Abstract
It is well accepted that ethanol is able to produce major health and economic problems associated to its abuse. Because of its intoxicating and addictive properties, it is necessary to analyze its effect in the central nervous system. However, we are only now learning about the mechanisms controlling the modification of important membrane proteins such as ligand-activated ion channels by ethanol. Furthermore, only recently are these effects being correlated to behavioral changes. Current studies show that the glycine receptor (GlyR) is a susceptible target for low concentrations of ethanol (5 to 100 mM). GlyRs are relevant for the effects of ethanol because they are found in the spinal cord and brain stem where they primarily express the α1 subunit. More recently, the presence of GlyRs was described in higher regions, such as the hippocampus and nucleus accumbens, with a prevalence of α2/α3 subunits. Here, we review data on the following aspects of ethanol effects on GlyRs: 1) direct interaction of ethanol with amino acids in the extracellular or transmembrane domains, and indirect mechanisms through the activation of signal transduction pathways; 2) analysis of α2 and α3 subunits having different sensitivities to ethanol which allows the identification of structural requirements for ethanol modulation present in the intracellular domain and C-terminal region; 3) Genetically modified knock-in mice for α1 GlyRs that have an impaired interaction with G protein and demonstrate reduced ethanol sensitivity without changes in glycinergic transmission; and 4) GlyRs as potential therapeutic targets.
Keywords: Glycine receptor, alcohol, G proteins, allosteric modulation
General Overview of the neuronal Glycine receptor
Neuronal excitability is a complex membrane phenomenon which is also controlled by receptors of the ligand-gated ion channel (LGIC) superfamily, which includes the Cys-loop family composed of the excitatory nicotinic acetyl choline receptors (nAchRs) and serotonin type 3 receptors (5HT3). Upon activation, they undergo conformational changes that increase permeability to cations and increase neuronal excitability. Inhibitory glycine receptors (GlyRs) and γ-aminobutyric acid receptors type A (GABAARs), on the other hand, increase membrane permeability to anions, primarily chloride ions, leading to a fast and potent inhibition of neuronal membranes [1,2]. These receptors have a similar structure and can form homo or heteropentameric channels, where each subunit is composed by: i) a large extracellular amino-terminal domain that provides the neurotransmitter-binding site and has a barrel-like structure formed predominantly by β-sheets accompanied with an alpha-helix in the amino-terminal region containing the ligand binding pocket which is conserved among the different members of LGICs. This neurotransmitter-binding site is located in the interface between two neighboring subunits and involves six regions of the extracellular domain, where the principal face, also called “(+)”, is formed by three loops (A, B, C) of one subunit whereas three β-strands (D, E, F) of the other subunit contribute to the formation of the complementary face denoted “(−)”; ii) four transmembrane domains (TM1–4) composed of amphipathic alpha-helices where TM2 contributes to form the channel pore while TM4 is located in the peripheral region interacting with lipid components of the cell membrane [3,4]; iii) a large intracellular loop domain between TM3 – TM4 important for the functional regulation of the receptors, including intracellular trafficking, sorting, insertion into cell membranes, clustering in postsynaptic regions and interaction with intracellular modulators from signaling pathways [5–7]; and iv) a small extracellular carboxy-terminal region [8].
Amino acids in TM2 determine the ion selectivity of the pore, thus defining their excitatory or inhibitory nature. For example, the selectivity region is determined by a ring of negatively charged residues in nAchRs, 5HT-3 or positively charged and proline redidues in GlyRs, GABAARs [9,10]. In particular, GlyR ion selectivity is controlled mainly by three residues in TM2 (P250, A251, T265) that when mutated change the ion selectivity from anionic to cationic (A251E, T265V, deletion P250) [10,11]. Indeed, anionic receptors from the LGICs family show a strict permeability to anions with an order of preference for SCN−>NO3−>I−>Br−>Cl−>F−, along with a significant permeability to HCO3−[12]. From the calculated diameters for each type of receptors (0.7 – 0.8 nm for the 5HT-3 and nAChRs, 0.5 – 0.6 nm in the case of GABAARs and GlyRs), it has been suggested that partially dehydrated ions are responsible for ion permeation [12].
Several studies have examined the role of the intracellular loop domain and confirmed its involvement in regulating channel conductance by way of positively charged residues acting as a weak filter [13,14]. Mutations in positively charged amino acids at the C-terminal of the intracellular loop domain decreased conductance of GlyRs [15]. Furthermore, this domain was associated to receptor desensitization [16] and ion selectivity involving the electrostatic influence of charged residues [17]. Despite all of these regulatory functions, the intracellular loop domain is not essential for channel formation, and truncated receptors generated functional receptors with macroscopic and pharmacological characteristics rather similar to native receptors [18].
Glycinergic neurotransmission
Glycine is the main inhibitory neurotransmitter in the mammalian spinal cord and brain stem. Activation of GlyRs by glycine leads to a fast increase in chloride conductance which produces hyperpolarization of the neuronal membrane known as an inhibitory postsynaptic potential (IPSP). This phenomenon is associated with a reduction in the excitability and firing properties of the spinal cord and brain stem neurons involved in the control of pain transmission, respiratory rhythms, motor coordination, reflex responses and sensory processing [19–21]. At the spinal and brain stem levels, glycinergic interneurons regulate the generation of action potentials on motoneurons through the presynaptic release of glycine and subsequent GlyR activation [22]. These spinal interneurons also control reciprocal inhibition in reflex circuits producing relaxation of antagonistic muscles during the coordinated contraction of agonist muscles, where Renshaw cells regulate motoneuron excitability by recurrent inhibition through a negative feedback loop [23].
Glycinergic synapses are formed by presynaptic vesicles containing glycine alone or in combination with the neurotransmitter GABA. These neurotransmitters are stored in the vesicles by the vesicular inhibitory amino acid transporter (VIAAT) [24]. Inactivation of VIAAT produces a drastic reduction in GABA and glycine release confirming that glycinergic neurons have a common vesicular transporter with GABA and compete for vesicular uptake in the synaptic cleft [25]. GlyT2, a transport protein responsible for the uptake of glycine from the synaptic cleft, is also found in this region [26,27]. In the postsynaptic membranes, GlyRs are found in the soma and primary processes of inhibitory neurons where their location dynamically changes between synaptic or extrasynaptic distribution, mainly defined by the presence of a microtubule-binding protein named gephyrin that anchors GlyRs in the synaptic cleft [28,29].
GlyRs are composed of α and β subunits that can associate and form homo (5α) or heteropentameric receptors in the conformations 2α–3β or 3α-2β (α/ β) which are possibly associated with non-synaptic and synaptic location, respectively [30,31]. Molecular and immunohistochemical studies have described the presence of 4 isoforms of the α subunit (α1 – α4) and only 1 β that are found widely distributed in the CNS[32–34]. This diversity is also increased by post-transcriptional modification of the α subunits such as alternative splicing of exons in the α1[35], α2[36], and α3[33] subunits. Also, the receptor undergoes RNA editing, including deamination of cytidines in α2 and α3 subunits [37,38] and post-translational modifications [38,39].
While α subunits are responsible for ion channel formation and contain binding sites for agonists and antagonists, the β subunit is related to structural and regulatory functions such as GlyR clustering in synaptic locations by interaction between intracellular loop domains with gephyrin [29,40], and regulation of the response to agonists or allosteric modulators due in part to the presence of interfaces α/ β and β/ β [41].
Expression of GlyRs in the Central Nervous System
Subunits forming the GlyR modify their temporal and spatial composition during development and maturation of the nervous system, suggesting different physiological functions for each receptor [42,43]. The α1 subunit is widely distributed in the CNS and expressed at low levels in the spinal cord in embryonic and newborn animals, and increases during development reaching high levels of expression at 15 days postnatal (P15) [42–44]. The α2 subunit, on the other hand, reaches a higher expression level during embryonic development in the spinal cord and brainstem, even in regions with no glycinergic activity such as the cortex, hippocampus and thalamus, and subsequently decreases its expression in advanced stages of development. However, the presence of α2 subunits was previously described associated to tonic inhibition in upper brain regions having sensitivity to ethanol [45–47].
During early development, membrane depolarization induced by the activation of α2 containing GlyRs leads to cortical tangential migration and promotes interneuron migration in the cortical wall [42–44,48]. In the case of cultured spinal neurons, it was determined that the expression of α1 and α2 subunits change significantly during development, which is directly related to changes in the pharmacological properties of GlyRs [49].
The GlyR α3 subunit is expressed later in development, around the third week of postnatal maturation, in regions such as the hippocampus, retina and spinal cord, thus contributing to the integration of sensory transmission [42–44]. Finally, the GlyR α4 subunit has been described as a pseudogene in humans [43], and its expression has been detected in spinal cord, dorsal root ganglion, sympathetic ganglia, adrenal gland, kidney, liver, spermatozoides and retina of mammals and birds [50]. This heterogeneity of isoforms and spatiotemporal patterns of expression yield glycine receptors with different conductances and sensitivity to agonists and positive or negative modulators, regulating different physiological functions in various regions and stages of development in the CNS [5].
General Pharmacology of GlyRs
The principal physiological agonist described for GlyRs is glycine, nevertheless, previous studies identified a series of compounds capable of activating these receptors in addition to glycine, including β-alanine, taurine and γ-amino butyric acid in decreasing order of potency [51,52]. Interestingly, there is significant variability in the response to these agonists depending on the expression systems. For example, it was found that glycine, β-alanine and taurine act as full agonists for α1 GlyR in HEK293 cells while in Xenopus laevis oocytes they were partial agonists [51,53].
GlyR function, on the other hand, is regulated by different molecules acting as antagonists, the most common of which is strychnine, an alkaloid derived from plants of the genus Strychnos that is a potent competitive antagonist of glycine, α-alanine and taurine. Since strychnine has a high selectivity for GlyRs, it is useful for discriminating between gabaergic or glycinergic synaptic currents because the latter are strongly inhibited by the action of strychnine [52,54]. Picrotoxin, a noncompetitive antagonist of LGICs and a pore blocker that alters the conformation of the TM2–3 loop which is essential for coupling the ligand binding in the extracellular domain and channel opening, inhibits GlyR to a lesser degree as compared to its effect on GABAAR [55,56]. The reduced sensitivity of GlyR to picrotoxin is due to the presence of the β subunit, where TM2 residues (F282 in β, T258 in α1 subunits) are essential for the antagonistic effects [56,57]. Furthermore, the α isoform regulates the effect of picrotoxin, and GlyRs containing α2 are more sensitive than the α1 containing GlyRs [58].
Modulators
Inorganic Allosteric Modulators (Zn2+, Ca2+, pH, Cl−)
A number of inorganic compounds are also capable of modulating GlyR function, including zinc, intracellular calcium, proton (hydrogen ions) concentration (pH) and chloride. In particular, Zn2+that is presynaptically stored with neurotransmitters is released from the synaptic vesicles in neuronal subpopulations present in several regions of the brain and spinal cord, being especially abundant in the hippocampus and olfactory bulb [59,60]. Zn2+has a biphasic concentration-dependent effect on GlyRs, producing a potentiation of glycinergic currents at low micromolar concentrations, by mainly increasing channel opening probability and burst duration [59,61]. On the other hand, concentrations higher than 10 μM can inhibit the glycine activated current by decreasing its efficacy and consequently modifying the inhibitory capacity of these receptors [62,63]. The binding sites for Zn2+ associated with potentiation of channel activity are found on the outer face of the amino-terminal domain of α1 GlyR subunits whereas residues involved in the inhibitory effects are on the opposite side of the same domain [64]. Additionally, the relationship of this ion with the effects of ethanol has recently been described. Zn2+ increases the potentiation of glycine evoked currents in α GlyRs in the presence of ethanol and this was associated with the development of alcohol addiction [39,65].
Ca2+ ions are also capable of affecting the activity of GlyRs. For example, an increase in intracellular Ca2+concentration causes a rapid augmentation in the amplitude of glycinergic currents proportional to the Ca2+ influx which may result from the activity regulated by depolarizing events that produce the opening of voltage-dependent calcium channels, or through ionotropic receptors such as N-methyl-D-aspartate receptors (NMDARs) [66,67]. This effect was fast and characterized by an apparent increase in glycine affinity in recombinant glycine receptors expressed in HEK293 and neurons from the spinal cord and hypoglossal nucleus [66,68,69].
Proton concentration (pH) corresponds to another ion capable of modulating GlyR activity. For example, extracellular pH acidification produced significant inhibition of glycine evoked currents in recombinant α1 and α1βreceptors expressed in HEK293 cells and spinal neurons. This was a voltage-independent effect and involved critical residues located in the amino-terminal region (T112, H109 in α1 subunits, T135 in β subunits) and it was accompanied by rapid receptor desensitization. The alkalinization of pH to 8.5and the reduction in proton concentration as a result of bicarbonate ion (HCO3−) efflux also produced an inhibitory effect [70,71].
Finally, due to the passive flow of chloride ions through GlyRs, intracellular Cl− concentration has a significant effect on GlyR function, where an increase leads to a slower deactivation of the receptor and augments the decay time of inhibitory currents generated by glycine (IPSCs) [72], which would be associated with chloride interaction to specific regions within the transmembrane 2 domains that delimit the channel pore, involving positively charged residues [73].
Modulation of GlyRs by signal transduction
GlyRs can be modulated through different intracellular signaling pathways involving the intracellular loop domain between transmembrane domains 3 and 4 (TM3–4), where basic amino acids are part of an internal portal that regulates ion selectivity and channel conductance [15]. Within this region, some residues are targets for phosphorylation by protein kinase C (PKC), cAMP-dependent protein kinase A (PKA) and protein tyrosine kinase (PTK) [74,75]. However, until now, the consequences of these modifications are extensively debated due to discrepancies in the response of the GlyR. PKC activation using phorbol myristate acetate (PMA) results in a decrease in chloride currents evoked by glycine in the spinal cord [76,77] and recombinant receptors expressed in Xenopus laevis [78]. Conversely, in hippocampal and dissociated sacral dorsal commissural neurons, an increase in glycinergic currents were found upon PKC activation, either through the use of PMA or indirectly by stimulation of serotonin receptor type 2 affecting in both cases serine 391 and 398 in α1 and β subunits, respectively [79,80].
PKA activation also produced divergent responses in GlyRs, finding increased glycinergic currents in spinal cord and recombinant receptors expressed in Xenopus laevis with cyclic AMP analogues [76,78]. Furthermore, single-channel analysis using dissociated neurons from the spinal trigeminal nucleus have shown that this increase in current amplitude mediated by PKA is produced by an increase in channel opening probability [74]. Meanwhile, in substantia nigra, dorsal spinal cord or ventromedial hypothalamic neurons, inhibition in receptor function was observed [81–83]. Additionally, in HEK293 cells expressing α3 GlyRs, PKA activation in response to prostaglandin A resulted in decreased glycine currents, with a critical role of serine 346[84,85]. Therefore, these divergent results were explained by regional differences, expression of specific GlyR isoforms and the experimental approach used [76].
Several studies using heterologous expression systems and neurons have shown that tyrosine kinases play a role in the maintenance and regulation of GlyR function. In this context, it was found that lavendustin A, a PTK inhibitor, reduced glycinergic currents in neurons from the hippocampus (CA1 region) and spinal cord. Furthermore, application of the intracellular tyrosine-protein kinase CSK caused an increase in the magnitude of the glycine currents by decreasing the apparent affinity [86]. These effects were also observed in recombinant α1βreceptors expressed in HEK293 cells and were mediated by phosphorylation of Y413 in βGlyRs [86].
Activation of the G protein signaling pathway is also involved in the modulation of GlyRs [87]. Activation of a G protein – coupled receptor (GPCR) catalyzes the exchange of GDP for GTP in the inactive trimeric Gα-GDP/Gβγ generating Gα-GTP and Gβγ, the active forms. The signal is terminated as a result of the intrinsic GTPase activity of the Gα subunit allowing the association of the inactive Gα-GDP with Gβγ, reconstituting the trimeric form and terminating the signal [88,89]. The dimer Gβγ can affect some forms of AC (AC-I) and PLC (PLC-β2), plus voltage-gated calcium channels (VGCCs) and GIRK channels [90,91]. In this context, it has been possible to define a region on the surface of Gβγ that interacts with some of these groups of effectors generating the hypotheses that on the Gβ surface there is a “hot spot” where different effectors bind [92].
GlyRs are also modulated by interaction with Gβγ [87]. Activation of G protein using non-hydrolyzable analogs of GTP, GTP-γ-S or GppNHp, produced a significant increase in the amplitude of the glycine-evoked currents in HEK293 cells expressing the α1 GlyR and spinal cord neurons. Further, activation of G protein enhanced the synaptically activated glycinergic current in spinal cord producing a change in the current decay phase without alterations in the amplitude of synaptic currents [87]. These effects were independent of phosphorylation, since experiments conducted in presence of 0 ATP and 1 μM staurosporine did not affect the potentiation produced by G protein activation. However, overexpression of the wild-type Gα subunits or the carboxy-terminal of G protein-coupled receptor kinase 2 (ct-GRK2) blocked the G protein effects by decreasing the availability of Gβγ [87].
This intracellular regulation by G protein has also been described in voltage-gated calcium channels (VGCCs) [93,94], G protein-gated inwardly rectifying potassium (GIRK) channels [95] and interestingly in nAchRs, confirming this type of modulation in other members of the LGICs [96].
A more detailed analysis revealed the molecular determinants for the modulation of GlyRs by G protein [97]. Two basic amino acid clusters in the intracellular loop domain of the α1 subunit,316RFRRK320 and 385KK386, are essential for the binding and modulation by Gβγ. Mutations of these residues to alanine significantly reduced the potentiation of glycine-evoked currents produced by GTP-γ-S associated with a decrease in binding of Gβγ dimers to the intracellular loop domain of α1 [97]. The presence of basic amino acids has been described as critical for the binding and modulation by G proteins. Interestingly, similar domains of basic residues were found in the intracellular regions of VGCCs, β-adrenergic receptor kinase 1 and phospholipase Cβ3[93,97–100].
Modulation of GlyRs by general anesthetics
General anesthetics are a group of allosteric GlyR modulators with pharmacological and therapeutic relevance since they cause strong CNS depression and sedation, relaxation, amnesia, analgesia, unconsciousness, and other clinical manifestations [101–103]. Volatile anesthetics are able to potentiate glycinergic currents in α1 homomeric GlyRs [104–106]. Isoflurane, enflurane and halothane have shown a potentiation in glycinergic activity with increases in the frequency of IPSC and decay time constants [107,108] and they cause an increase in glycine evoked currents in recombinant receptors [60,109]. These anesthetics share binding sites on GlyRs which consist of amino acids located in transmembrane domains 2 and 3 that form an intra-subunit cavity involving residues S267 (TM2) and A288 (TM3) as important for binding and modulation of these receptors [110–112]. Intravenous anesthetics also potentiate GlyRs, but these studies have been controversial. For example, analyses using propofol showed the presence of the hydrophobic cavity in transmembrane domains where interaction with anesthetics produce small local disturbances in the receptor structure, and the mutation S267I in α1 inhibits the effects of propofol probably by preventing access to the cavity [113]. Additionally, α1 GlyRs have a second binding site located in the intracellular loop domain in which the amino acid F380 is essential for receptor modulation [60,106,114]. This intracellular F380 site is highly specific because its replacement significantly reduces the potentiation produced by propofol, but does not affect the regulation by other general anesthetics like etomidate and isoflurane [114].
Effect of ethanol on glycinergic transmission
Ethanol is one of the most popular drugs and its excessive use creates serious social problems. Acute alcohol affects many functions of the nervous system and peripheral organs, ranging from a decrease in reflexes and disinhibition in social behavior to mental incoherence, coma and death [115,116]. Initially, alcohol consumption induces a disinhibition, followed by euphoria and depression, but chronic use can lead to problems of addiction and cardiac arrhythmia, with hepato-pancreatic and neurological disorders. The effects of alcohol abuse and dependence increase the risk for hypertension, heart diseases, obesity and fatty liver among other pathologies, thus increasing morbidity in the global population [115,116]. Despite the widespread use of alcohol in society and its adverse effects, mechanisms of action have been very complicated to analyze and several theories have been proposed to explain its effects.
Ethanol is a CNS depressant drug, which at intoxicating concentrations (5 – 100 mM) alters most brain functions. Early studies described the first effects of alcohol on LGICs receptors and showed evidence linking this receptor group to the actions of ethanol. Ethanol inhibited the activity of N-methyl-D-aspartate (NMDA) receptors in neurons from hippocampus and nucleus accumbens [117,118], α-amino-3-hydroxyisoxazolepropionic acid (AMPA) receptors [119] and nAchR expressed in Xenopus laevis oocytes [120], whereas 5-HT3 receptors [121], GABARs [122] and GlyRs [123] are potentiated by this alcohol in receptors expressed in HEK293 cells or Xenopus laevis oocytes and spinal cord, ganglionic, cortical and hippocampal neurons [124–126].
It has been described that 10 mM ethanol potentiates glycinergic currents in GlyRs from cultured spinal neurons by increasing the apparent affinity of GlyR, without changing its efficacy [105,123,127,128]. In a synaptic context, ethanol also positively modulates synaptic glycinergic events increasing inhibitory effects in mature neurons [129]. Subsequent studies have shown that the ability of physiological concentrations of ethanol (<100 mM) to potentiate the GlyR depends on the arrangement of their subunits, demonstrating different responses according to their conformation, and in the case of GlyRs, it has been confirmed that the α1 subunit is essential for the effects observed in the presence of ethanol [42,130].
The available information demonstrates that modulation of GlyRs by ethanol is associated with various pathological and behavioral effects observed during consumption and alcohol intoxication [115,116]. Studies using microdialysis of glycine together with reuptake inhibitors or strychnine in the nucleus accumbens suggest that GlyRs are important for regulating the voluntary intake of alcohol and participate in the excitability of this brain reward region, a key point for the development of addiction [116,131,132]. These data suggest that modulators of glycine mediated neurotransmission might serve as potential therapeutic agents for alcoholism. However, better ligands are needed because the clinical expectations for glycine reuptaker inhibitors such as acamprosate, Org25935 and Org24598have not been met [133–135].
Mechanisms of ethanol modulation on GlyRs
Direct interaction with the protein
The first theory proposed to explain the effects of ethanol on the function of GlyRs is related to the disturbance caused by alcohol in the plasma membrane, i.e. increasing the fluidity. However, this phenomenon is described with high concentrations of ethanol, practically lethal to living organisms (> 200 mM) [136]. This theory was discarded because i) changes in temperature that caused alterations in membrane fluidity were unable to induce the intoxicating effects of ethanol; and ii) A2C (2-(2-methoxyethoxy) ethyl-8-(cis-2-n-octylcyclopropyl) octane), a fatty acid derivative that produces a disturbance in the plasma membrane, was also unable to reproduce the effects of ethanol [137,138]. Currently, it is widely accepted that the most critical effects of ethanol are related to the specific modulation of different proteins, including glycine and GABAA receptors [116,139,140].
One proposed mechanism involves the interaction of ethanol with a group of amino acids that form a binding site or “pocket” for ethanol [Figure 1]. This notion is the result of mutagenesis studies that demonstrated loss of GlyR potentiation induced by ethanol. More recently, the model was refined by structural studies [110,140–143]. In any case, the loss of an ethanol sensitive phenotype might not be strong enough evidence to support a binding site involved in the potentiation of the receptor. Alanine 52 located in the N-terminal, serine 267 (TM2) and alanine 288 (TM3) have been defined as crucial for modulating ethanol at high concentrations (200 mM) [105,127,144], accompanied by their neighboring amino acids Q266 and M287 [142]. Furthermore, residues located in the TM4, isoleucine 409, tyrosine 410 and lysine 411 that form a hydrophilic cavity involved in the action of ethanol have also been described as important [145]. Initial data were obtained using chimeric receptors between ethanol sensitive α1 GlyR subunits and insensitive ρ1 GABAAR, from which the two residues A267 and S288 were identified as critical for the modulation of GlyRs by high concentrations of ethanol and volatile anesthetics [105]. Subsequently, using propanethiol and propyl methane thiosulfonate (PMTS) and the S267C mutation, it was shown that these compounds are covalently attached to the cysteine residue introduced into TM2, resulting in increased GlyR function [104]. Studies to determine the TM4 domain functionality and its possible participation in forming the hydrophobic binding pocket for ethanol evaluated the interaction capability of mutations W407C, I409C, Y410C and K411C with methanethiosulfonate (MTS) reagent observing that all these substitutions produced loss of modulation by ethanol [145,146]. Together, this evidence suggests the existence of a hydrophobic binding pocket for general anesthetics and alcohol formed by the residues previously described. However, the lack of pharmacological specificity of this site and the dramatic alterations in physiological properties of the channel caused by mutations in the S267, including desensitization, changes in apparent affinity and pharmacological selectivity could complicate the analysis of this mechanism [144,147]. In addition, this site might be responsible for the inhibition of these receptors at higher concentrations of ethanol.
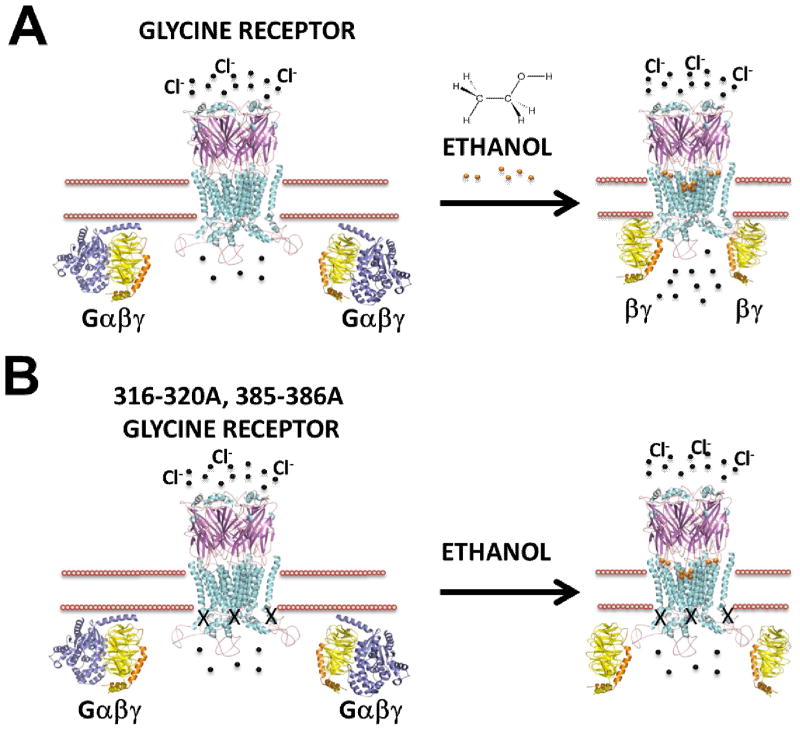
Figure 1. Schematic representation of GlyR modulation by ethanol.
After glycine binds to the receptor, a series of conformational changes lead to ion channel opening and an increase in chloride ion (black spheres) permeability. Ethanol (orange spheres) enhances glycine-evoked currents and these effects would be caused by its direct interaction with specific regions in TM2 – 3, loop 2 and/or intracellular modulation mediated by Gβγ dimers. (B) Mutated α1 GlyR 316–320A, 385–386A or GlyRs expressed in Knock-in KK385/386AA mice are not potentiated by ethanol due to a reduced interaction with Gβγ dimers. Unlike the mutations in the TM regions, intracellular mutations do not affect normal channel physiology.
Indirect mechanism for ethanol modulation
More recently, an intracellular mechanism has been postulated to explain the effects of ethanol at physiological and pharmacological relevant concentrations (10–100 mM)[148]. It is recognized that ethanol at low concentrations can modulate specific intracellular signaling pathways [149,150], including the modulation by G protein through interaction with two groups of basic amino acids in the intracellular loop domain of the α1 subunit, 316RFRRK320 and 385KK386[Figure 1][97].
Ethanol (10 mM) enhanced the amplitude of the glycine-evoked current [5,19,105,123], but mutations in clusters of basic amino acids 316 – 320, 385 – 386significantly reduced this effect making the receptor insensitive to modulation by ethanol [148]. Single-channel analysis showed that 10 mM ethanol strongly modulated wild-type GlyRs enhancing the open-channel probability, while the mutant 385 – 386 was not modulated by ethanol and did not exhibit changes in channel function [148], in contrast to previous studies in “ethanol-resistant” GlyRs with mutated amino acids in transmembrane domains that showed altered openings and bursts [151,152]. Interestingly, intracellular modulation by ethanol is specific and did not affect the regulation of other allosteric modulators described for GlyRs, for example, the mutants 312 – 320A and 385 – 386A were modulated by anesthetics, neurosteroid, trichloroethanol and zinc similar to wild-type receptors [148]. Conversely, ethanol binding sites located in the transmembrane domains are nonspecific and shared for general anesthetics and alcohols [105,110,143].
Since glycine receptors are modulated by Gβγ dimers, a significant positive correlation was found between the effects of ethanol on wild-type and mutant receptors and Gβγ activation. Ethanol effects can also be inhibited by overexpression of Gβγ scavengers like ct-GRK2 and ct-GRK3, Gαo or an antibody against Gβ subunits, confirming the involvement of G protein signaling in potentiation of glycine-evoked currents by low ethanol concentrations in α1 GlyRs [148,153].
Some studies showed that the sensitivity of α1 GlyR to ethanol was quite different to that of theα2 subunit, with the latter being resistant to ethanol [128,154,155]. The α2 subunit presents different physiological properties such as channel activation, conductance, apparent agonist affinity, and kinetic of desensitization [43,156]. For example, analysis of single-channel recordings from α2 GlyR showed a low opening probability after application of glycine [156], while whole-cell patch clamp studies described different sensitivities to other allosteric modulators such as neurosteroids, zinc or ethanol [39,46,61,157,158]. These differences between α1 and α2 led to the identification of the requirements for potentiation of glycine-evoked currents in the presence of a low ethanol concentration. The intracellular loop domain of the α2 subunit was able to interact with Gβγ, but this binding was not associated with changes in electrophysiology recordings of receptors expressed in HEK293 cells [154]. Since the differential sensitivity of α2 to ethanol could not be explained by any differences in the intracellular loop domain, consequent analyses focused on the upstream regions, the transmembranes and extracellular domains, and showed three divergent amino acids with respect to α1 at positions A52, G254 and S296 [154]. Previously, several studies proposed that A52 located in the region of loop 2 was associated with receptor activation and ethanol sensitivity [127,155], while residue G254 regulated the channel conductance [159]. Additional residues essential for ethanol and general anesthetic effects described in GlyRs, such as S267 and A288, were fully conserved between α1 and α2 subunits [110,144,160]. Triple-mutant α2 T52A/A254G/A296S GlyRs recovered a positive modulation with a significant potentiation of glycine-evoked current after dialysis with GTP-γ-S and 100 mM ethanol, accompanied by an increase in the open-channel probability in presence of ethanol, similar to α1 GlyRs. These results confirmed that insensitivity to ethanol and Gβγ modulation of α2 GlyRs was associated to the absence of elements forming part of the transmembrane domains 2 – 3 and loop 2 that are present in the α1 subunit (A52, G254 and S296) [154]. The three amino acids described as essential for ethanol effects and Gβγ modulation would be associated with the molecular transition between the resting closed state and a pre-opened closed state or “flipped” state of the glycine-bound receptors [161] facilitating the open channel after Gβγ interaction [154].
Gβγ modulation of GlyRs contributes to a differential mechanism in response to ethanol for the different receptor isoforms. For instance, α3 GlyRs differ in their modulation by ethanol with regards to α1 GlyRs. The α3 GlyRs expressed in HEK293 cells were not potentiated by low concentrations of ethanol (≤100 mM) or by Gβγ [162]. The structural elements of ethanol insensitivity present in α3 subunits revealed interesting information about the mechanisms involved in the modulation of GlyR by ethanol. Thus, insertion of the intracellular α3L splice cassette into the α1 GlyR inhibited the potentiation of the glycine evoked-current in the presence of 100 mM ethanol or after the dialysis of GTP-γ-S. This indicates that the 15 amino acids encoded by this splice cassette act as a negative regulator in the response to ethanol, likely associated to the formation of an α-helix and change in flexibility of the intracellular loop domain [162,163]. Meanwhile, the A254G mutation and the addition of the C-terminus of α1 into the α3 subunit converted the receptor into one capable of being potentiated by ethanol, which indicates that the absence of these elements along with the splice cassette are responsible for the α3 GlyR insensitivity to ethanol and Gβγ [162]. Additionally, based on the full conservation of amino acids between α3 and α1 subunits that generate a binding pocket in the transmembrane domains for ethanol, the loss of sensitivity for this alcohol in α3 GlyRs is not related to this particular region [162].
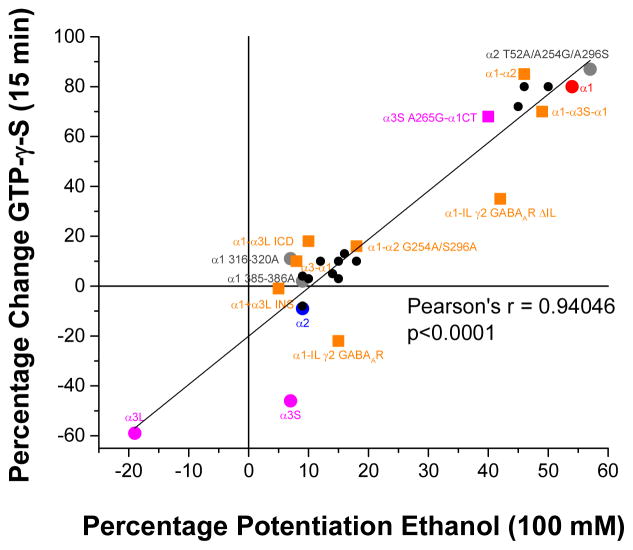
Taken together, the sensitivity to ethanol and intracellular modulation of the different isoforms of glycine receptors are related to residues in the extracellular region (A52), transmembrane domains (G254, S296), intracellular loop domain (316RFRRK320, 385KK386) and the lack of the splice cassette of α3 [60,148,153,154,162,164]. Because ethanol sensitivity is related to intracellular modulation of glycine receptors by Gβγ from activation of G proteins, the results obtained using chimeric receptors, site-directed mutations and wild type receptors from different regions of the CNS confirmed a highly significant correlation between the sensitivity to ethanol (100 mM) and G protein modulation [Figure 2] and showed the role of Gβγ signaling pathway in the effects of ethanol in the CNS [87,97,123,148,154,162,165–169].
Figure 2. Ethanol sensitivity correlates with G protein modulation in GlyRs.
Correlation plot comparing the percentage of the potentiation of the glycine-evoked currents in the presence of ethanol (100 mM) and after 15 minutes of dialysis with GTP-γ-S. The correlation coefficient was highly significant, confirming the correlation between ethanol effects and Gβγ modulation for all GlyRs tested. We highlighted the WTs α1 (red), α2 (blue) and α3 (magenta), some relevant mutated (gray) and chimeric receptors (orange).
Structure of glycine receptors and ethanol sensitivity
Presently, there is no information derived from crystallographic structures or nuclear magnetic resonance containing the intracellular loop domain. Originally, structural data was obtained from prokaryotic receptors of LGICs homologues such as cationic proton-gated ion channel from Gloeobacter violaceus (GLIC) [170,171], the Erwiniachrysanthemi ligand-gated ion channel activated by GABA (ELIC) [172,173] and the Caenorhabditis elegans glutamate-gated chloride channel (GluCl) [174,175]. Subsequently, structures were obtained of the GLIC receptor associated to allosteric modulators, or with mutations such as GLIC F14’A which is sensitive to ethanol [176–178], and structures of chimeric receptors derived from GLIC and TM domains of α1 GlyRs [112,179]. However, all these structures do not present the intracellular loop domain and only contain a small linker between transmembrane domains 3 and 4. Recently, additional structures were obtained for the first eukaryotic structure of GABAA receptors, which corresponds to a homopentamer β3 with the intracellular loop domain removed to facilitate purification and crystallization [180], the 5-HT3 receptor which presents only the C-terminal of the intracellular loop domain but nevertheless has allowed confirmation of the “portal” region or intracellular vestibule [181], and the transmembrane domains of a homopentamer α1 GlyR[182].
Using these new structures, homology models of GlyR and GABAA receptors were generated for the analysis of binding sites for general anesthetics and ethanol [183–185]. All these structures and molecular models have provided valuable information about the events leading to the opening of the channels upon ligand binding in the extracellular or TM regions. However, similar studies in GlyRs have been more difficult because all available related structures lack the intracellular loop domain hindering a more detailed analysis of its function and intracellular modulation.
Most of the α1 GlyR models available use the structure of GluCl as the template, making it possible to analyze the events associated with binding of glycine and strychnine and elucidate the conformational changes leading to channel opening [185]. Furthermore, the comparative analysis between the structures of GluCl in open and closed states has helped to describe the conformational changes of the receptor quaternary structure necessary to open the channel and that involve displacements of the extracellular and transmembrane domains [175]. Many of the gating events described in GluCl will likely occur in GlyRs given their relative structural conservation.
Recently, a full model of the α1 GlyR was published in which the presence of an α helical conformation in the N- and C-terminus of the intracellular loop domain were associated with intracellular modulation by Gβγ and ethanol effects [167]. Data generated using circular dichroism to validate this model was used [167]. Interestingly, the helical structure content was slightly lower than that previously reported for the intracellular loop domain of the α3 subunit suggesting a high conservation [163,167]. The helical structure described in the intracellular loop domain of α1 GlyRs may be required for protein/protein interactions as previously described for other protein complexes [186,187] or for peptides having the helical structure [168,169,187]. In this regard, numerous studies have reported the involvement of helical structures in the interaction sites such as complexes between Gβγ and peptides SlGK, GRK2 or phosducin [89,92,188]. Therefore, the helical structure in the α1subunit might be required for interaction with the helices present in the “hot spot” of Gβγ and thus modulate the function of GlyRs [87,92,148,154,189].
Glycine receptors and ethanol related behavior
Studies in different strains of mice with mutations in the α1 GlyR subunit (spasmodic and oscillators) or in the β GlyR subunit (spastic) have demonstrated a critical role of glycinergic inhibition on transmission and sensory processing [190–192]. These mice not only display increased muscle tone, but also a strong hyperekplexia phenotype, very similar to epileptogenic conditions [193]. Indeed, mutations in the α1 GlyR coding gene has been found in patients with hyperekplexia/seizure disease [194]. It is now accepted that GlyR functions can be affected by inherited mutations, altered RNA editions or post-translational modifications [195,196]. Furthermore, LGICs are targets of a wide range of chemical compounds. For example, several effects of ethanol on α1 GlyRs are believed to be relevant to human health because ethanol causes motor, respiratory and cardiovascular alterations [197–200], likely by altering these chloride currents [129,130,139].
Recent studies have demonstrated that agonists and antagonists of GlyRs affect the brain reward system [201]. Interestingly, while alcohol acutely activates the mesolimbic dopaminergic system, its chronic administration induces functional and structural alterations, similar to other drugs of abuse [202,203]. Somewhat surprising because of their lower location, GlyRs have also been implicated in the release of dopamine in the nucleus accumbens (nAc)[203,204]. Glycine stimulates the release of dopamine in a strychnine-dependent manner, both in the absence and presence of ethanol [134]. Furthermore, the administration of GlyT1 inhibitors increased the extracellular level of glycine, together with an elevation in dopamine in the nAc [132]. The increase in dopamine reduced the ethanol intake and preference [134]. Also, recent studies showed that glycine microinjections in the rat VTA selectively reduced ethanol intake in rats chronically exposed to ethanol [202].
The use of mice bearing mutations in the α1 subunit have provided relevant new information on the role of GlyRs in ethanol behaviors. A study found that the homozygous KI mice with the S267Q mutation, an ethanol resistant phenotype in recombinant GlyRs, exhibited seizures and convulsions and died at 3 weeks. The heterozygous mice, on the other hand, displayed a hyperekplexia phenotype demonstrating the physiological importance of GlyRs [151,152]. Two other mutations in the α1 GlyR, M287L and Q266I [142], similarly caused early death in homozygous mice, possibly caused by GlyR hypo function [142]. Interestingly, ethanol-induced loss of righting reflex (LORR) was reduced in M287L KI mice and increased in heterozygous Q266I mice [205]. In addition, a reduced ethanol withdrawal response was found in the M287l, but not in the Q266I KI mice [205]. These results suggest a differential ethanol modulation in both KI mice, but also brings into question the significance of the findings considering their altered basal behaviors, even in the absence of ethanol.
Recently, another KI mice model was generated with the mutations KK385/386AA [165]. Unlike mice with mutations in TM domains, these animals were overtly normal. Also, no differences were found when the acoustic startle test was examined [165]. More interestingly, the mice displayed a 30% shorter LORR duration with ethanol (3.5 g/Kg) than the WT mice. This study confirmed the important function of α1 GlyRs on the locomotor-stimulating actions at low doses, and sedative effects of ethanol at high doses [165].
Studies done in mice lacking Glra2 showed that these animals displayed a reduced ethanol consumption and lower ethanol preference demonstrating the significance of α2 GlyRs in ethanol behaviors, whereas the α3 KO showed unchanged ethanol consumption and preference [46]. In addition, no differences were found in ethanol-induced LORR in α2 and α3 KO [46]. Therefore, these results suggest that α2 GlyR subunits have a role in consumption and preference, but not in motor control. These results are in agreement with studies that showed a significant expression of α2 in mesolimbic dopaminergic regions [206,207].
In summary, α1 and α2 GlyR subunits have an important role regulating the excitatory-inhibitory balance, controlling motor actions, modulating sedative ethanol effects and probably regulating ethanol preference and consumption. Those functions make α1 and α2 GlyR subunits interesting and relevant targets for acute alcohol intoxication and alcohol abuse treatment.
Conclusions
Two main hypotheses have been proposed to explain the effects of ethanol on the GlyR. First, a direct interaction of ethanol with specific regions in the receptor structure, the transmembrane domains 2, 3 and loop 2 located in the extracellular region. This binding region is shared for alcohol and general anesthetics. Second, an indirect mechanism mediated by Gβγ dimers released by ethanol that interact with the intracellular loop domain of GlyRs, a specific mechanism for ethanol that does not affect their sensitivity to other allosteric modulators.
Modulation of GlyRs by Gβγ and ethanol is different in α1 and α2 subunits and because the distribution of these subunits is spatially and temporarily regulated in the CNS this could be relevant for the effects of low ethanol concentrations. Furthermore, the expression and regulation of GlyRs by ethanol in higher regions of the CNS, such as the nucleus accumbens, would be associated with the development of addiction to alcohol. This hypothesis still needs to be proven in genetically modified mice where it is expected that potentiation of GlyRs is associated with a decrease in voluntary intake and psycho-motor effects produced by ethanol.
The inhibitory effects of GlyRs in the CNS are greatly enhanced when they are potentiated by ethanol. Interestingly, the range of ethanol concentrations that affect the receptor vary from those that produce euphoria to those that induce death. Thus, depending on the ethanol concencentration and the location of GlyRs in the CNS, their enhanced function might cause effects that range from reward, muscle relaxation and respiratory arrest. Therefore, the development of inhibitors that can block the effects of ethanol on GlyRs could be a potential treatment for addiction or acute intoxication.
Acknowledgments
The authors thank Mrs. Lauren Aguayo for revising the article.
Work supported by National Institutes of Health Grant R01AA15150, Fondo Nacional de Desarrollo Científico y Tecnológico grant 1131004 and Comisión Nacional de Investigación Científica y Tecnológica grant DPI20140008.
Abbreviations
- GlyR
glycine receptor
- LGIC
ligand-gated ion channel
- Gβγ
βγ dimer of G protein
- TM
transmembrane domain
- IL
large intracellular loop domain
- GTP-γ-S
guanosine 5′-O-(3-thiotriphosphate)
- GST
glutathione-S-transferase
- HEK
human embryonic kidney
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: New twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Miller PS, Smart TG. Binding, activation and modulation of cys-loop receptors. Trends Pharmacol Sci. 2010;31:161–174. doi: 10.1016/j.tips.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Ma D, Liu Z, Li L, Tang P, Xu Y. Structure and dynamics of the second and third transmembrane domains of human glycine receptor. Biochemistry. 2005;44:8790–8800. doi: 10.1021/bi050256n. [DOI] [PubMed] [Google Scholar]
- 4.Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 5.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Zhu Y, Heinemann SF. Identification of sequence motifs that target neuronal nicotinic receptors to dendrites and axons. J Neurosci. 2006;26:9780–9793. doi: 10.1523/JNEUROSCI.0840-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ZW, Olsen RW. Gabaa receptor associated proteins: A key factor regulating gabaa receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- 8.Thompson AJ, Lester HA, Lummis SC. The structural basis of function in cys-loop receptors. Q Rev Biophys. 2010;43:449–499. doi: 10.1017/S0033583510000168. [DOI] [PubMed] [Google Scholar]
- 9.Corringer PJ, Bertrand S, Galzi JL, Devillers-Thiery A, Changeux JP, Bertrand D. Mutational analysis of the charge selectivity filter of the alpha7 nicotinic acetylcholine receptor. Neuron. 1999;22:831–843. doi: 10.1016/s0896-6273(00)80741-2. [DOI] [PubMed] [Google Scholar]
- 10.Keramidas A, Moorhouse AJ, French CR, Schofield PR, Barry PH. M2 pore mutations convert the glycine receptor channel from being anion- to cation-selective. Biophys J. 2000;79:247–259. doi: 10.1016/S0006-3495(00)76287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng MH, Cascio M, Coalson RD. Theoretical studies of the m2 transmembrane segment of the glycine receptor: Models of the open pore structure and current-voltage characteristics. Biophys J. 2005;89:1669–1680. doi: 10.1529/biophysj.105.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keramidas A, Moorhouse AJ, Schofield PR, Barry PH. Ligand-gated ion channels: Mechanisms underlying ion selectivity. Prog Biophys Mol Biol. 2004;86:161–204. doi: 10.1016/j.pbiomolbio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Gee VJ, Kracun S, Cooper ST, Gibb AJ, Millar NS. Identification of domains influencing assembly and ion channel properties in alpha 7 nicotinic receptor and 5-ht3 receptor subunit chimaeras. Br J Pharmacol. 2007;152:501–512. doi: 10.1038/sj.bjp.0707429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Toole KK, Jenkins A. Discrete m3-m4 intracellular loop subdomains control specific aspects of gamma-aminobutyric acid type a receptor function. J Biol Chem. 2011;286:37990–37999. doi: 10.1074/jbc.M111.258012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carland JE, Cooper MA, Sugiharto S, Jeong HJ, Lewis TM, Barry PH, Peters JA, Lambert JJ, Moorhouse AJ. Characterization of the effects of charged residues in the intracellular loop on ion permeation in alpha1 glycine receptor channels. J Biol Chem. 2009;284:2023–2030. doi: 10.1074/jbc.M806618200. [DOI] [PubMed] [Google Scholar]
- 16.Hu XQ, Hayrapetyan V, Gadhiya JJ, Rhubottom HE, Lovinger DM, Machu TK. Mutations of l293 in transmembrane two of the mouse 5-hydroxytryptamine3a receptor alter gating and alcohol modulatory actions. Br J Pharmacol. 2006;148:88–101. doi: 10.1038/sj.bjp.0706685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters JA, Cooper MA, Carland JE, Livesey MR, Hales TG, Lambert JJ. Novel structural determinants of single channel conductance and ion selectivity in 5-hydroxytryptamine type 3 and nicotinic acetylcholine receptors. J Physiol. 2010;588:587–596. doi: 10.1113/jphysiol.2009.183137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen M, Bali M, Akabas MH. Modular design of cys-loop ligand-gated ion channels: Functional 5-ht3 and gaba rho1 receptors lacking the large cytoplasmic m3m4 loop. J Gen Physiol. 2008;131:137–146. doi: 10.1085/jgp.200709896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dresbach T, Nawrotzki R, Kremer T, Schumacher S, Quinones D, Kluska M, Kuhse J, Kirsch J. Molecular architecture of glycinergic synapses. Histochem Cell Biol. 2008;130:617–633. doi: 10.1007/s00418-008-0491-y. [DOI] [PubMed] [Google Scholar]
- 21.Zeilhofer HU, Wildner H, Yevenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callister RJ, Graham BA. Early history of glycine receptor biology in mammalian spinal cord circuits. Front Mol Neurosci. 2010;3:13. doi: 10.3389/fnmol.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez FJ, Benito-Gonzalez A, Siembab VC. Principles of interneuron development learned from renshaw cells and the motoneuron recurrent inhibitory circuit. Ann N Y Acad Sci. 2013;1279:22–31. doi: 10.1111/nyas.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller E, Le-Corronc H, Legendre P. Extrasynaptic and postsynaptic receptors in glycinergic and gabaergic neurotransmission: A division of labor? Front Mol Neurosci. 2008;1:3. doi: 10.3389/neuro.02.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of gaba and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: Essential regulators of neurotransmission. Trends in biochemical sciences. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Dohi T, Morita K, Kitayama T, Motoyama N, Morioka N. Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol Ther. 2009;123:54–79. doi: 10.1016/j.pharmthera.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Meier J, Vannier C, Serge A, Triller A, Choquet D. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci. 2001;4:253–260. doi: 10.1038/85099. [DOI] [PubMed] [Google Scholar]
- 29.Calamai M, Specht CG, Heller J, Alcor D, Machado P, Vannier C, Triller A. Gephyrin oligomerization controls glyr mobility and synaptic clustering. J Neurosci. 2009;29:7639–7648. doi: 10.1523/JNEUROSCI.5711-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Durisic N, Godin AG, Wever CM, Heyes CD, Lakadamyali M, Dent JA. Stoichiometry of the human glycine receptor revealed by direct subunit counting. J Neurosci. 2012;32:12915–12920. doi: 10.1523/JNEUROSCI.2050-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker CM, Betz H. Alpha subunit variants of the human glycine receptor: Primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990;9:771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolic Z, Laube B, Weber RG, Lichter P, Kioschis P, Poustka A, Mulhardt C, Becker CM. The human glycine receptor subunit alpha3. Glra3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J Biol Chem. 1998;273:19708–19714. doi: 10.1074/jbc.273.31.19708. [DOI] [PubMed] [Google Scholar]
- 34.Miller PS, Harvey RJ, Smart TG. Differential agonist sensitivity of glycine receptor alpha2 subunit splice variants. Br J Pharmacol. 2004;143:19–26. doi: 10.1038/sj.bjp.0705875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malosio ML, Grenningloh G, Kuhse J, Schmieden V, Schmitt B, Prior P, Betz H. Alternative splicing generates two variants of the alpha 1 subunit of the inhibitory glycine receptor. J Biol Chem. 1991;266:2048–2053. [PubMed] [Google Scholar]
- 36.Kuhse J, Kuryatov A, Maulet Y, Malosio ML, Schmieden V, Betz H. Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett. 1991;283:73–77. doi: 10.1016/0014-5793(91)80557-j. [DOI] [PubMed] [Google Scholar]
- 37.Eichler SA, Kirischuk S, Juttner R, Schaefermeier PK, Legendre P, Lehmann TN, Gloveli T, Grantyn R, Meier JC. Glycinergic tonic inhibition of hippocampal neurons with depolarizing gabaergic transmission elicits histopathological signs of temporal lobe epilepsy. J Cell Mol Med. 2008;12:2848–2866. doi: 10.1111/j.1582-4934.2008.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legendre P, Forstera B, Juttner R, Meier JC. Glycine receptors caught between genome and proteome - functional implications of rna editing and splicing. Front Mol Neurosci. 2009;2:23. doi: 10.3389/neuro.02.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCracken LM, Trudell JR, McCracken ML, Harris RA. Zinc-dependent modulation of alpha2- and alpha3-glycine receptor subunits by ethanol. Alcohol Clin Exp Res. 2013;37:2002–2010. doi: 10.1111/acer.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Zundert B, Castro P, Aguayo LG. Glycinergic and gabaergic synaptic transmission are differentially affected by gephyrin in spinal neurons. Brain Res. 2005;1050:40–47. doi: 10.1016/j.brainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Dutertre S, Drwal M, Laube B, Betz H. Probing the pharmacological properties of distinct subunit interfaces within heteromeric glycine receptors reveals a functional betabeta agonist-binding site. J Neurochem. 2012;122:38–47. doi: 10.1111/j.1471-4159.2012.07755.x. [DOI] [PubMed] [Google Scholar]
- 42.Aguayo LG, van Zundert B, Tapia JC, Carrasco MA, Alvarez FJ. Changes on the properties of glycine receptors during neuronal development. Brain Res Brain Res Rev. 2004;47:33–45. doi: 10.1016/j.brainresrev.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mrnas in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avila A, Nguyen L, Rigo JM. Glycine receptors and brain development. Frontiers in cellular neuroscience. 2013;7:184. doi: 10.3389/fncel.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blednov YA, Benavidez JM, Black M, Leiter CR, Osterndorff-Kahanek E, Harris RA. Glycine receptors containing alpha2 or alpha3 subunits regulate specific ethanol-mediated behaviors. J Pharmacol Exp Ther. 2015;353:181–191. doi: 10.1124/jpet.114.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chau P, Hoifodt-Lido H, Lof E, Soderpalm B, Ericson M. Glycine receptors in the nucleus accumbens involved in the ethanol intake-reducing effect of acamprosate. Alcohol Clin Exp Res. 2010;34:39–45. doi: 10.1111/j.1530-0277.2009.01063.x. [DOI] [PubMed] [Google Scholar]
- 48.Avila A, Vidal PM, Tielens S, Morelli G, Laguesse S, Harvey RJ, Rigo JM, Nguyen L. Glycine receptors control the generation of projection neurons in the developing cerebral cortex. Cell death and differentiation. 2014;21:1696–1708. doi: 10.1038/cdd.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapia JC, Aguayo LG. Changes in the properties of developing glycine receptors in cultured mouse spinal neurons. Synapse. 1998;28:185–194. doi: 10.1002/(SICI)1098-2396(199803)28:3<185::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 50.Harvey RJ, Schmieden V, Von Holst A, Laube B, Rohrer H, Betz H. Glycine receptors containing the alpha4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur J Neurosci. 2000;12:994–1001. doi: 10.1046/j.1460-9568.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 51.Lewis TM, Schofield PR, McClellan AM. Kinetic determinants of agonist action at the recombinant human glycine receptor. J Physiol. 2003;549:361–374. doi: 10.1113/jphysiol.2002.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pless SA, Dibas MI, Lester HA, Lynch JW. Conformational variability of the glycine receptor m2 domain in response to activation by different agonists. J Biol Chem. 2007;282:36057–36067. doi: 10.1074/jbc.M706468200. [DOI] [PubMed] [Google Scholar]
- 53.De Saint Jan D, David-Watine B, Korn H, Bregestovski P. Activation of human alpha1 and alpha2 homomeric glycine receptors by taurine and gaba. J Physiol. 2001;535:741–755. doi: 10.1111/j.1469-7793.2001.t01-1-00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brams M, Pandya A, Kuzmin D, van Elk R, Krijnen L, Yakel JL, Tsetlin V, Smit AB, Ulens C. A structural and mutagenic blueprint for molecular recognition of strychnine and d-tubocurarine by different cys-loop receptors. PLoS Biol. 2011;9:e1001034. doi: 10.1371/journal.pbio.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dibas MI, Gonzales EB, Das P, Bell-Horner CL, Dillon GH. Identification of a novel residue within the second transmembrane domain that confers use-facilitated block by picrotoxin in glycine alpha 1 receptors. J Biol Chem. 2002;277:9112–9117. doi: 10.1074/jbc.M111356200. [DOI] [PubMed] [Google Scholar]
- 56.Hawthorne R, Lynch JW. A picrotoxin-specific conformational change in the glycine receptor m2-m3 loop. J Biol Chem. 2005;280:35836–35843. doi: 10.1074/jbc.M506645200. [DOI] [PubMed] [Google Scholar]
- 57.Shan Q, Haddrill JL, Lynch JW. A single beta subunit m2 domain residue controls the picrotoxin sensitivity of alphabeta heteromeric glycine receptor chloride channels. J Neurochem. 2001;76:1109–1120. doi: 10.1046/j.1471-4159.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 58.Wang DS, Mangin JM, Moonen G, Rigo JM, Legendre P. Mechanisms for picrotoxin block of alpha2 homomeric glycine receptors. J Biol Chem. 2006;281:3841–3855. doi: 10.1074/jbc.M511022200. [DOI] [PubMed] [Google Scholar]
- 59.Trombley PQ, Blakemore LJ, Hill BJ. Zinc modulation of glycine receptors. Neuroscience. 2011;186:32–38. doi: 10.1016/j.neuroscience.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yevenes GE, Zeilhofer HU. Allosteric modulation of glycine receptors. Br J Pharmacol. 2011;164:224–236. doi: 10.1111/j.1476-5381.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller PS, Topf M, Smart TG. Mapping a molecular link between allosteric inhibition and activation of the glycine receptor. Nat Struct Mol Biol. 2008;15:1084–1093. doi: 10.1038/nsmb.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller PS, Beato M, Harvey RJ, Smart TG. Molecular determinants of glycine receptor alphabeta subunit sensitivities to zn2+-mediated inhibition. J Physiol. 2005;566:657–670. doi: 10.1113/jphysiol.2005.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eto K, Arimura Y, Nabekura J, Noda M, Ishibashi H. The effect of zinc on glycinergic inhibitory postsynaptic currents in rat spinal dorsal horn neurons. Brain Res. 2007;1161:11–20. doi: 10.1016/j.brainres.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 64.Miller PS, Da Silva HM, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005;280:37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- 65.McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. Zinc enhances ethanol modulation of the alpha1 glycine receptor. Neuropharmacology. 2010;58:676–681. doi: 10.1016/j.neuropharm.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fucile S, De Saint Jan D, de Carvalho LP, Bregestovski P. Fast potentiation of glycine receptor channels of intracellular calcium in neurons and transfected cells. Neuron. 2000;28:571–583. doi: 10.1016/s0896-6273(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 67.Xu TL, Dong XP, Wang DS. N-methyl-d-aspartate enhancement of the glycine response in the rat sacral dorsal commissural neurons. Eur J Neurosci. 2000;12:1647–1653. doi: 10.1046/j.1460-9568.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 68.Diana MA, Bregestovski P. Calcium and endocannabinoids in the modulation of inhibitory synaptic transmission. Cell Calcium. 2005;37:497–505. doi: 10.1016/j.ceca.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 69.Mukhtarov M, Ragozzino D, Bregestovski P. Dual ca2+ modulation of glycinergic synaptic currents in rodent hypoglossal motoneurones. J Physiol. 2005;569:817–831. doi: 10.1113/jphysiol.2005.094862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z, Dillon GH, Huang R. Molecular determinants of proton modulation of glycine receptors. J Biol Chem. 2004;279:876–883. doi: 10.1074/jbc.M307684200. [DOI] [PubMed] [Google Scholar]
- 71.Song YP, Schlesinger F, Ragancokova D, Calixto R, Dengler R, Krampfl K. Changes in extracellular ph affect glycine receptor channels expressed in hek 293 cells. Eur J Pharmacol. 2010;636:59–64. doi: 10.1016/j.ejphar.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 72.Pitt SJ, Sivilotti LG, Beato M. High intracellular chloride slows the decay of glycinergic currents. J Neurosci. 2008;28:11454–11467. doi: 10.1523/JNEUROSCI.3890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moroni M, Biro I, Giugliano M, Vijayan R, Biggin PC, Beato M, Sivilotti LG. Chloride ions in the pore of glycine and gaba channels shape the time course and voltage dependence of agonist currents. J Neurosci. 2011;31:14095–14106. doi: 10.1523/JNEUROSCI.1985-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song YM, Huang LY. Modulation of glycine receptor chloride channels by camp-dependent protein kinase in spinal trigeminal neurons. Nature. 1990;348:242–245. doi: 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- 75.Ruiz-Gomez A, Vaello ML, Valdivieso F, Mayor F., Jr Phosphorylation of the 48-kda subunit of the glycine receptor by protein kinase c. J Biol Chem. 1991;266:559–566. [PubMed] [Google Scholar]
- 76.Tapia JC, Espinoza F, Aguayo LG. Differential intracellular regulation of cortical gaba(a) and spinal glycine receptors in cultured neurons. Brain Res. 1997;769:203–210. doi: 10.1016/s0006-8993(97)00672-0. [DOI] [PubMed] [Google Scholar]
- 77.Albarran FA, Roa JP, Navarrete R, Castillo R, Nualart F, Aguayo LG. Effect of protein kinase c activation on the glycine evoked cl(−) current in spinal cord neurons. Brain Res. 2001;902:1–10. doi: 10.1016/s0006-8993(01)02255-7. [DOI] [PubMed] [Google Scholar]
- 78.Vaello ML, Ruiz-Gomez A, Lerma J, Mayor F., Jr Modulation of inhibitory glycine receptors by phosphorylation by protein kinase c and camp-dependent protein kinase. J Biol Chem. 1994;269:2002–2008. [PubMed] [Google Scholar]
- 79.Schonrock B, Bormann J. Modulation of hippocampal glycine receptor channels by protein kinase c. Neuroreport. 1995;6:301–304. doi: 10.1097/00001756-199501000-00019. [DOI] [PubMed] [Google Scholar]
- 80.Xu TL, Nabekura J, Akaike N. Protein kinase c-mediated enhancement of glycine response in rat sacral dorsal commissural neurones by serotonin. J Physiol. 1996;496 (Pt 2):491–501. doi: 10.1113/jphysiol.1996.sp021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nabekura J, Xu TL, Rhee JS, Li JS, Akaike N. Alpha2-adrenoceptor-mediated enhancement of glycine response in rat sacral dorsal commissural neurons. Neuroscience. 1999;89:29–41. doi: 10.1016/s0306-4522(98)00303-0. [DOI] [PubMed] [Google Scholar]
- 82.Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Muller U. Glyr alpha3: An essential target for spinal pge2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 83.Agopyan N, Tokutomi N, Akaike N. Protein kinase a-mediated phosphorylation reduces only the fast desensitizing glycine current in acutely dissociated ventromedial hypothalamic neurons. Neuroscience. 1993;56:605–615. doi: 10.1016/0306-4522(93)90360-r. [DOI] [PubMed] [Google Scholar]
- 84.Heindl C, Brune K, Renner B. Kinetics and functional characterization of the glycine receptor alpha2 and alpha3 subunit. Neurosci Lett. 2007;429:59–63. doi: 10.1016/j.neulet.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Han L, Talwar S, Wang Q, Shan Q, Lynch JW. Phosphorylation of alpha3 glycine receptors induces a conformational change in the glycine-binding site. ACS Chem Neurosci. 2013;4:1361–1370. doi: 10.1021/cn400097j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caraiscos VB, Mihic SJ, MacDonald JF, Orser BA. Tyrosine kinases enhance the function of glycine receptors in rat hippocampal neurons and human alpha(1) beta glycine receptors. J Physiol. 2002;539:495–502. doi: 10.1113/jphysiol.2001.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yevenes GE, Peoples RW, Tapia JC, Parodi J, Soto X, Olate J, Aguayo LG. Modulation of glycine-activated ion channel function by g-protein betagamma subunits. Nat Neurosci. 2003;6:819–824. doi: 10.1038/nn1095. [DOI] [PubMed] [Google Scholar]
- 88.Oldham WM, Hamm HE. Heterotrimeric g protein activation by g-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 89.Lin Y, Smrcka AV. Understanding molecular recognition by g protein betagamma subunits on the path to pharmacological targeting. Mol Pharmacol. 2011;80:551–557. doi: 10.1124/mol.111.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Digby GJ, Sethi PR, Lambert NA. Differential dissociation of g protein heterotrimers. J Physiol. 2008;586:3325–3335. doi: 10.1113/jphysiol.2008.153965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of gbetagamma subunits in the organization, assembly, and function of gpcr signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis TL, Bonacci TM, Sprang SR, Smrcka AV. Structural and molecular characterization of a preferred protein interaction surface on g protein beta gamma subunits. Biochemistry. 2005;44:10593–10604. doi: 10.1021/bi050655i. [DOI] [PubMed] [Google Scholar]
- 93.Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- 94.Tedford HW, Zamponi GW. Direct g protein modulation of cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 95.Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier k+ channels. Eur J Biochem. 2000;267:5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- 96.Fischer H, Liu DM, Lee A, Harries JC, Adams DJ. Selective modulation of neuronal nicotinic acetylcholine receptor channel subunits by go-protein subunits. J Neurosci. 2005;25:3571–3577. doi: 10.1523/JNEUROSCI.4971-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yevenes GE, Moraga-Cid G, Guzman L, Haeger S, Oliveira L, Olate J, Schmalzing G, Aguayo LG. Molecular determinants for g protein betagamma modulation of ionotropic glycine receptors. J Biol Chem. 2006;281:39300–39307. doi: 10.1074/jbc.M608272200. [DOI] [PubMed] [Google Scholar]
- 98.Zamponi GW, Currie KP. Regulation of ca(v)2 calcium channels by g protein coupled receptors. Biochim Biophys Acta. 2013;1828:1629–1643. doi: 10.1016/j.bbamem.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Canti C, Page KM, Stephens GJ, Dolphin AC. Identification of residues in the n terminus of alpha1b critical for inhibition of the voltage-dependent calcium channel by gbeta gamma. J Neurosci. 1999;19:6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barr AJ, Ali H, Haribabu B, Snyderman R, Smrcka AV. Identification of a region at the n-terminus of phospholipase c-beta 3 that interacts with g protein beta gamma subunits. Biochemistry. 2000;39:1800–1806. doi: 10.1021/bi992021f. [DOI] [PubMed] [Google Scholar]
- 101.Grasshoff C, Antkowiak B. Effects of isoflurane and enflurane on gabaa and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats. Br J Anaesth. 2006;97:687–694. doi: 10.1093/bja/ael239. [DOI] [PubMed] [Google Scholar]
- 102.Eckle VS, Hauser S, Drexler B, Antkowiak B, Grasshoff C. Opposing actions of sevoflurane on gabaergic and glycinergic synaptic inhibition in the spinal ventral horn. PLoS One. 2013;8:e60286. doi: 10.1371/journal.pone.0060286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen HT, Li KY, daGraca RL, Delphin E, Xiong M, Ye JH. Behavior and cellular evidence for propofol-induced hypnosis involving brain glycine receptors. Anesthesiology. 2009;110:326–332. doi: 10.1097/ALN.0b013e3181942b5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci U S A. 2000;97:9305–9310. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on gaba(a) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 106.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 107.Yamashita M, Ueno T, Akaike N, Ikemoto Y. Modulation of miniature inhibitory postsynaptic currents by isoflurane in rat dissociated neurons with glycinergic synaptic boutons. Eur J Pharmacol. 2001;431:269–276. doi: 10.1016/s0014-2999(01)01421-2. [DOI] [PubMed] [Google Scholar]
- 108.Cheng G, Kendig JJ. Pre- and postsynaptic volatile anaesthetic actions on glycinergic transmission to spinal cord motor neurons. Br J Pharmacol. 2002;136:673–684. doi: 10.1038/sj.bjp.0704760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jenkins A, Lobo IA, Gong D, Trudell JR, Solt K, Harris RA, Eger EI., 2nd General anesthetics have additive actions on three ligand gated ion channels. Anesth Analg. 2008;107:486–493. doi: 10.1213/ane.0b013e31817b70c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lobo IA, Harris RA. Sites of alcohol and volatile anesthetic action on glycine receptors. Int Rev Neurobiol. 2005;65:53–87. doi: 10.1016/S0074-7742(04)65003-3. [DOI] [PubMed] [Google Scholar]
- 111.Roberts MT, Phelan R, Erlichman BS, Pillai RN, Ma L, Lopreato GF, Mihic SJ. Occupancy of a single anesthetic binding pocket is sufficient to enhance glycine receptor function. J Biol Chem. 2006;281:3305–3311. doi: 10.1074/jbc.M502000200. [DOI] [PubMed] [Google Scholar]
- 112.Duret G, Van Renterghem C, Weng Y, Prevost M, Moraga-Cid G, Huon C, Sonner JM, Corringer PJ. Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci U S A. 2011;108:12143–12148. doi: 10.1073/pnas.1104494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahrens J, Leuwer M, Stachura S, Krampfl K, Belelli D, Lambert JJ, Haeseler G. A transmembrane residue influences the interaction of propofol with the strychnine-sensitive glycine alpha1 and alpha1beta receptor. Anesth Analg. 2008;107:1875–1883. doi: 10.1213/ane.0b013e3181875a31. [DOI] [PubMed] [Google Scholar]
- 114.Moraga-Cid G, Yevenes GE, Schmalzing G, Peoples RW, Aguayo LG. A single phenylalanine residue in the main intracellular loop of alpha1 gamma-aminobutyric acid type a and glycine receptors influences their sensitivity to propofol. Anesthesiology. 2011;115:464–473. doi: 10.1097/ALN.0b013e31822550f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 116.Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther. 2010;127:53–65. doi: 10.1016/j.pharmthera.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lovinger DM, White G, Weight FF. Ethanol inhibits nmda-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 118.Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. Darpp-32 and regulation of the ethanol sensitivity of nmda receptors in the nucleus accumbens. Nat Neurosci. 2002;5:641–648. doi: 10.1038/nn877. [DOI] [PubMed] [Google Scholar]
- 119.Moykkynen T, Korpi ER, Lovinger DM. Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (ampa) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther. 2003;306:546–555. doi: 10.1124/jpet.103.050666. [DOI] [PubMed] [Google Scholar]
- 120.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- 121.Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- 122.Aguayo LG. Ethanol potentiates the gabaa-activated cl− current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187:127–130. doi: 10.1016/0014-2999(90)90349-b. [DOI] [PubMed] [Google Scholar]
- 123.Aguayo LG, Tapia JC, Pancetti FC. Potentiation of the glycine-activated cl− current by ethanol in cultured mouse spinal neurons. J Pharmacol Exp Ther. 1996;279:1116–1122. [PubMed] [Google Scholar]
- 124.Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position alpha267. J Biol Chem. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]
- 126.Celentano JJ, Gibbs TT, Farb DH. Ethanol potentiates gaba- and glycine-induced chloride currents in chick spinal cord neurons. Brain Res. 1988;455:377–380. doi: 10.1016/0006-8993(88)90098-4. [DOI] [PubMed] [Google Scholar]
- 127.Crawford DK, Trudell JR, Bertaccini EJ, Li K, Davies DL, Alkana RL. Evidence that ethanol acts on a target in loop 2 of the extracellular domain of alpha1 glycine receptors. J Neurochem. 2007;102:2097–2109. doi: 10.1111/j.1471-4159.2007.04680.x. [DOI] [PubMed] [Google Scholar]
- 128.Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Targets for ethanol action and antagonism in loop 2 of the extracellular domain of glycine receptors. J Neurochem. 2008;106:1337–1349. doi: 10.1111/j.1471-4159.2008.05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eggers ED, Berger AJ. Mechanisms for the modulation of native glycine receptor channels by ethanol. J Neurophysiol. 2004;91:2685–2695. doi: 10.1152/jn.00907.2003. [DOI] [PubMed] [Google Scholar]
- 130.Sebe JY, Eggers ED, Berger AJ. Differential effects of ethanol on gaba(a) and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90:870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- 131.Molander A, Lof E, Stomberg R, Ericson M, Soderpalm B. Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res. 2005;29:38–45. doi: 10.1097/01.alc.0000150009.78622.e0. [DOI] [PubMed] [Google Scholar]
- 132.Molander A, Lido HH, Lof E, Ericson M, Soderpalm B. The glycine reuptake inhibitor org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol. 2007;42:11–18. doi: 10.1093/alcalc/agl085. [DOI] [PubMed] [Google Scholar]
- 133.Lido HH, Marston H, Ericson M, Soderpalm B. The glycine reuptake inhibitor org24598 and acamprosate reduce ethanol intake in the rat; tolerance development to acamprosate but not to org24598. Addict Biol. 2012;17:897–907. doi: 10.1111/j.1369-1600.2011.00367.x. [DOI] [PubMed] [Google Scholar]
- 134.Lido HH, Ericson M, Marston H, Soderpalm B. A role for accumbal glycine receptors in modulation of dopamine release by the glycine transporter-1 inhibitor org25935. Frontiers in psychiatry / Frontiers Research Foundation. 2011;2:8. doi: 10.3389/fpsyt.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Bejczy A, Nations KR, Szegedi A, Schoemaker J, Ruwe F, Soderpalm B. Efficacy and safety of the glycine transporter-1 inhibitor org 25935 for the prevention of relapse in alcohol-dependent patients: A randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res. 2014;38:2427–2435. doi: 10.1111/acer.12501. [DOI] [PubMed] [Google Scholar]
- 136.Harris RA. Ethanol actions on multiple ion channels: Which are important? Alcohol Clin Exp Res. 1999;23:1563–1570. [PubMed] [Google Scholar]
- 137.Harris RA, Schroeder F. Ethanol and the physical properties of brain membranes: Fluorescence studies. Mol Pharmacol. 1981;20:128–137. [PubMed] [Google Scholar]
- 138.Buck KJ, Allan AM, Harris RA. Fluidization of brain membranes by a2c does not produce anesthesia and does not augment muscimol-stimulated 36cl− influx. Eur J Pharmacol. 1989;160:359–367. doi: 10.1016/0014-2999(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 139.Mariqueo TA, Agurto A, Munoz B, San Martin L, Coronado C, Fernandez-Perez EJ, Murath P, Sanchez A, Homanics GE, Aguayo LG. Effects of ethanol on glycinergic synaptic currents in mouse spinal cord neurons. J Neurophysiol. 2014;111:1940–1948. doi: 10.1152/jn.00789.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Trudell JR, Messing RO, Mayfield J, Harris RA. Alcohol dependence: Molecular and behavioral evidence. Trends Pharmacol Sci. 2014;35:317–323. doi: 10.1016/j.tips.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Howard RJ, Trudell JR, Harris RA. Seeking structural specificity: Direct modulation of pentameric ligand-gated ion channels by alcohols and general anesthetics. Pharmacol Rev. 2014;66:396–412. doi: 10.1124/pr.113.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Borghese CM, Blednov YA, Quan Y, Iyer SV, Xiong W, Mihic SJ, Zhang L, Lovinger DM, Trudell JR, Homanics GE, Harris RA. Characterization of two mutations, m287l and q266i, in the alpha1 glycine receptor subunit that modify sensitivity to alcohols. J Pharmacol Exp Ther. 2012;340:304–316. doi: 10.1124/jpet.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harris RA, Trudell JR, Mihic SJ. Ethanol’s molecular targets. Science signaling. 2008;1:re7. doi: 10.1126/scisignal.128re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lobo IA, Mascia MP, Trudell JR, Harris RA. Channel gating of the glycine receptor changes accessibility to residues implicated in receptor potentiation by alcohols and anesthetics. J Biol Chem. 2004;279:33919–33927. doi: 10.1074/jbc.M313941200. [DOI] [PubMed] [Google Scholar]
- 145.Lobo IA, Trudell JR, Harris RA. Accessibility to residues in transmembrane segment four of the glycine receptor. Neuropharmacology. 2006;50:174–181. doi: 10.1016/j.neuropharm.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 146.McCracken LM, McCracken ML, Gong DH, Trudell JR, Harris RA. Linking of glycine receptor transmembrane segments three and four allows assignment of intrasubunit-facing residues. ACS Chem Neurosci. 2010;1:482. doi: 10.1021/cn100019g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sine SM, Engel AG. Recent advances in cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 148.Yevenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG. A selective g betagamma-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc Natl Acad Sci U S A. 2008;105:20523–20528. doi: 10.1073/pnas.0806257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mailliard WS, Diamond I. Recent advances in the neurobiology of alcoholism: The role of adenosine. Pharmacol Ther. 2004;101:39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 151.Findlay GS, Harris RA, Blednov YA. Male transgenic glycine receptor alpha1 (s267q) mutant mice display a hyperekplexia-like increase in acoustic startle responses. Pharmacology, biochemistry, and behavior. 2005;82:215–222. doi: 10.1016/j.pbb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 152.Findlay GS, Phelan R, Roberts MT, Homanics GE, Bergeson SE, Lopreato GF, Mihic SJ, Blednov YA, Harris RA. Glycine receptor knock-in mice and hyperekplexia-like phenotypes: Comparisons with the null mutant. J Neurosci. 2003;23:8051–8059. doi: 10.1523/JNEUROSCI.23-22-08051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yevenes GE, Moraga-Cid G, Romo X, Aguayo LG. Activated g protein alpha s subunits increase the ethanol sensitivity of human glycine receptors. J Pharmacol Exp Ther. 2011;339:386–393. doi: 10.1124/jpet.111.184408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yevenes GE, Moraga-Cid G, Avila A, Guzman L, Figueroa M, Peoples RW, Aguayo LG. Molecular requirements for ethanol differential allosteric modulation of glycine receptors based on selective gbetagamma modulation. J Biol Chem. 2010;285:30203–30213. doi: 10.1074/jbc.M110.134676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perkins DI, Trudell JR, Asatryan L, Davies DL, Alkana RL. Charge and geometry of residues in the loop 2 beta hairpin differentially affect agonist and ethanol sensitivity in glycine receptors. J Pharmacol Exp Ther. 2012;341:543–551. doi: 10.1124/jpet.111.190942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mangin JM, Baloul M, Prado De Carvalho L, Rogister B, Rigo JM, Legendre P. Kinetic properties of the alpha2 homo-oligomeric glycine receptor impairs a proper synaptic functioning. J Physiol. 2003;553:369–386. doi: 10.1113/jphysiol.2003.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kirson D, Cornelison GL, Philpo AE, Todorovic J, Mihic SJ. Physiological concentrations of zinc reduce taurine-activated glyr responses to drugs of abuse. Neuropharmacology. 2013;75C:286–294. doi: 10.1016/j.neuropharm.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiong W, Wu X, Li F, Cheng K, Rice KC, Lovinger DM, Zhang L. A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors. J Neurosci. 2012;32:5200–5208. doi: 10.1523/JNEUROSCI.6347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bormann J, Rundstrom N, Betz H, Langosch D. Residues within transmembrane segment m2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 1993;12:3729–3737. doi: 10.1002/j.1460-2075.1993.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheng MH, Coalson RD, Cascio M. Molecular dynamics simulations of ethanol binding to the transmembrane domain of the glycine receptor: Implications for the channel potentiation mechanism. Proteins. 2008;71:972–981. doi: 10.1002/prot.21784. [DOI] [PubMed] [Google Scholar]
- 161.Plested AJ, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. Single-channel study of the spasmodic mutation alpha1a52s in recombinant rat glycine receptors. J Physiol. 2007;581:51–73. doi: 10.1113/jphysiol.2006.126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sanchez A, Yevenes GE, San Martin L, Burgos CF, Moraga-Cid G, Harvey RJ, Aguayo LG. Control of ethanol sensitivity of the glycine receptor alpha3 subunit by transmembrane 2, the intracellular splice cassette and c-terminal domains. J Pharmacol Exp Ther. 2015;353:80–90. doi: 10.1124/jpet.114.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Breitinger HG, Villmann C, Melzer N, Rennert J, Breitinger U, Schwarzinger S, Becker CM. Novel regulatory site within the tm3–4 loop of human recombinant alpha3 glycine receptors determines channel gating and domain structure. J Biol Chem. 2009;284:28624–28633. doi: 10.1074/jbc.M109.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Castro PA, Figueroa M, Yevenes GE, San Martin LS, Aguayo LG. The basic property of lys385 is important for potentiation of the human alpha1 glycine receptor by ethanol. J Pharmacol Exp Ther. 2012;340:339–349. doi: 10.1124/jpet.111.185140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aguayo LG, Castro P, Mariqueo T, Munoz B, Xiong W, Zhang L, Lovinger DM, Homanics GE. Altered sedative effects of ethanol in mice with alpha1 glycine receptor subunits that are insensitive to gbetagamma modulation. Neuropsychopharmacology. 2014;39:2538–2548. doi: 10.1038/npp.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aguayo LG, Pancetti FC. Ethanol modulation of the gamma-aminobutyric acida- and glycine-activated cl− current in cultured mouse neurons. J Pharmacol Exp Ther. 1994;270:61–69. [PubMed] [Google Scholar]
- 167.Burgos CF, Castro PA, Mariqueo T, Bunster M, Guzman L, Aguayo LG. Evidence for alpha-helices in the large intracellular domain mediating modulation of the alpha1-glycine receptor by ethanol and gbetagamma. J Pharmacol Exp Ther. 2015;352:148–155. doi: 10.1124/jpet.114.217976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guzman L, Moraga-Cid G, Avila A, Figueroa M, Yevenes GE, Fuentealba J, Aguayo LG. Blockade of ethanol-induced potentiation of glycine receptors by a peptide that interferes with gbetagamma binding. J Pharmacol Exp Ther. 2009;331:933–939. doi: 10.1124/jpet.109.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.San Martin L, Cerda F, Jimenez V, Fuentealba J, Munoz B, Aguayo LG, Guzman L. Inhibition of the ethanol-induced potentiation of alpha1 glycine receptor by a small peptide that interferes with gbetagamma binding. J Biol Chem. 2012;287:40713–40721. doi: 10.1074/jbc.M112.393603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Prevost MS, Sauguet L, Nury H, Van Renterghem C, Huon C, Poitevin F, Baaden M, Delarue M, Corringer PJ. A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nat Struct Mol Biol. 2012;19:642–649. doi: 10.1038/nsmb.2307. [DOI] [PubMed] [Google Scholar]
- 171.Sauguet L, Poitevin F, Murail S, Van Renterghem C, Moraga-Cid G, Malherbe L, Thompson AW, Koehl P, Corringer PJ, Baaden M, Delarue M. Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels. EMBO J. 2013;32:728–741. doi: 10.1038/emboj.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 173.Zimmermann I, Dutzler R. Ligand activation of the prokaryotic pentameric ligand-gated ion channel elic. PLoS Biol. 2011;9:e1001101. doi: 10.1371/journal.pbio.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Althoff T, Hibbs RE, Banerjee S, Gouaux E. X-ray structures of glucl in apo states reveal a gating mechanism of cys-loop receptors. Nature. 2014;512:333–337. doi: 10.1038/nature13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mowrey D, Chen Q, Liang Y, Liang J, Xu Y, Tang P. Signal transduction pathways in the pentameric ligand-gated ion channels. PLoS One. 2013;8:e64326. doi: 10.1371/journal.pone.0064326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer PJ, Harris RA, Delarue M. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun. 2013;4:1697. doi: 10.1038/ncomms2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pan J, Chen Q, Willenbring D, Mowrey D, Kong XP, Cohen A, Divito CB, Xu Y, Tang P. Structure of the pentameric ligand-gated ion channel glic bound with anesthetic ketamine. Structure. 2012;20:1463–1469. doi: 10.1016/j.str.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moraga-Cid G, Sauguet L, Huon C, Malherbe L, Girard-Blanc C, Petres S, Murail S, Taly A, Baaden M, Delarue M, Corringer PJ. Allosteric and hyperekplexic mutant phenotypes investigated on an alpha1 glycine receptor transmembrane structure. Proc Natl Acad Sci U S A. 2015;112:2865–2870. doi: 10.1073/pnas.1417864112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miller PS, Aricescu AR. Crystal structure of a human gabaa receptor. Nature. 2014;512:270–275. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, Moreau C, Li XD, Poitevin F, Vogel H, Nury H. X-ray structure of the mouse serotonin 5-ht3 receptor. Nature. 2014;512:276–281. doi: 10.1038/nature13552. [DOI] [PubMed] [Google Scholar]
- 182.Mowrey DD, Cui T, Jia Y, Ma D, Makhov AM, Zhang P, Tang P, Xu Y. Open-channel structures of the human glycine receptor alpha1 full-length transmembrane domain. Structure. 2013;21:1897–1904. doi: 10.1016/j.str.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bertaccini EJ, Wallner B, Trudell JR, Lindahl E. Modeling anesthetic binding sites within the glycine alpha one receptor based on prokaryotic ion channel templates: The problem with tm4. J Chem Inf Model. 2010;50:2248–2255. doi: 10.1021/ci100266c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Olsen RW, Li GD, Wallner M, Trudell JR, Bertaccini EJ, Lindahl E, Miller KW, Alkana RL, Davies DL. Structural models of ligand-gated ion channels: Sites of action for anesthetics and ethanol. Alcohol Clin Exp Res. 2014;38:595–603. doi: 10.1111/acer.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yu R, Hurdiss E, Greiner T, Lape R, Sivilotti L, Biggin PC. Agonist and antagonist binding in human glycine receptors. Biochemistry. 2014;53:6041–6051. doi: 10.1021/bi500815f. [DOI] [PubMed] [Google Scholar]
- 186.Shim JY, Ahn KH, Kendall DA. Molecular basis of cannabinoid cb1 receptor coupling to the g protein heterotrimer galphaibetagamma: Identification of key cb1 contacts with the c-terminal helix alpha5 of galphai. J Biol Chem. 2013;288:32449–32465. doi: 10.1074/jbc.M113.489153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Everitt AB, Seymour VA, Curmi J, Laver DR, Gage PW, Tierney ML. Protein interactions involving the gamma2 large cytoplasmic loop of gaba(a) receptors modulate conductance. FASEB J. 2009;23:4361–4369. doi: 10.1096/fj.09-137042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thal DM, Yeow RY, Schoenau C, Huber J, Tesmer JJ. Molecular mechanism of selectivity among g protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol. 2011;80:294–303. doi: 10.1124/mol.111.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Smrcka AV. G protein betagamma subunits: Central mediators of g protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Buckwalter MS, Cook SA, Davisson MT, White WF, Camper SA. A frameshift mutation in the mouse alpha 1 glycine receptor gene (glra1) results in progressive neurological symptoms and juvenile death. Hum Mol Genet. 1994;3:2025–2030. doi: 10.1093/hmg/3.11.2025. [DOI] [PubMed] [Google Scholar]
- 191.Mulhardt C, Fischer M, Gass P, Simon-Chazottes D, Guenet JL, Kuhse J, Betz H, Becker CM. The spastic mouse: Aberrant splicing of glycine receptor beta subunit mrna caused by intronic insertion of l1 element. Neuron. 1994;13:1003–1015. doi: 10.1016/0896-6273(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 192.Ryan SG, Buckwalter MS, Lynch JW, Handford CA, Segura L, Shiang R, Wasmuth JJ, Camper SA, Schofield P, O’Connell P. A missense mutation in the gene encoding the alpha 1 subunit of the inhibitory glycine receptor in the spasmodic mouse. Nat Genet. 1994;7:131–135. doi: 10.1038/ng0694-131. [DOI] [PubMed] [Google Scholar]
- 193.Koch M, Kling C, Becker CM. Increased startle responses in mice carrying mutations of glycine receptor subunit genes. Neuroreport. 1996;7:806–808. doi: 10.1097/00001756-199602290-00030. [DOI] [PubMed] [Google Scholar]
- 194.Rees MI, Lewis TM, Vafa B, Ferrie C, Corry P, Muntoni F, Jungbluth H, Stephenson JB, Kerr M, Snell RG, Schofield PR, Owen MJ. Compound heterozygosity and nonsense mutations in the alpha(1)-subunit of the inhibitory glycine receptor in hyperekplexia. Hum Genet. 2001;109:267–270. doi: 10.1007/s004390100569. [DOI] [PubMed] [Google Scholar]
- 195.Yevenes GE, Zeilhofer HU. Allosteric modulation of glycine receptors. British Journal of Pharmacology. 2011;164:224–236. doi: 10.1111/j.1476-5381.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dworakowska B, Dolowy K. Ion channels-related diseases. Acta Biochim Pol. 2000;47:685–703. [PubMed] [Google Scholar]
- 197.Schmid K, Bohmer G, Gebauer K. Glycine receptor-mediated fast synaptic inhibition in the brainstem respiratory system. Respir Physiol. 1991;84:351–361. doi: 10.1016/0034-5687(91)90129-7. [DOI] [PubMed] [Google Scholar]
- 198.Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3721–3730. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Krowicki ZK, Kapusta DR. Microinjection of glycine into the hypothalamic paraventricular nucleus produces diuresis, natriuresis, and inhibition of central sympathetic outflow. The Journal of pharmacology and experimental therapeutics. 2011;337:247–255. doi: 10.1124/jpet.110.175398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chang Q, Martin LJ. Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2815–2827. doi: 10.1523/JNEUROSCI.2475-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Söderpalm B, Löf E, Ericson M. Mechanistic studies of ethanol’s interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry. 2009;42 (Suppl 1):S87–94. doi: 10.1055/s-0029-1220690. [DOI] [PubMed] [Google Scholar]
- 202.Li J, Nie H, Bian W, Dave V, Janak PH, Ye JH. Microinjection of glycine into the ventral tegmental area selectively decreases ethanol consumption. J Pharmacol Exp Ther. 2012;341:196–204. doi: 10.1124/jpet.111.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Molander A, Söderpalm B. Accumbal strychnine-sensitive glycine receptors: An access point for ethanol to the brain reward system. Alcoholism: Clinical & Experimental Research. 2005;29:27–37. doi: 10.1097/01.alc.0000150012.09608.81. [DOI] [PubMed] [Google Scholar]
- 204.Molander A, Löf E, Stomberg R, Ericson M, Söderpalm B. Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcoholism: Clinical & Experimental Research. 2005;29:38–45. doi: 10.1097/01.alc.0000150009.78622.e0. [DOI] [PubMed] [Google Scholar]
- 205.Blednov YA, Benavidez JM, Homanics GE, Harris RA. Behavioral characterization of knockin mice with mutations m287l and q266i in the glycine receptor alpha1 subunit. J Pharmacol Exp Ther. 2012;340:317–329. doi: 10.1124/jpet.111.185124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Soderpalm B. Changes in glycine receptor subunit expression in forebrain regions of the wistar rat over development. Brain Res. 2012;1446:12–21. doi: 10.1016/j.brainres.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 207.Jonsson S, Kerekes N, Hyytia P, Ericson M, Soderpalm B. Glycine receptor expression in the forebrain of male aa/ana rats. Brain Res. 2009;1305 (Suppl):S27–36. doi: 10.1016/j.brainres.2009.09.053. [DOI] [PubMed] [Google Scholar]