Abstract
Objective
To image subretinal neovascularization in proliferative macular telangiectasia type 2 (MacTel2) using swept source optical coherence tomography based microangiography (OMAG).
Study Design
Patients with MacTel2 were enrolled in a prospective, observational study known as the MacTel Project and evaluated using a high-speed 1050nm swept-source OCT (SS-OCT) prototype system. The OMAG algorithm generated en face flow images from three retinal layers, as well as the region bounded by the outer retina and Bruch’s membrane, the choriocapillaris, and the remaining choroidal vasculature. The en face OMAG images were compared to images from fluorescein angiography (FA) and indocyanine green angiography (ICGA).
Results
Three eyes with neovascular MacTel2 were imaged. The neovascularization was best identified from the en face OMAG images that included a layer between the outer retinal boundary and Bruch’s membrane. OMAG images identified these abnormal vessels better than FA and were comparable to the images obtained using ICGA. In all three cases, OMAG identified choroidal vessels communicating with the neovascularization, and these choroidal vessels were evident in the two cases with ICGA imaging. In one case, monthly injections of bevacizumab reduced the microvascular complexity of the neovascularization, as well as the telangiectatic changes within the retinal microvasculature. In another case, less frequent bevacizumab therapy was associated with growth of the subretinal neovascular complex.
Conclusions
OMAG imaging provided detailed, depth-resolved information about subretinal neovascularization in MacTel2 eyes demonstrating superiority to FA imaging and similarities to ICGA imaging for documenting the retinal microvascular changes, the size and extent of the neovascular complex, the communications between the neovascular complex and the choroidal circulation, and the response to monthly bevacizumab therapy.
Keywords: SSOCT, Angiography, OMAG, Neovascular, MacTel
INTRODUCTION
Macular telangiectasia type 2 (MacTel2) is a bilateral, progressive retinal vascular disease that arises within the temporal, juxtafoveal region of the macula.1, 2 Initially, the microvascular features of non-proliferative MacTel2 include telangiectatic abnormalities within the deep retinal capillary plexus temporal to the fovea and these changes progress to involve the superficial retinal capillary plexus with subsequent extension circumferentially to involve the perifoveal microvasculature. Additional fundus manifestations of non-proliferative MacTel2 include a loss of retinal transparency resulting in a grayish coloration of the juxtafoveal region, the deposition of superficial retinal crystals, and the formation of right-angled retinal vessels.3-7 Optical coherence tomography (OCT) imaging of the retina often shows characteristic changes that include cystic cavities arising within the inner and outer regions of the juxtafoveal retina with eventual loss of the photoreceptor inner/outer/ellipsoid (IS/OS/E) region followed by outer retinal atrophy.1, 2, 8-15 In the later proliferative stages of MacTel2, prominent intraretinal anastomoses develop within the temporal, juxtafoveal region and neovascularization may arise and extend under the retina often leading to pigment deposition and disciform scar formation. Gass and Oyakawa16 were the first to propose that this neovascularization associated with MacTel2 could develop anastomoses with the choroidal circulation based on the presence of dilated retinal arterioles and subretinal blood in these eyes. Irrespective of whether neovascularization arises, late stage MacTel2 is characterized by depigmentation of the retinal pigment epithelium (RPE), RPE atrophy, and foveal thinning with significant parafoveal or central visual function impairment.2-7, 17 While MacTel2 is thought to be a complex, genetic disorder, its pathogenesis is unknown and no proven treatment exists.
In its early stages, the pathognomonic feature of MacTel2 is the bilateral temporal, juxtafoveal leakage detected by fluorescein angiography (FA).1, 2 Optical coherence tomography angiography (OCTA) has identified retinal microvascular abnormalities that correlate with these areas of abnormal FA leakage.18, 19 These abnormal microvascular changes observed by OCTA appear to arise first within the deep capillary plexus of the retina and extend to the superficial plexus and then extend circumferentially around the fovea with concomitant formation of telangiectatic, microaneurysmal-like changes. As these microvascular abnormalities evolve, it appears as though prominent anastomoses between retinal layers arise with concomitant thinning of the retina. In a minority of cases, neovascularization develops, which can be visualized by the presence of a complex microvascular network that occupies the outer retinal layer, which is usually avascular.18 These neovascular lesions are often associated with an increase in retinal thickness, which usually does not occur in MacTel2 unless neovascularization is present. The neovascularization and the associated retinal thickening in proliferative Mactel2 has been shown to respond well to intravitreal inhibitors of vascular endothelial growth factor (VEGF), and intravitreal anti-VEGF therapy can improve vision in these eyes with neovascularization.20-28 Anti-VEGF therapy has been shown to reduce fluorescein angiographic leakage in non-proliferative MacTel2 as well, but the use of anti-VEGF therapy in eyes without neovascularization has not been shown to improve vision or slow disease progression.
In this study, we report on three cases with neovascular MacTel2 imaged using a prototype swept source OCT (SS-OCT) instrument and analyzed with an OCT microangiography (OMAG) algorithm that uses variations in both the intensity and phase information between sequential B-scans at the same position when creating an en face blood flow image.29 We demonstrate that OCTA is superior to FA and comparable to indocyanine green angiography (ICGA) for visualizing these neovascular lesions. Moreover, we show evidence that the choroidal vasculature appears to communicate with the MacTel2 subretinal neovascularization, and we document changes that occur to these neovascular lesions and the retinal microvasculature in response to anti-VEGF therapy.
METHODS
Patients were enrolled with the diagnosis of MacTel2 in a prospective, observational study at the Bascom Palmer Eye Institute as part of the MacTel Project and also enrolled in a prospective OCT imaging study. The Institutional Review Board of the University Of Miami Miller School Of Medicine approved both studies, and informed consents to participate in both the MacTel Project and the prospective OCT studies were obtained from all patients. The studies were performed in accordance with the tenets of the Declaration of Helsinki and compliant with the Health Insurance Portability and Accountability Act of 1996.
To be included in the MacTel2 study, patients needed to be diagnosed with MacTel2 in at least one eye and confirmed by the Moorfields Eye Hospital Reading Centre. Information about previous medical conditions and ocular treatments was obtained by reviewing the medical charts. In addition to a comprehensive ocular examination, all patients underwent color fundus imaging (Topcon, Tokyo, Japan), digital fundus autofluorescence imaging (Topcon, Tokyo, Japan and Heidelberg Engineering, Heidelberg, Germany), FA imaging (Heidelberg Engineering, Heidelberg, Germany), and spectral domain OCT (SD-OCT) imaging (CIRRUS, Carl Zeiss Meditec,Inc., Dublin, CA). SD-OCT imaging included both the 200X200 and the 512X128 macular raster scan patterns. In addition, patients were imaged using a modified CIRRUS prototype containing a swept source laser provided by Carl Zeiss Meditec Inc., (Dublin, CA) with a central wavelength of 1050nm (1000-1100 nm full width) and a speed of 100,000 A-scans per second. The ZEISS 1050nm swept source OCT (SS-OCT) prototype had a full width at half maximum (FWHM) axial resolution of ~5 μm in tissue and a lateral resolution at the retinal surface estimated at ~14 μm. To achieve OMAG imaging of the retinal and choroidal vasculatures, a repeated B-mode scan protocol was used to acquire volumetric datasets.30, 31,29 The OMAG scan was centered on the fovea and measured 3 mm × 3 mm on the retina. In the fast, transverse, x-axis scanning direction, 300 A-lines were used to form one single B-scan. Four consecutive B-scans were performed at each fixed location before proceeding to the next transverse location on the retina. In the slow, vertical, y-axis scanning direction, there were 300 positions over a 3-mm distance. The spacing between adjacent B-scan positions was 10 μm. The time difference between two successive B-scans was roughly 3.8 ms, which corresponded to a B-scan acquisition rate of 263 B-scans per second.
The OMAG algorithm was applied to the acquired volumetric dataset, and angiographic images representing the microvasculature within the scanned retinal tissue were extracted. 29-33 The OMAG algorithm utilizes the complex OCT signal, and is described in detail elsewhere. 30, 31. Briefly, complex OCT signals are first obtained by fast Fourier transformation of the dispersion-compensated k-space spectral data. Then, significant displacement occurring between repeated B-scans caused by the involuntary movement of the human eye or head was compensated for by using a 2-D cross correlation method. 34 Additional compensation of sub-pixel small motions of the eye could be achieved by using a high order phase compensation scheme as described in a previous publication.31 Finally, a direct differentiation of OCT signals between two consecutive B-scans was calculated and averaged for all repetitions at the same location to detect the signal change induced by red blood cells moving within patent vessels:
| (1) |
where i is the index for the repeated time of B-scans at each y-scan location, x is the fast axis scan position, z is the depth, C is the motion corrected complex OCT signal and N is the number of repeated B-scans in each location (N=4 in this study). After the OMAG processing of each set of repeated scans, an averaged structural (N=4) B-scan and OMAG B-scan was generated at each position.
The application of the OMAG algorithm to the acquired volumetric datasets produced OCT angiograms containing the retinal, choriocapillaris, and choroidal microvasculature. To enhance vascular visualization, a semi-automated retinal layer segmentation algorithm was used to create an en face projection of the vascular networks in individual layers. Using the OCT cross-sectional structural images based on the intensity differences, we developed an algorithm to segment the retina and choroid into different layers. 35 Three distinct physiological layers were segmented within the retina: an inner retinal layer from the ganglion cell layer to the inner plexiform layer (GCL + IPL), a middle retinal layer from the inner nuclear layer to the outer plexiform layer (INL + OPL), and an outer retinal layer from outer nuclear layer to the external limiting membrane (ONL + ELM layer). To visualize the subretinal neovascularization in patients, three additional layers were segmented representing a slab from the outer retinal layer to the choriocapillaris (CC), which is about 8 um beneath Bruch’s membrane layer; a slab from 8 um to 20 um beneath Bruch’s membrane, which includes the CC and inner choroid; and a slab encompassing the choroidal layers beneath the CC to the boundary of sclera. Colors were used to code for different layers to give a better depth-encoded visualization of the microvasculature. The microvasculature from the inner retina (superficial capillary plexus) is colored red, the microvasculature from the middle retinal layer (deep capillary plexus) is colored green, and any microvascular structures with flow in the outer retina are colored blue. The microvasculature from the outer retinal layer to the CC is colored purple, the remainder of the CC is colored red, and the rest of the choroidal vasculature is colored green. The three-dimensional structure of the retina and microvasculature were rendered and projected using Matlab software. The segmentation allowed for the visualization of the microvasculature in different layers of the retina and choroid, and a maximum projection method within the layer was used to create the en face images of interest. The qualitative OMAG en face images were compared with early and late-phase FA/ICGA images. Abnormalities in the OMAG images were identified qualitatively based on deviations from the expected location, shape, size, and distribution of the microvasculature in the various layers. The averaged OCT B-scans were also examined in the usual manner, and the cross-sectional retinal images were compared with the en face intensity and flow images.
RESULTS
A total of 82 patients with MacTel2 have been enrolled in the MacTel Project and 37 patients (74 eyes) have been imaged using the 1050nm prototype SS-OCT instrument. Of the 82 patients enrolled, 31 eyes had a presumed history of subretinal neovascularization and were treated between 2003 and 2015. Since mid-2013, when the prototype SS-OCT instrument became available for imaging, the diagnosis of subretinal neovascularization was confirmed in five eyes, and three of these eyes underwent OCT angiographic imaging. These three cases are described in this report.
Case 1: Neovascular MacTel2 in a 51-year-old woman
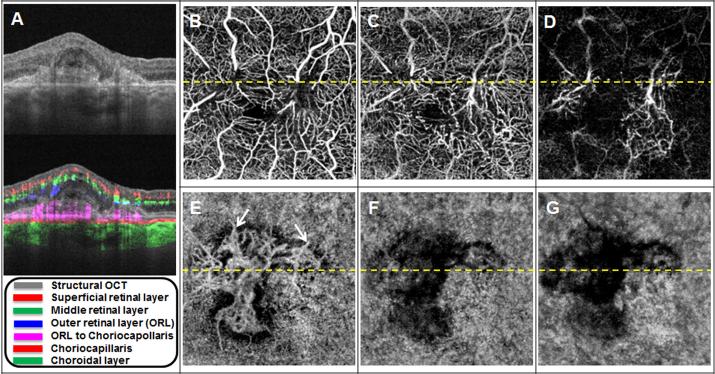
This 51-year-old woman presented with BCVA of 20/640 in the left eye. Her angiographic images from this initial encounter were published previously.18 However, the segmentation boundaries of the en face macular images have been changed to better visualize the subretinal neovascularization. Figure 1A shows the cross-sectional B-scan through her left macula, and the dotted horizontal yellow lines depicted on panels B through G represent the location of the B-scan. The layers segmented for OCT angiographic analyses are depicted by colors in the legend in panel A. The improved segmentation isolates the layer between the outer retinal boundary and the innermost aspect of the choriocapillaris. This slab is referred to as the outer retinal layer (ORL) to choriocapillaris layer. The en face image from this layer, which is depicted in panel E, shows the subretinal neovascular complex. The overlying retinal layers, depicted in panels B through D, show circumferential microvascular dropout around the fovea with prominent anastomoses between the superficial and deep retinal vascular plexi. The areas of apparent flow impairment within the choroidal layers under the neovascular complex (Fig. 1 F,G) are due to a lack of signal from these layers as documented by the absence of reflected light from the choroid as shown in in panel A by the intensity B-scan images. Thus, no conclusion can be drawn regarding the choroidal blood blow underlying the neovascularization. Panels A and B from Figure 2 show magnified views of early and late transit frames from FA images that correspond to color-encoded composite OCT angiographic images from the retina and the choroid (Fig. 2 C,D). The OCT angiographic images show the retinal and choroidal vasculature in greater detail than can be appreciated from the FA images alone. While vascular leakage cannot be detected by OCTA, the increase in retinal thickness and macular fluid seen on the cross-sectional B-scan images in Figure 1A suggest the presence of of vascular leakage.
Figure 1. Baseline OCT microangiography (OMAG) images of a 51-year-old woman with neovascular macular telangiectasia type 2 (Case #1).
(A) Horizontal B-scan averaged from four repeated B-scans with and without color-coded blood flow information. The layer and the corresponding color code is shown in the legend. The dotted yellow line in B-G represents the location of the B-scan. (B) En face OCT flow image of the superficial retinal layer. (C) En face OCT flow image of the middle retinal layer. (D) En face OCT flow image of the outer retinal layer (ORL). (E) En face OCT flow image from the ORL to the choriocapillaris (CC). (F) En face OCT flow image from the CC. (G) En face OCT flow image from the remaining choroidal vasculature.
Figure 2. Comparison of fluorescein angiography (FA) images with color-coded en face OCT microangiography (OMAG) flow images from the retina and choroid (Case #1).
(A) Early phase magnified FA image of the central macula. (B) Late phase FA image of the central macula. (C) Composite, color-coded en face OCT flow image of the retina. (D) Composite, color-coded en face OCT flow image from the boundary of the outer retinal layer to the sclera.
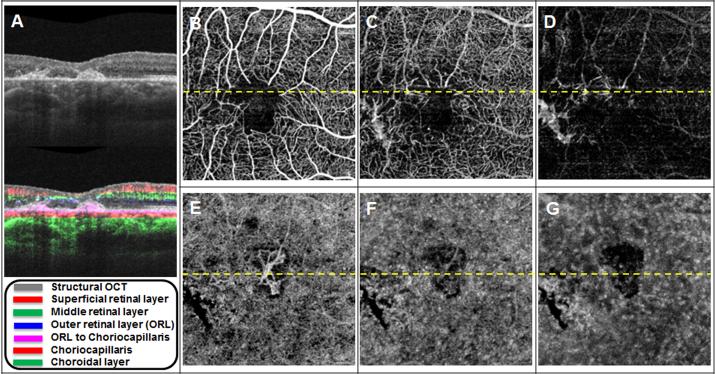
While the patient was enrolled in the MacTel Project at the Bascom Palmer Eye Institute, her treatment with anti-VEGF therapy had to be performed by an outside retina specialist due to insurance limitations. From April 2014 to March 2015 she received a total of three intravitreal bevacizumab injections with the last injection given in November of 2014. When she returned four months later for an annual visit, her BCVA had decreased to count fingers at two feet. Figure 3A shows a representative B-scan from this visit with evidence of increased retinal thickness and macular fluid, as well as an increase in the thickness of the subretinal neovascular complex. The increased size of this complex is shown in Figure 3E. In addition, arrows point to at least two vessels contributing to the neovascular complex that appear to arise from the choroidal circulation and do not correspond to overlying retinal vessels. Since retinal vessels could appear as projection artifacts on the underlying en face images, it is important to exclude them as masquerading as potential choroidal vessels. The two vessels indicated do not show an overlying structure that could create such a projection artifact, so it may be reasonable to conclude that they represent real signal from the choroidal vasculature. Figure 4 shows the magnified early and late FA images and the corresponding color-encoded en face images from the retina and choroidal vasculature, once again demonstrating how OCT angiographic imaging is superior to FA for visualizing the retinal microvasculature and the neovascular complex. The white arrows designate two of several locations where there appears to be anatomoses with the choroidal circulation, which cannot be appreciated with FA imaging. Figure 5 depicts representative images from the visits one year apart to better appreciate the microvascular changes and the growth of the subretinal neovascular complex.
Figure 3. One year follow-up OCT microangiography (OMAG) images of a 51-year-old woman with neovascular macular telangiectasia type 2 (Case #1).
(A) Horizontal B-scan averaged from four repeated B-scans with and without color-coded blood flow information. The layer and the corresponding color code is shown in the legend. (B) En face OCT flow image of the superficial retinal layer. (C) En face OCT flow image of the middle retinal layer. (D) En face OCT flow image of the outer retinal layer (ORL). (E) En face OCT flow image from the ORL to the choriocapillaris (CC). The white arrows point to choroidal vessels communicating with the subretinal neovascular complex. (F) En face OCT flow image from the CC. (G) En face OCT flow image from the remaining choroidal vasculature.
Figure 4. One year follow-up comparison of fluorescein angiography (FA) images with color-coded en face OCT microangiography (OMAG) flow images from the retina and choroid (Case #1).
(A) Early phase magnified FA image of the central macula. (B) Late phase FA image of the central macula. (C) Composite, color-coded en face OCT flow image of the retina. (D) Composite, color-coded en face OCT flow image from the boundary of the outer retinal layer to the sclera. The white arrows point to choroidal vessels communicating with the subretinal neovascular complex
Figure 5. Comparison of OCT structure and flow information after one year and three intravitreal bevacizumab intravitreal injections in a 51-year-old woman with neovascular macular telangiectasia type 2 (Case #1).
(A) Baseline: Horizontal B-scan averaged from four repeated B-scans with color-coded blood flow information. (B) Baseline: Composite, color-coded en face OCT flow image of the retina. (C) Baseline: En face OCT flow image from the layer between the outer retina to the inner choriocapillaris (CC). (D) One year follow-up: Horizontal B-scan comprised of four averaged B-scans with color-coded blood flow information. (E) One year follow-up: Composite, color-coded en face OCT flow image of the retina. (F) One year follow-up: En face OCT flow image from the layer between the outer retina to the inner choriocapillaris (CC).
Case 2: Neovascular MacTel2 in a 52-year-old man
A 52-year-old man with MacTel2 and a history of subretinal neovascularization presented with a BCVA of 20/60 in his right eye. He had received a total of seven intravitreal bevacizumab injections between December 2011 and July 2012. At the time of his enrollment into the MacTel Project, he had not received an injection for 35 months. Of note, he had an identical twin brother with the diagnosis of MacTel2 followed at another study site. Figure 6A shows the cross-sectional B-scan through his right macula, and the dotted horizontal yellow lines depicted on panels B through G show the location of the B-scan. The layers segmented for OCT angiographic analyses are depicted by colors in the legend in panel A. The en face image from the layer between the outer retinal boundary and the inner choriocapillaris depicted in panel E shows the subretinal neovascular complex. The overlying retinal layers, depicted in panels B through D, show the microvascular involvement of the foveal avascular zone in the superficial capillary plexus with microvascular dropout in the deeper layer, along with prominent anastomoses between the layers. The areas of apparent flow impairment within the choroidal layers under the neovascular complex (Fig. 6 F,G) are due to a lack of signal from these layers as documented by the absence of reflected light from within the choroid as seen in the intensity B-scan images shown in panel A. Thus, no conclusion can be drawn regarding the choroidal blood blow underlying the neovascularization. Figure 7 (panels A, B, D, and E) shows magnified views of early and late transit frames from FA and ICGA images that correspond to color-encoded composite OCT angiographic images from the retina and choroid (Fig. 7C,F). The composite OCT angiographic images depict the retinal and choroidal vasculature in greater detail than can be appreciated from the FA and ICGA images. While vascular leakage cannot be detected by OCT angiography, the cross-sectional B-scan shown in Fig. 6A does not reveal an increase in retinal thickness or macular fluid, which suggests that there is no active neovascular complex and no treatment was required. This conclusion could not have been reached if the only imaging modality used was FA due to the significant leakage shown in Figure 7B, which arises from the incompetent perifoveal microvasculature in MacTel2. Without additional information from the structural OCT B-scan, the source of this leakage could have been confused with leakage from the neovascular complex. Thus, it would appear that the use of both OCT structural and flow imaging provides all the information needed to manage this patient with neovascular MacTel2. Moreover, the white arrows on figures 6E and 7F designate at least one likely choroidal vessel that is contributing to the neovascular complex, and this vessel does not correspond to any of the overlying retinal vessels that could appear as projection artifacts on the underlying en face images. Further evidence that this vessel is contributing from the choroid is that it can be identified by the white arrow on the early ICG angiographic image shown in Figure 7D.
Figure 6. OCT microangiography (OMAG) images of a 52-year-old man with neovascular macular telangiectasia type 2 (Case #2).
(A) Horizontal B-scan averaged from four repeated B-scans with and without color-coded blood flow information. The layer and the corresponding color code are shown in the legend. The dotted yellow line in B-G represents the location of the B-scan. (B) En face OCT flow image of the superficial retinal layer. (C) En face OCT flow image of the middle retinal layer. (D) En face OCT flow image of the outer retinal layer (ORL). (E) En face OCT flow image from the ORL to the choriocapillaris (CC). The white arrow points to a choroidal vessel communicating with the subretinal neovascular complex. (F) En face OCT flow image from the CC. (G) En face flow image from the remaining choroidal vasculature.
Figure 7. Comparison of fluorescein angiography (FA) and indocyanine green (ICGA) images with color-coded en face OCT microangiography (OMAG) flow images from the retina and choroid (Case #2).
(A) Early phase magnified FA image of the central macula. (B) Late phase FA image of the central macula. (C) Composite, color-coded en face OCT flow image of the retina. (D) Early phase magnified ICGA image of the central macula. The white arrow points to a choroidal vessel communicating with the subretinal neovascular complex. (E) Late phase ICGA image of the central macula. (F) Composite, color-coded en face OCT flow image from the boundary of the outer retinal layer to the sclera. The white arrow points to a choroidal vessel communicating with the subretinal neovascular complex.
Case 3: Neovascular MacTel2 in a 56-year-old man
A 56-year-old man with MacTel2 presented with new onset vision loss in his right eye and BCVA of 20/400. Figure 8A shows the cross-sectional B-scan through his right macula, and the dotted horizontal yellow lines depicted on panels B through G represent the location of the B-scan. The layers segmented for OCT angiographic analyses are depicted by colors in the legend in panel A. The en face image from the layer between the outer retinal boundary and the inner choriocapillaris depicted in panel E shows two independent subretinal neovascular complexes with vascular connectivity to both the retinal and choroidal circulations. The choroidal vessels are shown with arrows. The overlying retinal layers, depicted in panels B through D, show telangiectatic, microaneurysmal-like changes in the deep capillary plexus (middle retinal layer) associated with microvascular dropout and prominent anastomoses with the inner retinal layer. The areas of apparent flow impairment within the choroidal layers under the neovascular complex (Fig. 8 F,G) are due to a lack of signal in these layers as documented by the absence of reflected light from within the choroid as represented by the intensity B-scan images shown in panel A. Thus, no conclusion can be drawn regarding the choroidal blood blow underlying the neovascularization. Figure 9 (panels A, B, D, and E) shows magnified views of early and late transit frames from FA and ICGA images that correspond to color-encoded composite OCT angiographic images from the retina and the choroid (Fig. 9 C,F). The composite OCT angiographic images depict the retinal and choroidal vasculature in greater detail than can be appreciated from the FA and ICGA images. While vascular leakage cannot be detected by OCT angiography, the cross-sectional B-scan shown in Fig. 8A does show an increase in retinal thickness and the presence of macular fluid, which suggests that the neovascular complex is active and requires treatment. The white arrows on Figures 8E and 9F designate at least two likely choroidal vessels that are contributing to the neovascular complex, and these vessels do not correspond to any of the overlying retinal vessels that could appear as projection artifacts on the underlying en face images. Moreover, these contributing choroidal vessels can be identified as well by the black arrows on the early ICGA image shown in Figure 9D. An intravitreal injection of bevacizumab was given at this visit.
Figure 8. OCT microangiography (OMAG) images of a 56-year-old man with neovascular macular telangiectasia type 2 (Case #3).
(A) Horizontal B-scan averaged from four repeated B-scans with and without color-coded blood flow information. The layer and the corresponding color code is shown in the legend. The dotted yellow line in B-G represents the location of the B-scan. (B) En face OCT flow image of the superficial retinal layer. (C) En face OCT flow image of the middle retinal layer. (D) En face OCT flow image of the outer retinal layer (ORL). (E) En face OCT flow image from the ORL to the choriocapillaris (CC). The white arrows point to choroidal vessels communicating with the subretinal neovascular complex (F) En face OCT flow image from the CC. (G) En face flow image from the remaining choroidal vasculature.
Figure 9. Comparison of fluorescein angiography (FA) and indocyanine green (ICGA) images with color-coded en face OCT microangiography (OMAG) flow images from the retina and choroid (Case #3).
(A) Early phase magnified FA image of the central macula. (B) Late phase FA image of the central macula. (C) Composite, color-coded en face OCT flow image of the retina. (D) Early phase magnified ICGA image of the central macula. The black arrows point to choroidal vessels communicating with the subretinal neovascular complex. (E) Late phase ICGA image of the central macula. (F) Composite, color-coded en face OCT flow image from the boundary of the outer retinal layer to the sclera. The white arrows point to choroidal vessel communicating with the subretinal neovascular complex.
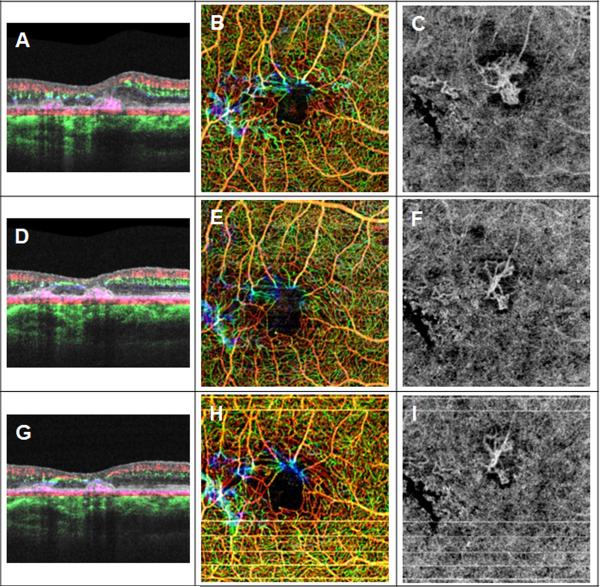
The patient returned for two additional, monthly bevacizumab injections. BCVA improved to 20/63 one month after the first injection. Figure 10A shows the response at this visit with the B-scan demonstrating a decrease in retinal thickness and macular fluid. The dilation of the retinal microvasculature appeared diminished and the telangiectatic changes were no longer as prominent (Fig. 10C). In addition, the two subretinal neovascular complexes appeared to decrease in size and complexity (Fig. 10E). A second injection of bevacizumab was given at this visit and one month later, the BCVA had improved to 20/40. The OCT B-scan showed a further decrease in retinal thickness (Fig. 11A), the retinal telangiectatic changes were no longer visible (Fig. 11C), and the subretinal neovascular complexes were even smaller with fewer vascular loops (Fig. 11E). Figure 12 shows the representative B-scans, color-encoded retinal vascular flow images, and the subretinal microvascular complexes at baseline and at each subsequent monthly follow-up to demonstrate the thinning of the retina, the changes in the retinal vasculature, and the improvements in the subretinal neovascular complex after monthly intravitreal injections of bevacizumab.
Figure 10. OCT microangiography (OMAG) images of a 56-year-old man with neovascular macular telangiectasia type 2 one month after the first intravitreal bevacizumab injection (Case #3).
(A) Horizontal B-scan averaged from four repeated B-scans with and without color-coded blood flow information. The layer and the corresponding color code is shown in the legend. The dotted yellow line in B-G represents the location of the B-scan. (B) En face OCT flow image of the superficial retinal layer. (C) En face OCT flow image of the middle retinal layer. (D) En face OCT flow image of the outer retinal layer (ORL). (E) En face OCT flow image from the ORL to the choriocapillaris (CC). (F) En face OCT flow image from the CC. (G) En face OCT flow image from the remaining choroidal vasculature.
Figure 11. OCT microangiography (OMAG) images of a 56-year-old man with neovascular macular telangiectasia type 2 one month after the second intravitreal bevacizumab injection (Case #3).
(A) Horizontal B-scan averaged from four repeated B-scans with and without color-coded blood flow information. The layer and the corresponding color code is shown in the legend. The dotted yellow line in B-G represents the location of the B-scan. (B) En face OCT flow image of the superficial retinal layer. (C) En face OCT flow image of the middle retinal layer. (D) En face OCT flow image of the outer retinal layer (ORL). (E) En face OCT flow image from the ORL to the choriocapillaris (CC). (F) En face OCT flow image from the CC. (G) En face OCT flow image from the remaining choroidal vasculature.
Figure 12. Comparison of OCT B-scans and microangiography (OMAG) flow images after monthly intravitreal injections of bevacizumab in a 56-year-old man with neovascular macular telangiectasia type 2 (Case #3).
(A) Baseline: Horizontal B-scan averaged from four repeated B-scans with color-coded blood flow information.. (B) Baseline: Composite, color-coded en face OCT flow image of the retina. (C) Baseline: En face OCT flow image from the layer between the outer retina to the inner choriocapillaris (CC). (D) One month after first bevacizumab injection: Horizontal B-scan comprised of four averaged B-scans with color-coded blood flow information. (E) One month after first bevacizumab injection: Composite, color-coded en face OCT flow image of the retina. (F) One month after first bevacizumab injection: En face OCT flow image from the layer between the outer retina to the inner choriocapillaris (CC). (G) One month after second bevacizumab injection: Horizontal B-scan comprised of four averaged B-scans with color-coded blood flow information. (H) One month after second bevacizumab injection: Composite, color-coded en face OCT flow image of the retina. (I) One month after second bevacizumab injection: En face OCT flow image from the layer between the outer retina to the inner choriocapillaris (CC).
DISCUSSION
In the three cases presented in this report, the subretinal neovascularization in these eyes with MacTel2 were well visualized with the ZEISS 1050nm SS-OCT prototype system with OCTA using the OMAG algorithm, and these OCTA images provided better detailed visualization of the neovascularization than fluorescein angiographic imaging and comparable to or even better visualization than ICG angiographic imaging. By using a segmentation strategy that isolated the layer between the outer retinal boundary and the inner most aspect of the choriocapillaris, we were able to observe the detailed microvascular structure of the subretinal neovascularization associated with MacTel2. In addition, the retinal microvasculature was visualized better with OCTA than with either angiographic strategy. The inability to visualize vascular leakage using OCTA is not a major deficiency since exudation from neovascularization can be detected on the traditional cross-sectional B-scans by the appearance of increased macular thickness, and these B-scans are acquired as part of the routine dataset for OMAG. When the OMAG dataset is acquired, each B-scan is repeated four times at the same position, so a representative intensity B-scan is an average of these four scans, each comprised of 300 A-scans over 3mm. Since averaging increases the signal to noise ratio, the image quality of this composite B-scan is better than it would be if only a single B-scan was acquired. Thus, OMAG has all the advantages of traditional OCT imaging, with the ability to generate B-scans and thickness maps, plus OMAG can generate high-density vascular flow maps of the retina and choroid.
MacTel2 is the ideal disease to study using OMAG since most of the pathology is contained within the central macula and the microvascular changes within the retina can be well visualized. The practical advantages of OMAG over traditional angiography are that it is faster, cheaper, and non-invasive, with none of nausea or anaphylactic risks associated with fluorescein and ICG angiography. In addition, OMAG can be repeated frequently, which is particularly useful when following patients undergoing anti-VEGF therapy. Another disadvantage of traditional fluorescein angiography is that the angiographic leakage associated with MacTel2 can obscure the presence and extent of the subretinal neovascularization. From our experience with ICG angiography in these patients, it is apparent that ICG angiography is better than fluorescein angiography in delineating the neovascularization, the response to neovascularization, and in identifying the choroidal vascular contributions into these subretinal neovascular complexes. In the past, ICG angiography was not routinely performed to diagnose and follow eyes with MacTel2 and this choroidal contribution to the subretinal neovascularization was not fully appreciated. However, like ICG angiography, OMAG imaging clearly demonstrates communications between these subretinal neovascular complexes and the choroidal circulation. Previously, subretinal neovascularization was thought to arise primarily from the retinal circulation, but now it would appear that the neovascular complex commonly communicates with both the retinal and choroidal circulations. It is unclear at what point the choroidal vasculature becomes involved in these subretinal neovascular complexes. While retinal anastomoses appear to play a prominent role in establishing these subretinal neovascular complexes, it may be that these neovascular lesions only start causing exudation-induced vision loss once the choroidal circulation contributes to the lesion. While Cases #1 and #2 represent more chronic examples of subretinal neovascularization, Case #3 was examined as soon as vision loss occurred and the choroidal component was evident even at the first visit when the neovascularization was diagnosed. It remains to be seen whether OMAG imaging can predict the formation of these neovascular complexes, but it is possible they may exist long before exudation causes vision loss, and we may be able to detect their formation as we follow patients longitudinally in the MacTel2 Study with OMAG based OCTA.
OMAG imaging also showed that there are changes that occur within the retinal microvasculature following the use of anti-VEGF therapy (Figure 12). It was known that anti-VEGF therapy decreased the exudation associated with MacTel2.20-28 However, OMAG also demonstrated that the caliber of the retinal vessels and their anastomoses decreased after anti-VEGF therapy and that the microaneurysmal changes associated with the telangiectatic vessels were no longer detectable. It remains to be determined whether anti-VEGF therapy actually caused regression of these lesions or whether the blood flow within them diminished beyond the level of detection.
The limitations of this study include the small number of eyes imaged using OMAG and the lack of a fully automated algorithm available on a commercial instrument. In addition, the high-density images were only achieved within a 3mm by 3mm scan area. Another limitation is that this prototype instrument is not yet commercially available, but that will hopefully be rectified in the near future. The goal is to provide a swept-source OCT instrument capable of high density imaging of both the retinal and choroidal vasculatures using a fully automated algorithm.
In summary, our results clearly demonstrate the utility of swept-source OCT angiography in eyes with neovascular MacTel2. SS-OCT imaging was able to identify vascular structures deep to the retina even in the presence of significant fluid. The combination of SS-OCT with the OMAG algorithm resulted in images of the retinal and choroidal vasculature that were sufficient for the management of eyes with neovascular MacTel2. We predict that with the use of SS-OCT angiography, traditional angiography will no longer be needed for these MacTel2 patients. The ability to non-invasively acquire both structure and flow information from a single dataset demonstrates that OCTA is all you need. Whether traditional angiography will be replaced by OCTA in other macular diseases remains to be determined, but the improved choroidal imaging associated with the longer wavelength light used in SS-OCT imaging would suggest that SS-OCT could become the gold standard for imaging diseases that involve the choroidal circulation.
Summary Statement.
Imaging subretinal neovascularization in proliferative macular telangiectasia type 2 (MacTel2) with swept source optical coherence tomography based microangiography (OMAG) revealed detailed, depth-resolved information that was superior to fluorescein angiographic imaging in showing retinal microvascular changes and similar to indocyanine green angiographic imaging in showing communications with the choroidal vasculature.
Acknowledgements
We appreciate all the exceptional efforts of Cristy Lage-Rodriguez and Monica Arango in coordinating patients for the MacTel Study.
Research supported by grants from Carl Zeiss Meditec, Inc. (Dublin, CA), the National Eye Institute (R01EY024158), the Macula Vision Research Foundation, the Emma Clyde Hodge Memorial Foundation, the Feig Family Foundation, an unrestricted grant from the Research to Prevent Blindness, Inc., New York, NY and the National Eye Institute Center Core Grant (P30EY014801) to the Department of Ophthalmology, University of Miami Miller School of Medicine
Footnotes
Disclosures:
Drs. Wang, Gregori, and Rosenfeld received research support from Carl Zeiss Meditec, Inc. Dr. Gregori and the University of Miami co-own a patent that is licensed to Carl Zeiss Meditec, Inc. Dr. Rosenfeld received research support from Acucela, Apellis, Genentech/Roche, GlaxoSmithKline, Neurotech, Ocata Therapeutics, and Tyrogenex. He is a consultant for Achillion, Acucela, Alcon, Bayer, Chengdu Kanghong Biotech,CoDa Therapeutics, Genentech/Roche, Healios K.K., Merck, Regeneron, Stealth, and Tyrogenex.
Dr. Wang and the Oregon Health & Science University co-own a patent that is licensed to Carl Zeiss Meditec, Inc. Dr. Wang received an innovative research award from Research to Prevent Blindness.
Dr. Zhang, Dr Chen, Dr. A. Legarreta, and Dr. J. Legarreta have no disclosures.
Drs. An, Durbin, Sharma, and Stetson are employed by Carl Zeiss Meditec, Inc.
REFERENCES
- 1.Yannuzzi LA, Bardal AM, Freund KB, et al. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124(4):450–60. doi: 10.1001/archopht.124.4.450. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, Evans T, Arevalo JF. Idiopathic macular telangiectasia type 2 (idiopathic juxtafoveolar retinal telangiectasis type 2A, Mac Tel 2) Surv Ophthalmol. 2013;58(6):536–59. doi: 10.1016/j.survophthal.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Aung KZ, Wickremasinghe SS, Makeyeva G, et al. The prevalence estimates of macular telangiectasia type 2: the Melbourne Collaborative Cohort Study. Retina. 2010;30(3):473–8. doi: 10.1097/IAE.0b013e3181bd2c71. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Blodi BA, Meuer SM, et al. The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. Am J Ophthalmol. 2010;150(1):55–62. doi: 10.1016/j.ajo.2010.02.013. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemons TE, Gillies MC, Chew EY, et al. Baseline characteristics of participants in the natural history study of macular telangiectasia (MacTel) MacTel Project Report No. 2. Ophthalmic Epidemiol. 2010;17(1):66–73. doi: 10.3109/09286580903450361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallo FB, Leung I, Chung M, et al. Retinal crystals in type 2 idiopathic macular telangiectasia. Ophthalmology. 2011;118(12):2461–7. doi: 10.1016/j.ophtha.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu M, Krilis M, Gillies MC. The relationship between inner retinal cavitation, photoreceptor disruption, and the integrity of the outer limiting membrane in macular telangiectasia type 2. Retina. 2013;33(8):1547–50. doi: 10.1097/IAE.0b013e318285cb9c. [DOI] [PubMed] [Google Scholar]
- 8.Surguch V, Gamulescu MA, Gabel VP. Optical coherence tomography findings in idiopathic juxtafoveal retinal telangiectasis. Graefes Arch Clin Exp Ophthalmol. 2007;245(6):783–8. doi: 10.1007/s00417-006-0432-1. [DOI] [PubMed] [Google Scholar]
- 9.Gaudric A, Ducos de Lahitte G, Cohen SY, et al. Optical coherence tomography in group 2A idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 2006;124(10):1410–9. doi: 10.1001/archopht.124.10.1410. [DOI] [PubMed] [Google Scholar]
- 10.Paunescu LA, Ko TH, Duker JS, et al. Idiopathic juxtafoveal retinal telangiectasis: new findings by ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113(1):48–57. doi: 10.1016/j.ophtha.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koizumi H, Iida T, Maruko I. Morphologic features of group 2A idiopathic juxtafoveolar retinal telangiectasis in three-dimensional optical coherence tomography. Am J Ophthalmol. 2006;142(2):340–3. doi: 10.1016/j.ajo.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Baumuller S, Charbel Issa P, Scholl HP, et al. Outer retinal hyperreflective spots on spectral-domain optical coherence tomography in macular telangiectasia type 2. Ophthalmology. 2010;117(11):2162–8. doi: 10.1016/j.ophtha.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Sallo FB, Peto T, Egan C, et al. The IS/OS junction layer in the natural history of type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2012;53(12):7889–95. doi: 10.1167/iovs.12-10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallo FB, Peto T, Egan C, et al. "En face" OCT imaging of the IS/OS junction line in type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2012;53(10):6145–52. doi: 10.1167/iovs.12-10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathew R, Sivaprasad S, Florea D, et al. Agreement between time-domain and spectral-domain optical coherence tomography in the assessment of macular thickness in patients with idiopathic macular telangiectasia type 2. Ophthalmologica. 2013;230(3):144–50. doi: 10.1159/000353455. [DOI] [PubMed] [Google Scholar]
- 16.Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100(5):769–80. doi: 10.1001/archopht.1982.01030030773010. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz-Valckenberg S, Ong EE, Rubin GS, et al. Structural and functional changes over time in MacTel patients. Retina. 2009;29(9):1314–20. doi: 10.1097/IAE.0b013e3181a4d2f1. [DOI] [PubMed] [Google Scholar]
- 18.Thorell MR, Zhang Q, Huang Y, et al. Swept-source OCT angiography of macular telangiectasia type 2. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):369–80. doi: 10.3928/23258160-20140909-06. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF, Klancnik JM, Jr., Cooney MJ. Retinal vascular layers in macular telangiectasia type 2 imaged by optical coherence tomographic angiography. JAMA Ophthalmol. 2015;133(1):66–73. doi: 10.1001/jamaophthalmol.2014.3950. [DOI] [PubMed] [Google Scholar]
- 20.Charbel Issa P, Holz FG, Scholl HP. Findings in fluorescein angiography and optical coherence tomography after intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Ophthalmology. 2007;114(9):1736–42. doi: 10.1016/j.ophtha.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 21.Charbel Issa P, Finger RP, Holz FG, Scholl HP. Eighteen-month follow-up of intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Br J Ophthalmol. 2008;92(7):941–5. doi: 10.1136/bjo.2007.129627. [DOI] [PubMed] [Google Scholar]
- 22.Gamulescu MA, Walter A, Sachs H, Helbig H. Bevacizumab in the treatment of idiopathic macular telangiectasia. Graefes Arch Clin Exp Ophthalmol. 2008;246(8):1189–93. doi: 10.1007/s00417-008-0795-6. [DOI] [PubMed] [Google Scholar]
- 23.Kovach JL, Rosenfeld PJ. Bevacizumab (avastin) therapy for idiopathic macular telangiectasia type II. Retina. 2009;29(1):27–32. doi: 10.1097/IAE.0b013e31818ba9de. [DOI] [PubMed] [Google Scholar]
- 24.Roller AB, Folk JC, Patel NM, et al. Intravitreal bevacizumab for treatment of proliferative and nonproliferative type 2 idiopathic macular telangiectasia. Retina. 2011;31(9):1848–55. doi: 10.1097/IAE.0b013e31820d3feb. [DOI] [PubMed] [Google Scholar]
- 25.Lira RP, Silva VB, Cavalcanti TM, et al. Intravitreous ranibizumab as treatment for macular telangiectasia type 2. Arch Ophthalmol. 2010;128(8):1075–8. doi: 10.1001/archophthalmol.2010.155. [DOI] [PubMed] [Google Scholar]
- 26.Charbel Issa P, Finger RP, Kruse K, et al. Monthly ranibizumab for nonproliferative macular telangiectasia type 2: a 12-month prospective study. Am J Ophthalmol. 2011;151(5):876–86. doi: 10.1016/j.ajo.2010.11.019. e1. [DOI] [PubMed] [Google Scholar]
- 27.Toy BC, Koo E, Cukras C, et al. Treatment of nonneovascular idiopathic macular telangiectasia type 2 with intravitreal ranibizumab: results of a phase II clinical trial. Retina. 2012;32(5):996–1006. doi: 10.1097/IAE.0b013e31824690a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do DV, Bressler SB, Cassard SD, et al. Ranibizumab for macular telangiectasia type 2 in the absence of subretinal neovascularization. Retina. 2014;34(10):2063–71. doi: 10.1097/IAE.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Zhang Q, Thorell MR, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):382–9. doi: 10.3928/23258160-20140909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang RK, An L, Francis P, Wilson DJ. Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Opt Lett. 2010;35(9):1467–9. doi: 10.1364/OL.35.001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An L, Subhush HM, Wilson DJ, Wang RK. High-resolution wide-field imaging of retinal and choroidal blood perfusion with optical microangiography. J Biomed Opt. 2010;15(2):026011. doi: 10.1117/1.3369811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang RK, Jacques SL, Ma Z, et al. Three dimensional optical angiography. Opt Express. 2007;15(7):4083–97. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
- 33.Wang RK, Hurst S. Mapping of cerebro-vascular blood perfusion in mice with skin and skull intact by Optical Micro-AngioGraphy at 1.3 mum wavelength. Opt Express. 2007;15(18):11402–12. doi: 10.1364/oe.15.011402. [DOI] [PubMed] [Google Scholar]
- 34.An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express. 2008;16(15):11438–52. doi: 10.1364/oe.16.011438. [DOI] [PubMed] [Google Scholar]
- 35.Yin X, Chao JR, Wang RK. User-guided segmentation for volumetric retinal optical coherence tomography images. J Biomed Opt. 2014;19(8):086020. doi: 10.1117/1.JBO.19.8.086020. [DOI] [PMC free article] [PubMed] [Google Scholar]