Abstract
Immunoglobulin E (IgE) functions as an Fc-receptor-bound antigen sensor for mast cells and basophils, the classical effector cells of allergy. A cell-bound IgE pool is formed when monomeric IgE binds to FcεRI, the high affinity IgE Fc receptor on these cells, and minor amounts of antigen are sufficient to trigger the pro-allergic innate IgE effector axis. Additionally, FcεRI is constitutively expressed on human dendritic cells (DCs), and thus the latter cell type also receives signals via cell-bound IgE. Notably, steady-state expression of FcεRI on DCs is absent in SPF-housed mice. How DCs integrate IgE/FcεRI-derived signals into their sentinel functions as gatekeepers of immunity was therefore only recently studied with transgenic mice that phenocopy human FcεRI expression. In this review, we summarize advances in our understanding of the functions of DC-bound IgE which demonstrate that IgE-mediated activation of DCs in allergic Th2-type inflammation appears to be immune regulatory rather than pro-inflammatory.
Keywords: IgE, antigen presentation, Fc receptor, dendritic cells
1. Introduction
Discrepancies of FcεRI expression between mice and humans have been noted since almost three decades ago (Gould and Sutton, 2008; Kinet, 1999; Maurer et al., 1994). Monocytes of allergic individuals, followed by dendritic cells (DCs), were the first cell types described to constitutively express FcεRI, whereas the receptor was not found on murine counterparts. In humans, inducible expression of FcεRI has been described in the context of allergy and helminth infections on several other innate cell types such as neutrophils, eosinophils, and even epithelial cells. In mice, although constitutive FcεRI expression by DCs has not been found, receptor expression is inducible by virus infection or house dust mites (Grayson et al., 2007; Hammad et al., 2010), In this review, we will focus our discuss on the physiological roles of constitutively expressed FcεRI (Figure 1), because this type of FcεRI contributes to the generation of a DC-specific IgE pool which is present in humans and humanized mouse models in the absence of allergic inflammation at steady state (Dehlink et al., 2010; Platzer et al., 2014; Vasudev et al., 2012).
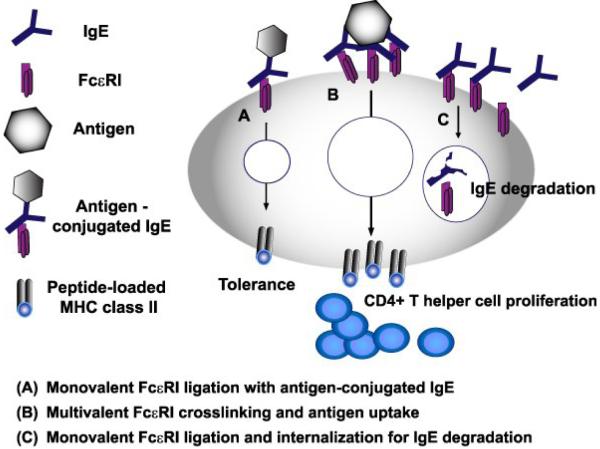
Figure 1. Functions of IgE and its high affinity receptor FcεRI on DCs.
So far DCs have been demonstrated to use IgE/FcεRI for antigen uptake via receptor crosslinking. Antigens taken up by this mechanism are shuttled efficiently into endo/lysosomal compartments for loading and presentation on MHC class II molecules. Notably, the induced proliferation and differentiation of naïve T cells is not skewed towards a specific T helper cell type. Furthermore, internalization of FcεRI after monovalent ligation of FcεRI with IgE or with an antigen-conjugated IgE fusion protein was reported. Interestingly, antigen uptake via monovalently ligated FcεRI leads to antigen-specific T cell tolerance. Whether antigen is shuttled and processed differently after mono- or multivalent FcεRI crosslinking for antigen presentation on MHC class-II molecules is not yet known.
The trimeric isoform of FcεRI, as expressed on human dendritic cells, is formed by the IgE-binding alpha chain and a dimer of the common Fc receptor gamma chain (Gould and Sutton, 2008; Kinet, 1999; Shin and Greer, 2015). DCs lack expression of the beta chain, a component of the tetrameric receptor found on mast cells and basophils, which is considered to be an amplifier for ITAM-based activating phosphorylation signals. While the signaling cascade of tetrameric FcεRI is as thoroughly studied as the T cell receptor and the B cell receptor, the DC-specific receptor signaling cascade downstream of trimeric FcεRI is not yet defined in great detail. Upon antigen-specific activation, SYK and ERK1/2 phosphorylation have been described despite the absence of the beta chain (Platzer et al., 2014), but additional components of the signaling network have yet to be determined. We also currently lack information with regards to the question of whether or not the signaling of inducible and constitutively expressed FcεRI are identical.
DCs are the most potent antigen presenting cells (APC) and function as innate gatekeepers of adaptive immune responses. These innate cells have the unique ability to induce proliferation of naive T cells and either direct them towards Th1, Th2, or Th17 immune responses or, alternatively, elicit regulatory T cell responses. During antigen presentation, DCs communicate the presence of exogenous antigen to T cells via peptide/major histocompatibility complexes (MHC) (Mellman, 2005; Mellman and Steinman, 2001). MHC class II-restricted activation of CD4+ T cells is the primary immune response to exogenous antigens (Roche and Furuta, 2015), which include the best characterized allergens for the induction of Th2-type allergic responses. To exert their APC function, DCs efficiently monitor their antigenic environment through pinocytosis and phagocytosis, as well as via surface receptors that allow for efficient antigen sampling. FcεRI on mast cells and basophils provides a unique sensitivity of IgE-mediated antigen sensing, and it has been demonstrated that DCs also constantly carry the immunoglobulin in humans and humanized mice (Platzer et al., 2014; Vasudev et al., 2012). Therefore, it is fair to conclude that FcεRI-expressing DCs use IgE for antigen sampling and that DCs are conditioned for immune surveillance by antigen sensing via surface-bound IgE in vivo at steady state and during inflammation.
FcεRI is a key element of the IgE network, an intricate group of proteins that regulate IgE-mediated immune responses (Gould and Sutton, 2008; Platzer et al., 2011). Despite exhibiting robust expression on human DCs (Dehlink et al., 2010; Maurer et al., 1996; Vasudev et al., 2012), FcεRI surface patterns had remained elusive for a significant amount of time because their detection requires antibodies that bind to IgE-loaded receptors; however, such reagents have not always been available for research purposes. While earlier studies focused on defining the expression of FcεRI in allergic individuals, it has been convincingly demonstrated that FcεRI is expressed early in life in humans without an allergic phenotype and serves to load the DCs with IgE irrespective of the allergic status of the individual. These observations challenge the perception of IgE/FcεRI-mediated antigen sensing as a strictly pro-inflammatory event, since all humans potentially receive IgE signals from their baseline polyclonal IgE pool via the DC-specific immunoglobulin fraction, yet humans do not uniformly develop allergies.
Using humanized mice to model consequences of IgE/FcεRI signals for DC function
Since healthy SPF mice do not express FcεRI constitutively, the opportunity to study physiologically relevant functions of the FcεRI-bound IgE pool depends on the availability of humanized mouse strains that express FcεRI on DCs. Murine models are considered to be humanized if the animals carry one or more functioning human genes, cells, tissues, and/or organs. Humanization is commonly achieved either by using a transgenic gene expression approach or by grafting human cells into immunodeficient mice (Ito et al., 2012; Legrand et al., 2009). Most FcεRI-humanized murine strains have been engineered to constitutively express a trimeric form of human FcεRI on DCs, while preserving FcεRI expression on mast cells and basophils. It is important to note here that the human FcεRI α-chain readily binds murine IgE and that IgE-effector functions appear to be similar whether human or murine IgE is used for mast cell activation. The high affinity of the human receptor for rodent IgE is a prerequisite for humanized animal models to yield meaningful results. In contrast, the murine alpha chain cannot interact with human IgE (Fung-Leung et al., 1996).
Several different strains of FcεRI humanized mice have been published. Two strains were independently generated in which the animals express human α-chain under its endogenous human promoter on a murine α-chain-deficient background (referred to as hpIgER-TG, (Dombrowicz et al., 1996; Fung-Leung et al., 1996)). Thus, mast cells, basophils, and DCs in hpIgER-TG mice express an identical human FcεRI alpha-chain for interactions with murine IgE. In contrast, mice that were generated using the mouse CD11c promoter for the transgene expression (IgER-TG animals, (Platzer et al., 2014; Platzer et al., 2015; Sallmann et al., 2011)) preserve the expression of endogenous murine FcεRI on mast cells and basophils.
All humanized strains constitutively express a trimeric chimeric version of FcεRI on DCs that consists of the human FcεRI α-chain and the dimer of the rodent common γ-chain for signal transduction. Attempts to express the murine α-chain constitutively in DCs have failed so far, likely because of the unique quality control mechanisms that restrict expression of functional FcεRI complexes (Fiebiger et al., 2005; Platzer and Fiebiger, 2010). Based on the published literature, it is fair to say that, despite having been generated by independent investigators with different promoters, humanized strains behave comparably. Importantly, none of the strains develop spontaneous allergy, which further justifies the hypothesis that IgE signals on DCs cannot be pro-inflammatory per definition.
An alternative approach to studying allergic responses in humanized animals was published using Nonobese diabetic (NOD)-scid-γc(−/−) mice that were reconstituted with human PBMCs from allergic donors (Weigmann et al., 2012). Primarily based on mast cell and basophil depletion experiments using a human FcεRI α-chain specific antibody, this study reached the conclusion that animals engrafted with human PBMCs are a good model for studying allergic gut inflammation. The specific role of IgE/FcεRI-bearing DCs was not addressed; thus, whether this model would also provide the opportunity to study functions of DC-bound IgE still needs to be evaluated.
IgE/FcεRI-mediated antigen presentation and priming of CD4+ T cell responses
Until recently, IgE/FcεRI-mediated antigen presentation by DCs has been considered to be a critical event during the establishment of allergy. Researchers assumed that this type of antigen uptake fosters the priming of Th2-type T cells and is one of the underlying mechanisms responsible for the uncontrolled expansion of the Th2-type effector T cell compartment. This working hypothesis was formulated based on the original description of the IgE-mediated presentation pathway with birch- and grasspollen-specific T cell clones and HLA-matched DCs from allergic donors (Maurer et al., 1996). However, within the research community, the conclusions in regards to the differentiation of naïve T cells towards effector T cells were still debated, given the fact that experimental settings in these studies were restricted to the use of established human T cell clones combined with DCs that were isolated or generated from the blood of allergic patients and thus likely already conditioned in vivo.
The recent availability of DCs from non-allergic FcεRI-humanized mice with naive antigen-specific T cells allowed a direct evaluation of the capacity of the IgE/FcεRI antigen presentation pathway to generate de novo Th2 effector cells. When T cell responses, elicited after antigen targeting via an IgE/FcεRI-crosslinking on DCs, were reevaluated, the observations that the IgE-mediated targeting pathway allows for highly efficient presentation were corroborated (Baravalle et al., 2014; Platzer et al., 2014). It was additionally shown that IgE/FcεRI-mediated antigen uptake induced T cell proliferation to otherwise sub-stimulatory antigen concentrations, supporting the unique sensitivity of the IgE-mediated presentation pathway for T cell stimulation. Importantly, in an attempt to more closely mimic physiological antigen sampling, the in vitro loading conditions for DCs used in the study by Platzer et al. (Platzer et al., 2014) were chosen in a way such that antigen was continuously available for DCs at 37°C. Thus, this experimental strategy allowed direct comparison of how DCs integrate IgE-dependent and independent antigen uptake pathways when occurring simultaneously. Based on these experiments, the IgE/FcεRI-mediated antigen focusing and subsequent presentation undoubtedly lowers the threshold for induction of primary T cell responses in vivo, where DCs constantly apply multiple antigen uptake pathways simultaneously. In line with previous reports describing enhanced Th2 cell priming after IgE/FcεRI-mediated antigen sampling, Platzer et al found that, in vitro after 3 to 4 days, the early primary T cell response was typified by Th2-type cytokine secretion, such as IL-4 and IL-13, but IFN-γ was likewise detected in the DC-T cell co-cultures. A critical assessment of the role of antigen concentrations for the induction of this mixed Th2/Th1 response led to the conclusion that the IgE-mediated antigen focusing of low antigen concentrations, rather than receptor signaling was responsible for the phenotype in early T cell cultures. This conclusion is in line with earlier demonstrations by the O’Garra laboratory, which showed that T cell responses that result from presentation of low-dose antigen typically present with a Th2-like phenotype (Boonstra et al., 2003). Importantly, intracellular cytokine staining showed that the T cells induced after IgE/FcεRI-dependent antigen sensing by the DCs failed to develop more efficiently into fully differentiated Th2-type effector T cells than those generated via IgE/FcεRI-independent antigen uptake. Since the primary T cells generated via the IgE/FcεRI pathway are not committed to differentiate into Th2-type effector cells, this pathway does not appear to be sufficient to initiate allergy.
The question of whether monovalently engaged FcεRI targets a pro-allergenic antigen presentation compartment was recently addressed using an ovalbumin-IgE fusion protein (Baravalle et al., 2014). In the absence of antigen-specific crosslinking, the IgE/FcεRI pathway was demonstrated to traffic antigens into intracellular compartments that allowed DCs to enhance their antigen presentation capacity 1000- to 2500-fold, confirming observations about the high sensitivity of the pathway for antigen recognition. However, enhanced Ag presentation by human FcεRIα-transgenic DCs did not result in enhanced Th2-type responses but, rather unexpectedly, had a tolerogenic outcome (Baravalle et al., 2014). With regards to the relevance of this pathway for the regulation of allergy in humans, it is important to note that an antigen equivalent to the monomeric IgE-antigen fusion as studied for its tolerogenic potential is unlikely to exist in vivo during an allergic response.
In summary, none of the recent antigen presentation studies with FcεRI-expressing DCs from humanized mice could formally prove that IgE-mediated antigen uptake contributes to the initiation of the allergic Th2-type T cell responses. Based on the current mechanistic evidences, it is thus unlikely for IgE/FcεRI-mediated antigen presentation by DCs to represent the causative event for the de novo generation of Th2-type effector cells during allergy. Therefore, the earlier hypothesis stating a strictly pro-allergic nature of the IgE-mediated presentation pathway needs to be revised.
Regulation of allergic tissue responses in vivo
The combined results from all antigen presentation experiments imply that it would be unlikely for IgER-Tg animals to present with a pro-allergic phenotype in vivo. Indeed, none of the published strains have been described to suffer from spontaneous onset of allergy. Evaluation of the total IgE pool demonstrated that FcεRI on DCs and monocytes is a critical contributor to the pool of cell-bound IgE and directly affects serum IgE levels.
Spontaneously produced IgE (i.e. pre-immune or natural IgE) is a constituent of the polyclonal humoral immune response at steady state in laboratory animals (Gould and Sutton, 2008). Similar to healthy non-allergic humans, DCs from non-allergic SPF-housed IgER-TG mice carry IgE at the cell surface, and, at steady state, serum IgE was significantly lower in the humanized strains than in wild type littermate controls (Platzer et al., 2014). When Th2-type sensitization protocols were employed, an increase of antigen-specific IgE was readily detected and resulted in an expansion of the DC-bound IgE pool in IgER-TG. Importantly, wild type animals still did not exhibit a cell-bound IgE-pool on DCs, demonstrating that even during allergic inflammation DC in wild type mice cannot respond to IgE-mediated signals. Interestingly, serum immune IgE levels, which are a common readout for the severity of allergy in clinical practice, appeared lower in the IgER humanized strains. Greer et al. recently described a mechanism for clearance of IgE that involves monovalent ligation of FcεRI by IgE on DCs and monocytes, resulting in internalization and degradation of the immunoglobulin in endo/lysosomal compartments (Greer et al., 2014). It is fair to speculate that this IgE-clearance pathway described for monovalently ligated FcεRI (Greer et al., 2014) is operative in IgER-TG mice, providing a possible explanation for the reduced serum IgE levels of IgER-TG mice when compared to WT controls. If IgE clearance by DCs is a major mechanism underlying diminished allergic inflammation, sensitization levels of mast cells and basophils should be reduced as well. However, experiments analyzing the severity of systemic anaphylaxis showed that mast cells and basophils were comparably sensitized in humanized and non-humanized strains, arguing against clearance of IgE by DCs as a key pathway to inhibit allergic responses in vivo (Platzer et al., 2014). An alternative, but not exclusive, explanation for the reduction of serum IgE in the IgER-TG animals would be the segregation of soluble IgE to the receptor-bound fraction on DCs.
Importantly, reduced Th2-type tissue inflammation was found in three independent allergy models. Food allergy experiments with a humanized strain that lacks FcεRI on mast cells and basophils but expresses the IgE receptor selectively on DCs support the conclusion that the DC-bound IgE pool alone is executing immune regulatory functions. It was demonstrated that IgE-crosslinking dampens the production of Th2-promoting mediators by DCs which appeared to exhibit large-scale effects on Th2-type tissue inflammation, including impaired recruitment of mast cells to sites of allergic tissue inflammation. Furthermore, although not directly addressed in the study, the tolerizing pathway downstream of antigen-targeting via non-crosslinked FcεRI might contribute via antigen-specific deletion of T cell clones to the reduction in allergic tissue inflammation in IgER-TGs animals. In summary, the overall diminished allergic phenotype argues in support of a DC-mediated IgE-antigen-specific feedback loop with the purpose to regulate the overshooting allergic response.
Finally, it is warranted to consider the immune protective characteristics of the DC-specific IgE pool in the context of an earlier report about exacerbation of Th2-type airway inflammation in IgER-TGs mice (Sallmann et al., 2011). The most likely explanation for the controversial findings derives from the fact that the earlier study preceded the current knowledge of microbial stimuli as critical regulators of IgE-mediated immune responses (Cahenzli et al., 2013; Stefka et al., 2014). Therefore, the study was not conducted in a littermate controlled setting and the microbiome, which has been shown to have a significant effect on immune responses, can most easily explain the divergent observations.
Conclusions and perspectives
Recent studies on the functions of DC-bound IgE revealed several so far unappreciated immune regulatory pathways of antigen sensing via IgE/FcεRI. With regards to antigen presentation, it is important to reiterate that the DC-bound IgE pool does not promote expansion of Th2-type effector T cells; therefore, this pathway of antigen recognition cannot causatively fuel the Th2-perpetuating immune machinery in allergy. Irrespectively, in vivo experiments with FcεRI-humanized mice, combined with epidemiologic data on allergy prevalence in humans, clearly show that the DC-derived IgE-signals are overridden during the development of allergy. Perhaps the local allergic tissue environment can reverse the regulatory character of IgE-mediated DC activation? This issue will require more research attention in the future. Currently, it is fair to conclude that a more detailed understanding of the mechanisms underlying DC-specific IgE signals have the potential to serve as the basis for novel therapeutic strategies for the prevention and treatment of allergy by exploiting the regulatory features of the human IgE network.
Highlights.
Constitutive expression of trimeric FcεRI on DCs modulates the serum and the cell-bound IgE pool at steady state and during allergy
DC-bound IgE does not lead to a spontaneous allergic phenotype
Fc-epsilon-RI on DCs contributes to IgE clearance, which is considered anti-inflammatory
Antigen-specific crosslinking of IgE/FcεRI on DCs induces immune regulatory responses that diminish tissue allergy in vivo
Acknowledgements
We acknowledge the large number of investigators in the field whose primary publications could not be cited due to space limitations. Funding for this work was received from NIH: grants K01DK093597 (to B.P) and RO1AI075037 (to E.F.). The work of the Fiebiger laboratory is further supported by the Harvard Digestive Diseases Center Grant P30DK034854 and an unrestricted gift from the Mead Johnson Company.
Abbreviations
- APC
antigen presenting cells
- DCs
dendritic cells
- FcεRI
the high affinity IgE Fc receptor
- IgE
Immunoglobulin E
- SPF
specific pathogen free
- MHC
major histocompatibility complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baravalle G, Greer AM, LaFlam TN, Shin JS. Antigen-conjugated human IgE induces antigen-specific T cell tolerance in a humanized mouse model. J Immunol. 2014;192:3280–3288. doi: 10.4049/jimmunol.1301751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O'Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. The Journal of experimental medicine. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell host & microbe. 2013;14:559–570. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlink E, Baker AH, Yen E, Nurko S, Fiebiger E. Relationships between levels of serum IgE, cell-bound IgE, and IgE-receptors on peripheral blood cells in a pediatric population. PloS one. 2010;5:e12204. doi: 10.1371/journal.pone.0012204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, Koller BH. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- Fiebiger E, Tortorella D, Jouvin MH, Kinet JP, Ploegh HL. Cotranslational endoplasmic reticulum assembly of Fc{varepsilon}RI controls the formation of functional IgE-binding receptors. The Journal of experimental medicine. 2005;201:267–277. doi: 10.1084/jem.20041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, Panakos JA, Chourmouzis E, Liu FT, Lau CY. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. The Journal of experimental medicine. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature reviews Immunology. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, Battaile JT, Alevy Y, Yan L, Agapov E. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. The Journal of experimental medicine. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer AM, Wu N, Putnam AL, Woodruff PG, Wolters P, Kinet JP, Shin JS. Serum IgE clearance is facilitated by human FcepsilonRI internalization. The Journal of clinical investigation. 2014;124:1187–1198. doi: 10.1172/JCI68964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. The Journal of experimental medicine. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cellular & molecular immunology. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annual review of immunology. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de Jong Y, Deng H, Di Santo JP. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell host & microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Fiebiger E, Ebner C, Reininger B, Fischer GF, Wichlas S, Jouvin M-H, Schmitt-Egenolf M, Kraft D, Kinet J-P. Peripheral blood dendritic cells express FceRI as a complex composed of FceRIa- and FceRIg-chains and can use this receptor for IgE-mediated allergen presentation. J Immunol. 1996;157:607–613. [PubMed] [Google Scholar]
- Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin M-H, Kilgus O, Kinet J-P, Stingl G. Expression of functional high affinity immunoglobulin E receptors (FceRI) on monocytes of atopic individuals. J Exp Med. 1994;179:745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Antigen processing and presentation by dendritic cells: cell biological mechanisms. Adv Exp Med Biol. 2005;560:63–67. doi: 10.1007/0-387-24180-9_9. [DOI] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Platzer B, Baker K, Vera MP, Singer K, Panduro M, Lexmond WS, Turner D, Vargas SO, Kinet JP, Maurer D. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal immunology. 2014 doi: 10.1038/mi.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer B, Elpek KG, Cremasco V, Baker K, Stout MM, Schultz C, Dehlink E, Shade KT, Anthony RM, Blumberg RS. IgE/FcepsilonRI-Mediated Antigen Cross-Presentation by Dendritic Cells Enhances Anti-Tumor Immune Responses. Cell reports. 2015 doi: 10.1016/j.celrep.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer B, Fiebiger E. The signal peptide of the IgE receptor alpha-chain prevents surface expression of an immunoreceptor tyrosine-based activation motif-free receptor pool. The Journal of biological chemistry. 2010;285:15314–15323.. doi: 10.1074/jbc.M110.104281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer B, Ruiter F, van der Mee J, Fiebiger E. Soluble IgE receptors--elements of the IgE network. Immunol Lett. 2011;141:36–44. doi: 10.1016/j.imlet.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nature reviews Immunology. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmann E, Reininger B, Brandt S, Duschek N, Hoflehner E, Garner-Spitzer E, Platzer B, Dehlink E, Hammer M, Holcmann M. High-Affinity IgE Receptors on Dendritic Cells Exacerbate Th2-Dependent Inflammation. J Immunol. 2011 doi: 10.4049/jimmunol.1003392. [DOI] [PubMed] [Google Scholar]
- Shin JS, Greer AM. The role of FcepsilonRI expressed in dendritic cells and monocytes. Cellular and molecular life sciences : CMLS. 2015 doi: 10.1007/s00018-015-1870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR. Commensal bacteria protect against food allergen sensitization. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudev M, Cheung DS, Pincsak H, Li SH, Yan K, Simpson P, Dasu T, Grayson MH. Expression of high-affinity IgE receptor on human peripheral blood dendritic cells in children. PloS one. 2012;7:e32556.. doi: 10.1371/journal.pone.0032556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann B, Schughart N, Wiebe C, Sudowe S, Lehr HA, Jonuleit H, Vogel L, Becker C, Neurath MF, Grabbe S. Allergen-induced IgE-dependent gut inflammation in a human PBMC-engrafted murine model of allergy. The Journal of allergy and clinical immunology. 2012;129:1126–1135. doi: 10.1016/j.jaci.2011.11.036. [DOI] [PubMed] [Google Scholar]