Abstract
Objectives
In contrast to peptic strictures, clinically significant strictures in patients with EoE may be subtle and go unrecognized at the time of endoscopy. We aimed to identify how often stricture was identified by endoscopy as compared to contrast esophagram.
Methods
We retrospectively reviewed esophagram and endoscopy examinations of all EoE patients with esophageal stricture seen at a tertiary care pediatric hospital over a 6 year period who had both procedures completed within a 3-month time frame. Medical charts were reviewed for clinicopathologic information including age, duration of symptoms, histology and treatment.
Results
Twenty-two children with EoE associated stricture completed both esophagram and endoscopic assessments. Esophageal strictures were identified by esophagram, and not endoscopy, in 55% of these children. Patients with stricture identified at esophagram alone had a shorter duration of symptoms (2.1 years duration vs. 5.4 years duration, p = 0.03) than the group identified by endoscopy. Pre-operative radiographic identification of a stricture was associated with dilation more often being performed.
Conclusions
Esophagram is a valuable test to assess esophageal anatomy in children with complicated EoE. Esophagram may be able to detect subtle fibrostenosis earlier in the natural history of the disease than endoscopy.
Keywords: esophageal stricture, children, eosinophil, fibrosis
Introduction
Esophageal strictures in children are uncommon and typically represent manifestations of peptic or surgical injury. Peptic strictures are classically associated with focal, distal esophageal narrowing. Endoscopy and fluoroscopic esophagram are the most commonly utilized tests for detection of peptic related strictures with endoscopy offering the capacity to provide concurrent therapeutic dilation. Eosinophilic esophagitis (EoE) is also associated with esophageal strictures, but differences exist between pathogenetic mechanisms when compared to those related to peptic injury.
While the exact pathogenesis of EoE associated stricture is not known, basic and translational studies suggest that longstanding eosinophilic inflammation contributes to upregulation of transforming growth factor beta resulting in subepithelial fibrosis, smooth muscle contraction, pathogenic remodeling and the symptoms of food impaction or dysphagia.1-5 Limited studies examining esophageal wall thickness localize inflammation to not only the epithelial surface but also to the deeper muscular layers and throughout the length of the esophagus.6, 7 Taken together, we speculate that the pathogenesis and clinical detection of esophageal luminal narrowing in pediatric EoE is different than previously accepted paradigms associated with gastroesophageal reflux disease (GERD). As opposed to the distinct, visible and isolated narrowing due to chronic surface injury observed with peptic stricture, inflammation of the mucosa and muscularis in EoE may lead to submucosal fibrosis of longitudinal portions of the esophageal wall with subtle segmental luminal narrowing.
In this regard, our clinical experience with pediatric EoE patients suggests that esophageal luminal narrowing can be subtle and therefore missed at endoscopy but may be captured during fluoroscopicesophagram. An incident that initiated this concern was a consultation for a 6-year old female with EoE with dysphagia and recurrent food impaction. Her past evaluation included three upper endoscopies, none of which detected esophageal stricture. As her dysphagia persisted despite treatment with topical steroids, she underwent a fourth endoscopy. Esophageal mucosal appearance and histological analysis were normal. Following endoscopy, an esophagram revealed circumferential narrowing of the mid esophagus. Following dilation, a longitudinal rent was identified in the mid esophagus and her dysphagia resolved.
While a number of studies have identified radiological and endoscopic features of esophageal remodeling in EoE, none have compared these tests in assessing for esophageal narrowing in pediatric EoE.8-16 Based on our clinical experience, we questioned how often esophageal stricture is not visualized at the time of endoscopy but is detected by esophagram.
Methods
A retrospective review of children with EoE was undertaken to compare detection of esophageal stricture by fluoroscopic esophagram and endoscopic visualization.
Study Population
Following IRB approval, the electronic medical record (EPIC) was searched to identify patients evaluated at Children's Hospital Colorado between July 2008 and July 2014 with a diagnosis code (ICD-9) and/or procedure code of EoE and esophageal stricture, esophageal foreign body, esophageal dilation or endoscopic foreign body removal. This cohort was then screened to identify those who had undergone both upper endoscopy and fluoroscopic esophagram within 3 months of each other. We chose a 3-month interval in order to limit the impact of treatment on esophagram and endoscopic findings; previous work suggests fibrostenosis may be diminished within three months of topical steroid treatment.16-20
Initial chart review confirmed the diagnosis of EoE using published Consensus Recommendation criteria: symptoms related to esophageal dysfunction and biopsy showing >15 eosinophils per high power field (hpf) with other causes ruled out.21, 22 All subjects confirmed to have EoE either failed proton pump inhibitor trial or had a normal pH-impedance study. Patients without confirmed EoE were excluded.
Subject Characterization
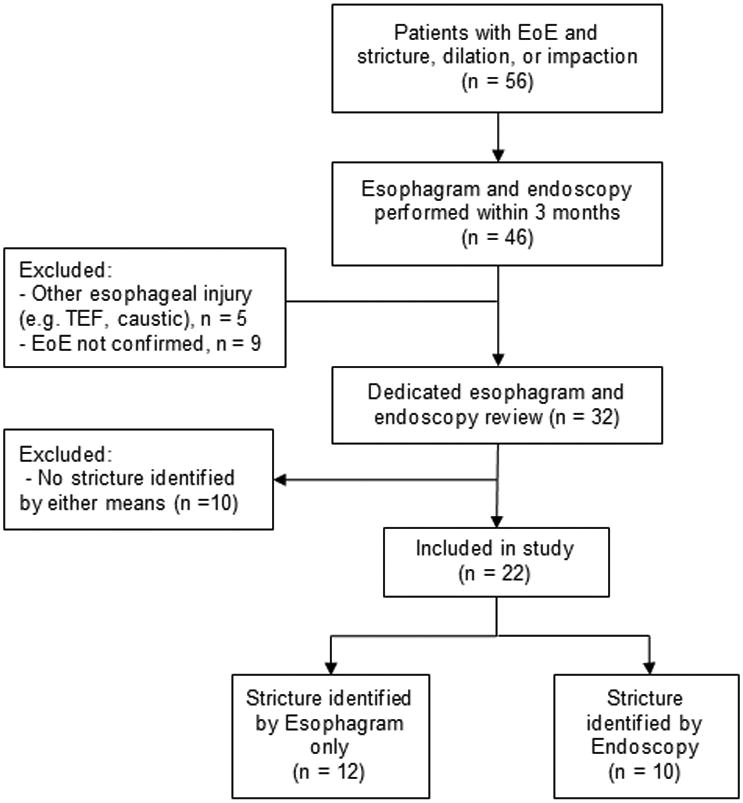
Following review of fluoroscopic images and endoscopic reports (see below), patients were divided into the following two groups, 1) stricture identified by esophagram only and 2) stricture identified by endoscopy (Figure 1).
Figure 1. Flow diagram of subject identification.
Endoscopic Assessment of Esophageal Lumen
Esophagogastroduodenscopy was performed by board certified pediatric gastroenterologists using age appropriate Olympus endoscopes. Endoscopic images were immediately obtained of the proximal and distal esophagus following esophageal insufflation. Endoscopic images and procedure reports were independently reviewed by two gastroenterologists (CMK, PM) who were blinded to esophagram results. Discrepancies were resolved by consensus. Endoscopic detection of esophageal stricture was defined as; 1) resistant to passage of a 9 mm scope, 2) luminal narrowing precluding passage of a 9 mm endoscope, 3) fixed isolated stricture or 4) longitudinal rent identified after the passage of the endoscope (crepe paper esophagus). If one or more of these were present in the endoscopic report, the patient was characterized as having stricture. The presence of other endoscopic features associated with EoE including exudates, rings, edema and furrows was also noted. 23
Fluoroscopic Assessment of Esophageal Lumen
Fluoroscopic esophagram was performed with single-contrast technique and low-density barium suspension (Liquid E-Zpaque, E-ZEM Canada, incorporated) by board certified pediatric radiologists on digital pulse fluoroscopic equipment (Philips Easy Diagnost Eleva and Toshiba 4580 G12G/EPSRF). Esophagrams were reviewed independently by two pediatric radiologists (MPS, LZF) with discrepancies resolved by consensus. All studies were reviewed for the following; 1) diffuse small caliber esophagus (age matched controls searched for if necessary to confirm), 2) focal luminal narrowing characterized as proximal (cervical to T2 level), mid (T3-T6) or lower (T7-thoracolumbar junction) esophagus relative to proximal or distal esophagus, 3) mucosal irregularity characterized as uneven surfaces, rings or discrete ulcer, 4) proximal esophageal dilatation, 5) delayed passage of an 13mm barium pill when performed (EZ disk barium sulfate tablet, manufactured for E-ZEM by Confab laboratories incorporated), 6) disordered esophageal motility (mentioned in the report rendered at the time of interpretation), 7) Schatzki ring and 8) hiatal hernia. Measurements of luminal diameter were precluded due to variable fluoroscopic magnification settings and height of the fluoroscopy tower above the table. Radiologists were blinded in their retrospective review to subject's clinical course and gastroenterologists' review of endoscopic findings.
Statistical analysis
Data analysis was completed using SPSS 22 to calculate mean, standard deviations and test for significance. Wilcoxon rank-sum was used to compare continuous variables and Chi-Square and Fisher's exact test to compare proportions as appropriate16. A p-value of <0.05 was considered significant.
Results
Characterization of children with EoE associated esophageal stricture
We identified 56 EoE patients with a diagnosis of esophageal stricture, esophageal foreign body or esophageal dilation. Following exclusions, 22 EoE patients had undergone esophagram and endoscopy within three months of each other (Figure 1). Esophageal strictures were identified by esophagram only (n=12), endoscopy only (n=1) or both studies (n=9). Clinical features of all children with strictures are shown in Table 1. Study subjects were predominantly Caucasian (86.4%) and male (81.8%), had an average age of 13.3 years, and presented most commonly with dysphagia (63.6%). The majority had history of food impaction (68.2%). Sixteen (64%) were on proton pump inhibitor. The majority of subjects were naïve to EoE directed treatment (77%). Five subjects were on EoE treatment at time of endoscopy (4 with swallowed topical steroids alone, 1 on dual therapy of swallowed topical steroids and 4 food elimination diet) for an average duration of 8.4 months (± 5.1 months, range 1 – 14 months). One subject was on treatment with swallowed steroids between endoscopy and subsequent esophagram. Therefore, a total of six subjects were on EoE directed treatment between studies; for these 6 subjects, studies were performed 1, 2, 3, 68, 75, and 83 days apart.
Table 1.
Characterization and fluoroscopic and endoscopic findings of EoE patients with esophageal stricture, stratified by stricture identification.
| Total (n=22) | Esophagram Only (n=12) | Endoscopy (n=10) | |
|---|---|---|---|
| Age, y (sd; min – max) | 13.3 (4.8; 2.2-20.8) | 11.4 (5.5; 2.2-17.0) | 15.6 (2.6; 12.0-20.8) |
| Male, n (%) | 18 (81.8) | 10 (83.3) | 8 (80) |
| White, n (%) | 19 (86.4) | 9 (75) | 10 (100) |
| Sx Duration, y (sd; min – max) | 3.6 (3.4; 0.0-12.7) | 2.1 (2.1; 0.0-6.9) | 5.4 (3.7; 1.1-12.7) |
| Presenting Symptom, n (%) | |||
| Dysphagia | 14 (63.6) | 6 (50) | 8 (80) |
| Impaction | 5 (22.7) | 3 (25) | 2 (20) |
| Pain | 1 (8.7) | 1 (8) | 0 (0) |
| Vomiting | 2 (9.1) | 2 (17) | 0 (0) |
| History of Impaction, n (%) | 15 (68.2) | 6 (50) | 6 (60) |
|
| |||
| Days between studies, (sd; min – max) | 30 (24; -74-83) | 24 (23; -75-37) | 41 (26; 3-83) |
|
| |||
| Fluoroscopy pre endoscopy, n (%) | 16 (73) | 9 (75) | 7 (70) |
|
| |||
| Endoscopic Findings, n (%) | |||
| Exudate | 13 (59) | 7 (58) | 6 (60) |
| Rings | 12 (53) | 6 (50) | 6 (60) |
| Edema | 16 (73) | 8 (67) | 8 (80) |
| Furrows | 20 (91) | 11 (92) | 9 (90) |
| Crepe | 4 (18) | 0 (0) | 4 (40) |
| Dilation Performed | 6 (27) | 1 (8) | 5 (50) |
| Resulting mucosal split | 4 | 1 | 3 |
| Peak eos/hpf (sd; min – max) | 56 (32.2; 0-120) | 58 (37.7; 0-120) | 54 (26.1; 0-90) |
|
| |||
| Fluoroscopic Findings | |||
| Stricture | 21 (95.5) | 12 (100) | 9 (90) |
| Stricture Location | |||
| Proximal | 7 | 4 | 3 |
| Mid | 4 | 3 | 1 |
| Distal | 8 | 4 | 4 |
| Panesophageal | 2 | 1 | 1 |
| Mucosal Irregularity | 20 (90.9) | 11 (92) | 9 (90) |
| Schatzki Ring | 5 (22.7) | 2 (17) | 3 (30) |
| Hiatal Hernia | 6 (27.3) | 3 (25) | 3 (30) |
| Reflux | 5 (23) | 3 (25) | 2 (20) |
Fluoroscopic Findings
The primary indication for esophagram was dysphagia (n = 15, 68%) followed by impaction (n = 5, 23%). On retrospective review of esophagram, stricture was identified in the proximal, midor distal esophagus and two subjects had pan esophageal narrowing. Ninety six percent of esophagrams had additional EoE associated abnormalities including mucosal irregularity (90.9%), hiatal hernia (27.3%) and Schatzki ring (22.7%) (Table 1). Of the 4patients who received a barium pill, 3 (75%) showed impaction or delayed pill transit. Figure 2 provides examples of fluoroscopic findings.
Figure 2.
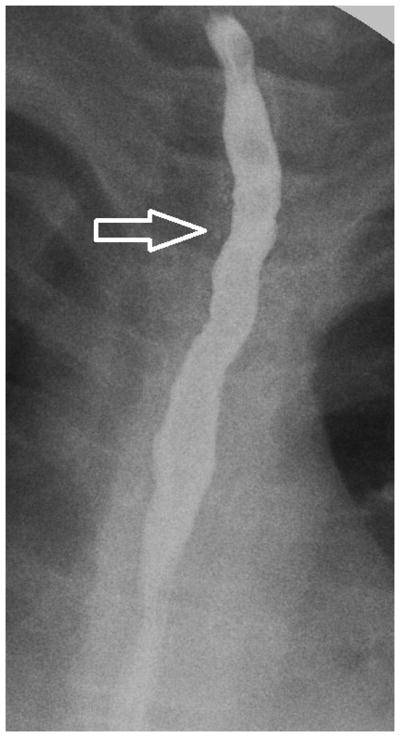
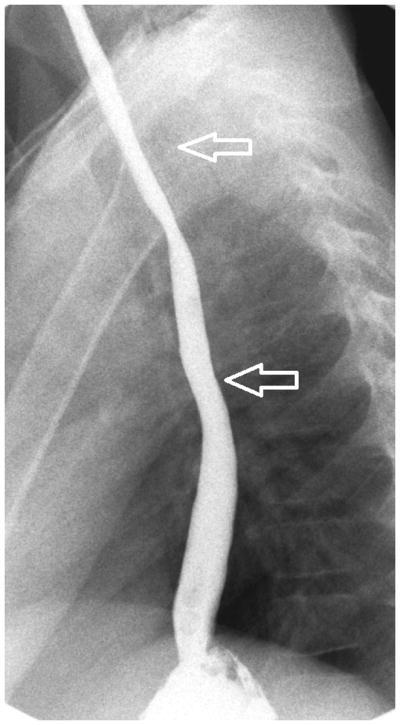
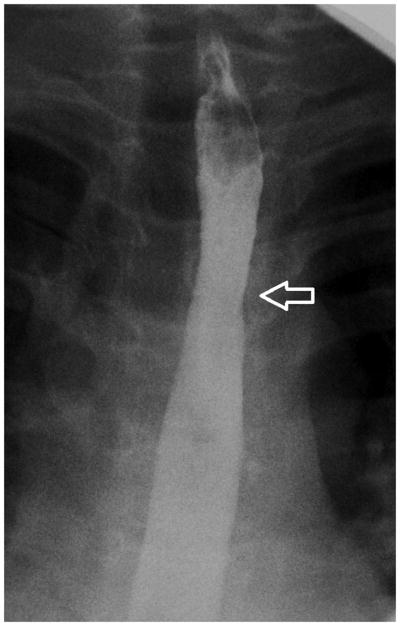
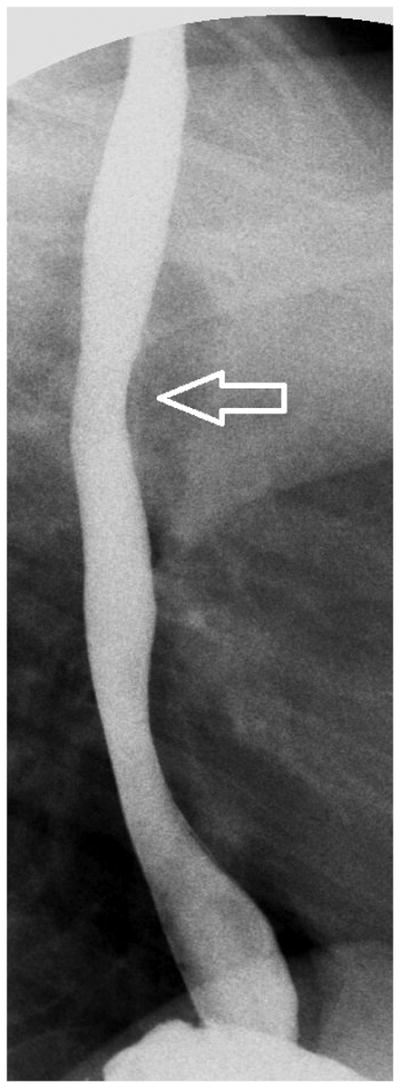
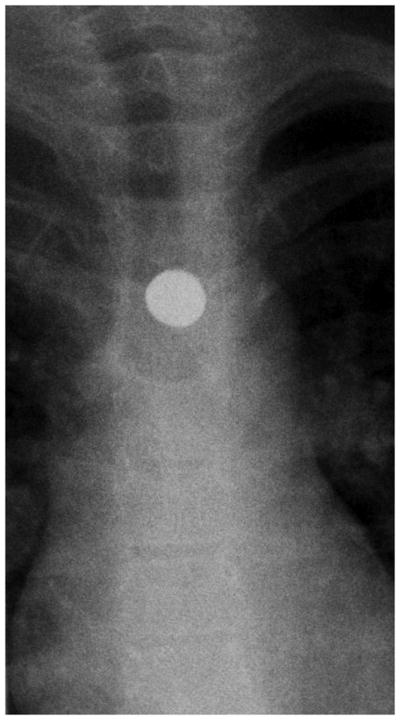
Esophagram findings in EoE with stricture in three children: Frontal (A) and lateral (B) views show mucosal irregularity, ulceration and long segment narrowing in the upper and mid esophagus (open arrows); C) frontal view shows focal short segment narrowing in the upper esophagus (open arrow); D) lateral view shows mild short segment focal narrowing in the upper esophagus (open arrow), confirmed by E) delayed transit of contrast pill at the same level on frontal view.
Endoscopic Findings
Stricture was identified by endoscopy in 10 patients; 4 of which with crepe paper esophagus (Table 1). Other endoscopic findings included linear furrows (91%), exudate (59%) and concentric rings (55%). A summary of endoscopic findings are presented in table 1.
Dilation was performed in six patients with either balloon (n=4) or Maloney dilators (n=2) and resulted in mucosal split in four. Long term follow up is not available on all patients however of those not initially dilated, three required future dilation and one had repeat food impaction. Initial endoscopy of these four patients did not identify stricture.
Histology Findings
With respect to the histopathological features, the mean peak eosinophil per hpf (eos/hpf) was 56 (median 58, ± 32, range 0 to 120). Of 8 patients with sufficient lamina propria for assessment, five (63%) had subepthelial fibrosis. There were 2 patients with normal histology who demonstrated crepe paper esophagus and split upon dilation, respectively.
Stricture Detection, Comparison between Methods
The average time between endoscopy and esophagram was 30 days. (Table 1) Six patients had studies within 1 week of each other. Esophagram was performed prior to endoscopy in 16 patients (73%) (mean 29 days, range same day to 74 days prior to endoscopy). In the remaining 6 (27%) patients, esophagram was performed a mean of 32 days after endoscopy (range 1 to 83 days). Stricture was identified prior to endoscopy by esophagram in 9 patients (41%).
Since EoE directed treatment could affect inflammation and stricture, we performed a subanalysis excluding patients (n=3) on treatment for more than 3 days between studies. Endoscopy identified 8 strictures of 19 detected by esophagram (42%).
Patients with strictures identified by esophagram only trended towards younger age (11.4 years vs. 15.6 years, p = NS) and had shorter duration of symptoms (2.1 years duration vs. 5.4 years duration, p = 0.03) compared to those in whom strictures were identified by endoscopy (Table 1). No other significant difference was found between the groups with respect to patient demographics, endoscopic or radiologic findings, histology, or treatment.
Endoscopic dilation in pediatrics often requires pre-operative planning. Thus, we next determined whether pre-operative radiographic identification of a stricture was more often associated with dilation being performed. Those dilated were more likely to have had a stricture identified by esophagram prior to the procedure (X2 = 7.5, p = 0.006). Dilation was not performed in the single patient with stricture identified initially at endoscopy.
Discussion
Our aim was to compare fluoroscopy and endoscopy in detection of esophageal strictures in children with EoE. We found that in 55% of patients, esophagram identified luminal narrowing not observed at endoscopy. Patients in whom esophagram alone discovered the stricture were both younger and had shorter duration of symptoms suggesting that fluoroscopy may detect subepithelial remodeling prior to onset of intraluminal narrowing.
In contrast to peptic or surgical strictures that are typically focal, EoE related strictures may be focal or diffuse. While a number of studies have described the endoscopic features in adult and pediatric EoE, few studies have evaluated fluoroscopic esophagram in complicated EoE. Emerging data from adult natural history studies document the progressive nature of fibrostenotic disease but it is unclear how these features develop in children.24, 25 Our clinical experience and these studies suggest that a time lag exists between diagnosis and the onset of clinically significant esophageal narrowing. In addition, while epithelial eosinophilia characterizes the disease histologically, submucosal inflammation may lead to underlying processes that are not captured visually on the mucosal surface.
As such, the pathogenesis of esophageal fibrosis and stricture formation in children and adults with EoE continues to evolve. In contrast to peptic disease, in which an epithelial injury leads to chronic scarring and stricture, we speculate that chronic subepithelial inflammation leads to a thickened, less pliable wall. A number of studies have identified that both children and adults with EoE have increased lamina propria fibrosis and decreased compliance may be predictive of future food impaction.1, 5, 19, 26, 27 Basic studies identify the contribution of not only epithelial cells but also smooth muscle cells and fibroblasts in the generation of contractile responses.1, 3, 4 Therefore, the majority of clinically relevant remodeling likely occurs in a micromilieu not apparent on the mucosal surface. We speculate that GERD related strictures represent an “outside-in” pattern of injury that is more apparent at endoscopy whereas some EoE related strictures are caused by “inside-out” pattern of injury with subepithelial fibrotic injury leading to occult narrowing that may not be evident endoscopically. Over time esophageal narrowing may become more conspicuous at endoscopy.
A recent study of adults assessed the role of esophagram in identification of adult EoE related stricture. Gentile et al performed a retrospective study of 58 well-defined adult patients ranging in age from 20-79 years with stricture identified by esophagram. In their previous adult work, they had developed radiographic maximal and minimal esophageal luminal measurements. They compared identification of esophageal stricture by esophagram with endoscopy. Their results showed that 85-66% of strictures identified by esophagram were not apparent by endoscopy.28 Specific factors contributing to esophageal narrowing included female sex and history of multiple food impactions.
Several key clinical points pertaining to the pediatric population deserve mention. As a clear history is often difficult to obtain from children in the face of occult coping mechanisms, a high level of suspicion coupled with meticulous esophagram technique and analysis is necessary in the ordering and interpretation of esophagram studies in EoE patients. Narrowing in EoE is often subtle on imaging. Of the 21 strictures identified by retrospective review of esophagram, 12 (57%) were identified prospectively at the initial read. Additionally, children with a fear of swallowing may have difficulty cooperating and thus may not be able to drink large enough bariumboluses to adequately distend the esophagus; leading to a falsely identified small caliber esophagus or narrowing. Transient peristalsis may also simulate luminal narrowing. In these cases, the dissolvable barium pill can be a useful fluoroscopic tool to aid in detecting subtle narrowing as evidenced by 3 of 4 children who had delayed pill transit at the site of stricture.29, 30 Inclusion of barium pill swallowing during the esophagram may be particularly helpful since, in contrast to the adult study, no published radiological reference standards exist defining the normal esophageal luminal diameter in children in general and especially not in those who have EoE.
Our study has several limitations. With regards to endoscopy, we were limited to retrospective review of endoscopic images and procedure reports and therefore unable to control for endoscopic technique or procedure documentation. For example, esophageal insufflation is critical to the appropriate detection of subtle mucosal abnormalities such as furrows, edema, rings and even stricture and we were unable to control for adequate esophageal inflation. We examined patients who received endoscopy and fluoroscopy in a relatively narrow 3-month time frame. We are confident that a clinically relevant stricture would not develop in the relatively short time frame; however EoE directed treatment could affect stricture treatment. The majority of subjects studied here though were naïve to EoE treatment between studies. When the three subjects on chronic (more than 3 days) treatment between studies were excluded, we found no significant differences in results, supporting the conclusion that over 50% of children with esophageal strictures detected by esophagram were not seen at the time of endoscopy.
In conclusion, assessment of children with EoE by endoscopy alone may miss up to 55% of esophageal strictures. This high number is consistent with that found in one adult study and supports exercising a low threshold for performing fluoroscopic esophagram in select children with EoE. In the era of radiation awareness, it is prudent to limit those examinations in children whenever possible; however esophagram may be beneficial to define esophageal narrowing not evident by endoscopy and provide therapeutic benefit in certain children with EoE when clinically indicated. Other endoscopic assessments may allow for improved endoscopic detection of strictures in EoE.27, 31, 32 Our findings contribute to a growing body of literature that characterizes complicated EoE and suggests EoE is a pan esophageal disease, which benefit from novel approach to diagnosis and monitoring of adverse outcomes.
Figure 3.
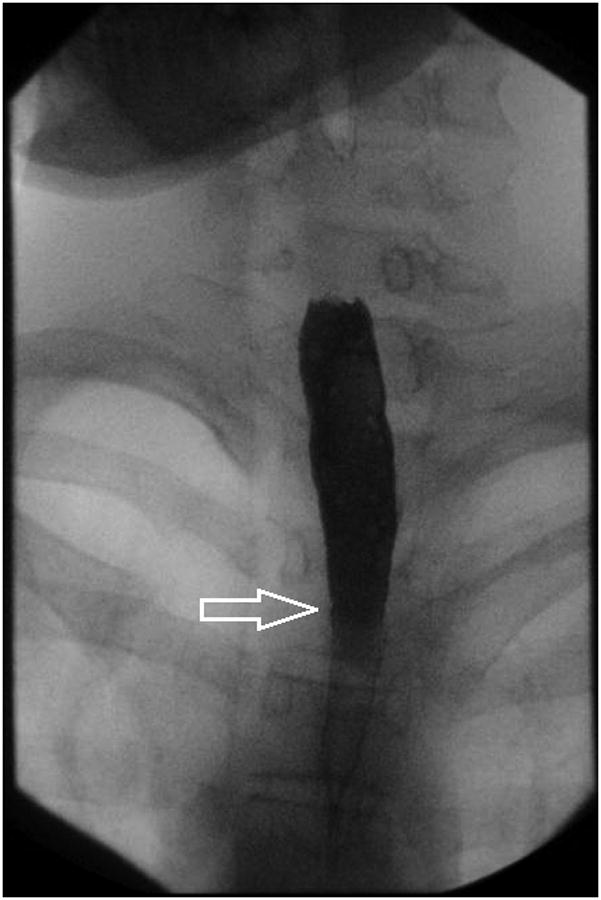
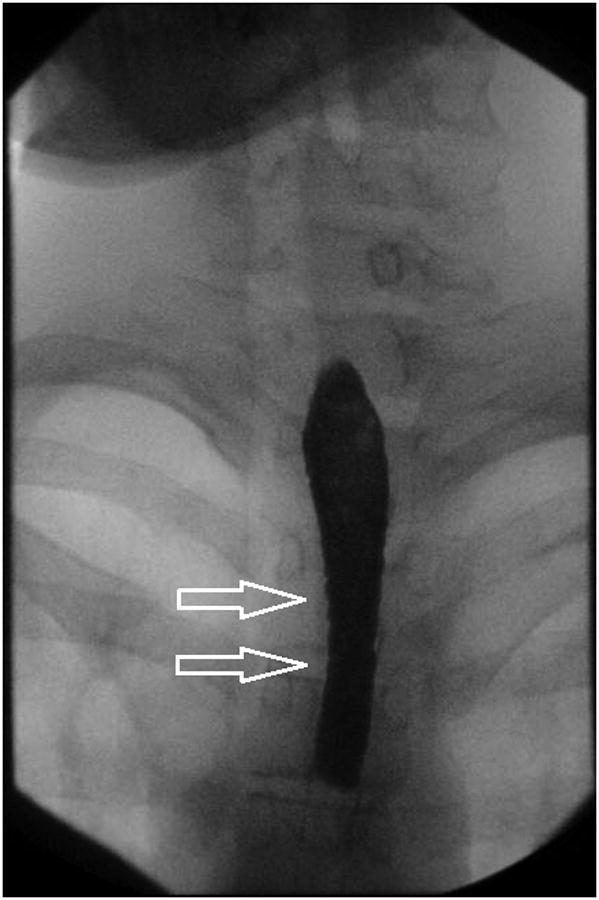
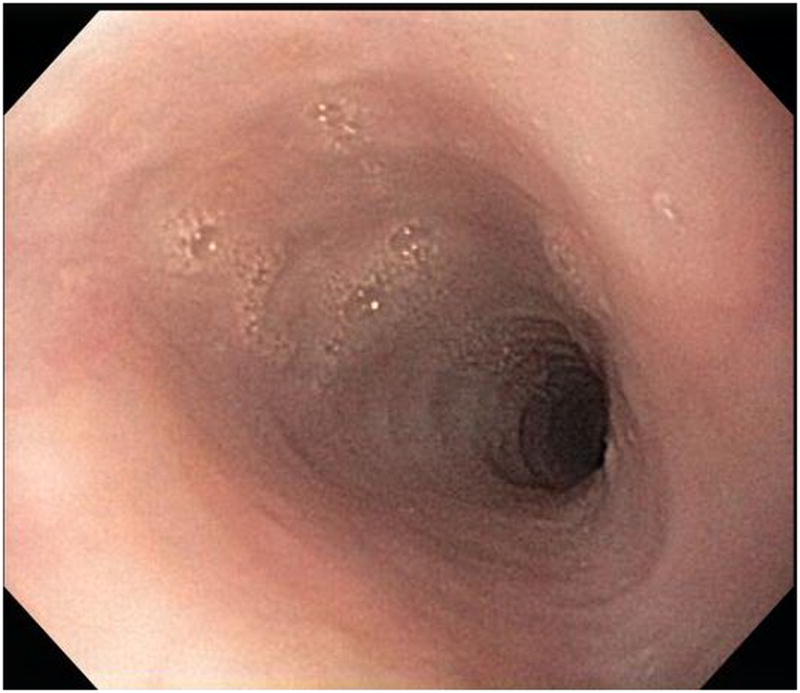
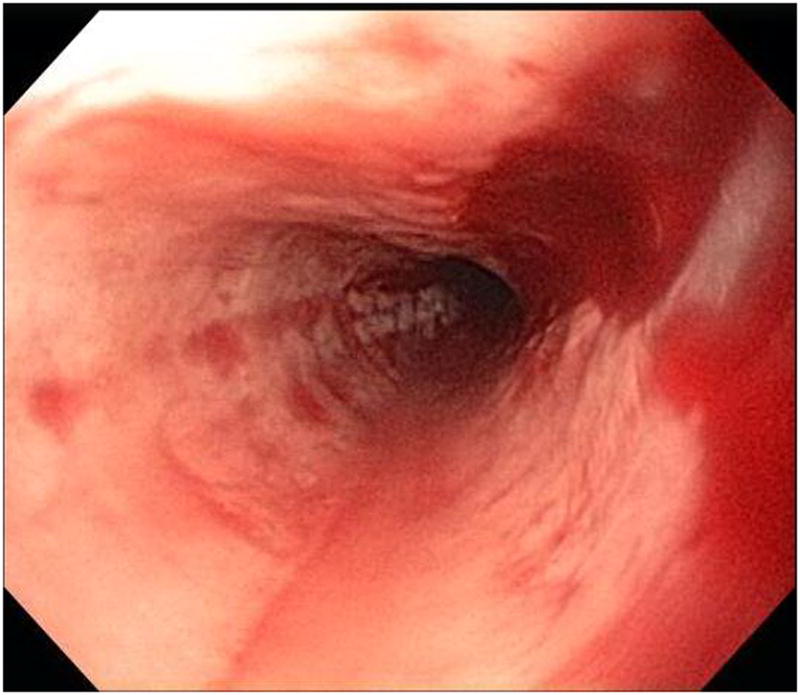
Stricture is not always appreciated on endoscopy when compared to esophagram. In this patient, A-B) esophagram detected short segment mild stricture of the esophagus just below the thoracic inlet associated with smooth ring-like indentations (arrows). C) Endoscopy detected edema and mild trachealization but did not detect stricture however D) upon dilation to 15 mm there was a resultant mucosal split.
What is known
Gastoesophageal reflux disease or post-operative associated esophageal strictures can typically be visualized at the time of endoscopy.
Children and adolescents with EoE can present with or develop esophageal strictures
Symptoms associated with EoE related strictures in children are often non-specific.
What is new
Endoscopic visualization may not be able to detect intraluminal narrowing in children with EoE.
Over 50% of children with esophageal strictures were not seen at the time of endoscopy but were detected by contrast esophagram.
Children in whom esophagram only identified strictures had shorter disease duration than those in who strictures were seen at the time of endoscopy.
Radiologic assessment in patients with dysphagia may assist in pre-endoscopic planning and decision regarding dilation.
Acknowledgments
This study was funded in part by NIH, 1K24DK100303 (Furuta GT). The authors thank Dresden Whitehead for her assistance in data entry.
Abbreviations
- EoE
eosinophilic esophagitis
References
- 1.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Kagalwalla AF, Akhtar N, Woodruff SA, et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol. 2012;129:1387–1396 e7. doi: 10.1016/j.jaci.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieder F, Nonevski I, Ma J, et al. T-Helper 2 Cytokines, Transforming Growth Factor beta1, and Eosinophil Products Induce Fibrogenesis and Alter Muscle Motility in Patients With Eosinophilic Esophagitis. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beppu LY, Anilkumar AA, Newbury RO, et al. TGF-beta1-induced phospholamban expression alters esophageal smooth muscle cell contraction in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134:1100–1107 e4. doi: 10.1016/j.jaci.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chehade M, Sampson HA, Morotti RA, et al. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–28. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 6.Stevoff C, Rao S, Parsons W, et al. EUS and histopathologic correlates in eosinophilic esophagitis. Gastrointest Endosc. 2001;54:373–7. doi: 10.1067/mge.2001.116569. [DOI] [PubMed] [Google Scholar]
- 7.Fox VL, Nurko S, Teitelbaum JE, et al. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc. 2003;57:30–6. doi: 10.1067/mge.2003.33. [DOI] [PubMed] [Google Scholar]
- 8.Binkovitz LA, Lorenz EA, Di Lorenzo C, et al. Pediatric eosinophilic esophagitis: radiologic findings with pathologic correlation. Pediatr Radiol. 2010;40:714–9. doi: 10.1007/s00247-009-1484-2. [DOI] [PubMed] [Google Scholar]
- 9.Diniz LO, Putnum PE, Towbin AJ. Fluoroscopic findings in pediatric eosinophilic esophagitis. Pediatr Radiol. 2012;42:721–7. doi: 10.1007/s00247-011-2329-3. [DOI] [PubMed] [Google Scholar]
- 10.Nurko S, Teitelbaum JE, Husain K, et al. Association of Schatzki ring with eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr. 2004;38:436–41. doi: 10.1097/00005176-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman SL, Levine MS, Rubesin SE, et al. Idiopathic eosinophilic esophagitis in adults: the ringed esophagus. Radiology. 2005;236:159–65. doi: 10.1148/radiol.2361041100. [DOI] [PubMed] [Google Scholar]
- 12.White SB, Levine MS, Rubesin SE, et al. The small-caliber esophagus: radiographic sign of idiopathic eosinophilic esophagitis. Radiology. 2010;256:127–34. doi: 10.1148/radiol.10091060. [DOI] [PubMed] [Google Scholar]
- 13.Vasilopoulos S, Murphy P, Auerbach A, et al. The small-caliber esophagus: an unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc. 2002;55:99–106. doi: 10.1067/mge.2002.118645. [DOI] [PubMed] [Google Scholar]
- 14.Aceves SS, Newbury RO, Dohil R, et al. Distinguishing eosinophilic esophagitis in pediatric patients: clinical, endoscopic, and histologic features of an emerging disorder. J Clin Gastroenterol. 2007;41:252–6. doi: 10.1097/01.mcg.0000212639.52359.f1. [DOI] [PubMed] [Google Scholar]
- 15.Kim HP, Vance RB, Shaheen NJ, et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988–96 e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Huprich J, Kujath C, et al. Esophageal diameter is decreased in some patients with eosinophilic esophagitis and might increase with topical corticosteroid therapy. Clin Gastroenterol Hepatol. 2012;10:481–6. doi: 10.1016/j.cgh.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 17.Straumann A, Conus S, Degen L, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400–9 e1. doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Dohil R, Newbury R, Fox L, et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy. 2010;65:109–16. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Sultaneh SM, Durst P, Maynard V, et al. Fluticasone and food allergen elimination reverse sub-epithelial fibrosis in children with eosinophilic esophagitis. Dig Dis Sci. 2011;56:97–102. doi: 10.1007/s10620-010-1259-5. [DOI] [PubMed] [Google Scholar]
- 21.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20 e6. doi: 10.1016/j.jaci.2011.02.040. quiz 21-2. [DOI] [PubMed] [Google Scholar]
- 22.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108:679–92. doi: 10.1038/ajg.2013.71. quiz 693. [DOI] [PubMed] [Google Scholar]
- 23.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2012 doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 24.Dellon ES, Kim HP, Sperry SL, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79:577–585 e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–6 e1. 2. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Aas IH. Guidelines for rating Global Assessment of Functioning (GAF) Ann Gen Psychiatry. 2011;10:2. doi: 10.1186/1744-859X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101–1107 e1. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther. 2014;40:1333–40. doi: 10.1111/apt.12977. [DOI] [PubMed] [Google Scholar]
- 29.Oh CH, Levine MS, Katzka DA, et al. Congenital esophageal stenosis in adults: clinical and radiographic findings in seven patients. AJR Am J Roentgenol. 2001;176:1179–82. doi: 10.2214/ajr.176.5.1761179. [DOI] [PubMed] [Google Scholar]
- 30.Ngo PD, Lee E, McCauley R, et al. Esophageal barium tablet impaction: A novel non-invasive diagnostic test for eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2011;53:E46. [Google Scholar]
- 31.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madanick RD, Shaheen NJ, Dellon ES. A novel balloon pull-through technique for esophageal dilation in eosinophilic esophagitis (with video) Gastrointest Endosc. 2011;73:138–42. doi: 10.1016/j.gie.2010.09.034. [DOI] [PubMed] [Google Scholar]


