Abstract
Background
Adults with Down syndrome (DS) are at risk of developing dementia and cognitive assessment is a fundamental part of the diagnostic process. Previously, we developed a Rapid Assessment for Developmental Disabilities (RADD), a brief, broadly focused direct test of cognition. In the current report, we assess whether the RADD is sensitive to dementia in DS and the degree to which it compares to other cognitive measures of dementia in this population.
Methods
In a sample of 114 individuals with DS, with dementia diagnosed in 62%, the RADDwas compared to the Dementia Questionnaire for Mentally Retarded Persons (DMR), the Bristol Activities of Daily Living Scale (BADLS), Severe Impairment Battery (SIB) and the Brief Praxis Test (BPT).
Results
The RADD showed predicted effects across intellectual disability (ID) levels and dementia status (p < .001). Six month test-retest reliability for the subset of individuals without dementia was high (r (41) = .95, p < .001). Criterion-referenced validity was demonstrated by correlations between RADD scores and ID levels based upon prior intelligence testing and clinical diagnoses (rs (114) = .67, p = .001) and with other measures of cognitive skills, such as the BPT, SIB and DMR-Sum of Cognitive scores (range .84 through .92). Using receiver operating characteristic (ROC) curves for groups varying in pre-morbid severity of ID, the RADD exhibited high sensitivity (.87) and specificity (.81) in discriminating among individuals with and without dementia, although sensitivity was somewhat lower (.73) for the subsample of dementia cases diagnosed no more than two years prior to their RADD assessment.
Conclusion
Taken together, findings indicated that the RADD, a relatively brief, easy to administer test for cognitive function assessment across ID levels and dementia status, would be a useful component of cognitive assessments for adults with DS, including assessments explicitly focused on dementia.
Keywords: Down Syndrome, dementia, rapid cognitive assessment
Introduction
Dementia is a risk for adults with Down syndrome (DS). The prevalence of dementia increases with age rising to near 75% after 65 years (Tyrell et al, 2001; Coppus et al, 2006). The average age of dementia onset in DS is now estimated to be in the mid-50s (Schupf and Sergievsky 2002), although neuropathology consistent with Alzheimer's disease (AD) is observed almost universally within this population from their mid-30s (Zigman and Lott, 2007). This disparity between the neuropathological and clinical presentations suggest that there may be a lengthy prodromal period with little or no cognitive decline despite progression of underlying AD pathology. Of the many factors that contribute to dementia risk in DS, the triplication and over-expression of the amyloid precursor protein gene on chromosome 21 appears to participate through a gene-dosage effect in virtually every individual with trisomy 21. Even so, there is no study showing that 100% of individuals with DS become demented (Zigman and Lott, 2007).
The diagnosis of dementia is predicated on a decline from a normal level of functioning. In the general population, the neuropsychological batteries for diagnosing dementia focus on determination of mental status, cognition and functional abilities, with often relying on population-normed neuropsychological tests (Albert et al, 2011; McKhann et al, 2011). For the majority of patients with AD, symptoms of dementia follow a progressive course in which impairment in episodic memory is followed by weaknesses in executive functioning, language and spatial abilities.
Since DS, as well as intellectual disability (ID) due to other causes, is associated with a baseline profile of cognitive impairments, including relative strengths and weaknesses that vary substantially among affected individuals (Silverman, 2007; Lott and Dierssen, 2010), the diagnosis of dementia is more challenging than in the general population. Recommendations for the diagnosis of dementia in people with ID have included the use of International Classification of Diseases, Tenth Edition (ICD-10; WHO, 2010) criteria to create a test battery that would ultimately reflect declines in performance (Aylward et al, 1997; Burt and Aylward, 2000). In people with ID other than DS, the prevalence of dementia may not be higher than that in the general population (Zigman et al, 2004; Krinsky-McHale and Silverman, 2013), although findings have been mixed (Silverman et al, 2013; Strydom et al, 2009). By contrast, adults with DS, being at dramatically higher risk for AD, present a much more common requirement for dementia diagnosis and the assessment of cognitive functioning is fundamental to that process.
In assessing cognitive decline during early stages of dementia in individuals with DS, numerous test methods have been reviewed (Krinsky-McHale and Silverman, 2013). The domains include memory, language, executive functioning, motor performance, new learning, personality and behavior. Over 30 individual psychological measures have been developed for these assessments offering a wide array of instruments for measuring cognitive functioning and its possible decline. In the general population, short screening instruments have been found to be accurate (Lin et al, 2013). Of these, the Mini Mental State Exam (Folstein et al, 1975) has been the best studied, with sensitivity and specificity in a memory clinic setting reported to be 79.8 % and 81.3%, respectively (Mitchell, 2009). But the measures used for cognitive assessment in the general population are rarely appropriate in their entirety for assessment of individuals with DS because of floor and ceiling effects secondary to the lifelong cognitive disability characteristic of this population.
For these reasons, we became interested in developing a brief and reliable instrument for measuring cognitive functioning in individuals with DS and ID due to other causes, referred to as the Rapid Assessment for Developmental Disabilities (RADD; Walsh et al, 2007). The RADD requires less than 25 minutes to administer and contains selected items from standardized tests used in assessing individuals with developmental disorders. Normative values for the RADD were obtained from a sample of 271 individuals with developmental disabilities ranging in severity from mild to profound ID. The data showed internal reliability and were particularly sensitive to the severity of ID. While individuals with DS were included in the original RADD standardization sample, the focus was not on the DS population per se, nor was the RADD developed originally for the express purpose of assessing cognitive manifestations of dementia.
The current study was designed to determine if the RADD would be sensitive to differences in cognitive functioning between demented and non-demented individuals with DS. Additional analyses examined the relationship between RADD scores and selected measures of cognitive functioning within this population.
Methods
Procedures
Participants were recruited through the use of flyers, public lectures and referrals from the Adult Down Syndrome Clinic at the University of California, Irvine (UCI). Participants resided in Southern California and lived semi-independently in their family home or within community care residential facilities. Data collection took place at ambulatory clinic sites of the UCI Institutes for Clinical and Translational Science. All participants were diagnosed with DS, based on blood karyotype diagnosis of trisomy 21. Participants provided data from prior standardized intelligence tests, which indicated their pre-dementia level of intellectual functioning. Individuals who were diagnosed as demented met the criteria from the ICD-10 (WHO, 2010) and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; APA, 2004). Medical conditions that might cause symptoms mimicking dementia were eliminated during medical and neurological examinations of each study participant. The final diagnosis of dementia was made by a board-certified neurologist, and was determined independent of RADD testing. Participants’ medical conditions were required to be stable for at least three months prior to the study. The neuropsychological and informant-based measures were administered by a neuropsychologist at the initial examination. A subsample completed a second RADD administration for reliability analyses. The study was approved by the Institutional Review Board at UCI. Data were analyzed with SPSS, Version 21.
Materials
As shown in Table 1, the RADD consists of items from the standardized Mini-Mental State Examination (Folstein et al, 1975), the Severe Mini-Mental State Examination (Harrell et al, 2000), the Expressive One Word Picture Vocabulary Test - Revised (Gardner, 1990), the Peabody Picture Vocabulary Test – Revised Form M (Dunn & Dunn, 1981), the Merrill-Palmer Scale of Mental Tests (Stutsman, 1948), the Hawaii Early Learning Profile (Parks, 1996), and the Wechsler Intelligence Scale for Children - Third Edition (Wechsler, 1991). The RADD previously demonstrated high criterion-based validity and internal reliability among individuals with ID, as well as the capacity to differentiate across the full range of ID severity (Walsh et al, 2007).
Table 1.
RADD Items and Original Test Sources
| RADD Subtest | Items | Source |
|---|---|---|
| Orientation | Week, month, year, location, state of residence | Mini-Mental State Examination (Folstein, et al. 1975) |
| Registration | Ball, flag, tree | |
| Recall | Ball, flag, tree | |
| Attention-forward | C-A-T | The Severe Mini-Mental State Examination (Harrell et al. 2000) |
| Attention-b ackward | T-A-C | |
| Self-identification | Language item 4 All or none item 10 |
Merrill-Palmer Scale of Mental Tests (Stutsman, 1948) |
| Movement | All or none items 13 and 14 | |
| Imitation | Gestural imitation section | Hawaii Early Learning Profile (Parks, 1996) |
| Expressive language | 1, 6, 11, 12, 25, 26, 31, 33, 42, 46, 54, 56, 60, 62, 70 and 73 | Expressive One-Word Picture Vocabulary Test - Revised (Gardner, 1990) |
| Receptive language | 1, 3, 16, 17, 45, 48, 65, 67, 81, 82, 91 and 92 | Peabody Picture Vocabulary Test - Revised, Form M (Dunn & Dunn, 1981) |
| Similarities | 1, 6, 7 and 8 | Wechsler Intelligence Scale for Children - Third Edition (Wechsler 1991) |
| Arithmetic | 6, 7, 9, 12 and 14 | |
| Comprehension | 1, 4, 6, 7 and 9 | |
| Digit span | 1, 2, 3 and 4 (both trials) |
For the present study, RADD assessments were compared to informant-based and direct measures of cognition and dementia. The informant-based measures consisted of the Dementia Questionnaire for Mentally Retarded Persons (DMR; Evenhuis, 1992; Evenhuis, 1996) and the Bristol Activities of Daily Living Scale (BADLS; Bucks et al, 1996). The direct cognitive measures included for comparison consisted of the Severe Impairment Battery (SIB; Panisset, 1994) and the Brief Praxis Test (BPT; Dalton, 1997).
Sample Characteristics
One hundred and fourteen individuals (n = 114) with DS participated in the study. Approximately 45% of participants were women, with an average age of 49.8 years (standard deviation; SD = 8.9). Approximately 35% were previously diagnosed with mild ID, 39% with moderate ID, 23% with severe ID and 3% with profound ID. Due to the low number of individuals with profound ID (n = 4), these individuals were merged with the severe ID group for some analyses. Approximately 62% of participants were diagnosed with dementia. The interval between dementia onset and date of testing ranged from 3.7 to 79.8 months, with a mean interval of 29.1 months (SD = 17). The demographic and clinical characteristics of the sample appear in Table 2.
Table 2.
Demographic and Clinical Characteristics (n = 114)
| Characteristics | n | % | % Floor | % Ceiling |
|---|---|---|---|---|
| Gender | ||||
| Men | 63 | 55 | 14.3 | 0 |
| Women | 51 | 45 | 15.7 | 0 |
| Age (years) | ||||
| <40 | 16 | 14 | 0 | 0 |
| 41-50 | 38 | 33.3 | 10.5 | 0 |
| 51-60 | 54 | 47.4 | 22.2 | 0 |
| 61 + | 6 | 5.3 | 16.6 | 0 |
| Intellectual Disability Level | ||||
| Mild | 40 | 35.1 | 2.5 | 0 |
| Moderate | 44 | 38.6 | 15.9 | 0 |
| Severe & Profound | 30 | 26.3 | .3 | 0 |
| Dementia | ||||
| Yes | 71 | 62.3 | 23.9 | 0 |
| No | 43 | 37.7 | 0 | 0 |
% Floor, percent of subjects with RADD score of 0; % Ceiling, percent of subjects with RADD score of 76.
Results
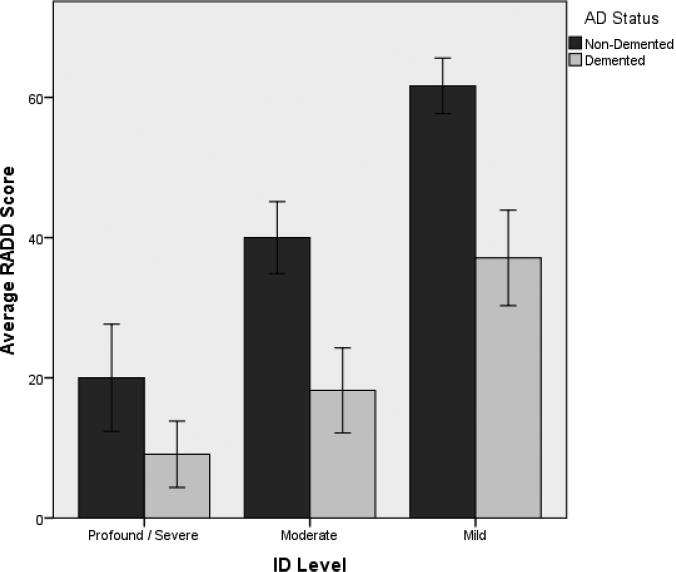
The mean RADD total score was 30.3 (SD = 21.6), with individual performance ranging between 0 and 73 (of a maximum possible score of 76). Descriptive statistics for the RADD and all other dependent measures are summarized in Table 3. Multivariate analyses of variance (MANOVA) were utilized to evaluate the possible effect of gender with the six outcome measures as dependent variables. Gender differences were not present [F (6, 100) = 1.05, p = .40]; therefore, data from men and women were combined for further analyses. MANOVA using dementia status as the independent variable and the six outcome measures as dependent variables was significant [F (6, 100) = 10.89, p < .001]; individuals with dementia exhibited more severe impairment on all measures. In order to set the RADD apart from other tests, a two-way analyses of variance (ANOVA) was completed with ID level and dementia status as independent variables and RADD scores as the dependent variable. The model was significant [F (5, 108) = 45.45, p < .001] and accounted for 67.8% of the overall variance. There were significant main effects for both ID level (p < .001) and dementia status (p < .001), with a non-significant interaction effect (p < .10). Post-hoc analyses for ID level found progressive gains from severe-profound to mild ID (Figure 1).
Table 3.
Descriptive Statistics for Direct and Informant-based Measures
| Test | Mean | SD |
|---|---|---|
| Rapid Assessment for Developmental Disabilities | 30.3 | 21.6 |
| Severe Impairment Battery | 61.1 | 33.1 |
| Brief Praxis Test | 54.6 | 24.4 |
| Dementia Scale for Mentally Retarded Persons - Sum of Cognitive Subscale | 20.7 | 13.3 |
| S Dementia Scale for Mentally Retarded Persons - Sum of Social Subscale | 15.1 | 12.5 |
| Bristol Activities of Daily Living | 22.5 | 13.5 |
SD, standard deviation
Figure 1.
RADD Scores Across ID Levels Among Individuals with DS Based on Dementia Status
Six month test re-test reliability of the RADD was demonstrated on a subset of individuals (r (41) = .95, p < .001). The mean scores from the first and second administrations 19.4 (SD = 18.7) and 17.6 (SD = 20.1), respectively, were not statistically different. In order to further illustrate the test's validity, RADD scores were correlated with scores from other direct and informant-based measures. As shown in Table 4, the RADD exhibited high correlations with other measures of cognitive skills, such as the BPT, SIB and DMR-Sum of Cognitive scores (SCS; range .84 through .92). Furthermore, the patterns of correlations between RADD and other measures remained consistent regardless of the presence or absence of dementia.
Table 4.
Correlations Between RADD and Other Direct and Informant-based Measures Among Individuals with DS Based on Dementia Status
| Measure | RADD Scores Total Sample (n = 114) | RADD Scores Non-Demented (n = 43) | RADD Scores Demented (n = 71) |
|---|---|---|---|
| Brief Praxis Test | .842** | .789** | .852** |
| Severe Impairment Battery | .921** | .862** | .930** |
| Dementia Scale for Mentally Retarded Persons - Sum of Cognitive Subscale | −.889** | −.855** | −.827** |
| Dementia Scale for Mentally Retarded Persons - Sum of Social Subscale | −.683** | −.337** | −.661** |
| Bristol Activities of Daily Living. | −.812** | −.675** | −.769** |
RADD, Rapid Assessment for Developmental Disabilities; DS, Down syndrome
Pearson correlations significant p < .01
significant p < 05;
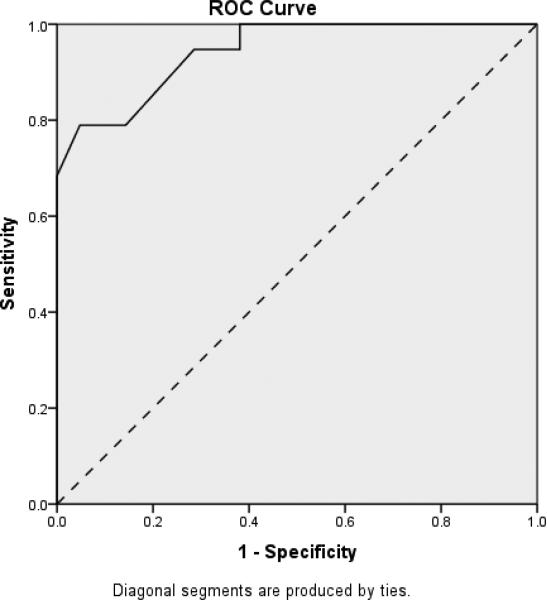
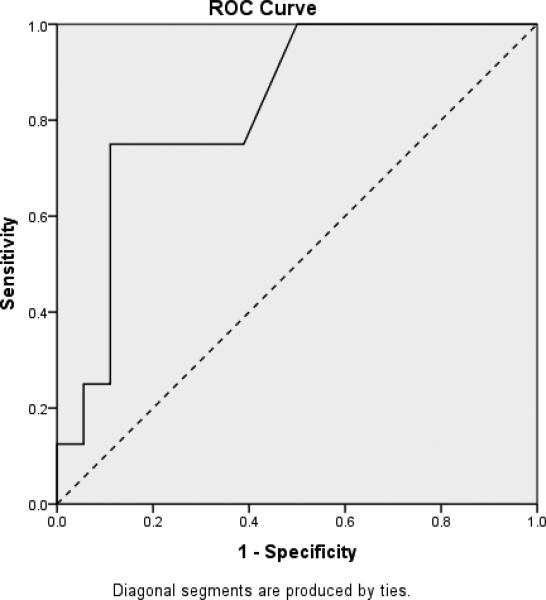
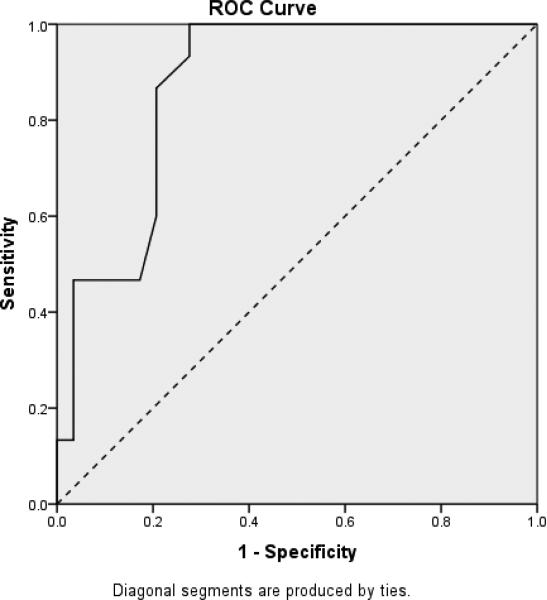
In order to demonstrate the RADD's ability to differentiate between participants with DS based upon their dementia status, three receiver operating characteristic (ROC) curves were calculated based on ID level. These curves plot sensitivity, which is the proportion of true dementia cases correctly identified, against 1-specificity, which is the proportion of false positives. ROC curves for individuals with mild, moderate and more severe levels of ID are plotted in Figures 2 through 4, respectively, with the accuracy of the RADD quantified as the area under the curve (AUC). Chance accuracy of 50% would be depicted by the diagonal (dotted) lines and perfect accuracy, reflecting sensitivity and specificity of 1.0 would be indicated by an ROC curve along the left and top boundaries of the ROC graph. As shown in Figure 2, results indicted the RADD effectively differentiated mild ID participants based on their dementia status (AUC = .944; p < .001). Sensitivity was 0.95%, specificity was 0.79, and 87.5% were correctly classified with the RADD cut-off score of under 60 indicating presence of dementia. Figure 3 provides comparable results for participants with moderate ID (AUC = .87; p < .001). Sensitivity was 0.79%, specificity was 0.87, and 81.8% were correctly classified with the RADD cut-off score of under 30 indicating presence of dementia. As shown in Figure 4, the RADD differentiated dementia status among severe ID participants (AUC = .83; p < .009). Sensitivity was 0.89, specificity was 0.75, and 84.6% of participants with severe ID were correctly classified with the RADD cut-off score of under 20 indicating presence of dementia. Participants with profound ID (n = 4) were excluded from the ROC analyses due to the limited sample size and performance at floor independent of dementia presence.
Figure 2.
Receiver Operating Characteristic Curve Depicting Differentiation of Dementia Status Among DS Participants with Mild ID (n = 40)
Figure 4.
Receiver Operating Characteristic Curve Depicting Differentiation of Dementia Status Among DS Participants with Severe ID (n = 26)
Figure 3.
Receiver Operating Characteristic Curve Depicting Differentiation of Dementia Status Among DS Participants with Moderate ID (n = 44)
To ascertain if these RADD criteria for classifying dementia status were sensitive to relatively early stages of AD, sensitivity was recalculated for only cases diagnosed within the two years immediately preceding RADD assessment (n = 30). Cases included 11 adults with mild ID, 10 with moderate ID, and 9 with severe ID. Overall sensitivity was 0.73, with the criteria for the mild ID group remaining quite high at 0.91 but with estimated sensitivity considerably lower for the moderate ID subgroup (0.50). However, these ID-level differences could reflect imprecision in estimates associated with small sample sizes rather than true effects.
Discussion
As reviewed by Edgin et al (2010), neuropsychological measures in DS should include features that measure a range of skills. Distributional properties should allow statistical analysis of potential treatment effects, adequate test-retest reliability, and resistance to confounding factors such as poor motivation and language impairment. Based on our present study, the RADD fulfilled these characteristics. For participants with mild, moderate and severe-profound ID, levels of performance were acceptably below ceiling and above floor for the vast majority of individuals, suggesting ample opportunity for performance to move up and down. The RADDdemonstrated construct validity via convergent correlations with other established measures of cognitive functioning among individuals with DS. Furthermore, the RADD exhibited criterion-referenced validity by way of strong correlations between RADD scores and premorbid IQ levels determined during prior standardized IQ testing, as well as by differentiation between participants with and without dementia.
In the present study, we utilized measures of dementia that we employed in our previous clinical trials (Lott et al, 2012; 2011; 2002), and compared those measures to the RADD. Comparisons between the RADD and other direct as well as informant-based measures indicated the RADD has efficacy for assessing cognitive functions relevant to AD in DS. The RADD could then be a measure of cognitive functioning that could be more broadly applied in treatment trials for individuals with DS, although the present findings should only be considered suggestive on this point.
There are other limitations of the present study. While the RADD reflected cognitive performance in individuals with DS over a wide spectrum of ability, the vast majority of individuals with profound intellectual limitations would be expected to perform at floor prior to developing dementia. As true for many other measures of cognition, the RADD would not be informative in these cases. In addition, the sensitivity of the RADD to the earliest stages of AD in adults with DS, comparable to “mild cognitive impairment” within the elderly population without ID, has not yet been assessed, nor was the sensitivity of the RADD to changes in cognition within individuals. Finally, the specific criterion scores for distinguishing presence from absence of dementia were selected based on inspection of the data and, therefore, independent replication is needed to validate the obtained estimates of their sensitivity and specificity. These important issues need to be addressed in future research.
The present findings are consistent with those of other studies of cognition in adults with DS. In a study assessing two informant-based measures sensitive to dementia, DMR subscales scores showed positive correlations with three factor scores on the Adaptive Behavior Scale (Kirk et al, 2006). In a study of 55 individuals with DS ranging in age from 19-58 years, data on measures of non-verbal cognition, language, and working memory (Iacona et al, 2010), first order correlations ranged from small to large, with aging effects interpreted as due to the presence of AD. In a study of 78 adults with DS who were non-demented, disinhibition scores predicted abnormalities in executive functioning and apathy scores predicted difficulties in spatial organization and prospective memory (Ball et al, 2010). Using the Prudhoe Cognitive Function Test and the Adaptive Behavior Scales, Margallo-Lana and colleagues (2007) showed correlations with cognitive decline over a 15 year period of follow-up in individuals with DS. In a large meta-analysis of screening tests for cognitive impairment in the general (non-DS) population, it was concluded that several brief instruments were capable of detecting the cognitive changes associated dementia, but that only a handful of instruments had been used in more than one clinical trial (Lis et al, 2013).
For people with DS, the RADD offers the advantage of a relatively brief and easy to administer assessment of cognition that correlates well with other measures. For adults with DS, the brevity, efficiency and score validity of the RADD may make it an attractive option for helping to inform diagnostic decisions, as well as a potentially useful outcome measure in clinical trials. Although the RADD may be somewhat less sensitive for detecting early dementia compared to its overall performance, it can still reduce diagnostic uncertainty to a considerable degree, suggesting that it could be usefully included as a component of any dementia assessment battery developed for adults with DS and, more generally, ID due to other causes.
Acknowledgements
We appreciate Thomas Granoff, Phd, Michelle McGregor-Grayden, PhD, and Deirdre Syms, MS, for their helpful consultations.
This study was supported by grant HD-065160 (NICHD), the Alzheimer Disease Research Center (P50-AG16573) at University of California-Irvine, the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR000153) and the Intellectual and Developmental Disabilities Research Center of the Kennedy Krieger Institute and Johns Hopkins University (NICHD grant P30 HD-024061).
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition, Text Revision APA; Washington, DC.: 2004. [Google Scholar]
- Aylward EH, Burt DB, Thorpe LU, Lai F, Dalton A. Diagnosis of dementia in individuals with intellectual disability. Journal of Intellectual Disability Research. 1997;41(2):152–164. doi: 10.1111/j.1365-2788.1997.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Ball SL, Holland AJ, Watson PC, Huppert FA. Theoretical exploration of the neural bases of behavioral disinhibition, apathy and executive dysfunction in preclinical Alzheimer's disease in people with Down's syndrome: potential involvement of multiple frontal-subcortical neuronal circuits. Journal of Intellectual Disability Research. 2010;54(4):320–326. doi: 10.1111/j.1365-2788.2010.01261.x. [DOI] [PubMed] [Google Scholar]
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age and Ageing. 1996;25:113–120. doi: 10.1093/ageing/25.2.113. [DOI] [PubMed] [Google Scholar]
- Burt DB, Aylward EH. Test battery for the diagnosis of dementia in individuals with intellectual disability. Journal of Intellectual Disability Research. 2000;44(2):175–180. doi: 10.1046/j.1365-2788.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- Coppus A, Evenhuis H, Verberne GJ, Visser F, van Gool P, Eikelenboom P, van Duijin C. Dementia and mortality in persons with Down's syndrome. Journal of Intellectual Disability Research. 2006;50(10):768–777. doi: 10.1111/j.1365-2788.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- Dalton A, Fedor B. Dyspraxia scale for adults with Down syndrome. Institute for Basic Research in Developmental Disabilities; Staten Island, NY: 1997. 2001. [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test – Revised Manual for Forms L and M. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Edgin JO, Pennington BF, Mervis CB. Neuropsychological components of intellectual disability: the contributions of immediate, working, and associative memory. Journal of Intellectual Disability Research. 2010;54(5):406–417. doi: 10.1111/j.1365-2788.2010.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenhuis HM. Evaluation of a screening instrument for dementia in ageing mentally retarded persons. Journal of Intellectual Disability Research. 1992;36(4):337–347. doi: 10.1111/j.1365-2788.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gardner MF. Expressive One-Word Picture Vocabulary Test – Revised Manual. Academic Therapy Publications; Novato, CA: 1990. [Google Scholar]
- Harrell LE, Marson D, Chatterjee A, Parrish JA. The Severe Mini-Mental State Examination for the Bedside Assessment of Severely Impaired Patients with Alzheimer's disease. Alzheimer Disease and Associated Disorders. 2000;14(3):168–175. doi: 10.1097/00002093-200007000-00008. [DOI] [PubMed] [Google Scholar]
- Iacono T, Torr J, Wong HY. Relationships amongst age, language and related skills in adults with Down syndrome. Research in Developmental Disabilities. 2010;31(2):568–576. doi: 10.1016/j.ridd.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Kirk LJ, Hick R, Laraway A. Assessing dementia in people with learning disabilities: the relationship between two screening measures. Journal of Intellectual Disability. 2006;10(4):357–364. doi: 10.1177/1744629506070053. [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale SJ, Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: Issues of diagnosis. Developmental Disabilities and Research Reviews. 2013;18(1):31–42. doi: 10.1002/ddrr.1126. [DOI] [PubMed] [Google Scholar]
- Lin JS, O'Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2013;159(9):601–612. doi: 10.7326/0003-4819-159-9-201311050-00730. [DOI] [PubMed] [Google Scholar]
- Lin JS, O'Connor E, Rossom RC. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; Rockville, Maryland: 2013. ( www.ncbi.nlm.nih.gov/books/NBK174643) [PubMed] [Google Scholar]
- Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurology. 2010;9(6):623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- Lott IT, Doran E, Nguyen VQ, Tournay A, Head E, Gillen DL. Down syndrome and dementia: a randomized, controlled trial of antioxidant supplementation. American Journal of Medical Genetics Part A. 2011;155A(8):1939–1948. doi: 10.1002/ajmg.a.34114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT, Doran E, Nguyen VQ, Tournay A, Movsesyan N, Gillen DL. Down syndrome and dementia: seizures and cognitive decline. Journal of Alzheimer's disease. 2012;29(1):177–185. doi: 10.3233/JAD-2012-111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT, Osann K, Doran E, Nelson L. Down syndrome and Alzheimer disease: response to donepezil. Archives of Neurology. 2002;59(7):1133–1136. doi: 10.1001/archneur.59.7.1133. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margallo-Lana ML, Moore PB, Kay DW, Perry RH, Reid BE, Berney TP, Tyrer SP. Fifteen-year follow-up of 92 hospitalized adults with Down's syndrome: incidence of cognitive decline, its relationship to age and neuropathology. Journal of Intellectual Disability Research. 2007;51(6):463–477. doi: 10.1111/j.1365-2788.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. Journal of Psychiatric Research. 2009;43(4):411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Panisset M, Roudier M, Saxton J, Boiler F. Severe Impairment Battery: A Neuropsychological Test for Severely Demented Patients. Archives of Neurology. 1994;51(1):41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- Parks S. Curriculum-Based Assessment Birth to Three Years: Adapted from the Hawaii Early Learning Profile. VORT Corporation; Palo Alto, California: 1996. [Google Scholar]
- Schupf N, Sergievsky GH. Genetic and host factors for dementia in Down's syndrome. The British Journal of Psychiatry. 2002;180:405–410. doi: 10.1192/bjp.180.5.405. [DOI] [PubMed] [Google Scholar]
- Silverman W. Down syndrome: cognitive phenotype. Mental Retardation and Developmental Disabilities Research Review. 2007;13(3):228–236. doi: 10.1002/mrdd.20156. [DOI] [PubMed] [Google Scholar]
- Silverman WP, Zigman WB, Krinsky-McHale SJ, Ryan R, Schrupf N. Intellectual Disability, Mild Cognitive Impairment, and Risk for Dementia. Journal of Policy and Practice in Intellectual Disabilities. 2013;10(3) doi: 10.1111/jppi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutsman R. Guide for Administering the Merrill-Palmer Scale of Mental Tests. Stoelting Company; Chicago: 1948. [Google Scholar]
- Strydom A, Hassiotis A, King M, Livingstong G. The relationship of dementia prevalence in older adults with intellectual disability (ID) to age and severity of ID. Psychological Medicine. 2009;39(1):13–21. doi: 10.1017/S0033291708003334. [DOI] [PubMed] [Google Scholar]
- Tyrrell J, Cosgrave M, McCarron M, McPherson J, Calvert J, Kelly A, McLaughlin M, Gill M, Lawlor BA. Dementia in people with Down's syndrome. International Journal of Geriatric Psychiatry. 2001;16(12):1168–1174. doi: 10.1002/gps.502. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Finwall J, McGregor M, Fernandez G, Lott I, Touchette PE, Sandman CA. Rapid Assessment of Severe Cognitive Impairment in individuals with developmental disabilities. Journal of Intellectual Disability Research. 2007;51(2):91–100. doi: 10.1111/j.1365-2788.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, Third Edition – Manual. Harcourt Brace and Company; San Antonio: 1991. [Google Scholar]
- World Health Organization . International Classification of Diseases and Health Related Problems. 10th Edition. Stylus Publishing, LLC.; Herndon, VA: 2010. [Google Scholar]
- Zigman WB, Lott IT. Alzheimer's disease in Down syndrome: neurobiology and risk. Mental Retardation and Developmental Disabilities Research Review. 2007;13(3):237–246. doi: 10.1002/mrdd.20163. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Devenny DA, Miezeieski C, Ryan R, Urv TK, Schubert R, Silverman W. Incidence and prevalence of dementia in elderly adults with mental retardation without Down syndrome. American Journal of Mental Retardation. 2004;109(2):126–141. doi: 10.1352/0895-8017(2004)109<126:IAPODI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]