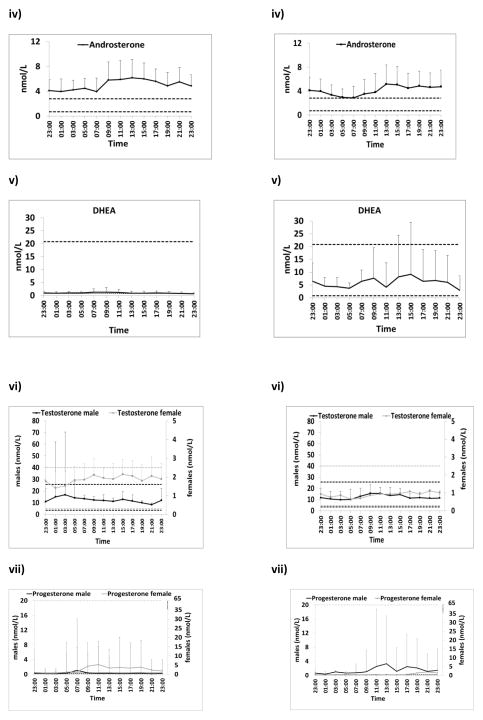
Figure 2. Serum Hormonal profiles for exogenous glucocorticoids and CAH-related hormones.
A) Eleven patients (six males; five females) receiving twice daily prednisone experienced hormonal escape mainly in the early morning for ACTH, androstenedione, androsterone and progesterone (males). 17OHP levels were higher in the morning but mainly remained within the desired range. In some patients a spontaneous reduction in hormone levels occurs before the evening dose.
B) Four patients (3 males; one female) receiving nocturnal dexamethasone had escape of most hormones including ACTH, 17OHP, androstenedione, androsterone and progesterone in the afternoon. Dotted segment of dexamethasone concentration-time profile (between 13:00h and 21:00h) represents undetectable levels
Geometric mean (SEM) is shown for prednisolone and dexamethasone and all other hormones. Horizontal dotted lines indicate normal ranges or optimal hormonal control. Only one female was taking dexamethasone.
Establishing hormonal monitoring times
For patients on prednisone, the time to the lowest and peak hormone concentrations following morning and evening doses were remarkably similar across the majority of adrenocortical hormones (Table 2). Maximal levels occurred approximately 10 hours following dose, while the lowest values occurred approximately 4 to 5 hours following dose. Time for measurement of peak 17OHP and androstenedione, the typical biomarkers of disease control, best corresponded to [mean (SD) TmaxAC 9.7 (3.5) hours] after the morning dose and [mean (SD) TmaxAC 10.3 (2.4) hours] for the evening dose, whereas monitoring for hormonal suppression corresponded to [mean (SD) TminAC 4.3 (2.3) hours] for the morning dose and [mean (SD) TminAC 4.5 (2.0) hours] for the evening dose. In support of these findings, these time-points correlated (P<0.001) with pre and post 8-hourly AUCs (data not shown). In a sub-analysis exploring whether TmaxAC and TminAC in patients on prednisone varied in the different sexes these were in general similar with no significant differences (Supplementary Table 2).
Table 2.
Target Monitoring Times Following Twice Daily Prednisone in 11 Patients with Classic CAH
| Morning Dose | Evening Dose | |||
|---|---|---|---|---|
|
| ||||
| TminAC (h)1 | TmaxAC (h)1 | TminAC (h)1 | TmaxAC (h)1 | |
| ACTH | 4.5 (1.3) | 11.0 (2.5) | 3.2 (1.5) | 10.9 (2.0) |
| 17OHP | 4.7 (2.4) | 9.7 (3.5) | 4.9 (1.8) | 10.7 (2.0) |
| Androstenedione | 3.8 (2.2) | 9.6 (3.6) | 4.1 (2.1) | 10.0 (2.8) |
| Androsterone | 7.4 (3.1) | 10.2 (3.2) | 4.2 (2.6) | 8.2 (3.5) |
| DHEA | 3.4 (4.1) | 12.7 (1.6) | 2.5 (3.8) | 10.1 (2.2) |
| Testosterone | 6.0 (4.4) | 11.5 (2.7) | 3.8 (1.3) | 11.0 (1.5) |
| Progesterone | 4.0 (1.9) | 10.2 (3.3) | 4.0 (2.5) | 11.2 (2.0) |
Prednisone (Dose Range – HC equiv: 12mg/m2/day – 33.7mg/m2/day)
Mean (SD)
TminAC, the number of hours from dose administration to minimal adrenocortical (AC) hormone level; TmaxAC, the number of hours from dose administration to the maximal AC hormone level
The time to lowest hormone concentrations and time to peak levels varied across the different adrenocortical hormones with once daily dexamethasone (Table 3). Time for measurement of 17OHP and androstenedione best corresponded to [mean (SD) TminAC 9.2 (3.5) hours] and [mean (SD) TmaxAC 16.4 (7.2) hours] for dexamethasone.
Table 3.
Target Monitoring Times Following Once Daily Dexamethasone in 5 patients with Classic CAH
| Once Daily Dose | ||
|---|---|---|
|
| ||
| TminAC (h)1 | TmaxAC (h)1 | |
| ACTH | 5.8 (1.9) | 20.2 (4.4) |
| 17OHP | 9.4 (1.8) | 17.4 (6.5) |
| Androstenedione | 9.0 (4.8) | 15.4 (8.4) |
| Androsterone | 6.2 (2.2) | 17.4 (8.7) |
| DHEA | 3.4 (2.9) | 21.4 (3.6) |
| Testosterone | 3.8 (2.4) | 20.6 (7.6) |
| Progesterone | 6.2 (1.9) | 11.8 (7.6) |
Dexamethasone (Dose Range - HC equiv: 9.6 – 21.3mg/m2/day)
Mean (SD)
TminAC, the number of hours from dose administration to minimal adrenocortical (AC) hormone level; TmaxAC, the number of hours from dose administration to the maximal AC hormone level
Hormonal concentrations at these time points (TminAC vs TmaxAC) show significant differences for 17OHP (median 6.4 vs 67.6nmol/L; P=0.005), and androstenedione (median 4.6 vs 9.5nmol/L; P=0.046) and no difference for testosterone (males median 9.3 vs 11.8nmol/L; P=0.31; females median 1.3 vs 1.8nmol/L; P=0.15).
Exploring hormonal inter-relationships to identify novel disease biomarkers
One of the hormones that was analyzed in our study and is not routinely measured was progesterone. AUC(0-24hr) Progesterone significantly correlated with ACTH and 17OHP in both males and females, whereas in males these relationships were stronger and progesterone was also associated with androstenedione (Table 4, Supplementary Figure 1). Progesterone AUC0-24hr significantly correlated with backdoor metabolites (pdiol: females, rho=0.83, P=0.042; males, rho=0.87, P=0.002; serum androsterone AUC0-24hr: females, rho=1.0, P=0.01; males, rho=0.714, P=0.047). DHEA showed no relationship with ACTH, 17OHP, androstenedione or any of the measured backdoor metabolites. 47% of patients had undetectable DHEA levels throughout the 24 hours.
Table 4.
Correlation between routinely measured biomarkers and other classic and backdoor pathway metabolites
| Biomarkers | ACTH Co-efficient (P-value) 1 | 17OHP Co-efficient (P-value) 1 | Androstenedione Co-efficient (P-value) 1 |
|---|---|---|---|
| Serum Steroids | |||
| Androsterone2 | 0.63 (0.02) | 0.75 (≤0.01) | 0.79 (<0.001) |
| DHEA | −0.09 | 0.31 | 0.17 |
| Testosterone – Male | −0.60 | −0.53 | −0.42 |
| Testosterone – Female | 0.83 (0.04) | 0.71 | 0.77 |
| Progesterone – Male | 0.73 (0.03) | 0.98 (<0.001) | 0.70 (0.04) |
| Progesterone – Female | 0.89 (0.02) | 0.83 (0.04) | 0.71 |
| Urinary Steroids | |||
| Pdiol | 0.62 (≤0.01) | 0.85 (<0.001) | 0.84 (<0.001) |
| 5β-pdiol | 0.68 (≤0.01) | 0.88 (<0.001) | 0.77 (<0.001) |
Correlations based on AUC0-24hr (serum) or 24-hour urine collection.
P-values > 0.05 not shown.
Androsterone produced by both classic and backdoor pathways
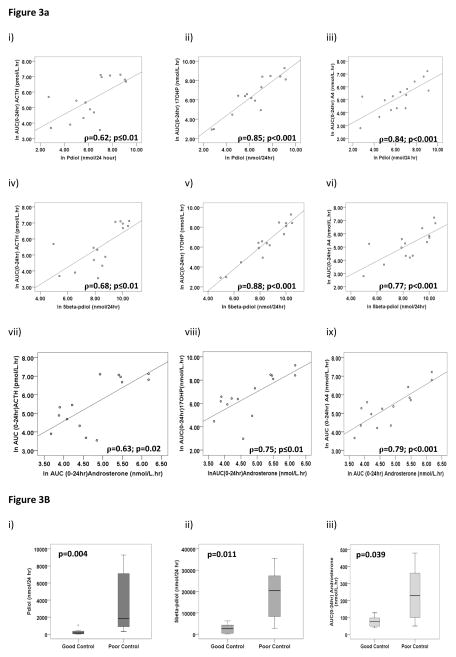
Twenty-four hour urine pdiol, a backdoor pathway metabolite, serum androsterone, a product of both classic and backdoor pathways, and 5β-pdiol, a precursor of pregnanetriol, were significantly correlated with ACTH, 17OHP and androstenedione (Figure 3a, Table 4). Twenty-four hour urine pdiol (Median: 1848 vs 150nmol /24 hrs; P=0.004), 5β-pdiol (Median: 20481 vs 2643nmol /24hrs; P=0.011) and androsterone AUC0-24hr (Median: 229 vs 75nmol/L; P=0.039) were significantly higher in patients with poor disease control (Figure 3b). Pdiol positively predicted androstenedione (unstandardized coefficient B=0.45 95%CI 0.22 – 0.68; P=0.001) and 17OHP (unstandardized coefficient B=0.89 95%CI 0.63 – 1.15; P<0.001) by linear regression.
Figure 3.
Figure 3A. Hormonal Inter-Relationships for pdiol, 5β-pdiol and androsterone
Correlations between backdoor pathway 24 hour urine pdiol (i – iii), 5β-pdiol (iv – vi) and AUC(0-24hr) androsterone (vii-ix) with major serum biomarkers
Figure 3B. Pdiol, 5β-pdiol and androsterone levels in CAH
Box and whisker plots demonstrate higher concentrations of pdiol, 5β-pdiol and androsterone in patients with classic CAH. Significantly higher levels are evident in patients with poor control. Poor control defined as 07:00h androstenedione level >6.0nmol/L.
Discussion
Our comprehensive hormonal analysis of adult patients with CAH reveals intact diurnal rhythms of multiple hormones and fluctuating androgen excess throughout the day that may not be detected with routine random single hormonal measurements. The androgen excess commonly experienced by patients with CAH resulted from both the classic and backdoor pathways to androgen production, albeit driven by the circadian secretion of ACTH. Twenty-four hour serial sampling allowed us to estimate when escape from hormonal suppression most commonly occurred and to evaluate this in relation to glucocorticoid administration. To our knowledge this is the most detailed study to date evaluating optimal monitoring times in patients on intermediate and long-acting glucocorticoids and the only study to also include backdoor pathway steroid metabolites.
In our study, detailed pharmacokinetic (PK) modelling revealed that maximal hormone levels in patients on morning and evening prednisone occur 10 hours after dose administration and 16 hours following dexamethasone. In addition, lowest levels corresponding to maximal suppression occurs around 4 to 5 hours after prednisone and 9 hours after a daily dexamethasone dose. Significant differences were observed between these highest and lowest levels of 17OHP and androstenedione, highlighting the importance of the timing of hormonal evaluation. Furthermore, we found that serum progesterone and androsterone and 24-hour urinary pdiol and 5β-pdiol correlated well with routinely monitored hormones and could be alternative markers of disease control. These findings provide insight into the importance of taking into consideration the type of glucocorticoid and the time of dose administration when monitoring hormone levels and also suggest that in poorly controlled CAH patients, both the classic and backdoor pathways are similarly activated.
The management of CAH changes throughout life. Although, the majority of children with CAH are treated with short-acting hydrocortisone 1, a significant number of CAH adults are treated with longer-acting glucocorticoids such as prednisone, prednisolone or dexamethasone 19, 20. These medications are often used for convenience because they can be dosed once or twice daily or to optimise compliance. Long- and intermediate-acting glucocorticoids have shown better adrenocortical suppression compared to hydrocortisone 21, 22, although they may increase risk of metabolic complications such as insulin resistance 19 and osteoporosis 23. Our data reveal that androgen suppression may be transient even for long- and intermediate-acting glucocorticoids with wide inter-individual variability. Prednisone is an inactive synthetic analogue of cortisone, and post-absorption is rapidly converted to biologically active prednisolone by hepatic 11-beta hydroxysteroid dehydrogenase Type 1. Although it is recognised that the biological half-life of prednisolone is approximately 18 – 36 hours 24, and the serum half-life is around 3 to 4 hours 25, 26, the biological half-life for corticosterone suppression has been shown to be 6.2 hours 27; Similarly, dexamethasone has a biological half-life of 36 – 54 hours 24, a serum half-life of around 4 to 5 hours and a biological half-life for corticosterone suppression of 7 hours 27. The corticosterone suppression data from Meikle et al 27 could explain why many of our patients on prednisone experienced maximal 17OHP and androstenedione within 10 hours following dose administration and patients on dexamethasone within 16 hours. Dexamethasone concentrations were detected in the circulation for around 10 hours. Our data also suggest that some patients receiving once daily dexamethasone could potentially benefit from twice daily dexamethasone though this might increase the risk for adverse metabolic effects. Other studies investigating monitoring times for hydrocortisone have shown a rapid fall in 17OHP levels after the morning hydrocortisone dose and early morning laboratory evaluation is generally suggested for patients receiving hydrocortisone 22, 28.
In most untreated patients, 17OHP and sex steroids maintain their circadian rhythms 3 and the circadian rhythm of 17OHP has been shown to develop as early as 3 months of age 29. Our analysis of 24-hour concentration-time profiles for androstenedione and 17OHP indicate that patients on long-acting glucocorticoids maintain hormonal rhythms and exhibit wide hormonal fluctuations. Interestingly, irrespective of a low glucocorticoid state, in some instances there was a decrease in 17OHP and androgen levels in the evening during the quiescent phase; an effect most likely related to circadian regulation of the endogenous timing system by the central clock in the suprachiasmatic nucleus. Thus, hormonal disturbances may only be appreciated when measuring multiple hormone samples during the day. Unfortunately this type of research based analysis may be too intensive, laborious and costly to be carried out as part of daily clinical management. To circumvent this problem we attempted to define the most suitable time to measure hormone levels related to poor disease control, this dependent on time of dose and glucocorticoid formulation. This may be an alternative strategy especially as some clinicians do measure adrenal androgens during other times of the day such as late in the afternoon 30. Our opinion is that aiming to measure maximal hormone levels is most useful in clinical management, corresponding to evaluation in the early morning for night time prednisone, or late afternoon for morning prednisone or night time dexamethasone. We also emphasize that hormone levels taken at recommended time points should not preclude a full clinical assessment of the patient. Management decisions should take all clinical features and biochemical data into consideration.
Comprehensive steroid panels were evaluated. DHEA was mostly suppressed or within normal range even when disease was uncontrolled, confirming that DHEA is not a useful biomarker in CAH. In addition, DHEA levels did not correlate with any of the other measured hormones. Low DHEA has previously been described in patients with classic CAH 31, 32. Our data support the notion that DHEA is not solely regulated by ACTH in CAH patients, but low levels may be due to abnormal adrenal gland development as patients with 21-hydroxylase deficiency have been shown to have lack of zonation with extensive intermingling of adrenocortical and adrenomedullary cells 7, 33. Low intra-adrenal cortisol in CAH patients also may result in low DHEA production 31, 34.
Conversely progesterone may be useful in the management of CAH. In CAH, an increase in ACTH stimulation presumably drives the production of progesterone; this is supported by the significant correlation between progesterone and ACTH in our study. Much is unknown about progesterone in CAH. However, high progesterone levels could contribute to female infertility 35. In a case-control study of 16 women with classic CAH, those patients with a low LH pulse amplitude and frequency had higher progesterone levels and worse hormonal control than those with LH pulsatility similar to normal controls 36. Further studies investigating the relevance of high progesterone in CAH and its impact on fertility are needed.
An intriguing alternative pathway to androgen production is the backdoor pathway, which produces the most potent androgen, dihydrotestosterone (DHT), without the intermediary androstenedione or testosterone. 11, 37. Kamrath et al 11 found that androgen biosynthesis via the backdoor pathway is active throughout postnatal life, with possibly less backdoor pathway contribution to androgenic steroid production post adrenarche 38. Prior studies suggest that backdoor pathway androgen production increases in adults with CAH as control of androgen excess deteriorates 39. We mostly found a proportional increase in classic and backdoor pathway metabolites in adults as disease control deteriorates, albeit in a small sample size. The relationship between clinical features and backdoor pathway metabolites, together with the use of these hormones as markers of disease status, requires further investigation.
Limiting factors in our study include the use of different doses of glucocorticoids that were administered at different times. Furthermore, a small number of patients were on dexamethasone. With regards to identifying minimal hormone levels or more specifically the maximal suppression following medication (TminAC), we did not account for the clock-induced physiological drop in hormones, and this should be considered. In addition TminAC after evening prednisone suggests monitoring during very early morning which would be impractical in clinical practice though possibly useful to investigators in the research setting.
Our study is unique in that we obtained detailed 24-hour circadian profiles of common and uncommon biomarkers of androgen excess in patients with classic CAH allowing us to approximate ideal monitoring times post dose administration. Achieving optimal metabolic control is difficult in CAH and we found that laboratory evaluation is best performed in relation to medication timing. Backdoor pathway metabolites and progesterone are alternative biomarkers of disease control and may be useful in the management of CAH patients. Future studies should investigate the association between these steroids and clinical features of hyperandrogenism. Our findings provide useful information regarding the management of the adult patient with CAH and thus may help guide future clinical practice.
Supplementary Material
Acknowledgments
Funding
This research was supported in part by the Intramural Research Program of the NIH and in part by Diurnal Ltd.
We thank our patients for participating in the study, our nursing staff of the 5SWN Metabolic Unit for their assistance in carrying out this study and their expertise in serial sampling, and the CARES Foundation for patient referrals.
Footnotes
Disclosure
Decleration of interest
D.P.M received research funds from Diurnal Ltd through NIH Cooperative Research and Development Agreement; R.J.R is Director of Diurnal Ltd.
References
- 1.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. Amplitude, but not frequency, modulation of adrenocorticotropin secretory bursts gives rise to the nyctohemeral rhythm of the corticotropic axis in man. J Clin Endocrinol Metab. 1990;71:452–463. doi: 10.1210/jcem-71-2-452. [DOI] [PubMed] [Google Scholar]
- 3.Frisch H, Parth K, Schober E, Swoboda W. Circadian patterns of plasma cortisol, 17-hydroxyprogesterone, and testosterone in congenital adrenal hyperplasia. Arch Dis Child. 1981;56:208–213. doi: 10.1136/adc.56.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young MC, Robinson JA, Read GF, Riad-Fahmy D, Hughes IA. 170H-progesterone rhythms in congenital adrenal hyperplasia. Arch Dis Child. 1988;63:617–623. doi: 10.1136/adc.63.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes IA, Read GF. Control in congenital adrenal hyperplasia monitored by frequent saliva 17OH-progesterone measurements. Horm Res. 1984;19:77–85. doi: 10.1159/000179870. [DOI] [PubMed] [Google Scholar]
- 6.Auchus RJ, Arlt W. Approach to the patient: the adult with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98:2645–2655. doi: 10.1210/jc.2013-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 8.Groschl M, Rauh M, Dorr HG. Cortisol and 17-hydroxyprogesterone kinetics in saliva after oral administration of hydrocortisone in children and young adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2002;87:1200–1204. doi: 10.1210/jcem.87.3.8297. [DOI] [PubMed] [Google Scholar]
- 9.Young MC, Cook N, Read GF, Hughes IA. The pharmacokinetics of low-dose dexamethasone in congenital adrenal hyperplasia. Eur J Clin Pharmacol. 1989;37:75–77. doi: 10.1007/BF00609429. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua JS, Rotenstein D, Lee PA. Duration of suppression of adrenal steroids after glucocorticoid administration. Int J Pediatr Endocrinol. 2010;2010:712549. doi: 10.1155/2010/712549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97:E367–375. doi: 10.1210/jc.2011-1997. [DOI] [PubMed] [Google Scholar]
- 12.Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP. Comprehensive genetic analysis of 182: unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96:E161–172. doi: 10.1210/jc.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12: steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Stolze BGV, Gu J, Soldin SJ. A408: Development and Validation of a High Performance Liquid Chromatography Tandem Mass Spectrometry 9 Steroid Panel using Minimal Sample Volume. AACC Annual meeting Abstracts. 2014:S133. [Google Scholar]
- 15.Hansen LK, Nielsen FA, Larsen J. Exploring fMRI data for periodic signal components. Artif Intell Med. 2002;25:35–44. doi: 10.1016/s0933-3657(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 16.Bretthorst GL. Bayesian spectrum analysis and parameter estimation. Springer-Verlag; Berlin: 1988. Lecture Notes in Statistics. [Google Scholar]
- 17.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cugini P. Chronobiology: principles and methods. Ann Ist Super Sanita. 1993;29:483–500. [PubMed] [Google Scholar]
- 19.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244: patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, Stimson RH, Walker BR, Connell JM, Ross RJ. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203: patients. J Clin Endocrinol Metab. 2010;95:5110–5121. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horrocks PM, London DR. A comparison of three glucocorticoid suppressive regimes in adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 1982;17:547–556. doi: 10.1111/j.1365-2265.1982.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 22.Dauber A, Feldman HA, Majzoub JA. Nocturnal Dexamethasone versus Hydrocortisone for the Treatment of Children with Congenital Adrenal Hyperplasia. Int J Pediatr Endocrinol. 2010;2010 doi: 10.1155/2010/347636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falhammar H, Filipsson Nystrom H, Wedell A, Brismar K, Thoren M. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2013;168:331–341. doi: 10.1530/EJE-12-0865. [DOI] [PubMed] [Google Scholar]
- 24.Repchinsky C. The Canadian Drug Reference for Health Professionals 2008. Ottawa N Canadian pharmacists Association; 2008. Compendium of Pharmaceuticals and Specialties; pp. 574–576. Corticosteroids. [Google Scholar]
- 25.Meikle AW, Weed JA, Tyler FH. Kinetics and interconversion of prednisolone and prednisone studied with new radioimmunogassays. J Clin Endocrinol Metab. 1975;41:717–721. doi: 10.1210/jcem-41-4-717. [DOI] [PubMed] [Google Scholar]
- 26.Disanto AR, Desante KA. Bioavailability and pharmacokinetics of prednisone in humans. J Pharm Sci. 1975;64:109–112. doi: 10.1002/jps.2600640122. [DOI] [PubMed] [Google Scholar]
- 27.Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63:200–207. doi: 10.1016/0002-9343(77)90233-9. [DOI] [PubMed] [Google Scholar]
- 28.Charmandari E, Matthews DR, Johnston A, Brook CG, Hindmarsh PC. Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: is current replacement therapy satisfactory? J Clin Endocrinol Metab. 2001;86:4679–4685. doi: 10.1210/jcem.86.10.7972. [DOI] [PubMed] [Google Scholar]
- 29.Solyom J. Diurnal variation in blood 17-hydroxyprogesterone concentrations in untreated congenital adrenal hyperplasia. Arch Dis Child. 1984;59:743–747. doi: 10.1136/adc.59.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivkees SA. Dexamethasone therapy of congenital adrenal hyperplasia and the myth of the “growth toxic” glucocorticoid. Int J Pediatr Endocrinol. 2010;2010:569680. doi: 10.1155/2010/569680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkl TM, Ohl L, Rauh M, Schofl C, Dorr HG. Adrenarche and puberty in children with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2011;76:400–410. doi: 10.1159/000333696. [DOI] [PubMed] [Google Scholar]
- 32.El-Maouche D, Collier S, Prasad M, Reynolds JC, Merke DP. Cortical Bone Mineral Density in Patients with Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency. Clin Endocrinol (Oxf) 2014 doi: 10.1111/cen.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, Van Wyk JJ, Bornstein SR. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343:1362–1368. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 34.Topor LS, Asai M, Dunn J, Majzoub JA. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J Clin Endocrinol Metab. 2011;96:E31–39. doi: 10.1210/jc.2010-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witchel SF, Azziz R. Nonclassic congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2010;2010:625105. doi: 10.1155/2010/625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachelot A, Chakhtoura Z, Plu-Bureau G, Coudert M, Coussieu C, Badachi Y, Dulon J, Charbit B, Touraine P. Influence of hormonal control on LH pulsatility and secretion in women with classical congenital adrenal hyperplasia. Eur J Endocrinol. 2012;167:499–505. doi: 10.1530/EJE-12-0454. [DOI] [PubMed] [Google Scholar]
- 37.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Kamrath C, Hartmann MF, Wudy SA. Androgen synthesis in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Metab Res. 2013;45:86–91. doi: 10.1055/s-0032-1331751. [DOI] [PubMed] [Google Scholar]
- 39.Auchus RJ, Buschur EO, Chang AY, Hammer GD, Ramm C, Madrigal D, Wang G, Gonzalez M, Steven Xu X, Smit JW, Jiao J, Yu MK. Abiraterone Acetate to Lower Androgens in Women With Classic 21-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2014:jc20141258. doi: 10.1210/jc.2014-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.