Abstract
Not everyone who tries tobacco or other nicotine-containing products becomes a long-term user. Certain traits or factors that are differentially present in these individuals must be able to help health care providers and researchers determine who is more likely to become chronic users of nicotine-containing products. Some of these factors, particularly sensation-seeking/novelty, impulsivity, and anxiety, lend themselves to the creation of animal models of reactivity to nicotine. These models of reactivity to nicotine can improve the translational aspects of preclinical animal research on nicotine-induced behaviors and treatments in order to help reduce negative outcomes in human populations. The goal of this review is to evaluate the current status of animal models of individual differences that serve to predict the later behavioral effects of nicotine. The limited utility and inconsistency of existing novelty models is considered, as well as the promise of impulsivity and anxiety models in preclinical animal populations. Finally, other models that could be employed to extend the benefit of the current research are examined.
Keywords: Individual differences, Nicotine, Novelty, Sensation-seeking, Impulsivity, Anxiety, Rats, Mice
1. Introduction
Tobacco causes the deaths of nearly 6 million people a year worldwide (World Health Organization, 2013). Yet, not everyone who tries tobacco products becomes a daily user (Substance Abuse and Mental Health Services Administration, 2012). Certainly, an important goal for researchers is to determine who is apt to become a regular tobacco user prior to negative outcomes. Indeed, researchers working with human populations have been interested in factors that predict individual differences to tobacco and nicotine dependence (e.g., Baker et al., 2007; Cosci et al., 2009; Donny et al., 2008). Clinical research has identified some potential factors that may influence individual differences such as psychopathology, personality traits, and parental drug use (e.g., Alvarado and Breslau, 2005; Breslau et al., 1993). Well-informed preclinical research is needed to establish the causal mechanisms behind such factors. Preclinical models with rodents have been successfully used to dissect human behaviors into component constructs for scientific study; chronic nicotine use and its associated dependence is no exception. For example, nicotine self-administration studies are a widely used and effective means of investigating the reinforcing effects of nicotine (e.g., Corrigall, 1999; Rose and Corrigall, 1997). Certainly, the behavioral and physiological variation shown by rats and mice could be leveraged to better understand potential contributors to human individual differences in chronic use of nicotine-containing products (e.g., Antoniou et al., 2008; Kabbaj, 2006; Thiel et al., 1999).
To date, the majority of preclinical animal research on individual differences in the effects of drugs of abuse has largely focused on amphetamine and cocaine. The research that has focused on individual differences in the later reactivity to nicotine has mostly mirrored the investigations with the more traditional psychostimulants. As detailed in this review, this approach has had limited utility. Perhaps part of the limitation reflects the well-documented differences between traditional psychostimulants and nicotine (e.g., Di Chiara, 2002; Sulzer, 2011). With this in mind, we believe it is time to ask whether nicotine individual differences research with rodents should continue to mimic other psychostimulant research, or if different avenues should be explored that may be more specific to nicotine?
The goal of this review is to evaluate the current status of preclinical animal models examining predictors of individual differences in the behavioral effects of nicotine. For background, we will first briefly discuss some individual correlates of interest in smokers. We will also provide a brief overview of the psychomotor stimulant research on individual differences and its relation to nicotine individual differences research given the parallel in the literature. Finally, connections between current behavioral models and future research will be discussed.
2. Human individual differences
The path or paths from casual drug use to addiction is not completely clear; certainly there is no one causative factor. Numerous biological, psychological, and social factors have been implicated in an individual's vulnerability to addiction (e.g., Grant et al., 2009; Koob, 2004; Sweitzer et al., 2012; Swendsen and Le Moal, 2011). In this section, we will discuss three psychological constructs in humans that have been repeatedly linked to vulnerability to chronic tobacco use and nicotine addiction. These constructs, sensation-seeking, impulsivity, and anxiety, have lent themselves to the creation of analogous rodent models to investigate individual differences in the behavioral reactivity to nicotine (e.g., Bevins and Besheer, 2001; Cryan and Sweeney, 2011; Jupp et al., 2013; Zuckerman, 1984).
2.1. Novelty/sensation-seeking
One factor linked to individual differences in humans to vulnerability to chronic nicotine use is proneness to novelty-or sensation-seeking. Sensation-seeking is a trait defined as the need for varied, novel, and complex sensations and experiences to maintain an optimal level of arousal (cf. Zuckerman et al., 1972). Sensation-seeking has been found to be proportionally linked to smoking status, with a larger percentage of high sensation-seekers being smokers than either moderate or low sensation-seekers (Carton et al., 1994; Doran et al., 2011; Harmsen et al., 2006; Zuckerman et al., 1990). High sensation-seekers are more likely to begin and continue smoking (Malmberg et al., 2013). In addition, high sensation-seekers inhale more cigarette smoke than low sensation-seekers (Zuckerman et al., 1990). Interestingly, there does not appear to be a consistent relation between sensation-seeking and readiness to change smoking behaviors (Hall et al., 2012; Harmsen et al., 2006; Kahler et al., 2009).
2.2. Impulsivity
Impulsivity appears to be a multi-dimensional construct, which broadly defined, refers to the tendency to engage in inappropriate or maladaptive behaviors, or to engage in poorly planned and risky behaviors (de Wit, 2008; Evenden, 1999; Jupp et al., 2013). Individuals who are regular smokers tend to score higher on measures of impulsivity than those who have never smoked (Mitchell, 1999). Urgency, a component of impulsivity, has been found to be related to higher tobacco craving and to be associated with higher rates of tobacco use (Billieux et al., 2007; Spillane et al., 2010). Smokers high in urgency and lack of perseverance report higher rates of negative affect craving, or craving that anticipates relief from aversive states (Doran et al., 2009). Individuals that score higher on impulsivity traits may also be more sensitive to the acute rewarding effects of nicotine (Perkins et al., 2008).
2.3. Anxiety
Anxiety has the highest lifetime prevalence of any psychiatric disorder in the United States and Europe (Kessler et al., 2005; Kessler, 2007). The prevalence of drug abuse disorders, including nicotine dependence, are much higher in individuals with anxiety disorders than in the general population (Compton et al., 2007; Grant et al., 2004; Kushner et al., 2012). Smokers with anxiety symptoms tend to exhibit increased motivation to smoke, increased withdrawal symptoms, lack of a response to current pharmacological interventions, and impaired ability to quit smoking (Piper et al., 2010a). In addition, anxiety and “anxiety sensitivity” (i.e., a fear of anxious emotions), are related to nicotine use. Notably, anxiety sensitivity correlates with negative reinforcement value of tobacco use (Battista et al., 2008). Individuals with higher levels of anxiety sensitivity appear to have longer persisting symptoms of withdrawal than individuals with lower levels of anxiety sensitivity (Langdon et al., 2013).
3. Modeling psychostimulant individual differences in animal populations
Though the initial studies of predictors of individual differences of later behavioral drug effects in rodents appear to start with nicotine (Rosecrans, 1970), the majority of research in the area of individual differences to drug response has utilized traditional psychostimulants. As a result, later efforts using nicotine appear to be modeled on research with traditional psychostimulants, with varying degrees of success. To provide some historical context, individual difference research with traditional psychostimulants will be briefly summarized first, before discussing in further detail the individual differences research with nicotine.
3.1. d-Amphetamine
The first rodent study examining individual vulnerability to traditional psychostimulants was conducted by Piazza et al. (1989). In that study, Piazza and colleagues asked whether reactivity to novelty predicted later behavioral sensitivity to amphetamine in rats (Piazza et al., 1989). Reactivity to novelty was defined as locomotor activity in an inescapable novel environment. The more active half of the rats were classified as high responders (HR) and the less active half of the rats were classified as low responders (LR). HR rats had a higher initial locomotor response to amphetamine than LR rats. In addition, across repeated amphetamine treatment, HR rats did not show a significant increase in locomotion, whereas LR rats showed a significant increase in locomotion (i.e., sensitization). After sensitization, saline-treated and amphetamine-treated rats were implanted with intravenous cannulas and allowed to acquire amphetamine self-administration (SA). All amphetamine pre-treated rats, HRs or LRs, acquired SA. This was not the case for rats that had saline in the locomotor phase of the study. In the saline-pretreated rats, HRs acquired SA, whereas LRs failed to develop reliable SA behavior. Reactivity to the inescapable novel environment correlated with amphetamine locomotor sensitization and to amphetamine SA.
This now widely cited study helped create a new area of study in behavioral pharmacology with rats and mice–individual predictors of later vulnerability to traditional psychostimulants, and eventually nicotine, in rodent models. In addition, this study began an approach to using reactivity to the inescapable novel environment as a proxy for the human trait of sensation-seeking (see Blanchard et al., 2009; Dellu et al., 1996; Kabbaj, 2006; Redolat et al., 2009). While the validity of this model is beyond the scope of this review, previous reviews have suggested that there is significant evidence to support the validity of the inescapable novel environment model. In short, the published behavioral and neural correlates of HR rats appear similar to that of high sensation-seeking humans in terms of drug seeking, anxiety/stress states, and dopamine and serotonin levels (Blanchard et al., 2009; Dellu et al., 1996; Kabbaj, 2006).
Additional research has investigated the predictive relation between novelty and drug reinforcement and reward with d-amphetamine. A number of studies have replicated Piazza and colleagues' original study with HRs self-administering amphetamine at higher rates than LRs (e.g., Klebaur et al., 2001; Pierre and Vezina, 1997). Whether or not reactivity to an inescapable novel environment predicts the conditioned rewarding effects of amphetamine in a place conditioning task remains controversial (e.g., Erb and Parker, 1994; Mathews et al., 2010). Other research has shown that rats separated into high and low impulsivity groups, based on impulsive choice in a delay discounting task, displayed differences in amphetamine place conditioning (Yates et al., 2012). High impulsivity (HiI) rats developed a preference for an environment paired with amphetamine, whereas low impulsivity (LoI) rats do not display this conditioned place preference.
3.2. Cocaine
Similar to amphetamine, rats classified as HRs to an inescapable novel environment acquired and maintained cocaine SA at higher rates than LRs (Mantsch et al., 2001; Piazza et al., 2000). In the place conditioning task, HR and LR rats do not differ in expression of cocaine conditioned place preference (Gong et al., 1996; Kosten and Miserendino, 1998). HiI rats escalated their cocaine SA and persisted in increased responding relative to the LoI rats (Anker et al., 2009). Individual differences screens that separated rats on the basis of performance in the elevated plus maze (EPM) and/or light/dark box (LDB) tests, purported tasks that index anxiety, found that high anxiety (HA) rats were more sensitive to the conditioned rewarding effects of cocaine in a place conditioning task and had greater escalation of cocaine SA in comparison to low anxiety (LA) rats (Dilleen et al., 2012; Pelloux et al., 2009).
3.3. Methamphetamine
Currently, there is very little research addressing individual differences to later methamphetamine-induced behavioral effects in animal models. Research examining the relation between reactivity to the inescapable novel environment and the later conditioned and unconditioned locomotor effects of methamphetamine found that HR rats were more responsive to acute and repeated treatment with methamphetamine in a locomotor sensitization regimen (Bevins and Peterson, 2004). LRs did not display a stimulant response to acute treatment with methamphetamine, but displayed locomotor sensitization with repeated treatment. Other research has shown that HR rats acquired methamphetamine SA, whereas LR rats did not (Gancarz et al., 2011).
4. Modeling individual differences to nicotine in rats and mice
As this brief summary suggests, factors such as reactivity to novelty, impulsivity, and anxiety can predict the subsequent effects of d-amphetamine, cocaine, and methamphetamine. The early success of this research has set precedent for the experimental approach and predictive screens used by investigators interested in individual differences and the later behavioral effects of nicotine. As detailed in the following sections, the limited work in individual differences and nicotine in rodents has met with mixed success. This record suggests to us that alternative approaches to evaluate potentially predictive constructs may be necessary.
4.1. Novelty studies
Studies with nicotine have attempted to use individual differences in the response to inescapable novelty to predict sensitivity to a variety of behavioral effects of nicotine. As noted earlier (see Section 3.1), and reviewed by others (see Blanchard et al., 2009; Dellu et al., 1996; Kabbaj, 2006; Redolat et al., 2009), the HR/LR model of reactivity to inescapable novelty has served as a proxy for the human trait of sensation-seeking. The HR/LR approach normally uses a median (or quartile) split of locomotor behavior in an inescapable novel environment; though other statistical approaches have been used; see each table for approach to designating high vs. low responders in each study. There are a few studies with nicotine that assess reactivity to novelty via apparatus other than an inescapable novel environment (e.g., hole-board apparatus) or via free-choice methods, where the animal can select to approach or avoid the novel stimulus (see later).
4.1.1. Nicotine-induced activity
Though much of the current theoretical framework involving high/low responders and drug response largely ignores findings from research using nicotine, some of the early research was pioneered by J.A. Rosecrans in his work on nicotine's locomotor effects in male and female rats (Rosecrans, 1971a; Rosecrans, 1971b). Rosecrans did not use the typical inescapable novel environment. Rather, he had an apparatus where rats could move between a familiar and a novel area in the environment. In these studies, male and female rats were divided into high activity and low activity rats based on exploration of the novel area of an apparatus over four 3-min sessions (Rosecrans, 1971a; Rosecrans, 1971b). Rosecrans found that high activity rats exhibited different rates of locomotor sensitization after 4 nicotine treatments than low activity rats, depending on sex. In male rats, high activity rats had higher levels of sensitization than low activity rats. However, in female rats, high activity rats had lower levels of sensitization than low activity rats. Rosecrans' studies built on an earlier observation that nicotine administration increased the activity of male rats that initially had lower levels of activity and decreased the activity of initially more active rats (Morrison and Lee, 1968).
Other than these studies, predictors of individual differences in responses to nicotine went largely unstudied until Piazza's work with amphetamine (Piazza et al., 1989) re-ignited empirical interest in identifying individual-level predictors of later drug effects. Table 1 summarizes the studies that used the inescapable novel environment as a predictor of later locomotor behavior with nicotine. As can be seen in Table 1 and discussed below, this research has revealed an inconsistent relation between reactivity to an inescapable novel environment and the subsequent locomotor effects of nicotine.
Table 1.
Studies showing predictive relation between inescapable novel environment and subsequent nicotine-induced locomotor behavior.
| Reference | Animals | Nicotine dose(s)/treatment | Outcome |
|---|---|---|---|
| Aydin et al. (2011)T | Adolescent male Sprague–Dawley rats | 0.35 mg/kg SC; injection every 3 days × 4 injections | HR > LR |
| Aydin et al. (2012)T | Adolescent male Sprague–Dawley rats | 0.35 mg/kg SC; injection every 3 days × 4 injections | HR > LR |
| Bernardi and Spanagel (2014)M | Adult male C57BI/6N mice | 0.5 mg/kg IP; 15 injections | HR > LR |
| Bevins and Besheer (2001)*,Z | Adult male Sprague–Dawley rats | 0.351 mg/kg SC; 7 injections | HR = LR |
| Coolon and Cain (2009)M | Adult male Sprague–Dawley rats | 0.2 or 0.8 mg/kg SC; 8 injections | HR = LR |
| Morrison and Lee (1968)†,C | Adolescent and adult Lister, adolescent Sprague–Dawley, and adolescent Wistar rats | 0.4 mg/kg SC; 4 injections | HR ↓; LR ↑ |
| Pastor et al. (2013)M | Adolescent male Sprague–Dawley rats | 0.4 mg/kg SC; 1 injection | HR = LR |
| Pehrson et al. (2008)F | Adult male and female Sprague–Dawley rats | 0.8 mg/kg SC × 2/day; 14 days | Males: HR > LR Females: HR = LR |
| Philpot et al. (2012)F | Adolescent male Sprague–Dawley rats | 0.14, 0.21, 0.42, or 0.56 mg/kg SC; 8 injections | HR > LR at low doses HR = LR at moderate/high doses |
| Prus et al. (2008)M | Adult male and female Lewis rats | 0.4 mg/kg SC × 2/day; 14 days | HR = LR both sexes |
| Rosecrans (1971a)M | Adult female Sprague–Dawley rats | 0.4 mg/kg SC; 5 injections | HR ↓; LR ↑ |
| Rosecrans (1971b)M | Adult male and female Sprague–Dawley rats | 0.4 mg/kg SC; 4 injections | HR > LR in males; HR < LR in females |
HR = high responder; LR = low responder; SC = subcutaneous; IP = intraperitoneal;
nicotine dose in salt form;
sex unknown;
= uses median split to divide into HR/LR;
= tertiary split;
= uses top/bottom 40%;
= uses correlations;
= uses z-scores.
For example, Pehrson et al. (2008) found that HR male Sprague– Dawley rats exhibited significant reductions in locomotor behavior on days 1 and 7, but not day 14, of nicotine administration. LR male rats showed a chronic increase in nicotine-induced locomotor behavior (i.e., consistent with sensitization). However, neither HR nor LR females displayed locomotor sensitization. Recall that Rosecrans' original studies found that in HR male rats displayed greater sensitization than LRs, whereas HR females displayed less sensitization than LRs. Perhaps this difference reflects the use of a novel environment task by Rosecrans that allowed rats to avoid the novel environment. Albeit possible, complicating the picture is the work by Prus et al. (2008) in which HR and LR male and female Lewis rats displayed an increase in locomotor behavior when treated repeatedly with nicotine (see Table 1).
The predictive relation between inescapable novelty and nicotine-induced locomotor behavior has been examined in adolescent rats as well. Unfortunately, the findings here are also inconsistent. Philpot and colleagues found that adolescent HR rats developed sensitization to lower doses of nicotine than adolescent LR rats, though this was not the case at higher doses of nicotine (Philpot et al., 2012). Other sensitization work has been conducted in adolescent rats utilizingan intermittent nicotine sensitization regimen in which nicotine was administered every 3 days for a total of 4 injections (Aydin et al., 2011). HR rats treated with nicotine had higher activity levels than saline-injected rats on all days. LR rats, in contrast, showed nicotine-induced hyper-locomotion only after injections 3 and 4. HR rats previously treated with nicotine showed a significant increase in activity to a nicotine challenge one week after sensitization training; LR rats did not display hyperactivity in the nicotine-challenge test. A subsequent publication by this research team in adolescent rats did not replicate the earlier data pattern (Aydin et al., 2012). In this later work, Aydin et al. (2012) reported that HRs and LRs showed increased locomotor activity only after the fourth nicotine injection rather than the earlier onset of increased locomotor behavior in HR rats in earlier publication (Aydin et al., 2011). The predictive utility of the inescapable novel environment for the locomotor effects of nicotine seem inconsistent, sometimes even within a laboratory.
Research has also suggested that administration of nicotine may alter HR/LR status in a non-systematic manner. Bevins and Besheer (2001) found that acute treatment with nicotine disrupted the HR/LR status in rats; this status was maintained in saline-treated rats. Among HRs treated with nicotine, some HRs continued to show high locomotor activity, but the locomotor activity of others became lower. A similar mixed shift was seen in the LRs making the overall nicotine-induced activity level similar in HRs and LRs. Similar work found that in adolescent rats, initial HR/LR populations became mixed with an acute administration of nicotine (Pastor et al., 2013). In addition to these studies, there are an additional set of studies where the inescapable novel environment fails to predict in a consistent manner later nicotine-induced loco-motor behavior (Coolon and Cain, 2009; Pastor et al., 2013). Finally, there appears to be a single study showing that HR mice had higher rates of acute nicotine-induced locomotor behavior, as well as higher rates of nicotine sensitization than LR mice (Bernardi and Spanagel, 2014); this work awaits replication.
Clearly, across study designs, the inescapable novel environment has an inconsistent relation with nicotine-induced locomotor behavior. At this point, it is difficult to isolate within-study factors as the culprit behind this inconsistency, though it may be that in-depth study and replications can assist. It may simply be that that as nicotine is functioning differently in the brain than traditional psychostimulants (e.g., Di Chiara, 2002; Sulzer, 2011), that the inescapable novel environment has less predictive value with nicotine-induced behaviors.
4.1.2. Other nicotine-induced behaviors
As depicted in Table 1, the majority of work using the inescapable novel environment to identify HRs and LRs model has been used to predict later locomotor behavior induced by nicotine. In contrast, as shown in Table 2, only a small handful of studies have examined the relation between inescapable novelty and other behavioral effects of nicotine. For example, we could only find two published studies that have examined initial HR/LR status and intravenous nicotine self-administration. This paucity of research is in sharp contrast to the research with traditional psychostimulants. These two studies show an inconsistent ability of HR/LR status to correlate to later nicotine self-administration. The first study found that in rats, HRs had a greater tendency to self-administer nicotine in comparison to LRs (Suto et al., 2001). Further, HR rats attained a higher level of nicotine intake and HR rats earned more nicotine infusions under a progressive schedule of reinforcement. The second study found that in rats, there was no relation between HR/LR status and initial response rates or infusions earned under a progressive schedule of reinforcement (Guillem et al., 2005).
Table 2.
Studies showing predictive relation between inescapable novel environment and other nicotine-induced behaviors.
| Reference | Animals | Dose(s)/treatment | Behavior | Outcome |
|---|---|---|---|---|
| Bernardi and Spanagel (2014)M | Adult male C57BI/6N mice | 0.5 mg/kg IP | Place conditioning | HR < LR |
| Besheer and Bevins (2000)*,M | Adult male Sprague–Dawley rats | 0.3, 1.0 mg/kg SC | Visual discrimination in T-maze | HR < LR |
| Bhatti et al. (2009)T | Adolescent male Sprague–Dawley rats | 0.35, 0.70, 1.0 mg/kg SC; injection every 3 days × 4 injections; bupropion (40 or 60 mg/kg SC) or AM251 (1 or 5 mg/kg IP) | Locomotor sensitization | HR > LR in sensitization and intervention |
| Guillem et al. (2005)T | Adult male Sprague–Dawley rats | 0.03 mg/kg IV; vehicle or tranylcypromine (1.5 mg/kg) or phenelzine (2 mg/kg) | IV self-administration | HR = LR |
| Pastor et al. (2013)M | Adolescent male Sprague–Dawley rats | 0.4 mg/kg SC | Place conditioning | HR < LR post-nicotine |
| Suto et al. (2001)M | Adult male Sprague–Dawley rats | 0.03 mg/kg IV | IV self-administration | HR > LR |
HR = high responder; LR = low responder; IP = intraperitoneal; SC = subcutaneous; IV = intravenous.
= uses median split to divide in HR/LR;
= uses tertiary split.
Nicotine dose in salt form.
Another lone study investigated whether reactivity to an inescapable novel environment predicted later nicotine altered acquisition of a visual discrimination task in a T-maze (Besheer and Bevins, 2000). In this study, nicotine-treated LR rats made fewer errors when learning the initial discrimination; this difference in learning was not seen upon reversal of the visual discrimination.
There are a few papers on HR/LR status and place conditioning with nicotine. In one study, mice were tested for basal locomotor activity using the inescapable novel environment and then tested in a place conditioning regimen with nicotine (Bernardi and Spanagel, 2014). Mice initially found to be LRs displayed a conditioned place preference with nicotine, whereas mice that were HRs did not show a preference for the nicotine-paired compartment. In another study, adolescent rats were first classified as high or low responders based on locomotor behavior in a novel environment induced by an initial injection of nicotine (Pastor et al., 2013). Rats classified as LRs exhibited a nicotineconditioned place preference. In contrast, rats that were classified as HRs did not exhibit an acquired preference for an environment paired with nicotine.
A study performed by Bhatti and colleagues investigated whether reactivity to an inescapable novel environment could predict the effects of nicotine cessation treatments in adolescent rats exposed to an intermittent nicotine sensitization regimen (Bhatti et al., 2009). After HR and LR rats were tested for locomotor sensitization, cohorts were pretreated with bupropion, currently approved as the smoking cessation drug Zyban® or AM251, an antagonist for the cannabinoid receptor CB1. Only HR rats displayed locomotor sensitization to nicotine. In addition, while pretreatment with bupropion was ineffective in attenuating nicotine locomotor sensitization in HRrats, this sensitization was blunted by pretreatment with AM251.
At this point, it is difficult to extrapolate across these studies as most of the studies just described need to be replicated. There is some indication that the trend of inconsistency in using the inescapable novel environment continues when applied to nicotine self-administration. The additional studies linking the inescapable novel environment and visual discrimination, place preference, or cessation treatments are lone studies that would need to be replicated before clear patterns emerge.
4.1.3. Other novelty models
The majority of individual differences research with rodents had utilized reactivity to aninescapable novel environment to relate to later reactivity to nicotine. However, there are a few published papers using other screens that include some facet of novelty (see Table 3). For instance, the number of head dips on the hole-board apparatus has been used to define male and female adolescent mice as high novelty (HN) or low novelty (LN). HN mice show increased consumption of a nicotine solution over time, whereas LN mice exhibit a steady intake of a nicotine solution (Abreu-Villaça et al., 2006). Additional research found that among male and female mice that had been exposed perinatally to nicotine, higher levels of rearing on the hole-board apparatus predicted greater consumption of nicotine solution during adolescence (Gyekis etal., 2010).
Table 3.
Studies showing predictive relation between other novelty tests and later nicotine-induced behaviors.
| Reference | Animals | Novelty screen | Dose (s)/treatment | Behavior | Outcome |
|---|---|---|---|---|---|
| Abreu-Villaça et al. (2006)T | Adolescent male and female C57BL/6 mice | Hole board test | 10 μg/ml PO | Nicotine solution consumption | HN > LN |
| Besheer and Bevins (2000)a,M | Adult male Sprague–Dawley rats | Novel object preference (elevated platform and enclosed environment), saccharin consumption | 0.3, 1.0 mg/kg SC | Visual discrimination in T-maze | HN = LN |
| Gyekis et al. (2010)Q | Adolescent male and female C57B6J mice exposed to nicotine perinatally | Hole board test | 50 μg/ml PO | Nicotine solution consumption | HN > LN |
| Pawlak and Schwarting (2002)M | Adult male Wistar rats | Rearing activity | 0.06, 0.12 mg/ml PO | Nicotine solution consumption | HRA = LRA |
| Pawlak and Schwarting (2005)a,M | Adult male Wistar rats | Rearing activity | 0.4 mg/kg IP | Locomotor sensitization place and conditioning | HRA = LRA |
HN = high novelty; LN = low novelty HI = high inhibitory; LI = low inhibitory HRA = high rearing activity; LRA = low rearing activity.
PO = per os (oral); SC = subcutaneous; IP = intraperitoneal.
= used a median split to divide;
= used a tertiary split;
= used a quartile split.
Nicotine dose in salt form.
Although behavior on the hole-board apparatus has had some initial success as an individual difference screen, other screens have not been as successful. For example, rats separated into high and low responders based on rearing in a novel environment did not predict nicotine-induced locomotor sensitization, nicotine place conditioning, or oral intake of nicotine (Pawlak and Schwarting, 2002; Pawlak and Schwarting, 2005). Finally, free-choice novelty screens, notably novel-object preference and intake of a novel tastant (i.e., saccharin) did not predict nicotine altered acquisition in a T-maze visual discrimination task (Besheer and Bevins, 2000).
4.1.4. Predictive abilities of inescapable novel environment and free-choice novelty
The inescapable novel environment has been validated as a model of human sensation-seeking when predicting the effects of “traditional” psychomotor stimulants (e.g., Blanchard et al., 2009; Dellu et al., 1996; Kabbaj, 2006; Redolat et al., 2009). In contrast, the present review prompts us to question its usefulness as a predictive screen for subsequent reactivity to nicotine (see Table 1). Given these inconsistencies, we recommend that other novelty screens should be explored for possible predictive utility. In our laboratory, we conducted a study utilizing a host of individual difference screens including the inescapable novel environment, and several screens where rats may or may not interact with novelty (so-called free-choice novelty) in an initial attempt to find other possible predictors of nicotine locomotor behavior. Adult male Sprague-Dawley rats were first assessed in the individual differences screens. The screens were reactivity to an inescapable novel environment, consumption of a novel tastant (sucrose), consumption of a novel food in an unfamiliar environment (L-maze), and approach and interaction with a novel object. Following screening, rats underwent a 10-day sensitization regimen. Rats were injected SC with either 0.4 mg base/kg nicotine or saline and placed in the locomotor chamber (dia. 30.5 cm) for 30 min (n = 16 nicotine; n = 15 saline). After sensitization, a drug-free test was conducted 24 h after the last day of sensitization to determine whether the chamber evoked enhanced locomotion (conditioned hyperactivity; see Bevins etal., 2001; Bevins and Palmatier, 2003). Subsequently, two nicotine (0.4 mg base/kg) challenge tests were conducted to assess the lasting effects of nicotine sensitization; the first 24 h after the conditioned hyperactivity test, and the second 7 days after the first challenge.
Locomotor sensitization developed in nicotine-treated rats (see Fig. 1). There was no difference between nicotine-treated and saline-treated rats in the conditioned hyperactivity test. Later challenge tests found that sensitization lasted up to 7 days without nicotine. For each individual difference screen, a median split was performed. Rats with performance above the median were defined as high responders (HR); rats below the median were low responders (LR). There were no locomotor differences between HR and LR rats treated with saline during the sensitization phase. This was not so for nicotine-sensitized rats. When reactivity to the inescapable novel environment was used to split HRs and LRs, nicotine-induced hyperactivity was significantly greater in the HRs than in LRs on day 10 [t(14) = 3.148, p = 0.007; Fig.2, panel A]. These differences persisted in the conditioned locomotor test [t(14) = 5.188, p ≤ 0.0001; Fig. 2, panel B] and both nicotine challenges [ts(14) ≥ 4.308, ps ≤ 0.0007; Fig. 2, panels C and D]. Interestingly, the only other novelty-related behavior that consistently predicted the locomotor effects of nicotine was duration of sniff of the novel object in the 2 min test. For this screen, HRs activity was significantly lower than LRs for day 10 [t(14) = 2.664, p = 0.0185; Fig. 2, panel A], the conditioned locomotor test [t(14) = 2.172, p = 0.0475; Fig. 2, panel B], and both nicotine challenges [ts(14) ≥ 2.667, ps ≤ 0.0184; Fig. 2, panels C and D].
Fig. 1.
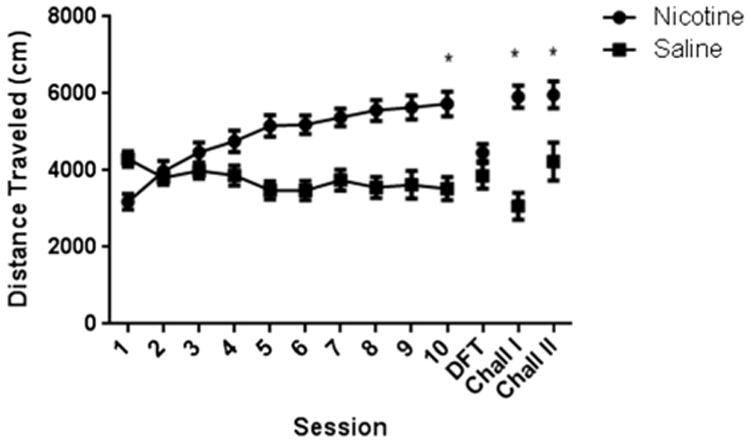
Adult male Sprague–Dawley rats were injected with 0.4 mg base/kg nicotine (n = 16) or saline (n = 15) SC and placed in circular locomotor chambers (dia = 30.5 cm) for 30 min for 10 sessions. In nicotine-treated rats, locomotor behavior was significantly higher on day 10 than on day 1, indicative of locomotor sensitization, t(14) = 6.713, p < 0.0001. Following 24 h after the last nicotine session, all rats received an injection of saline SC in a drug-free test (DFT) to assess conditioned hyperactivity. Rats then received two nicotine challenges (0.4 mg/kg, SC); one 24 h after the drug-free test and the other 7 days after the first challenge. In both nicotine challenges, the locomotor behavior of nicotine-treated rats was significantly higher than saline-treated rats (ts(29) ≥ 2.869, ps ≤ 0.0076).
Fig. 2.
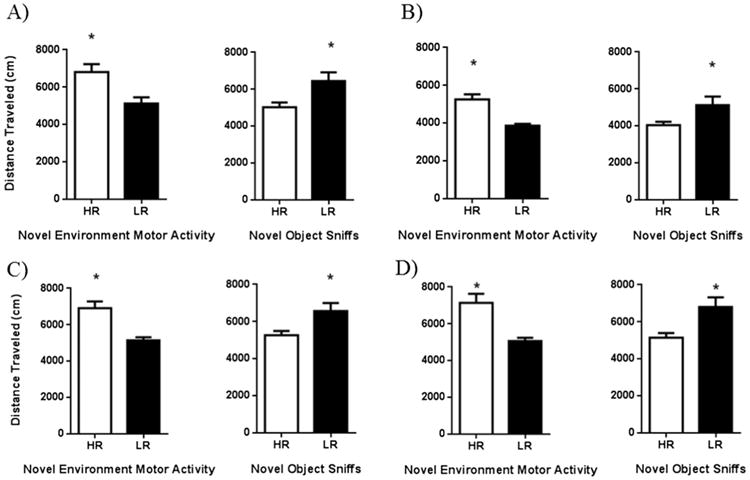
In a sample of adult male Sprague–Dawley rats, two individual differences screens predicted nicotine locomotor behaviors–the activity in an inescapable novel environment and sniff duration of a novel object. Overall, in the inescapable novel environment, HRs treated repeatedly with nicotine had greater levels of activity than LRs on day 10 (A), the conditioned locomotor test (B), and on both nicotine challenge days (C–challenge I; D–challenge II). For sniff duration in the novel-object screen, LRs had greater levels of activity than HRs on day 10 (A), the conditioned locomotor test (B), and on both nicotine challenge days (C–challenge I; D–challenge II).
This study continues to suggest that reactivity to an inescapable novel environment is an inconsistent predictor, even within a laboratory. Here, we found that reactivity to an inescapable novel environment predicted a number of locomotor behaviors in nicotine-treated rats, yet previous a publication from this laboratory found no differences between HR and LR rats in nicotine-induced locomotor behaviors (Bevins and Besheer, 2001). Though the negative findings involving the use of a novel tastant as an individual differences screen for nicotine-induced behaviors are consistent with our previous work (Besheer and Bevins, 2000), this publication stands alone in using the duration of a sniff of a novel object as a screen for later nicotine locomotor behaviors. Though Besheer and Bevins (2000) used object interactions as a screen, there may be a notable difference between operationalizing interactions and sniffs. Sniffing primarily utilizes the olfactory system which research in albino rodents has shown is an important modality for exploration (Misslin and Ropartz, 1981; Welker, 1964). The finding that rats that were LRs in the duration of sniffing a novel object had higher levels of locomotor behavior after repeated nicotine treatment needs replication. However, this result nudges us toward considering a greater array of free-choice novelty screens for future investigations.
4.2. Impulsivity
Impulsivity has been correlated with drug abuse in clinical populations (de Wit, 2008; Evenden, 1999; Jupp et al., 2013). To date, there has been extensive modeling of impulsivity in rodents, including as a correlate of addiction-related behaviors (e.g., Jupp et al., 2013). Unfortunately, for the most part, these studies have not examined nicotine (see Table 4). In one exception, rats were separated on impulsive choice measured by the delayed reward task and by the 5-choice serial reaction time task (Diergaarde et al., 2008). Impulsive choice and impulsive action were predictive of nicotine SA behaviors. Rats with higher levels of impulsive action reached higher nicotine intake levels than less impulsive rats. However, rats that were high on impulsive choice acquired nicotine SA at the same levels as less impulsive counterparts, and earned similar amounts of rewards on lower levels of response ratios during progressive ratio behaviors. Further work by this group has suggested that highly impulsive rats, as determined by a delay discounting task, initially take as many reinforcements as less impulsive rats (Diergaarde et al., 2012). However, as the response requirements to gain a nicotine infusion increased, highly impulsive rats perseverated longer in self-administration behavior than the less impulsive rats.
Table 4.
Studies showing predictive relation between impulsivity or anxiety measures and subsequent nicotine-induced behaviors.
| Reference | Animals | Dose(s)/treatment | Behavior | Outcome |
|---|---|---|---|---|
| Diergaarde et al. (2008)Q | Adult male Wistar rats | 0.03 mg/kg/infusion IV | IV self-administration | HiI > LoI |
| Diergaarde et al. (2012)Q | Adult male Wistar rats | 0.03 mg/kg/infusion IV | IV self-administration | HiI > LoI in nicotine demand |
| Abreu-Villaça et al. (2006)T | Adolescent male and female C57BL/6 mice | 10 μg/ml PO | Nicotine solution consumption | HA = LA |
| Falco et al. (2014)M | Adolescent male Sprague–Dawley rats | 0.5 mg/kg SC | Place conditioning | HA > LA |
| Manhães et al. (2008)M | Adolescent male and female C57BL/6 mice | 50 μg/ml PO | Nicotine solution consumption | HA < LA after withdrawal |
HiI = high impulsivity; LoI = low impulsivity HA = high anxiety; LA = low anxiety.
IV = intravenous; PO = per os (oral); SC = subcutaneous;
= used median split to divide;
= used tertiary split;
= used quartile split.
4.3. Anxiety
Anxiety status has been postulated to be a potential factor in the risk of nicotine use and dependence in humans (Grant et al., 2004; Piper et al., 2010b). To date, a few attempts have been made to determine whether anxiety-like behavior can determine the behavioral effects of nicotine in rodents, though only using adolescent populations (see Table 4). When the hole board apparatus was used to divide male and female adolescent mice into high anxiety (HA) and low anxiety (LA) groups, HA and LA mice did not show differences in oral nicotine consumption (Abreu-Villaça et al., 2006). In a later study, male and female adolescent mice were split into HA and LA groups using the elevated-plus maze during a period of withdrawal after a priming period of consumption of either water or nicotine solution (Manhães et al., 2008). HA animals consumed less nicotine solution than LA animals, irrespective of treatment during the priming period. Anxiety-like behavior may also predict other nicotine-induced behavioral regimens. Division of adolescent rats into HA and LA groups on the basis of pretesting in a biased place conditioning apparatus found that only HA rats expressed a nicotine-conditioned place preference (Falco et al., 2014). In contrast, adolescent rats that were classified as LA did not exhibit an acquired preference for an environment paired with nicotine. In addition, Falco and colleagues found that baclofen, a GABAB agonist that may have promise as a treatment for drug addiction, did not alter acquisition of nicotine-conditioned place preference. These few studies suggest that anxiety-like behavior may play a predictive role in various nicotine-induced behaviors in rodent populations.
5. Research directions
As reviewed here, there is a foundation of research in rats and mice investigating individual differences screen and their utility at predicting later behavioral reactivity to nicotine. This empirical foundation is much smaller and appears more inconsistent than the parallel literature with traditional psychostimulants (cf. Bardo et al., 2013). As we move forward, we will need to develop more reliable and replicable animal models to examine predictive constructs related to individual differences in sensitivity to nicotine. The following section reflects on some important areas of focus and direction for these efforts.
5.1. Populations at risk
Research has suggested that adolescents (e.g., Breslau and Peterson, 1996; Chen and Millar, 1998; Kandel and Chen, 2000) and females (e.g., Perkins et al., 2009; Schnoll et al., 2007) have unique risks for beginning and maintaining chronic tobacco use and nicotine dependence. As a result, it is necessary for individual differences research to consider developmental trajectory and sex-specific factors. Recent reviews and commentaries have highlighted the need for sex differences research in the neurosciences and in behavioral pharmacology (Beery and Zucker, 2011; Bevins and Charntikov, 2015; Prendergast et al., 2014). The area of focus for this review appears to be no different. Only about 1/3 of the discussed studies provided a female comparison group. As this field moves forward, research will need to incorporate female counterparts to truly distinguish the interactions between sex differences and individual differences in the behavioral effects of nicotine.
Adolescence marks a critical period for the development of smoking behaviors. Preclinical rodent models have shown that adolescents have a higher vulnerability to the rewarding effects of nicotine (Adriani et al., 2003; Belluzzi et al., 2004; Brielmaier et al., 2007; Shram et al., 2006; Torres et al., 2008). Individual differences research with nicotine will need to further incorporate age differences into the models. However, due to the relatively few studies presently published to date, the goal to study adolescent groups may be counterproductive without adult counterparts. For example, studies using a purported anxiety-related task as a screen for nicotine-induced behaviors only used adolescent rodents. Adolescents with anxiety disorders are a particularly important group to model given that they have higher rates of smoking behavior than non-anxious counterparts (Henry et al., 2012; Marmorstein et al., 2010; McKenzie et al., 2010; Sonntag et al., 2000). However, the lack of an adult comparisons leaves open the question of whether this is a developmentally specific effect.
5.2. Tasks currently used as screens
5.2.1. Inescapable novel environment
The inescapable novel environment is the most common screen of individual differences to relate to the later effects of nicotine. The use of the inescapable novel environment as a predictor is understandable given that this screen has been quite successful in predicting responses to traditional psychostimulants in the published literature (for reviews, see Bardo et al., 2013; Blanchard et al., 2009; Kabbaj, 2006). As detailed earlier, the evidence is mixed as to whether reactivity to an inescapable novel environment predicts the subsequent behavioral effects of nicotine. While some studies have found that reactivity to an inescapable novel environment predicts the later effects of nicotine (Aydin et al., 2011; Aydin et al., 2012; Bernardi and Spanagel, 2014; Bhatti et al., 2009; Pehrson et al., 2008; Philpot et al., 2012; Suto et al., 2001), others have found no relation (Besheer and Bevins, 2000; Bevins and Besheer, 2001; Coolon and Cain, 2009; Guillem et al., 2005; Pastor et al., 2013; Prus et al., 2008). Who knows how many other studies were never published because of null effects. Variation in methods and materials across studies is one factor that makes determining the utility of this screen difficult to evaluate. Such variation includes different types of apparatus, different strains of animals, different methods to split animals into HRs and LRs, and different treatment regimens to examine the effects of nicotine. At this point, it is nearly impossible to determine what factors may be behind predictive value of the inescapable novel environment, if any of them are. Nearly every study uses a different apparatus and method for dividing animals into HR/LRs. Tables 1 and 2 label the statistical methods used to divide animals into HR and LRs. As can be seen, there is no clear relation between what type of statistical split is used and which studies have predictive value. More careful parametric research is needed to determine which factors affect the predictive value of the inescapable novel environment task, and why.
Perhaps a better question to ask is what is unique about nicotine? The research with amphetamine and cocaine has the same problems surrounding discrepancies between species, apparatus, statistical splits, etc. However, discovery of more consistent predictive individual differences has emerged with those drugs. Is the difference due to the relative volume of conducted and hence published research on the more traditional psychostimulants in comparison to nicotine? Or, is it that nicotine is fundamentally acting in a different way in the brain that makes the inescapable novel environment a less apt screen for determining later reactivity to nicotine. As was examined in Sections 3.1 to 3.3, the inescapable novel environment predicts the locomotor effects of amphetamine, cocaine, and methamphetamine quite well (particularly amphetamine and cocaine). This outcome may be due, in part, to the physiological mechanisms underlying the behavioral effects of these drugs. Initially, Piazza et al. (1989) postulated that dopaminergic mechanisms were playing a role in the ability of the inescapable novel environment to relate to amphetamine induced behaviors. Comparing and contrasting the neural action of nicotine and amphetamine or cocaine is beyond the scope of this review. However, nicotine's indirect pathway from binding to nicotinic acetylcholine receptors to eventually affecting the dopamine system (for a discussion see Sulzer, 2011) should be taken in consideration regarding the inconsistent relation between reactivity to an inescapable novel environment and the later behavioral effects of nicotine.
Another important issue here is replication and reproducibility within and between laboratories for all individual difference screens. For example, there are only two published study using the inescapable novel environment screen to predict later nicotine intravenous SA, which present contrary findings (Guillem et al., 2005; Suto et al., 2001). Currently, there is wide spread use of SA in the more typical psychomotor stimulant individual differences literature. This extensive use, combined with the notion that SA is the “gold standard” model for studying drug reinforcement (Corrigall, 1999; Belin and Deroche-Gamonet, 2012), poses a notable need to replicate these effects with nicotine. If reproduced within and across laboratories, then understanding the behavioral and neural processes mediating the relation between inescapable novelty and self-administration will be of much interest. For now, what we know is that the inescapable novel environment does not consistently predict the behavioral effects of nicotine; even the same behavior (see Tables 1 and 2). Perhaps this finding should prompt investigators to search for other individual difference screens that have better ethological relevance and better approximate factors known to predict smoking behavior and nicotine dependence in humans.
5.2.2. Free-choice novelty screens
Free-choice novelty screens are tasks where the rodent's behavior is not forced by the design of the experiment or apparatus to interact or be exposed to novelty. Free-choice novelty screens have been found to have some predictive ability of drug-induced behaviors of traditional psychostimulants, particularly during self-administration behaviors (Belin et al., 2011; Cain et al., 2005; Cain et al., 2006; Pelloux et al., 2004). To date, only one published study to our knowledge has examined the relation between free-choice screens (novel object preference and saccharin consumption) and nicotine-induced changes in visual discrimination performance in the T-maze (Besheer and Bevins, 2000). Neither screen predicted performance in the discrimination task. In contrast, we presented evidence earlier in this review that the average duration of sniffing a novel object was able to predict locomotor effects of nicotine. If Besheer and Bevins (2000) had used duration of sniffs of the novel object as a predictor within the novel-object preference screen, perhaps they would have found a predictive relation with visual discrimination in the T-maze. The adaptation of other screens, such novelty-induced hypophagia or the two-compartment maze, where rats can choose between novel and familiar domains (cf. Rosecrans, 1971a), may also have a predictive relation with the behavioral effects of nicotine.
5.2.3. Impulsivity
Thus far, only two studies have examined the relation between performance in tasks purportedly indexing impulsivity and nicotine SA behaviors (Diergaarde et al., 2008; Diergaarde et al., 2012). These two studies have several notable strengths. Between the two of them, there are three measures of impulsivity (the delayed reward task, 5-choice reaction time task, and delay discounting task) used to divide rats into high and low impulsivity groups. Impulsive behaviors were used to predict nicotine SA, a method which is commonly employed in the nicotine literature (see earlier). Given the promising findings of the research conducted by Diergaarde and colleagues, there is a great need for replication and extension of this research to other aspects self-administration behavior. Additional screens can also be implemented to divide rats (or mice) into high and low impulsivity groups in future research, including the rodent gambling task (rGT; for review see van den Bos et al., 2014) and tasks using differential reinforcement of low rate (DRL) schedules (see Kirshenbaum et al., 2011; Mangabeira et al., 2015). Impulsivity screens need to be used in attempts to predict other behavioral effects of nicotine, including locomotor effects and place conditioning in order to broaden the current base of research findings.
5.2.4. Anxiety
Anxiety has been described in the clinical literature as an important trait in the development of drug use and abuse (e.g., Compton et al., 2007; Grant et al., 2004). However, only a handful of studies in the animal literature use anxiety-like behavior as a predictive screen for later drug effects (e.g., Dilleen et al., 2012; Lehner et al., 2014; Pelloux et al., 2009; Vautrin et al., 2005). The use of anxiety-like behavior as a predictive factor of subsequent behavioral effects of nicotine is promising. The hole-board apparatus and elevated-plus maze have been used as screens for oral consumption of nicotine in male and female adolescent mice (Abreu-Villaça et al., 2006; Manhães et al., 2008). Further, initial preference for the black side of a biased place conditioning chamber, presumed to reflect avoidance of the brighter white side, was found to predict later nicotine conditioned place preference in adolescent rats (Falco et al., 2014). These studies need to be replicated and extended. For example, these studies used only adolescent cohorts. Moving forward, it will be important to include adult comparison groups to determine whether the effects are developmentally specific to adolescence. The number of anxiety-like behaviors that are used as predictors needs to be increased to include tests such as elevated-plus maze, light–dark box, open field, and social anxiety in order to provide a better idea of the nature of the anxiety-like behaviors that serve as reliable predictors. Further, anxiety-like behaviors will need to be examined for their predictive relation to other behavioral effects of nicotine, including, but not limited to, locomotor effects, place conditioning, and intravenous nicotine self-administration.
5.2.5. Withdrawal and treatment regimens
Withdrawal from nicotine and the treatment of nicotine dependence are important components of reactivity to nicotine. Surprisingly, there are only a few studies using individual differences screens in the behavioral effects to nicotine withdrawal or sensitivity to approved or potential treatment compounds. One study examined the relation between anxiety-like behavior during nicotine withdrawal and oral nicotine consumption in male and female adolescent mice (Manhães et al., 2008). Only two studies investigated the effects of approved or investigational compounds on individual nicotine-induced behavioral effects. The effects of bupropion and AM251 have been studied on nicotine locomotor sensitization in adolescent rats (Bhatti et al., 2009). Additionally, the effects of baclofen have been studied on acquisition of nicotine-induced place conditioning in adolescent rats (Falco et al., 2014).
Again, there is a need to replicate and extend this work, as well as to include adult counterparts. Examination of withdrawal and treatment of effects of nicotine is an area where many individual differences screens can be employed–whether they utilize individual difference screens involving novelty, impulsivity, or anxiety. There is also a large pool of approved and potential compounds that can be examined in treatment effects of nicotine or in the withdrawal side effects (e.g., cognitive deficits). At this time, we are only aware of one individual differences study using bupropion and no studies using varenicline (Chantix®) in order to predict the behavioral effects of approved treatments for smoking cessation.
5.2.6. Other screens/techniques
There are a few other examples of other screens or techniques being used to predict or correlate with nicotine-induced behavior. A study examining the interaction between pre-pulse inhibition and locomotor sensitization found that low inhibitory rats (i.e., rats that had lower pre-pulse inhibition scores) were more prone to developing nicotine sensitization (Kayir et al., 2011). Additionally, there are a number of reports using an individual rodent's nicotine consumption or self-administration behavior as a data analysis tool (Adriani et al., 2002; Cao et al., 2012; Dadmarz and Vogel, 2003; Harris et al., 2009; Harris et al., 2011; Maehler et al., 2000; Nesil et al., 2011). These techniques bear mentioning here as they may be used in conjunction with other models to give more information about the individual rodents which have a higher reactivity to nicotine.
6. Conclusion
In all likelihood, smokers and other chronic users of nicotine-containing products (e.g., e-cigarettes) have different traits or factors that play a role in the formation or expression of their drug use and abuse behavior. These traits can be extremely difficult to isolate in clinical populations as health care providers may not always be aware of or have full access to behavioral and/or psychological treatment information. However, isolation of these traits may change the treatment picture for many individuals. Creating valid and reliable models of behavioral predictors of nicotine-induced behaviors is needed with the promise of creating translational models to improve quit success. While some of these animal models are still early in their development, continued research will collect evidence that shows there is a basis for valid behavioral modeling. These animal models should continue to be developed as another tool to generate treatments to aid in nicotine dependence.
Acknowledgments
A. Falco and R. Bevins were at least partially supported by USPHS grant DA034389 while preparing this manuscript.
References
- Abreu-Villaça Y, Queiroz-Gomez FE, Dal Monte AP, Filgueiras CC, Manahães AC. Individual differences in novelty-seeking behavior but not in anxiety response to a new environment can predict nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2006;167:175–182. doi: 10.1016/j.bbr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Adriani W, Macrì S, Pacifici R, Laviola G. Restricted daily access to water and voluntary nicotine oral consumption in mice: methodological issues and individual differences. Behav Brain Res. 2002;134:21–30. doi: 10.1016/s0166-4328(01)00448-x. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado GF, Breslau N. Smoking and young people's mental health. Curr Opin Psychiatry. 2005;18:397–400. doi: 10.1097/01.yco.0000172058.48154.14. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Papathanasiou G, Papalexi E, Hyphantis T, Nomikos GG, Syraki C, Papadopoulou-Daifoti Z. Individual responses to novelty are associated with differences in behavioral and neurochemical profiles. Behav Brain Res. 2008;187:462–472. doi: 10.1016/j.bbr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Nicotine-induced anxiety-like behavior in a rat model of the novelty-seeking phenotype is associated with long-lasting neuropeptidergic and neuroplastic adaptations in the amygdala: effects of the cannabinoid receptor 1 antagonist AM251. Neuropharmacology. 2012;63:1335–1345. doi: 10.1016/j.neuropharm.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Vulnerability to nicotine abstinence-related social anxiety-like behavior: molecular correlates in neuropeptide Y, Y2 receptor and corti-cotropin releasing factor. Neurosci Lett. 2011;490:220–225. doi: 10.1016/j.neulet.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Conti D, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Research. 2007;9(Suppl 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SR, Stewart SH, Fulton HG, Steeves D, Darredeau C, Gavric D. A further investigation of the relations of anxiety sensitivity to smoking motives. Addict Behav. 2008;33:1402–1408. doi: 10.1016/j.addbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012;2:a011940. doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Spanagel R. Basal activity level in mice predicts the initial and sensitized locomotor response to nicotine only in high responders. Behav Brain Res. 2014;264:143–150. doi: 10.1016/j.bbr.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Besheer J, Bevins RA. Nicotine enhances acquisition of a T-maze visual discrimination: assessment of individual differences. Behav Pharmacol. 2000;11:613–620. doi: 10.1097/00008877-200011000-00008. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Individual differences in rat locomotor activity are diminished by nicotine through stimulation of central nicotinic acetylcholine receptors. Physiol Behav. 2001;72:237–244. doi: 10.1016/s0031-9384(00)00413-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Charntikov S. We know very little about the subjective effects of drugs in females. ACS Chem Neurosci. 2015 doi: 10.1021/acschemneuro.5b00018. http://dx.doi.org/10.1021/acschemneuro.5b00018. [DOI] [PMC free article] [PubMed]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: assessment of the US-preexposure effect. Behav Brain Res. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Peterson JL. Individual differences in rats' reactivity to novelty and the unconditioned and conditioned effects of methamphetamine. Pharmacol Biochem Behav. 2004;79:65–74. doi: 10.1016/j.pbb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Bhatti AS, Aydin C, Oztan O, Ma Z, Hall P, Tao R, Isgor C. Effects of a cannabinoid receptor (CB) 1 antagonist AM251 on behavioral sensitization to nicotine in a rat model of novelty-seeking behavior: correlation with hippocampal 5HT. Psychopharmacology. 2009;203:23–32. doi: 10.1007/s00213-008-1366-6. [DOI] [PubMed] [Google Scholar]
- Billieux J, Van der Linden M, Ceschi G. Which dimensions of impulsivity are related to cigarette craving? Addict Behav. 2007;32:1189–1199. doi: 10.1016/j.addbeh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–1154. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Vulnerability to psychopathology in nicotine-dependent smokers: an epidemiologic study of young adults. Am J Psychiatry. 1993;150:941–946. doi: 10.1176/ajp.150.6.941. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Cain ME, Dotson WF, Bardo MT. Individual differences in the effect of novel environmental stimuli prior to amphetamine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2006;14:389–401. doi: 10.1037/1064-1297.14.3.389. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharm. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Cao J, Gautier NM, Li MD. CD-1 mice show individual differences in nicotine preference in a modified two-bottle oral self-administration model. Front Psychiatry. 2012;3:28. doi: 10.3389/fpsyt.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carton S, Jouvent R, Widlöcher D. Sensation seeking, nicotine dependence, and smoking motivation in female and male smokers. Addict Behav. 1994;19:219–227. doi: 10.1016/0306-4603(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Coolon RA, Cain ME. Individual differences in response to novelty and the locomotor effects of nicotine. Behav Pharmacol. 2009;20:322–329. doi: 10.1097/FBP.0b013e32832f0176. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nic Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Cosci F, Corlando A, Fornai E, Pistelli F, Paloletti P, Carrozzi L. Nicotine dependence, psychological distress and personality traits as possible predictors of smoking cessation. Results of a double-blind study with nicotine patch. Addict Behav. 2009;34:28–35. doi: 10.1016/j.addbeh.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Sweeney FF. The age of anxiety: role of animal models of anxiolytic action in drug discovery. Brit J Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadmarz M, Vogel WH. Individual self-administration of nicotine by rats. Pharmacol Biochem Behav. 2003;76:425–432. doi: 10.1016/j.pbb.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats-biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2008;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer ANM, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol. 2012;17:576–587. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, et al. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration, in rats. Psychopharmacology. 2012;222:89–97. doi: 10.1007/s00213-011-2626-4. [DOI] [PubMed] [Google Scholar]
- Donny EC, Griffin KM, Shiffman S, Sayette MA. The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers. Nicotine Tob Res. 2008;10:934–942. doi: 10.1080/14622200802133681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, Spring B. Impulsivity and cigarette craving: differences across subtypes. Psychopharmacology. 2009;207:365–373. doi: 10.1007/s00213-009-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Sanders PE, Bekman NM, Worley MJ, Monreal TK, McGee E, et al. Mediating influences of negative affect and risk perception on the relationship between sensation seeking and adolescent cigarette smoking. Nicotine Tob Res. 2011;13:457–465. doi: 10.1093/ntr/ntr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict strength of amphetamine-induced place conditioning. Pharmacol Biochem Behav. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Falco AM, McDonald CG, Smith RF. Anxiety status affects nicotine-and baclofen-induced locomotor activity, anxiety, and single-trial conditioned place preference in adolescent male rats. Dev Psychobiol. 2014;56:1352–1364. doi: 10.1002/dev.21217. [DOI] [PubMed] [Google Scholar]
- Gancarz AM, San George MA, Ashrafioun L, Richards JB. Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low-dose of methamphetamine in rats. Behav Process. 2011;86:295–304. doi: 10.1016/j.beproc.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav. 1996;53:191–196. [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the wave 2 national epidemiologic survey on alcohol and related conditions. Mol Psychiatry. 2009;14:1051–1066. doi: 10.1038/mp.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou P, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyekis J, Foreman JE, Anthony K, Klein LC, Vandenbergh DJ. Activity-related behaviors in the hole-board predict nicotine consumption in C57B6 mice perinatally exposed to nicotine. Behav Brain Res. 2010;206:139–142. doi: 10.1016/j.bbr.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Hall PA, Fong GT, Yong HH, Sansone G, Borland R, Siahpush M. Do time perspective and sensation-seeking predict quitting activity among smokers? Findings from the international tobacco control (ITC) four country survey. Addict Behav. 2012;37:1307–1313. doi: 10.1016/j.addbeh.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen H, Bischof G, Brooks A, Hohagen F, Rumpf HJ. The relationship between impaired decision-making, sensation seeking and readiness to change in cigarette smokers. Addict Behav. 2006;31:581–592. doi: 10.1016/j.addbeh.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, LeSage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology. 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Correlates of individual differences in compensatory nicotine self-administration in rats following a decrease in nicotine unit dose. Psychopharmacology. 2009;205:599–611. doi: 10.1007/s00213-009-1567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SL, Jamner LD, Whalen CK. I (should) need a cigarette: adolescent social anxiety and cigarette smoking. Ann Behav Med. 2012;43:383–393. doi: 10.1007/s12160-011-9340-7. [DOI] [PubMed] [Google Scholar]
- Jupp B, Caprioli D, Dalley JW. Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction. Dis Model Mech. 2013;6:302–311. doi: 10.1242/dmm.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets. 2006;5:513–520. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J, Leventhal AM, Monti PM. Sensation seeking as a predictor of treatment compliance and smoking cessation treatment outcomes in heavy social drinkers. Pharmacol Biochem Behav. 2009;93:285–290. doi: 10.1016/j.pbb.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob Res. 2000;2:263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- Kayir H, Goktalay G, Yavuz O, Uzbay TI. Impact of baseline prepulse inhibition on nicotine-induced locomotor sensitization in rats. Behav Brain Res. 2011;216:275–280. doi: 10.1016/j.bbr.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The global burden of anxiety and mood disorders: putting the European study of the epidemiology of mental disorders (ESEMeD)findings into perspective. J Clin Psychiatry. 2007;68(Suppl 2):10–19. [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Jackson ER, Brown SJ, Fuchs JR, Miltner BC, Doughty AH. Nicotine-induced impulsive action: sensitization and attenuation by mecamylamine. Behav Pharmacol. 2011;22:207–221. doi: 10.1097/FBP.0b013e328345ca1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Allostatic view of motivation: implications for psychopathology. Neb Symp Motiv. 2004;50:1–18. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD. Dissociation of novelty-and cocaine-conditioned locomotor activity from cocaine place conditioning. Pharmacol Biochem Behav. 1998;60:785–791. doi: 10.1016/s0091-3057(97)00388-2. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Menary KR, Maurer EW, Thuras P. Greater elevation in risk for nicotine dependence per pack of cigarettes smoked among those with an anxiety disorder. J Stud Alcohol Drugs. 2012;73:920–924. doi: 10.15288/jsad.2012.73.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: prospective relationships to nicotine withdrawal symptoms during smoking cessation. J Stud Alcohol Drugs. 2013;74:469–478. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner MH, Taracha E, Kaniuga E, Wislowska-Stanek A, Wróbel J, Sobolewska A, et al. High-anxiety rats are less sensitive to the rewarding affects of amphetamine on 50 kHz USV. Behav Brain Res. 2014;275:234–242. doi: 10.1016/j.bbr.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Maehler R, Dadmarz M, Vogel WH. Determinants of the voluntary consumption of nicotine by rats. Neuropsychobiology. 2000;41:200–204. doi: 10.1159/000026660. [DOI] [PubMed] [Google Scholar]
- Malmberg M, Kleinjan M, Overbeek G, Vermulst AA, Lammers J, Engels RCME. Are there reciprocal relationships between substance use risk personality profiles and alcohol or tobacco use in early adolescence? Addict Behav. 2013;38:2851–2859. doi: 10.1016/j.addbeh.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Manhães AC, Guthierrez MCS, Figueiras CC, Abreu-Villaça Y. Anxiety-like behavior during nicotine withdrawal predict subsequent nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2008;193:216–224. doi: 10.1016/j.bbr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Mangabeira V, Garcia-Mijares M, Silva MT. Sugar withdrawal and differential reinforcement of low rate (DRL) performance in rats. Physiol Behav. 2015;139:468–473. doi: 10.1016/j.physbeh.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology. 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, White HR, Loeber R, Stouthamer-Loeber M. Anxiety as a predictor of age at first use of substances and progression to substance use problems among boys. J Abnorm Child Psychol. 2010;38:211–224. doi: 10.1007/s10802-009-9360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews IZ, Morrissey MD, McCormick CM. Individual differences in activity predict locomotor activity and conditioned place preference to amphetamine in both adolescent and adult rats. Pharmacol Biochem Behav. 2010;95:63–71. doi: 10.1016/j.pbb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Olsson CA, Jorm AF, Romaniuk H, Patton GC. Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: findings from a 10-year longitudinal study. Addiction. 2010;105:1652–1659. doi: 10.1111/j.1360-0443.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- Misslin R, Ropartz P. Olfactory regulation of responsiveness to novelty in mice. Behav Neural Biol. 1981;33:230–236. doi: 10.1016/s0163-1047(81)91677-0. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Morrison CF, Lee PN. A comparison of the effects of nicotine and physostigmine on a measure of activity in the rat. Psychopharmacologia. 1968;13:210–221. doi: 10.1007/BF00401401. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61:189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor V, Andrés ME, Bernabeu RO. The effect of previous exposure to nicotine on nicotine place preference. Psychopharmacology. 2013;226:551–560. doi: 10.1007/s00213-012-2928-1. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Schwarting RKW. Object preference and nicotine consumption in rats with high vs. low rearing activity in a novel open field. Pharmacol Biochem Behav. 2002;73:679–687. doi: 10.1016/s0091-3057(02)00852-3. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Schwarting RKW. Repeated nicotine treatment in rats with high versus low rearing activity: analyses of behavioural sensitisation and place preference. Psychopharmacology. 2005;178:440–450. doi: 10.1007/s00213-004-2024-2. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Philibin SD, Gross D, Robinson SE, Vann RE, Rosecrans JA, James JR. The effects of acute and repeated nicotine doses on spontaneous activity in male and female Sprague Dawley rats: analysis of brain area epibatidine binding and cotinine levels. Pharmacol Biochem Behav. 2008;89:424–431. doi: 10.1016/j.pbb.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Anxiety increases the place conditioning induced by cocaine in rats. Behav Brain Res. 2009;197:311–316. doi: 10.1016/j.bbr.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Differential effects of novelty exposure on place preference conditioning to amphetamine and its oral consumption. Psychopharmacology. 2004;171:277–285. doi: 10.1007/s00213-003-1584-x. [DOI] [PubMed] [Google Scholar]
- Perkins K, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology. 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Coddington SB, Karelitz JL, Jetton C, Scott JA, Wilson AS, Lerman C. Variability in initial nicotine sensitivity due to sex, history of other drug use, and parental smoking. Drug Alcohol Depend. 2009;99:47–57. doi: 10.1016/j.drugalcdep.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Engberg ME, Wecker L. Effects of nicotine exposure on locomotor activity and pCREB levels in the ventral striatum of adolescent rats. Behav Brain Res. 2012;230:62–68. doi: 10.1016/j.bbr.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rogue-Pont F, Le Moal M. Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology. 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2010a;106:418–427. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J Consult Clin Psychol. 2010b;78:13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Vann RE, Rosecrans JA, James JR, Pehrnson AL, O'Connell MM, Philibin SD, Robinson SE. Acute nicotine reduces and repeated nicotine increases spontaneous activity in male and female Lewis rats. Pharmacol Biochem Behav. 2008;91:150–154. doi: 10.1016/j.pbb.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redolat R, Pérez-Martínez A, Carrasco MC, Mesa P. Individual differences in novelty-seeking and behavioral responses to nicotine: a review of animal studies. Curr Drug Abuse Rev. 2009;2:230–242. doi: 10.2174/1874473710902030230. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Forebrain biogenic amine function in high and low active female rats. Physiol Behav. 1970;5:453–458. doi: 10.1016/0031-9384(70)90250-7. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Effects of nicotine on behavioral arousal and brain 5-hydroxtryptamine function in female rats selected for differences in activity. Eur J Pharmacol. 1971a;14:29–37. doi: 10.1016/0014-2999(71)90119-1. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Effects of nicotine on brain area 5-hydroxytryptamine function in male and female rats separated for differences of activity. Eur J Pharmacol. 1971b;16:123–127. doi: 10.1016/0014-2999(71)90067-7. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Women's Health. 2007;16:1211–1218. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Sonntag H, Wittchen HU, Höfler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? Eur Psychiatry. 2000;15:67–74. doi: 10.1016/s0924-9338(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Spillane NS, Smith GT, Kahler CW. Impulsivity-like traits and smoking behaviors in college students. Addict Behav. 2010;35:700–705. doi: 10.1016/j.addbeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH report: trends in cigarette use among adolescents and young adults. 2012 Access at: http://www.samhsa.gov/data/2k12/NSDUH047/SR047CigaretteTrends2012.htm.
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology. 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Hariri AR. Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug Alcohol Depend. 2012;123S:S59–S71. doi: 10.1016/j.drugalcdep.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Le Moal M. Individual vulnerability to addiction. Ann N Y Acad Sci. 2011;1216:73–85. doi: 10.1111/j.1749-6632.2010.05894.x. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Müller CP, Huston JP, Schwarting RKW. High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neurosci. 1999;93:243–251. doi: 10.1016/s0306-4522(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O'Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Koot S, de Visser L. A rodent version of the Iowa gambling task: 7 years of progress. Front Psychol. 2014;5:203. doi: 10.3389/fpsyg.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautrin S, Pelloux Y, Costentin J. Preference for caffeine appears earlier in non-anxious than in anxious mice. Neurosci Lett. 2005;386:94–98. doi: 10.1016/j.neulet.2005.05.071. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing by the albino rat. Behaviour. 1964;22:223–244. [Google Scholar]
- World Health Organization. WHO Report on the Global Tobacco Epidemic 2013: Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship. WHO Press; 2013. [Google Scholar]
- Yates JR, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. High impulsivity in rats predicts amphetamine place preference. Pharmacol Biochem Behav. 2012;100:370–376. doi: 10.1016/j.pbb.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: a comparative approach. Behav Brain Sci. 1984;7:413–471. [Google Scholar]
- Zuckerman M, Ball S, Black J. Influences of sensation seeking, gender, risk appraisal, and situational motivation on smoking. Addict Behav. 1990;15:209–220. doi: 10.1016/0306-4603(90)90064-5. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Bone RN, Neary R, Mangelsdorff D, Brustman B. What is the sensation seeker? Personality trait and experience correlates of the sensation-seeking scales. J Consult Clin Psychol. 1972;39:308–321. doi: 10.1037/h0033398. [DOI] [PubMed] [Google Scholar]


