Abstract
Background
The worldwide epidemic of type 2 diabetes requires effective prevention. We determined the long-term extent of beneficial effects of lifestyle intervention and metformin on diabetes prevention, originally demonstrated during the 3-year Diabetes Prevention Program (DPP), and whether diabetes-associated microvascular complications are reduced.
Methods
The DPP (1996–2001) was a randomized trial comparing an intensive lifestyle intervention or masked metformin with placebo in a cohort selected to be at very high risk to develop diabetes. All participants were offered lifestyle training at DPP-end. 2776 (88%) of the surviving DPP cohort were followed in the DPP Outcome Study (DPPOS 2002–2013) and analyzed by intention-to-treat based on original DPP assignment. During DPPOS, the lifestyle group was offered lifestyle reinforcement semi-annually and the metformin group received unmasked metformin.
Findings
During 15 years of average follow-up, lifestyle intervention and metformin reduced diabetes incidence rates by 27% (p<0.0001) and 18% (p=0.001), respectively, compared with the placebo group, with declining between group differences over time. At year 15, the cumulative incidences of diabetes were 55, 56 and 62%, respectively. The prevalences at study-end of the aggregate microvascular outcome, composed of nephropathy, neuropathy, and retinopathy, were not significantly different among the treatment groups (11–13%) in the total cohort. However, in women (n=1887) lifestyle intervention was associated with a lower prevalence (8.7%) than in the placebo (11%) and metformin (11.2%) groups, with 21% (p=0.03) and 22% (p=0.02) reductions with lifestyle compared with placebo and metformin, respectively. Compared with participants who progressed to diabetes, those who didn’t progress had a 28% lower prevalence of microvascular complications (p<0.0001).
Interpretation
Lifestyle intervention or metformin significantly reduce diabetes development over 15 years. There were no overall differences in the aggregate microvascular outcome among treatment groups; however, those who did not progress to diabetes had a lower prevalence of microvascular complications than those who progressed.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Introduction
The most recent US data show that 12.3% of the adult population has diabetes, with 1.7 million new cases diagnosed per year.1 The vast majority has type 2 diabetes and the cost of diabetes and prediabetes was estimated to be $322 billion in 2012.2 When the Diabetes Prevention Program (DPP: 1996–2001) was planned in the mid-1990s,3 the goal was to determine whether a behavioural lifestyle intervention program designed to address the two major “environmental” risk factors for type 2 diabetes, overweight/obesity and sedentary lifestyle, or the most commonly used drug to treat diabetes, metformin, would reduce the development of the disease in a high-risk population. The large beneficial short-term effects demonstrated in DPP4 and in other studies5,6 prompted numerous translation projects internationally.7
The ultimate worth of diabetes prevention is in the reduction of long-term morbidity or mortality, compared with waiting for the disease to develop and then treating it. The DPP Outcomes Study (DPPOS: 2002–2013) was designed to examine the effects of the original DPP interventions, beyond the 3 years average treatment during DPP, on the further development of diabetes and on microvascular complications.8 The limited 3-year duration of the DPP precluded an understanding of longer-term effects of the interventions on diabetes prevention or on the development of complications associated with diabetes. Understanding the time course of the development of complications has been hampered in prior studies by a poor ascertainment of the actual time of diabetes onset, as the prevalence of complications is related to diabetes duration and exposure to hyperglycemia. Longer follow-up of the DPP cohort was necessary to determine whether preventing or delaying diabetes onset would reduce the development of complications.
We now report the main outcomes of the DPPOS, focusing on the long-term prevention of diabetes and the effects of the original, randomly assigned DPP interventions and the development of diabetes on microvascular complications over a total mean follow-up of 15 years.
Methods
Participants
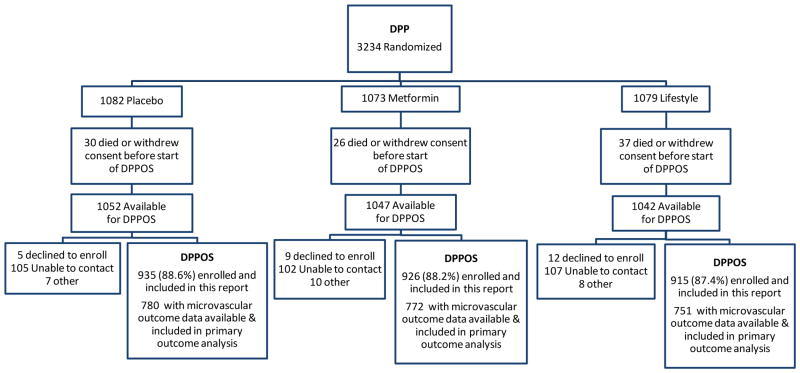
The Diabetes Prevention Program was a randomized controlled clinical trial, conducted in 27 centers across the U.S. All surviving members (n= 3149) of the three original DPP treatment arms (placebo, metformin, and intensive lifestyle intervention) who had not withdrawn consent, and regardless of diabetes status, were invited to join DPPOS and 2776 (88%) joined (Figure 1). The characteristics of the DPPOS cohort at DPP baseline, DPP-end, and at DPPOS-end are shown in Table 1. A similar fraction of each DPP treatment arm joined DPPOS.8 Moreover, there were no significant differences in the baseline characteristics of those who joined DPPOS and those who did not.8 At DPP-end, there were significant differences in several clinical characteristics among the treatment arms, reflecting the salutary effects of the DPP interventions.4 Specifically, the prevalence and duration of diabetes were different among the three treatment groups. Written informed consent was obtained from all participants and the studies were approved by each clinical center’s institutional review board. An independent data safety monitoring board, appointed by the sponsoring National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), oversaw the study.
Figure 1.
Diabetes Prevention Program Outcomes Study consort diagram
Table 1.
Characteristics of DPPOS Study Cohort
| DPP baseline | DPP End (2001)+ | DPPOS End (2013)# | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Total 2776 |
PLBO 935 |
MET 926 |
ILS 915 |
PLBO 780 |
MET 772 |
ILS 751 |
Non-DM 1226 |
DM 1550 |
| Age (y) | 51 (10) | 54 (10) | 54 (10) | 54 (11) | 65 (9.5) | 66 (9.5)† | 66 (10.5)† | 67 (10) | 65 (10)† |
| Sex (% women) | 68 | 69 | 67 | 68 | 69 | 67 | 68 | 68 | 68 |
| Race (%) | |||||||||
| Caucasian | 54 | 54 | 56 | 54 | 54 | 56 | 54 | 58 | 51† |
| African American | 20 | 21 | 21 | 19 | 21 | 21 | 19 | 17 | 23 |
| Hispanic American | 15 | 16 | 15 | 15 | 16 | 15 | 15 | 15 | 16 |
| American Indian | 6 | 4 | 3 | 6 | 6 | 5 | 6 | 6 | 6 |
| Asian Am/PI | 5 | 6 | 5 | 6 | 4 | 3 | 6 | 4 | 5 |
| Weight (kg) | 94 (20) | 94 (20) | 92 (21)† | 89 (21)†‡ | 91 (20.3) | 90 919.2) | 89 (19.4)† | 87 (18) | 92 (20)† |
| BMI (kg/m2) | 34 (7) | 34 (7) | 33 (7)† | 32 (7)†‡ | 33 (7) | 32 (7)† | 32 (7)† | 31 (6) | 33 (7)† |
| Type 2 diabetes (%) | 0 | 30 | 21† | 14†‡ | 60 | 55† | 52† | 0 | 100 |
| Diabetes duration (y) | |||||||||
| Total cohort& | 0 | 0.5 (0.9) | 0.3 (0.8)† | 0.2 (0.6)†‡ | 6.5 (6.0) | 5.5 (5.8)† | 4.8 (5.3)†‡ | 0 | 5.6 (5.8) |
| Participants who developed diabetes | 0 | 1.5 (1.0) | 1.5 (1.0) | 1.3 (1.1)† | 10.3 (4.3) | 9.7 (4.3) | 8.6 (4.3)†‡ | 0 | 9.6 (4.4) |
| FPG (mmol/L) | 5.9 (0.5) | 6.2 (1.1) | 5.9 (0.8)† | 5.9 (0.8)† | 6.8 (1.9) | 6.6 (1.9)† | 6.8 (2.0)‡ | 5.7 (0.5) | 7.4 (2.3)† |
| HbA1c (%) | |||||||||
| Total cohort | 5.9 (0.5) | 6.1 (0.7) | 6.0 (0.5)† | 5.9 (0.5)†‡ | 6.3 (1.2) | 6.1 (1.1)† | 6.2 (1.2)‡ | 5.6 (0.4) | 6.6 (1.4)† |
| Participants who developed diabetes | -- | 6.6 (0.9) | 6.3 (0.7)† | 6.4 (0.7)† | 6.7 (1.4) | 6.5 (1.3) | 6.7 (1.4)‡ | -- | 6.6 (1.4)† |
Characteristics of the 2776 DPPOS participants at DPP baseline, DPP End, and DPPOS end. Results are means (standard deviations) of continuous variables or percents as indicated. Asian-Am- Asian American, PI-Pacific Islanders
DPP end: last study annual visit; Diabetes assessed through masked phase.
DPPOS End is the final annual visit and the numbers reflect the number of subjects who had microvascular measurements included in primary outcome anaysis.
Diabetes duration for participants who remain non-diabetic calculated as 0 years
p<0.05 lifestyle or metformin vs. placebo or diabetes (DM) vs. non-DM;
p<0.05 vs. metformin. Statistical comparisons based on chi-square tests for categorical variables and t-tests for continous varaibles for ILS or MET vs. PLBO.
Procedures
DPP compared metformin at 850 mg twice per day or an individual behavioural lifestyle intervention program with placebo. The lifestyle program included a 16-session curriculum with individual sessions aimed at achieving a 7% weight loss through a healthy low-fat, low calorie diet and 150 minutes per week of moderate intensity physical activity. After the first 24 weeks, individual and group sessions were used to reinforce the lifestyle modification behaviors.9 The metformin and placebo treatment groups were double-masked but, for practical reasons, the lifestyle group was not.3,9 If diabetes was diagnosed by oral glucose tolerance test (OGTT) or fasting plasma glucose (FPG), and confirmed, participants and their health care providers were informed. Study metformin or placebo was still provided until hyperglycemia worsened to FPG≥7.78 mmol/L at which time study drugs were discontinued and diabetes management transferred to the participant’s own health care provider. At DPP-end, following a brief metformin and placebo washout study10, the placebo and metformin groups were subsequently unmasked to their treatment assignment and placebo stopped. Given the clear evidence of benefit of the lifestyle intervention, all participants were offered the lifestyle intervention in a group format during a one-year bridge period.11
During DPPOS, as in DPP, metformin was provided to the group originally assigned to it; however, metformin was now unmasked. The same transition from study drug and care to the patient’s care provider occurred as during DPP except that study metformin was continued until HbA1c was > 7%. Maintenance group lifestyle sessions, offered quarterly to all DPPOS participants, reinforced the basic lifestyle content and the weight loss and physical activity goals. In addition to the maintenance sessions, original lifestyle participants were offered supplementary group programs, reinforcing behavioural self-management activities, and an individual lifestyle “check-in”, each twice per year.
Outcomes
As in the DPP, the development of diabetes was determined with 75 gram OGTT performed annually and FPG tests every 6 months.3 For diagnosis of diabetes, FPG≥7.0 mmol/L or 2-hour levels ≥11.1 mmol/L had to be confirmed by a repeat test within 6 weeks. HbA1c levels were measured annually by high performance liquid chromatography but were not used to diagnose diabetes.
The aggregate microvascular disease outcome was defined by protocol to include the following three components. Nephropathy was defined as albuminuria ≥30 mg/g creatinine in a spot urine collection on 2 consecutive tests or an estimated GFR <45 ml/min/1.73 m2, based on annual serum creatinine using the CKD-EPI equation12 on 2 consecutive tests, or renal failure (end-stage renal disease, dialysis or transplantation). Participants taking antihypertensive drugs at the final assessment who did not meet albuminuria or estimated GFR criteria at that time were considered to have reached the nephropathy outcome if the nephropathy criteria were met at 2 consecutive past visits. Retinopathy was diagnosed on 7-field stereoscopic fundus photography as an ETDRS grade 20 or greater13 in either eye or treatment of retinopathy with laser or intravitreal injections. The presence of neuropathy was based on loss of light touch sensation (<8 of 10 applications detected on the dorsum of the great toe) measured with a 10-gram Semmes-Weinstein monofilament.14 Kidney function and neuropathy were measured annually during DPPOS, while retinopathy was measured during the final year of DPPOS (2012–13). Adverse events were documented at semi-annual visits using a standardized questionnaire
Statistical Analysis
The outcomes reported in these analyses are based on data entered as of January 2, 2014 for the 2776 DPP participants who enrolled in DPPOS. The primary DPPOS analytic outcomes, defined a priori, included the further development of diabetes, as defined above, and the prevalence of microvascular disease analyzed during the final year of DPPOS by intention-to-treat. Time to diabetes compared each intervention with placebo on a modified product-limit life-table distribution with a log-rank test statistic.15 Follow-up was “censored” at their last visit if diabetes had not developed.
As specified in the protocol, the aggregate microvascular outcomes were analyzed with the global test using general estimating equation models (GEE) 16 to estimate average prevalence and account for correlations among the 3 components. The study was powered based on the global test17, 18 which provided 91% power to detect a 25% reduction in microvascular complications due to an intervention with 2-sided α=0.025, from a projected placebo group average prevalence of 12.1%. Each of the 2 pairwise comparisons (ILS vs PLBO and MET vs PLBO) were set at α=0.025 to maintain an overall α=0.05 for multiple comparisons using Bonferroni adjustment. All secondary analyses were not adjusted for multiple comparisons and are nominally significant at α=0.05. A detailed explanation of the analyses is in the supplement (Supplement 2). GEE models were also used to assess differences in intervention effects using interactions across pre-specified subgroups that included sex, age, and racial-ethnic groups. Fixed-effects models with the assumption of normally distributed errors19 were used to assess differences in body weight over time among the three groups. An important issue in evaluating any treatment comparison is the amount of missing data. DPP and DPPOS generally have had low rates of missing data. The completion rates (87% of those enrolled) of the microvascular components did not differ among the three treatment groups and missing data were assumed to be missing at random. The global test17 used to test the composite microangiopathy outcome is less affected by incomplete ascertainment of one or more of its components than a traditional collapsed test. All analyses were conducted using SAS version 9.3.
Role of the Funding Source
The sponsor of this study, NIDDK, NIH, was represented on the Steering Committee and played a part in the study design, how the study was conducted, and publications. The sponsor was not represented in the writing group. All authors in the writing group had access to the data.
Results
Weight loss and Adherence to Metformin
The cohort continued to maintain differences in weight loss among the three treatment groups until approximately 4 years after randomization (Supplementary Figure 1). Subsequently, weight regain in the lifestyle group and sustained long-term weight loss with metformin led to almost identical weight loss in these two groups compared with the original placebo group. Weight in the placebo group fell slightly after the introduction of the group lifestyle intervention during the bridge between DPP and DPPOS and began to fall from DPP levels after 8 to 9 years of combined follow-up. Adherence to metformin in the metformin group, measured by pill count and defined as taking at least 80% of the pills assigned, was approximately 70% during DPP and fell to approximately 55%, and remained very stable, during DPPOS. By 15 years after randomization, 37% of the placebo and 29% of the lifestyle treatment groups were treated with metformin by their health-care providers, almost all in the setting of diabetes development. The mean exposure to metformin, including study and non-study treatment, remained widely separated during the combined DPP and DPPOS with 10.7, 2.3 and 1.7 metformin-years, in the metformin, placebo and lifestyle treatment arms, respectively.
Incidence of Diabetes
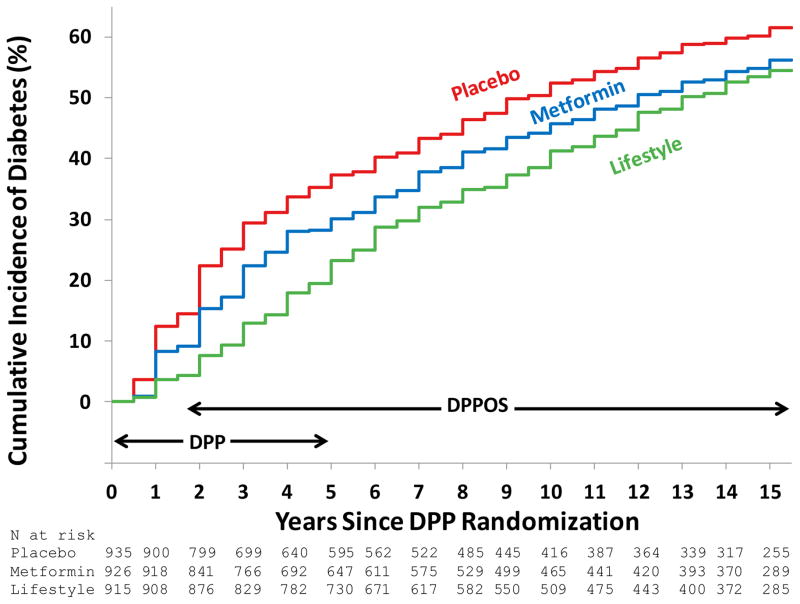
Among the 2776 DPPOS participants, 611 developed diabetes during DPP and 939 developed diabetes during DPPOS, compared with 1226 who did not develop diabetes during the entire study period. Diabetes incidence rates after an average follow-up of 15 years were significantly lower by 27 and 18% with lifestyle intervention (hazard ratio, HR [95%CI] 0.73[0.65, 0.83] and metformin (HR [95%CI] 0.82[0.72, 0.93], respectively, compared with the placebo group (Table 1 and Figure 2). These reductions are lower than the 58 and 31%, respectively, observed after the first 2.8 years of DPP2 (58% and 32% in the subgroup of the DPP cohort that continued in DPPOS). The reduced differences during DPPOS reflect a reduction in the incidence of diabetes in the placebo and metformin groups to the approximately 5% per year rate observed in the lifestyle group, which remained relatively constant during the entire DPP/DPPOS. The number of cases of diabetes and cumulative incidence calculated from the lifetables by year 15 in the placebo, metformin and lifestyle groups were 560 (62%), 499 (56%) and 480 (55%), respectively. The diabetes outcome did not include 9 persons who had a diabetes diagnosis “trigger” without a confirmation visit (because of death or refusal). Including these as diabetes cases in a sensitivity analysis had no effect on the results (details not shown). The mean duration of diabetes in those who developed diabetes was 10.3, 9.7, and 8.6 years, respectively (Table 1). The incidence rates over time among the three treatment arms were similar for men and women (Supplementary Figure 2), with no significant interaction between treatment and sex.
Figure 2.
Cumulative incidence of diabetes by treatment group among the 2776 DPPOS participants. The DPP and DPPOS periods, and the overlap between them, are indicated. Over the entire study, the incidence rates for participants were 7.0%, 5.7% and 5.2% per year for placebo, metformin and lifestyle, respectively, 27% and 18% lower for lifestyle and metformin vs. placebo, respectively (p<0.0001 and p= 0.001). The difference between lifestyle and metformin was not significant (p=0.10). The number at risk at each time point is listed by treatment group.
Microvascular Outcomes
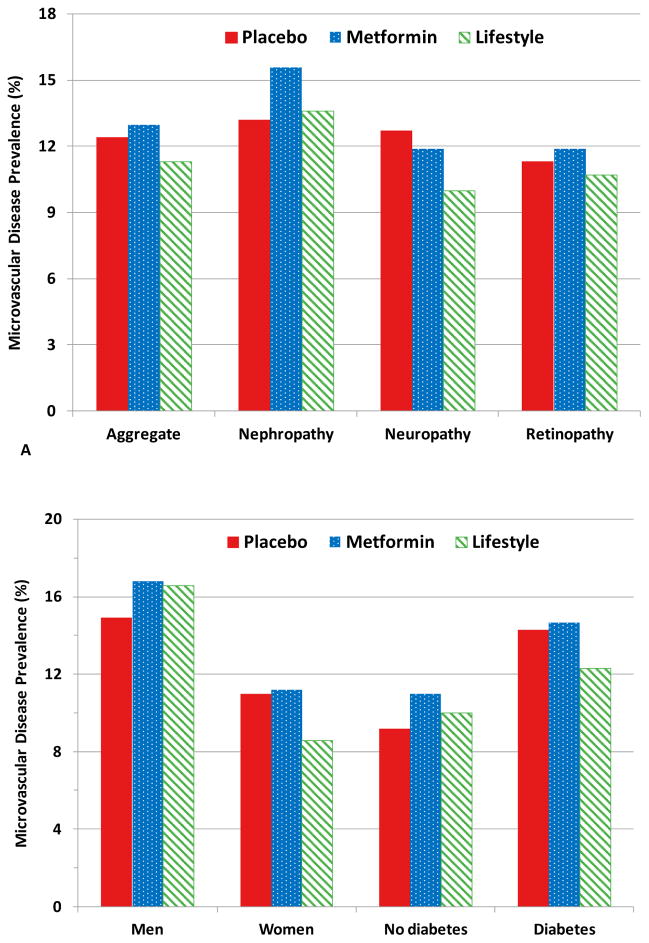
The average prevalence of the microvascular outcomes at DPPOS-end did not differ significantly among the three treatment groups (Figure 3a, Table 2), despite the group differences in diabetes incidence. The aggregate microvascular outcome prevalence was approximately 50% higher in men than women (Figure 3b), and increased with increasing age, but was similar across racial/ethnic groups (Table 2). The pre-specified sex-specific analysis showed a significant sex-by-lifestyle vs. placebo treatment interaction, with a benefit only in women (Table 2). This sex interaction was not seen with metformin which did not reduce microvascular disease in either sex (Table 2). In women but not in men, lifestyle intervention reduced microvascular disease significantly by 21% (relative risk 0.79) compared with placebo and 22% (relative risk 0.78) compared with metformin (Table 2, Figure 3b). There were no differences in the treatment effects on aggregate microvascular complications in other pre-specified subgroups defined by age and race or ethnicity, except that Hispanic Americans had a significantly lower microvascular disease prevalence in the lifestyle group compared with metformin and placebo (relative risk 0.42 and 0.43, respectively) (Table 2). Participants who did not develop diabetes during DPP/DPPOS had a statistically significant 28% lower (relative risk 0.72) aggregate microvascular disease prevalence than those who did develop diabetes for all treatment groups combined, with similar patterns in each treatment group (Table 2, Figure 3b).
Figure 3.
Prevalence of aggregate microvascular complication and individual microvascular components by DPPOS end (2012–2013). Placebo (red solid), metformin (blue dotted pattern), lifestyle (green diagonal stripes). 3A. By treatment group. None of the treatment group differences achieved statistical significance for the aggregate or the microvascular components. The aggregate microvascular is expressed as the average prevalence among the 3 components of nephropathy, retinopathy and neuropathy. 3B. By pre-specified subgroups according to sex and diabetes status. Prevalence was greater in men than in women in each of the three treatment groups. In women, the prevalence of the aggregate microvascular outcome was 22% (relative risk 0.78, p=0.02) lower in the lifestyle intervention group compared with the metformin group and 21% (relative risk 0.79, p=0.03) lower than in the placebo group. There were no significant differences among the treatment groups in men. The prevalence of microvascular disease in participants who did not develop diabetes was 28% lower than that in those who developed diabetes in a treatment group adjusted model (p<0.0001).
Table 2.
Model-based prevalence and relative risk of the aggregate microvascular outcome at DPPOS end among treatment groups, stratified by baseline DPP characteristics and diabetes status at the time of the microvascular outcomes assessment.
| Pooled relative risk (95% CI) among subgroup(s)† | Model Based Prevalence (95% CI) of Aggregate Microvascular Disease | Relative risk# (95% CI) for the aggregate microvascular disease | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Stratification Variable | Subgroups | Placebo | Metformin | Lifestyle | Lifestyle vs Metformin | Lifestyle vs Placebo | Metformin vs Placebo | |
|
| ||||||||
| Overall | -- | 12.4%(11.1, 13.8) | 13.0% (11.7, 14.5) | 11.3% (10.1, 12.7) | 0.87 (0.74, 1.02) | 0.91 (0.78, 1.07) | 1.05 (0.91, 1.23) | |
|
| ||||||||
| p = 0.08 | p = 0.28 | p = 0.50 | ||||||
| Sex | Female | 1.0 | 11.0% (9.6, 12.6) | 11.2% (9.7, 12.9) | 8.7% (7.4, 10.2) | 0.78 (0.62, 0.96) | 0.79 (0.64, 0.98) | 1.02 (0.84, 1.24) |
| Male | 1.58 (1.38, 1.80) | 15.1% (12.5, 18.2) | 16.8% (14.3, 19.7) | 16.6% (14.0, 19.7) | 0.99 (0.78, 1.25) | 1.10 (0.86, 1.42) | 1.11 (0.87, 1.43) | |
| p < 0.0001 | p = 0.14 | p = 0.04 | p = 0.55 | |||||
| Age at randomization (y) | 25–44 | 1.0 | 8.9% (6.8, 11.7) | 7.7% (5.7, 10.5) | 6.9% (4.9, 9.5) | 0.89 (0.57, 1.39) | 0.77 (0.50, 1.18) | 0.87 (0.58, 1.30) |
| 45–59 | 1.40 (1.15, 1.70) | 11.8% (10.2, 13.7%) | 11.7% (10.0, 13.7) | 9.5% (7.9, 11.5) | 0.82 (0.64, 1.04) | 0.81 (0.64, 1.03) | 0.99 (0.80, 1.23) | |
| ≥60 | 2.40 (1.94, 2.94) | 16.9% (13.5, 21.3) | 22.2% (18.8, 26.3) | 17.1% (13.9, 21.1) | 0.77 (0.59, 1.01) | 1.01 (0.74, 1.38) | 1.31 (0.99, 1.74) | |
| p < 0.0001 | p = 0.86 | p = 0.47 | p = 0.18 | |||||
| Race / ethnicity‡ | Caucasian | 1.0 | 13.5% (11.7, 15.6) | 14.3% (12.4, 16.4) | 13.0% (11.1, 15.1) | 0.91 (0.74, 1.12) | 0.96 (0.78, 1.19) | 1.06 (0.87, 1.29) |
| African Am | 0.85 (0.71, 1.0) | 12.5% (9.9, 15.7) | 11.6% (8.8, 15.2) | 10.2% (7.5, 13.9) | 0.89 (0.59, 1.34) | 0.82 (0.56, 1.21) | 0.93 (0.65, 1.33) | |
| Hispanic | 0.63 (0.51, 0.79) | 10.5% (7.7, 14.2) | 10.7% (7.7, 14.9) | 4.5% (2.2, 8.9) | 0.42 (0.19, 0.90) | 0.43 (0.20, 0.91) | 1.02 (0.65, 1.60) | |
| Asian | 0.83 (0.59, 1.17) | 9.7% (4.7, 19.9) | 11.8% (6.1, 22.6) | 11.0% (6.6, 18.4) | 0.94 (0.41, 2.15) | 1.14 (0.47, 2.78) | 1.22 (0.46, 3.23) | |
| p < 0.0001 | p = 0.29 | p = 0.20 | p = 0.92 | |||||
| Baseline BMI (kg/m2) | 22 to <30 | 1.0 | 10.1% (8.1, 12.6) | 12.8% (10.6, 15.4) | 11.7% (9.7, 14.2) | 0.91 (0.70, 1.20) | 1.15 (0.86, 1.54) | 1.26 (0.94, 1.69) |
| 30 to <35 | 1.0 (0.85, 1.19) | 11.1% (8.9, 13.8) | 13.6% (11.3, 16.3) | 10.3% (8.2, 13.0) | 0.76 (0.57, 1.02) | 0.93 (0.68, 1.27) | 1.23 (0.92, 1.63) | |
| ≥35 | 1.12 (0.96, 1.31) | 14.9% (12.8, 17.3) | 12.8% (10.6, 15.5) | 11.4% (9.2, 14.0) | 0.89 (0.67, 1.17) | 0.76 (0.59, 0.99) | 0.86 (0.68, 1.10) | |
| p = 0.27 | p = 0.62 | p = 0.12 | p = 0.08 | |||||
| Baseline fasting glucose (mmol/L) | 5.3–6.0 | 1.0 | 11.7% (10.2, 13.4) | 11.9% (10.4, 13.7) | 9.8% (8.4, 11.5) | 0.82 (0.67, 1.01) | 0.84 (0.68, 1.04) | 1.02 (0.84, 1.24) |
| 6.1–6.9 | 1.29 (1.13, 1.47) | 13.5% (11.3, 16.1) | 15.1% (12.7, 18.1) | 14.0% (11.6, 16.8) | 0.92 (0.72, 1.19) | 1.04 (0.80, 1.34) | 1.12 (0.87, 1.44) | |
| p = 0.0002 | p = 0.34 | p = 0.16 | p = 0.65 | |||||
| Baseline 2h glucose (mmo/L) | 7.8–8.5 | 1.0 | 11.8% (9.7, 14.4) | 13.2% (10.9, 16.1) | 10.8% (8.8, 13.3) | 0.82 (0.62, 1.08) | 0.92 (0.69, 1.22) | 1.12 (0.85, 1.48) |
| 8.6–9.5 | 0.97 (0.83, 1.14) | 11.5% (9.5, 14.0) | 12.5% (10.3, 15.1) | 11.0% (9.0, 13.5) | 0.89 (0.67, 1.17) | 0.96 (0.72, 1.27) | 1.08 (0.82, 1.42) | |
| 9.6–11.0 | 1.08 (0.92, 1.26) | 13.6% (11.4, 16.2) | 13.2% (11.1, 15.8) | 12.0% (9.7, 14.7) | 0.91 (0.69, 1.19) | 0.88 (0.67, 1.16) | 0.97 (0.76, 1.25) | |
| p = 0.42 | p = 0.86 | p = 0.93 | p = 0.74 | |||||
| Baseline HbA1c (%) | 3.2–<5.7 | 1.0 | 8.3% (6.4, 10.7) | 9.8% (7.8, 12.4) | 9.8% (7.7, 12.6) | 1.00 (0.71, 1.40) | 1.19 (0.83, 1.69) | 1.19 (0.84, 1.67) |
| 5.7–<6.0 | 1.33 (1.10, 1.60) | 12.2% (9.9, 15.2) | 14.4% (11.8, 17.5) | 11.0% (8.7, 13.9) | 0.77 (0.56, 1.04) | 0.90 (0.65, 1.24) | 1.17 (0.87, 1.57) | |
| 6.0–8.5 | 1.46 (1.24, 1.73) | 14.7% (12.7, 17.0) | 14.2% (12.1, 16.6) | 12.3% (10.4, 14.5) | 0.86 (0.69, 1.09) | 0.83 (0.67, 1.04) | 0.96 (0.78, 1.19) | |
| p < 0.0001 | p = 0.52 | p = 0.25 | p = 0.44 | |||||
| Diabetes at assessment | DM | 1.0 | 14.4% (12.7, 16.4) | 14.5% (12.6, 16.6) | 12.3% (10.5, 14.4) | 0.85 (0.69, 1.04) | 0.85 (0.70, 1.04) | 1.00 (0.83, 1.21) |
| Non-DM | 0.72 (0.63, 0.83) | 8.6% (7.0, 10.7) | 10.9% (9.1, 13.0) | 10.0% (8.3, 12.0) | 0.92 (0.71, 1.18) | 1.16 (0.88, 1.54) | 1.27 (0.96, 1.67) | |
| p < 0.0001 | p = 0.61 | p = 0.07 | p = 0.17 | |||||
All values expressed as point estimates (95% confidence intervals) and bolded values, p<0.05, for relative risks among subgroups and treatment groups.
The number of events among American Indian participants was too small to allow models to be evaluated.
Differences among subgroups. Since there were no significant heterogeneity among treatment groups based on the interaction term for subgroup*treatment (with age, BMI, and fasting glucose, 2 hour glucose and HbA1c levels analyzed as continuous variables), the relative risk among subgroups were assessed using treatment group-adjusted estimates of the aggregate prevalence. The reference group is noted with relative risk of 1.0. P-value reflects the overall test of significance among the subgroups.
Relative risk were compared across subgroups using an interaction for treatment effect x subgroup and resulting p-value is listed for each subgroup and treatment effect.
For lifestyle vs placebo, the relative risks differed significantly across subgroups defined by sex (p=0.04) and diabetes status (p=0.07).
For metformin vs placebo, the relative risks differed across baseline BMI (p=0.08).
Glycemia and microvascular disease
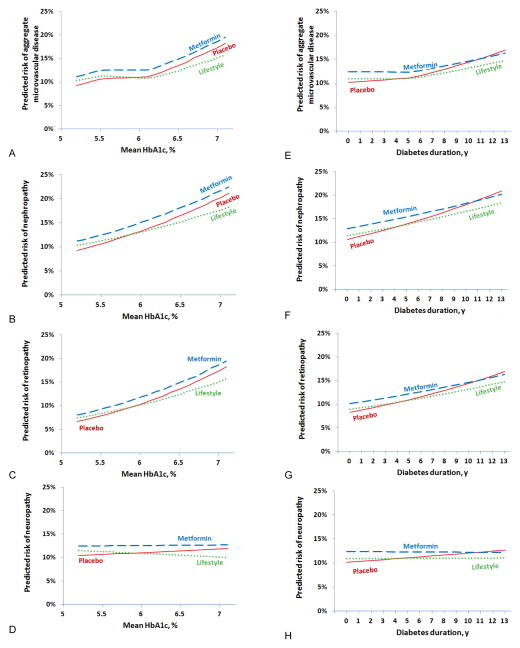
Higher levels of baseline fasting glucose and HbA1c were associated with higher prevalence of microvascular complications (Table 2). During the combined DPP/DPPOS, mean HbA1c levels, while statistically different between treatment groups, were generally low with mean levels of 6.1, 6.0 and 5.9% in the placebo, metformin and lifestyle groups, respectively (Table 1). Mean glycemia over the observation interval was higher in participants who developed diabetes compared with those who had not, and differed by treatment assignment (Table 1). Diabetes duration and mean HbA1c levels were correlated with retinopathy and nephropathy, but not with neuropathy (Figure 4). The pre-specified aggregate microvascular outcome exhibited a nonlinear relationship with HbA1c, with a suggestion of an inflection point at HbA1c ~6.2%. In a post-hoc analysis among those whose latest HbA1c was ≥6.5%, representing ~25% of the cohort, lifestyle showed significant reductions compared with placebo in the aggregate microvascular outcome (relative risk 0.59), retinopathy (relative risk 0.51), and neuropathy (relative risk 0.39) (Supplementary Figure 3), with no significant differences between the metformin and placebo groups.
Figure 4.
The role of HbA1c and diabetes duration on microvascular disease and its components were assessed in separate GEE models which included 2 interactions terms for treatment group*glycemia measure and microvascular component*glycemia measure. The interactions of HbA1c with the individual microvascular components were significantly different (p <0.0001). HbA1c (panels a–d) was associated with nephropathy and retinopathy (both p<0.0001) but not neuropathy (p=0.69). The interactions of diabetes duration also differed among the microvascular components (panel e–h) (p=0.01) with longer diabetes duration associated with nephropathy and retinopathy (both p<0.0001) but not with neuropathy (p=0.57).
Figure 4A. Aggregate
Figure 4B. Nephropathy
Figure 4C. Retinopathy
Figure 4D. Neuropathy
Figure 4E. Aggregate
Figure 4F. Nephropathy
Figure 4G. Retinopathy
Figure 4H. Neuropathy
Adverse events
Sprains or fractures requiring medical attention were predefined as a non-severe adverse event of special interest owing to the increased activity and exercise in the lifestyle intervention. The lifestyle intervention was not associated with an increase in risk for these events compared with the placebo or metformin groups (3.7, 4.3 and 4.1 events per 100 patient-years, respectively). No cases of lactic acidosis were reported in approximately 40,000 patient years of follow-up. There were no significant differences among the three treatment groups in any of the other severe adverse events collected over the course of the study.
Discussion
DPPOS has shown durable effects of the original DPP interventions on the cumulative incidence of diabetes, with the majority of the prevention or delay having occurred during the first three years of DPP, but with between-group differences persisting over the subsequent 12 years of follow-up. Despite the difference in diabetes development with the lifestyle and metformin interventions and a significantly lower prevalence of the aggregate microvascular outcome in those who remained free of diabetes compared with those who developed diabetes, there was no significant difference in the aggregate microvascular outcome among the three treatment groups. There was a significant reduction with lifestyle compared with placebo or metformin in the prespecified analysis in women.
The similar annual incidence of diabetes among the three treatment groups during DPPOS, with the original metformin and placebo groups achieving similar rates as the lifestyle group, suggests that either offering group lifestyle intervention to all of the participants was effective in reducing the further development of diabetes or that a vulnerable subset of the cohort was exhausted during DPP.20 Despite the reduction in relative efficacy of the interventions over time, the very long-term reduction in diabetes development remains substantial.
The ultimate benefits of prevention/delay of diabetes, or of earlier intervention during the course of dysglycemia, include potential reduction of the development of long-term complications, which cause major morbidity and mortality and contribute the largest fraction of total diabetes costs.21 The current results do not demonstrate a difference in the effect of the three interventions on the prevalence of aggregate microvascular outcome in the total cohort 15 years after randomization. However, the men had a substantially higher prevalence of microvascular complications than the women, while in women lifestyle intervention was associated with a 21% reduction in microvascular outcomes compared with placebo. These sex-specific findings, for which we have no explanation, are of great interest. Some22, 23, but not all,24 previous studies in type 1 and type 2 diabetes have found sex differences in incidence or prevalence of diabetic nephropathy or retinopathy. The recently reported Look AHEAD clinical trial of a lifestyle intervention patterned after DPP in adults with type 2 diabetes, showed sex effects and interactions similar to the present DPPOS findings for nephropathy, with a treatment benefit seen only in women.23
Also notable is the 28% lower prevalence of the aggregate microvascular outcome in those who did not develop diabetes compared with those who did. The lack of an effect of the active treatments on the microvascular outcome in the total cohort, even though diabetes development was significantly reduced, may be owing to limited power, the gender disparity in the effects described above, or the small separation in HbA1c levels among the three treatment groups.
The prevalence of individual microvascular complications was related (retinopathy, nephropathy) or unrelated (neuropathy) to the degree of hyperglycemia present in our population, with the aggregate microvascular complications more strongly related to glycemia in the HbA1c range greater than 6.5% than in the range less than 6.5%. Notably, the post hoc analysis of the cohort using this HbA1c threshold showed a significantly reduced microvascular disease among those treated with lifestyle (Figure 6), consistent with our observation of differences by diabetes status in year 15.
The strong relationship between duration of diabetes and HbA1c levels with the prevalence of complications in this study and in others25 suggests that further follow-up may reveal a differential effect of the original DPP interventions on complications. The Da Qing study reported benefits of lifestyle interventions on diabetes prevention, retinopathy and cardiovascular and all-cause mortality after 23 years of follow-up, with mortality benefits seen only in women.26
Benefits of the lifestyle intervention and metformin in the DPPOS cohort, in addition to the prevention or delay of diabetes observed to date, include a reduction in CVD risk factors27 and metabolic syndrome,28 reduced prevalence of lower urinary tract symptoms associated with obesity and diabetes,29 and improved quality of life.30 An economic analysis after 10 years, comparing all out-of-study medical costs with the costs of the interventions, revealed that metformin was cost-saving and lifestyle intervention was cost-effective.31 Longer-term follow-up of the DPP/DPPOS cohort is planned and should shed light on the effects of the interventions on cardiovascular disease and mortality and provide a more complete assessment of the economic impact of diabetes prevention.
Although the DPPOS had many strengths including a highly engaged cohort and consistent follow-up with standardized interventions and complete data collection, it had limitations as well. Limitations included the therapeutic cross-over through the offering of lifestyle change instruction to all three groups during the one-year bridge period at the end of DPP. Moreover, the application of the lifestyle intervention after the first 24 weeks of DPP and during DPPOS was less intensive, likely contributing to the weight regain in that group. The use of metformin by participants in the DPP lifestyle and placebo groups may also have diminished the relative effects of metformin; however, the difference in metformin exposure between the original metformin group and the other two groups remained substantial. These factors may have reduced the magnitude of putative microvascular benefits among the three original treatment groups. Although the factors above may temper our conclusions drawn from DPPOS, the intention-to-treat analyses, based on the original DPP treatment assignments, support the validity of our conclusions.
Another limitation may be the combination of three different microvascular outcomes in the aggregate outcome, two of which are objective measurements and masked and one of which, neuropathy measured by monofilament, is more subjective and less sensitive for early neuropathy. The combination of these outcomes was based on their being in the same pathogenic stream for diabetes complications and to improve power. Finally, the generalizability of the findings of any clinical trial, with selected populations and protocol-driven interventions often implemented in academic clinical centers, may be questioned. However, the DPP-protocol has been translated successfully into numerous settings7 and for the first time in several decades the annual incidence of diabetes in the US has begun to fall32, suggesting that the DPP findings are generalizable.
In conclusion, DPPOS has demonstrated very-long term effects of lifestyle intervention and metformin to reduce the incidence of diabetes in a very high-risk population. By 15 years after enrollment, however, the majority of persons in each treatment group had developed diabetes. Therefore more effective interventions for diabetes prevention are still needed. The two interventions did not reduce the prevalence of aggregate microvascular complications compared with placebo after a total of 15 years of follow-up in the total cohort, although there was a significant 21–22% reduction with lifestyle compared with placebo and metformin in the women. Participants who did not develop diabetes had a 28% lower prevalence of the aggregate microvascular complication compared with participants who did. This result supports the importance of diabetes prevention.
Supplementary Material
Acknowledgments
The Research Group gratefully acknowledges the commitment and dedication of the DPP and DPPOS participants.
During the DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data (U01 DK048489). The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and later the Clinical Translational Science Centers Programs of the National Center for Advancing Translational Sciences, NIH, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
Industry contributors have had no role in the planning or conduct of DPP/DPPOS but Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, and Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and DPPOS.
Footnotes
Contributors
The Writing Group for this paper included: D. M. Nathan, M.D. (Chair), E. Barrett-Connor, M.D., J.P. Crandall, M.D., S. L. Edelstein, Sc.M., R.B. Goldberg, M.D., E. S. Horton, M.D., W.C. Knowler, M.D., Dr.P.H., K. J. Mather, M.D., T. J. Orchard, M.D., X. Pi-Sunyer, M.D., D. Schade, M.D., M. Temprosa, Ph.D.
The Writing Group had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Accessed August 21, 2014]; http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
- 2.Dall TW, Yang W, Halder P, Pang B, Massoudi M, Wintfeld N, Semilla AP, Franz J, Hogan PF. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37:3172. doi: 10.2337/dc14-1036. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Prevention Program Research Group. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 6.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 7.Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med. 2011;1:460–91. doi: 10.1007/s13142-011-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. 2009;14:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diabetes Prevention Program Research Group. The diabetes prevention program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group. Effect of withdrawal from metformin on the development of diabetes in the Diabetes Prevention Program. Diabetes Care. 2003;26:977–980. doi: 10.2337/diacare.26.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venditti EM, Bray GA, Carrion-Petersen ML, Delahanty LM, Edelstein SL, Hamman RF, Hoskin MA, Knowler WC, Ma Y Diabetes Prevention Program Research Group. First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes. 2008;32:1537–44. doi: 10.1038/ijo.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. Chronic Kidney Disease Epidemiology Collaboration: A new equation to estimate glomerular filtration rate. Ann Int Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS Report Number 12. Ophthalmology. 1991;83:823–33. [PubMed] [Google Scholar]
- 14.Olaleye D, Perkins B, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Research and Clinical Practice. 2001;54:115–128. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 15.Lachin JM. Biostatistical methods: the assessment of relative risks. Chapter 9 New York: John Wiley; 2000. [Google Scholar]
- 16.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 17.Lefkopoulou M, Ryan L. Global tests for multiple binary outcomes. Biometrics. 1993;49:975–988. [PubMed] [Google Scholar]
- 18.Pan W. Sample size and power Calculations with Correlated Binary Data. Controlled Clinical Trials. 2001;22:211–227. doi: 10.1016/s0197-2456(01)00131-3. [DOI] [PubMed] [Google Scholar]
- 19.Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press; 1994. [Google Scholar]
- 20.Hamman RF, Horton E, Barrett-Connor E, Bray GA, Christophi C, Crandall J, Florez J, Fowler S, Goldberg R, Kahn SE, Knowler WC, Lachin J, Murphy M, Venditti E. Factors Affecting the Decline in Incidence of Diabetes in the Diabetes Prevention Program Outcome Study (DPPOS) Diabetes. 2015;64:989–98. doi: 10.2337/db14-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harjutsalo V, Maric C, Forsblom C, Thorn L, Waden J, Groop PH FinnDiane Study Group. Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia. 2011;54:1992–99. doi: 10.1007/s00125-011-2144-2. [DOI] [PubMed] [Google Scholar]
- 23.Look AHEAD Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomized clinical trial. Lancet Diabetes and Endocrinology. 2014;2:801–809. doi: 10.1016/S2213-8587(14)70156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson RG, Kunzelman CL, Pettitt DJ, Saad MF, Bennett PH, Knowler WC. Albuminuria in type 2 (non-insulin-dependent) diabetes and impaired glucose tolerance in Pima Indians. Diabetologia. 1989;32:870–876. doi: 10.1007/BF00297452. [DOI] [PubMed] [Google Scholar]
- 25.Stratton IM, Adler AI, Neil AW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Homan RR on behalf of the UK Prospective Diabetes Study Group. . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J, Engelgau MM, Li H, Roglic G, Hu Y, Bennett PH. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing diabetes prevention study: A 23-year follow-up study. Lancet Diabetes and Endocrinology. 2014;2:474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 27.DPP Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors. Diabetes Care. 2005;28:888–894. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diabetes Prevention Program Research Group. Metformin and intensive lifestyle intervention on the prevention of the metabolic syndrome. Ann Int Med. 2005;142:611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown JS, Wing R, Barrett-Connor E, Nyberg LM, Kusek JW, Orchard TJ, Ma Y, Vittinghoff E, Kanaya AM Diabetes Prevention Program Research Group. Lifestyle intervention is associated with lower prevalence of urinary incontinence: The Diabetes Prevention Program. Diabetes Care. 2006;29:385–90. doi: 10.2337/diacare.29.02.06.dc05-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Florez H, Pan Q, Ackermann RT, Marrero DG, Barrett-Connor E, Delahanty L, Kriska A, Saudek CD, Goldberg RB, Rubin RR Diabetes Prevention Program Research Group. Impact of lifestyle intervention and metformin on health-related quality of life: the diabetes prevention program randomized trial. J Gen Intern Med. 2012;27:1594–601. doi: 10.1007/s11606-012-2122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention. Diabetes Care. 2012;35(4):723–730. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–26. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.