Abstract
Objectives
Omega (n)-3 and n-6 polyunsaturated fatty acids (PUFA) are molecular modulators of neurotransmission and inflammation. We hypothesized that plasma concentrations of n-3 PUFA would be lower and of n-6 PUFA higher in subjects with bipolar disorder (BD) compared to healthy controls (HC), and would correlate with symptom severity in subjects with BD, and that effective treatment would correlate with increased n-3 but lower n-6 PUFA levels. Additionally, we explored clinical correlations and group differences in plasma levels of saturated and monounsaturated fatty acids.
Methods
This observational, parallel group study compared biomarkers between HC (n = 31), and symptomatic subjects with BD (n = 27) when ill and after symptomatic recovery (follow-up). Plasma concentrations of five PUFA [linoleic acid (LA), arachidonic acid (AA), alpha-linolenic acid (ALA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA)], of two saturated fatty acids (palmitic acid and stearic acid) and of two monounsaturated fatty acids (palmitoleic acid, oleic acid) were measured in esterified (E) and unesterified (UE) forms. Calculated ratios included UE:E for the five PUFA, ratios of n-3 PUFA (DHA:ALA, EPA:ALA, EPA:DHA), and the ratio of n-6:n-3 AA:EPA. Comparisons of plasma fatty acid levels and ratios between BD and HC groups were made with Student t-tests, between the BD group at baseline and follow-up using paired t-tests. Comparison of categorical variables was performed using Chi-square tests. Pearson’s r was used for bivariate correlations with clinical variables, including depressive and manic symptoms, current panic attacks, and psychosis.
Results
UE EPA was lower in BD than HC, with a large effect size (Cohen’s d = 0.86, p < 0.002), however, it was not statistically significant after correction for multiple comparisons. No statistically significant difference was seen in any plasma PUFA concentration between BD and HC after Bonferroni correction for 40 comparisons, at p < 0.001. Neither depressive severity nor mania severity was correlated significantly with any PUFA concentration. Exploratory comparison showed lower UE:E EPA in BD than HC (p < 0.0001). At follow-up in the BD group, UE, E DHA:ALA, and UE EPA:ALA were decreased (p < 0.002). Exploratory correlations of clinical variables revealed that mania severity and suicidality were positively correlated with UE:E EPA ratio, and that several plasma levels and ratios correlated with panic disorder and psychosis. Depressive severity was not correlated with any ratio. No plasma fatty acid level or ratio correlated with self-reported n-3 PUFA intake or use of medication by class.
Conclusions
A large effect size of reduced UE EPA, and a lower plasma UE:E concentration ratio of EPA in the symptomatic BD state may be an important factor in vulnerability to a mood state. Altered n-3 PUFA ratios could indicate changes in PUFA metabolism concurrent with symptom improvement. Our findings are consistent with preclinical and postmortem data and suggest testing interventions that increase n-3 and decrease n-6 dietary PUFA intake.
Keywords: bipolar disorder, eicosapentaenoic acid, inflammation, omega-3
Bipolar disorder (BD) is an episodic illness affecting 1.0–4.4% of the population (1). It is a highly heritable polygenic disorder (2), which has a complex, incompletely understood etiology (3). BD type I is characterized by manic episodes of elevated mood, energy and cognition, and by major depressive episodes of lowered mood, energy and cognition. BD type II is characterized by hypomanic episodes of less severity than manic episodes, and by major depressive episodes that are often longer and more difficult to treat than BD type I (4–7). Improvements in acute and maintenance treatments are needed to prevent relapses and reduce burden of disability.
Alterations of metabolism of lipids and resultant changes in cell signaling pathways have been hypothesized to perturb neurotransmitter systems in mood disorders (8, 9). Arachidonic acid [(AA) 20:4n-6] and docosahexaenoic acid [(DHA) 22:6n-3] are polyunsaturated fatty acids (PUFA) that compose over 90% of essential fatty acids in the mammalian brain (10). AA and DHA are derived from the diet or are synthesized in the liver from their respective, nutritionally essential, shorter-chain PUFA, linoleic acid [(LA) 18:2n-6] and alpha-linolenic acid [(ALA) 18:3n-3]. AA, DHA, and their metabolites function as intracellular second messengers during neurotransmission and as modulators of neuroinflammation and other pathological processes (8, 11).
Finding common changes in neurobiological systems among effective mood stabilizing medications, proven effective in Phase III trials, is a potential window into understanding etiological and treatment mechanisms in BD. One suggested common mechanism of action of mood stabilizers, derived from pre-clinical studies, is downregulation of brain AA metabolism (12–14). Epidemiological and clinical data support the hypothesis that altered PUFA metabolism is present in BD, including reduced n-3 PUFA metabolism (15–21).
We hypothesized that plasma concentrations of n-3 PUFA would be lower and plasma concentrations of n-6 PUFA would be higher in subjects with BD who experience manic or depressive symptoms than in healthy controls (HC), and that n-3 PUFA would increase and n-6 PUFA decrease after naturalistic treatment. We also hypothesized that lower n-3 and higher n-6 PUFA concentrations would be associated with manic and depressive symptoms, regardless of medication status. To test these hypotheses, we studied subjects with BD who were in a symptomatic episode while on medication.
In an exploratory analysis, we investigated associations between plasma n-3 and n-6 PUFA concentrations and clinical symptoms of panic, psychosis, and suicidality. We measured both unesterified (UE) and esterified (E) concentrations of nine fatty acids in plasma. We studied plasma concentrations because the UE form of the PUFA passes through the blood-brain barrier into brain more readily than does the E form (22, 23); thus the UE plasma concentration is the major peripheral form that represents PUFA metabolism in brain.
Methods
Participants and measures
Participants were recruited when presenting for care during mood episodes at the Pennsylvania Psychiatric Institute, under Institutional Review Board Protocol No. 39164EP and NIH Office of Human Subject Research Exemption #11509 (7/16/2012). Participants were screened and consented for the study. Patients with BD (i.e., bipolar disorder type I and bipolar disorder type II) and HC with no personal or family history of mood or psychotic disorder were included. Exclusion criteria included daily use of anti-inflammatory medications, active substance intoxication or withdrawal, or pregnancy. The Mini Neuropsychiatric Interview (24), a DSM-IV-TR-based structured interview, was performed, and current mood state was assessed with the Hamilton Depression Rating Scale-21 (HDRS) plus Atypical (25) and the Clinician-Administered Rating Scale for Mania (CARS-M) (26). Subjects completed rating scales including a Food Frequency Questionnaire (FFQ) for assessment of intake of n-3 PUFA (27). Demographic variables of interest included age and sex. Clinical variables of interest included depression severity (HDRS-21 + Atypical) and mania severity (CARS-M). Use of tobacco by smoking in the past month, alcohol use in the past month above recommended sex-specific limits (as recommended by the National Institute of Alcohol Abuse and Alcoholism: men, > 14 drinks/week or four drinks per occasion, and women, > 7 drinks/week or three drinks per occasion) were recorded from subject response (28). Use of antidepressant, antipsychotic, or mood-stabilizing medications was recorded from subject response. Height and weight were measured and recorded. Clinical features of illness including presence of current panic disorder, current suicidality, and current psychosis were obtained from the relevant MINI modules. Current suicidality accounted for history of suicidal attempts, and current intent and plan. A dietary report of ALA, DHA, and EPA was calculated from self-reported intake of omega-3 rich foods recorded on the FFQ.
Subjects were followed and treated as usual. At the start of the study, subjects were taking mood stabilizers (n = 21, 78%: lithium n = 14, carbamazepine n = 3, valproic acid n = 3, and lamotrigine n = 3), antipsychotics (n = 15, 56%: aripiprazole n = 1, haloperidol n = 2, risperidone n = 6, quetiapine n = 6, perphenazine n = 1, and olanzapine n = 2), antidepressants (n = 13, 48%), and/or sedatives (n = 17, 63%). At follow-up, subjects were taking mood stabilizers (n = 9, 69%: lithium n = 4, carbamazepine n = 1, valproic acid n = 1, and lamotrigine n = 1), antipsychotics (n = 7, 54%: aripiprazole n = 2, risperidone n = 3, perphenazine n = 1, and olanzapine n = 2), antidepressants (n = 4, 31%), and/or sedatives (n = 6, 46%). After discharge from the hospital, subjects were followed each week by phone and assessed for clinical improvement. A return visit was scheduled with repeat measures when the subject was asymptomatic, or after three months had elapsed. However, due to irregular contact with some subjects, the maximum number of days for follow-up was 187 (median length of follow-up = 22 days, mean length of follow-up = 52 days).
Sample collection and biochemical analysis
Participants fasted for at least six hours, and blood was drawn in vacutainers containing EDTA for biomarkers in the AM. After centrifugation for 10 min at 3000 rpm, the plasma supernatant was transferred to plastic tubes and maintained at −80°C or on dry ice until processed. Samples were processed for fatty acids after thawing in a blinded fashion at the Brain Physiology and Metabolism Section, NIA.
Each plasma sample (0.5 mL) was extracted using the method described by Folch et al. (29) and Sublette (17). The plasma was mixed with a partition system of 3.0 mL chloroform:methanol (2:1) and extracted with 0.6 mL 0.1 M KCl. Organic extracts were concentrated under N2 at 45°C, and then suspended in 0.5 mL chloroform. Standards and samples were applied to Silica gel 60 TLC plates and the lipids were separated using heptane:diethyl ether:acetic acid (60:40:3) (30). Plates were sprayed with 0.03% TNS (6-p- toluidine-2-naphthalene sulfonic acid) in 50 mM Tris buffer (pH 7.4) and lipid bands were visualized under UV light. Bands corresponding to UE fatty acids were scraped off and then directly methylated using 1% H2SO4 in methanol (v/v) and fatty acid methyl esters (FAMEs) were extracted with heptane (31). Prior to methylation, heptadecanoic acid (17:0) was added as an internal standard. For E fatty acid determinations, 50 μL plasma extract was concentrated under N2 at 45°C, then directly methylated as above. FAMEs were separated using a gas chromatograph (GC) (Model 6890 N; Agilent Technologies, Palo Alto, CA, USA) with a capillary column (SP 2330, 30 m × 0.32 mm i.d.; Supelco, Bellefonte, PA, USA) and a flame ionization detector (32). Plasma fatty acid concentrations (nmol/mL) were calculated by direct proportional comparison of GC peak areas with that of the added 17:0 internal standard.
Data and statistical analysis
SPSS version 20, IBM SPSS Statistics was used for all statistical calculations. Values of continuous variables were compared between BD and HC groups using two-sample t-tests, and categorical variables were compared using the Pearson chi-square test. Paired t-tests were used for baseline and follow-up measurements.
Hypothesis-driven analysis
Outcome variables included the E and UE plasma forms of five PUFA, including LA, AA, ALA, DHA, and eicosapentaenoic acid (EPA, 22:5n-3). Comparisons of (i) plasma fatty acid levels were made between the BD and HC groups using Student t-tests, (ii) between BD baseline and follow-up of plasma fatty acid concentrations using paired t-tests, and (iii) Pearson’s r correlations between mean PUFA concentrations and mania severity, and between mean PUFA concentrations and depression severity, also were performed. Thus, 40 comparisons were investigated in a hypothesis-driven manner. An alpha = 0.05/40 = 0.001 was used as a significance level for these comparisons.
In an exploratory analysis, comparisons were made between the BD and HC groups, and between baseline and follow-up of plasma concentrations in the UE and E forms of the saturated fatty acids, palmitic acid (16:0) and stearic acid (18:0), and of the monounsaturated fatty acids, palmitoleic acid (16:1 n-7) and oleic acid (18:1 n-9). Additionally, the ratios of UE to E concentrations of the five PUFA were calculated to determine the relations between unbound to bound forms; the unbound form crosses the blood-brain barrier more easily (22, 23). Ratios of n-3 PUFA concentrations to each other (DHA to ALA, EPA to ALA and EPA to DHA) in the UE and E forms were constructed to estimate progression in the n-3 metabolism pathway. The UE and E AA:EPA ratios were calculated to estimate the n-6/n-3 ratio, an indication of the balance of the overall body PUFA cascade and their bioactive metabolites involved in neurotransmission and the resolution of inflammation (11, 33, 34). All calculated ratios were compared between the HC and BD groups, and between baseline and follow-up.
Additional exploratory analysis was used to investigate associations between demographic and clinical variables and the fatty acid concentrations and ratios described above. Pearson’s r was used for bivariate correlations. Bivariate correlations were made between the fatty acid UE to E concentration ratios and n-3, n-3/n-6 ratios described above and the following demographic and clinical variables: age, sex, depression severity (HDRS-21 + Atypical), mania severity (CARS-M), smoking (past month), alcohol (use in the past month above recommended guidelines), current panic disorder (MINI), current suicidality (MINI), dietary report of ALA, DHA and EPA (FFQ), and use of antidepressant, antipsychotic or mood-stabilizing medications.
Results
Demographic and clinical description of the sample
A cohort of 27 patients with BD was recruited while in a symptomatic mood episode, and a parallel HC group was recruited for this study. Patients with BD had an average of 35 years at entry to the study and did not differ in age, gender distribution, race, or ethnicity from the HC group (n = 31) (Table 1). The subjects with BD were less likely to be married, and less likely to be employed or a student than the HC group (Table 1).
Table 1.
Demographics
| Healthy controls (n = 31) | Bipolar disorder (n = 27)a | p-value | |
|---|---|---|---|
| Sex, female, n (%) | 18 (60) | 12 (44) | 0.30 |
| Race, n (%) | |||
| Asian | 4 (13) | 0 (0) | 0.19 |
| Black | 1 (3) | 1 (3.7) | |
| White | 26 (84) | 25 (93) | |
| Mixed | 0 (0) | 1 (3.7) | |
| Ethnicity, n (%) | 0.18 | ||
| Hispanic | 2 (6) | 0 (0) | |
| Not Hispanic | 29 (94) | 27 (100) | |
| Marital status, n (%) | 0.03 | ||
| Married | 16 (52) | 5 (19) | |
| Separated | 1 (3) | 1 (3) | |
| Divorced | 0 (0) | 3 (11) | |
| Never married | 14 (46) | 18 (67) | |
| Employment, n (%) | < 0.01 | ||
| Unemployed | 2 (6) | 14 (54) | |
| Disabled | 0 (0) | 5 (19) | |
| Employed | 17 (55) | 6 (23) | |
| Student | 11 (36) | 1 (4) | |
| Retired | 1 (3) | 0 (0) | |
| Smoking, n (%) | 1 (3) | 14 (56) | NA |
| Heavy alcohol use, n (%) | 1 (3) | 4 (15) | |
| Drug use, n (%) | 1 (3) | 10 (39) | |
| Suicidality, n (%) | 0 (0) | 22 (82) | |
| Psychosis, n (%) | 0 (0) | 14 (52) | |
| Panic, n (%) | 0 (0) | 5 (19) | |
| Age, years, mean (SD) | 32 (11) | 35 (11) | 0.31 |
| BMI, mean (SD) | 24.9 (4.7) | 30.5 (6.0) | 1.7 × 10−4 |
| Mania (CARS-M), mean (SD) | 15.3 (12.5) | 0 (0) | |
| Depression (HDRS-21 + Atypical), mean (SD) | 34.4 (14.5) | 0.3 (0.6) |
SD = standard deviation; BMI = body mass index; CARS-M = Clinician-Administered Rating Scale for Mania; HDRS = Hamilton Depression Rating Scale-21.
At baseline.
The subjects with BD were significantly heavier than the HC subjects, with a mean body mass index (BMI) in the obese range [BD: 30.5 (6.0), HC: 24.9 (4.7); p = 1.7 × 10−4). At baseline, the BD group had a severe level of depressive symptoms [mean HDRS-21 + Atypical questions = 34.4 (14.5)] and a moderate level of manic symptoms [mean CARS-M = 15.3 (12.5)]. Severity of manic and depressive symptoms was significantly reduced at follow-up compared with entry [mean HDRS-21 + Atypical = 9.00 (8.98), p < 0.01; mean CARS-M 3.46 (5.36), p = 0.01]. However, more than 50% of the subjects with BD were lost to follow-up. Many subjects had an unstable living situation upon discharge from the hospital and this may have contributed to poor follow-up. Self-report of n-3 EPA, DHA, or ALA consumption using the FFQ did not differ between the HC and BD groups at baseline [EPA HC: 0.03 (0.03), BD: 0.03 (0.04), p = 0.88; DHA HC: 0.06 (0.07), BD: 0.04 (0.06), p = 0.36; ALA HC: 0.33 (0.50), BD: 0.32 (0.65), p = 0.96]; or in the BD group from baseline (BL) to follow-up (FU): [EPA BD BL: 0.02 (0.03), BD FU: 0.02 (0.03), p = 0.28; DHA BD BL: 0.04 (0.05), BD FU: 0.04 (0.05), p = 0.22; ALA HC: 0.28 (0.64), BD: 0.28 (0.23), p = 0.83].
The subjects with BD predominantly were diagnosed with bipolar disorder type I (n = 21, 78%) and a smaller number were diagnosed with bipolar disorder type II (n = 6, 22%). About one-half of the subjects with BD had psychosis with the current episode, the majority (n = 22, 82%) had suicide risk, and 19% (n = 5) had current panic disorder. At the time of assessment, more than one-half of the BD sample smoked (n = 14, 56%), 15% (n = 4) drank above the sex-specific recommended limit within the month prior to assessment, and 39% (n = 10) used an illicit drug within the month prior to assessment. At baseline presentation, 78% (n = 21) of the BD subjects were taking mood-stabilizing medication, 48% (n = 13) were taking antidepressants, and 56% (n = 15) were taking antipsychotic medication. Treatment was not controlled for this study, and on follow-up, 69% of the subjects were taking a mood-stabilizing medication, 54% were taking an antipsychotic, and 31% were taking an antidepressant.
We report our results below categorized by fatty acid or fatty acid group. Unesterified (UE) forms of the FA were measured because they are better able to cross the blood-brain barrier (22, 23), and a difference in the UE:E ratio may signal a difference in availability of the PUFA to the brain, or a slowed production of the UE from the E plasma PUFA due to hydrolysis by circulating or tissue lipases.
Plasma n-3 concentrations, UE:E ratios, n-3 ratios, and clinical correlations
EPA
The mean concentration of UE EPA was lower in the BD than HC group. Although the effect size was large (Cohen’s d = 0.86), the difference bordered on statistical significance after consideration of multiple testing (p = 0.002, alpha set at 0.001). No statistically significant difference was found in the E EPA levels at baseline, nor in either form of EPA in the BD group between baseline and follow-up (Table 1). Neither mania nor depression severity correlated with UE or E EPA plasma concentrations at baseline in the BD group.
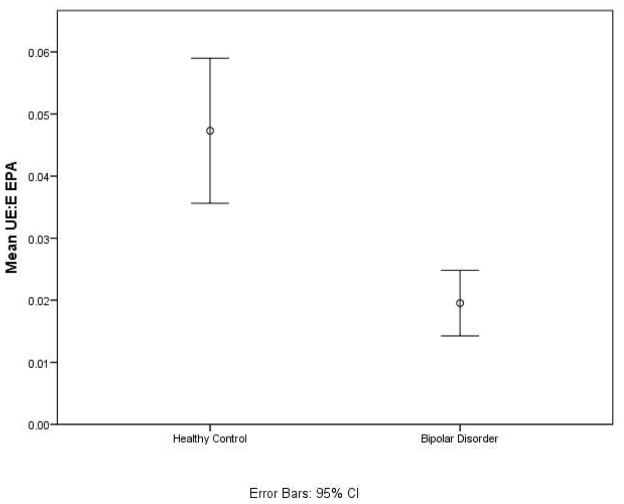
Exploratory analysis revealed that at baseline, the mean ratio of UE:E EPA was significantly lower in the BD than HC group (Table 2 and Fig. 1) (p = 9 × 10−5), but did not differ between BD at baseline or follow-up.
Table 2.
N-3 polyunsaturated fatty acid plasma concentrations and ratios in the bipolar disorder and healthy control groups at baseline and follow-up (nmol/mL)
| Baseline | Bipolar disorder, follow-up | |||||
|---|---|---|---|---|---|---|
| Healthy controls (n = 31) | Bipolar disorder (n = 27) | p-value | Baseline (n = 13) | Follow-up (n = 13) | p-value | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| ALA | ||||||
| E | 19.89 (25.64) | 18.86 (12.81) | 0.85 | 18.48 (17.30) | 25.45 (15.42) | 0.18 |
| UE | 2.10 (2.80) | 1.40 (1.77) | 0.27 | 0.88 (1.06) | 2.28 (1.72) | 0.02 |
| DHA | ||||||
| E | 220.93 (93.79) | 221.37 (83.10) | 0.99 | 227.46 (84.10) | 177.71 (161.19) | 0.28 |
| UE | 1.07 (0.64) | 1.04 (1.04) | 0.89 | 0.73 (0.38) | 0.55 (0.26) | 0.11 |
| EPA | ||||||
| E | 8.91 (6.94) | 13.91 (13.39) | 0.07 | 14.62 (18.98) | 12.68 (6.86) | 0.75 |
| UE | 0.32 (0.18) | 0.20 (0.08) | 0.002 | 0.21 (0.06) | 0.31 (0.26) | 0.16 |
| UE:E | ||||||
| ALA | 0.14 (0.13) | 0.08 (0.10) | 0.05 | 0.08 (0.13) | 0.12 (0.10) | 0.27 |
| DHA | 0.006 (0.005) | 0.005 (0.003) | 0.34 | 0.004 (0.003) | 0.005 (0.003) | 0.32 |
| EPA | 0.05 (0.03) | 0.02 (0.01) | 9 × 10−5 | 0.02 (0.02) | 0.04 (0.07) | 0.24 |
| DHA:ALA | ||||||
| E | 18.20 (11.34) | 14.27 (6.05) | 0.10 | 16.15 (6.05) | 7.44 (5.36) | 0.001 |
| UE | 0.92 (0.86) | 1.13 (0.77) | 0.33 | 1.12 (0.43) | 0.31 (0.17) | 4 × 10−5 |
| EPA:ALA | ||||||
| E | 0.61 (0.23) | 0.71 (0.18) | 0.07 | 0.72 (0.19) | 0.54 (0.20) | 0.06 |
| UE | 0.28 (0.18) | 0.26 (0.17) | 0.78 | 0.36 (0.17) | 0.19 (0.16) | 0.002 |
| EPA:DHA | ||||||
| E | 0.05 (0.03) | 0.08 (0.12) | 0.13 | 0.09 (0.17) | 0.10 (0.07) | 0.86 |
| UE | 0.39 (0.29) | 0.30 (0.21) | 0.18 | 0.37 (0.24) | 0.58 (0.29) | 0.03 |
SD = standard deviation; E = esterified; UE = unesterified; ALA = alpha-linolenic acid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid.
Fig. 1.
At baseline, the mean ratio of unesterified (UE) to esterified (E) concentration of eicosapentaenoic acid (EPA) was significantly lower in the bipolar disorder group when compared to the healthy control group. CI = confidence interval.
Significant exploratory clinical correlations for U or E EPA or UE:E EPA ratios included a positive correlation between age and a higher mean UE EPA concentration (r = 0.46, p = 0.02). Panic attacks and suicidality correlated negatively and significantly with mean UE EPA plasma concentrations (panic attacks: r = −0.41, p = 0.03; suicidality: r = −0.49, p = 0.009). However, mania was positively correlated with the UE:E EPA ratio (mania: r = 0.51, p = 0.006).
DHA
No significant difference was found between UE or E DHA plasma concentrations in the BD and HC groups at baseline or between BD group at follow-up and baseline, using an adjusted alpha for multiple testing (p = 0.001) (Table 2). Neither mania nor depression severity correlated with UE or E DHA plasma concentrations at baseline in the BD group.
Exploratory analysis revealed no differences in mean ratios of UE:E DHA at baseline, nor between BD at baseline or follow-up (Table 2).
Significant exploratory clinical correlations for U or E DHA or UE:E DHA included a positive correlation between panic attacks and UE DHA (r = 0.46, p = 0.02), and between panic attacks and UE:E DHA (r = 0.42, p = 0.03).
ALA
No significant difference was found between UE or E ALA plasma concentrations in the BD and HC groups at baseline or between BD group at follow-up and baseline, using an adjusted alpha for multiple testing (p = 0.001) (Table 2). Neither mania nor depression severity correlated with UE or E ALA plasma concentrations at baseline in the BD group.
Exploratory analysis revealed no differences in mean ratios of UE:E ALA at baseline, nor between BD at baseline or follow-up (Table 2).
Significant exploratory clinical correlations for U or E ALA or UE:E ALA included a negative correlation between psychosis and E ALA (r = −0.46, p = 0.03).
N-3 ratios (DHA:ALA, EPA:ALA, EPA:DHA)
Differences in the ratios of n-3 species between BD and HC, and BD at baseline and follow-up were tested in an exploratory fashion (Table 2). No group difference was found at baseline in the three ratios. At the follow-up time point, investigation of PUFA ratios revealed the UE EPA:ALA was decreased significantly (p = 0.002), and the UE EPA:DHA increased significantly (p = 0.03). Additionally, the E and UE DHA:ALA both decreased significantly (p ≤ 0.001).
Clinical correlations with these ratios revealed that panic attacks correlated negatively and significantly with the EPA:DHA ratio (r = −0.40, p = 0.04), and panic attacks were positively correlated with UE DHA:ALA (r = 0.47, p = 0.01).
Plasma n-6 PUFA concentrations and clinical correlations
No significant difference was found between UE or E LA or UE or E AA concentrations in the BD and HC groups at baseline or between BD group at follow-up and baseline using an adjusted alpha for multiple testing (p = 0.001) (Table 3). There were no significant correlations between UE or E LA, or UE or E AA and mania or depression severity.
Table 3.
N-6 and n-6:n-3 polyunsaturated fatty acid plasma concentrations and ratios in the bipolar disorder and healthy control groups at baseline and follow-up (nmol/mL)
| Baseline | Bipolar disorder, follow-up | |||||
|---|---|---|---|---|---|---|
| Healthy controls (n = 31) | Bipolar disorder (n = 27) | p-value | Baseline (n = 13) | Follow-up (n = 13) | p-value | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| LA | ||||||
| E | 2684.88 (794.62) | 2624.80 (638.07) | 0.75 | 2548.78 (724.90) | 3808.82 (2030.68) | 0.02 |
| UE | 54.07 (30.18) | 62.81 (57.70) | 0.48 | 53.45 (47.66) | 36.66 (27.46) | 0.07 |
| AA | ||||||
| E | 676.94 (202.42) | 720.07 (196.35) | 0.42 | 739.90 (244.02) | 950.11 (449.19) | 0.03 |
| UE | 2.73 (1.64) | 2.85 (2.15) | 0.82 | 2.28 (1.67) | 1.98 (0.84) | 0.46 |
| UE:E | ||||||
| LA | 0.02 (0.01) | 0.03 (0.02) | 0.39 | 0.02 (0.02) | 0.01 (0.01) | 0.02 |
| AA | 0.004 (0.002) | 0.004 (0.003) | 0.85 | 0.003 (0.002) | 0.002 (0.001) | 0.18 |
| AA: EPA | ||||||
| E | 100.69 (55.32) | 68.05 (26.48) | 0.005 | 78.68 (31.02) | 88.05 (39.86) | 0.54 |
| UE | 10.96 (7.59) | 18.59 (22.13) | 0.10 | 11.83 (9.62) | 9.65 (7.18) | 0.28 |
SD = standard deviation; E = esterified; UE = unesterified; LA = linoleic acid; AA = arachidonic acid; EPA = eicosapentaenoic acid.
In exploratory analysis, group differences in the UE:E LA significantly decreased between follow-up and baseline in the BD group that completed follow-up (Table 3).
Investigation for clinical correlations revealed that panic attacks were significantly positively correlated with UE AA (r = 0.43, p = 0.03) and mean UE:E ratios of LA (r = 0.42, p = 0.03) and of AA (r = 0.44, p = 0.02). Psychosis was negatively correlated with E LA (r = −0.45, p = 0.02).
N-6 to n-3 ratio and clinical correlations
The mean ratio of E AA:EPA was significantly lower in BD compared to HC (Table 3) (p = 0.005). There was a medium effect size difference of 0.46 calculated by Cohen’s d in the group difference between HC and BD of UE:E AA:EPA, but the difference was not statistically significant (p = 0.10). Panic (r = 0.59, p = 0.001) and psychosis (r = 0.43, p = 0.03) were significantly and positively correlated with UE AA:EPA ratio.
Saturated and monounsaturated fatty acids
No significant difference was found in saturated or monounsaturated fatty acid concentrations between BD and HC at baseline, or in BD between follow-up and baseline (see Supplementary Table S1). Panic attacks were positively correlated with mean concentrations of several UE fatty acids, including palmitic (r = 0.46, p = 0.03), palmitoleic (r = 0.46, p = 0.02), and oleic (r = 0.44, p = 0.022) acids. Psychosis was negatively correlated with mean E concentrations of palmitic (r = −0.53, p = 0.004) and stearic (r = −0.44, p = 0.02) acids.
Clinical correlations with PUFA concentrations and ratios were explored with age, sex, smoking, alcohol, current panic disorder, current suicidality, and use of antidepressant, antipsychotic, or mood-stabilizing medications. No fatty acid level or ratio correlated significantly with sex (p > 0.07), depression (p > 0.14), smoking (p > 0.06), or alcohol use (p > 0.07). There was no significant correlation between any fatty acid level and use of a medication class (antidepressant p > 0.24, mood stabilizer p > 0.12, or antipsychotic p > 0.08).
Discussion
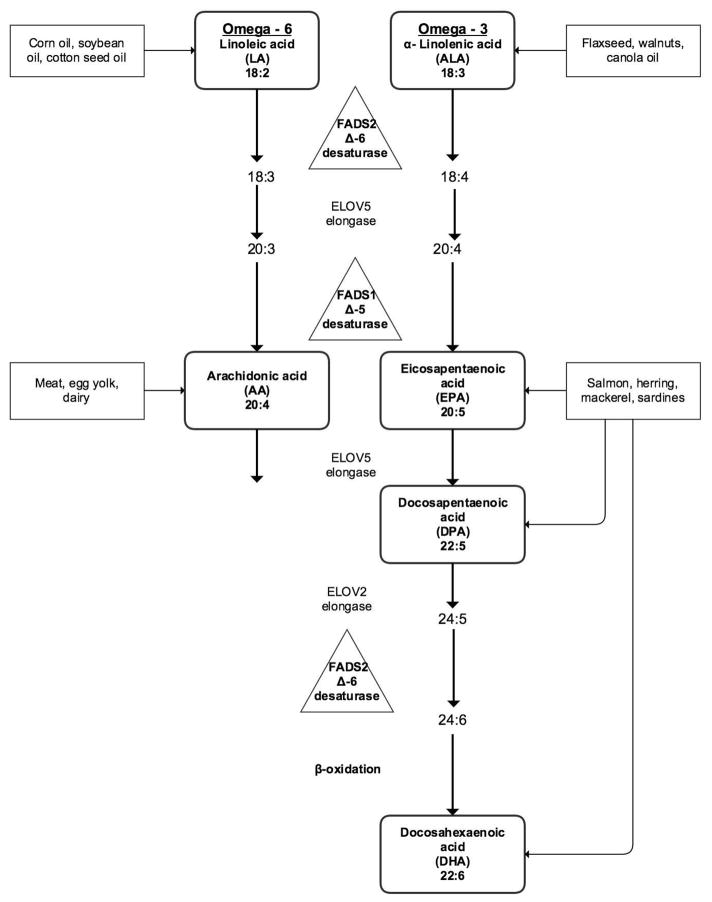
We determined differences in plasma concentrations and ratios of nine esterified (E) and unesterified (UE) long-chain fatty acids, five of which were PUFAs, between 27 BD patients and 31 HC. Two PUFAs belonged to the n-6 series, AA and its nutritionally essential shorter-chain precursor LA, three to the n-3 series, EPA, DHA, and their nutritionally essential shorter chain precursor ALA (illustrated in Fig. 2).
Fig. 2.
The metabolic pathway of the polyunsaturated fatty acids linoleic acid (the n-6 pathway) and alpha-linoenic acid (the n-3 pathway) to arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA).
Based on the literature, we hypothesized that (i) plasma concentrations of n-3 PUFA would be lower and concentrations of n-6 PUFA would be higher in patients with BD with manic or depressive symptoms; (ii) n-3 PUFA would increase and n-6 PUFA decrease after naturalistic treatment of the patients; and (iii) lower n-3 and higher n-6 PUFA concentrations would be associated with manic and depressive symptoms, regardless of medication status.
After correcting for 40 comparisons, we did not find a significant difference between the plasma E or UE concentration of any of the fatty acids, although the effect size indicating a reduced EPA in BD compared with HC was high and the p-value was 0.002, suggesting a robust effect. This suggestion is particularly relevant because this study should be considered exploratory in a unique patient cohort, and the multiple comparison restriction should be taken with reservations considering the likelihood of a Type 2 error (35).
Some evidence indicates that EPA is the active n-3 PUFA responsible for mood-altering effects in n-3 PUFA supplementation studies of depression (36, 37). Low EPA may be a vulnerability factor to experiencing mood episodes, and alteration of its elongation from ALA may influence effective treatment. Other studies of patients with BD also have reported reduced plasma EPA (38), and efficacy of nutritional supplementation with EPA (27, 39), although the issue is far from settled.
EPA metabolites include bioactive cyclopentenone-IsoP molecules, termed A(3)/J(3)-IsoPs, formed by EPA peroxidation (40), and anti-inflammatory resolvin E1 (41). EPA has positive effects in mitochondria (42), a protective effect in cardiac myocytes (43), and a regulatory effect on brain capillary tight junctions (44) and other capillary endothelium (45). In this regard, while unesterified EPA is largely β-oxidized after entering rat brain, as compared with the incorporation of intact DHA into brain membrane phospholipids (46), EPA’s mechanism of action in BD may be at the capillary endothelium, which has a high mitochondrial content (47) and may be disturbed due to neuroinflammation in BD (48).
Lower n-3 PUFA have been seen in a number of clinical studies of BD, particularly studies that investigate erythrocyte concentrations. McNamara et al. (19) found differences in erythrocyte EPA + DHA levels, and three studies reported lower erythrocyte DHA in symptomatic BD groups (18, 19, 49). However, Sublette et al. (17) reported on plasma concentrations of UE and E PUFA, and found no between-group differences in UE PUFA levels or the UE AA:EPA ratio. The critical differences may lie in the different methods of measuring PUFA, in the different symptomatic states of the subject populations, and in the different dietary intake status.
While plasma PUFA concentrations may vary more over time than erythrocyte concentrations, due to the four-month mean life time of circulating erythrocytes, studying plasma concentrations allows us to evaluate circulating UE and E PUFA separately. Additionally, plasma concentrations reflect immediate consequences of changing diet and liver metabolism. Esterase activity on circulating lipoproteins releases UE PUFA, the form that preferably enters the brain, likely by a diffusional mechanism (50, 51). After entering brain, the largest percentage of UE shorter-chain PUFA (e.g. LA, ALA, EPA) is rapidly beta-oxidized by mitochondria (38), whereas the elongated AA and DHA forms preferentially enter the sn-2 position of brain membrane phospholipids to replace the respective PUFAs that have been hydrolyzed and lost by metabolism.
The suggested beneficial effects of certain PUFAs may be peripheral as well as central. Increased UE fatty acids triggered by fasting have been shown in rodents to drive immunosuppression (52). Alternately, conversion of EPA to DHA in the liver (46) may drive the treatment effect. One intervention study using UE PUFA showed that administration of fish oil increased brain UE DHA and a neuroprotective DHA metabolite, neuroprotectin D-1, in a rodent model of Alzheimer’s disease (54). Additionally, phospholipase A2 genes and enzyme levels may differ between BD and HC groups, which may affect production of plasma PUFA (52–58). A recent trial of n-3 PUFA for reduction of plasma triglycerides showed a phospholipase A2 genotype x treatment interaction (59). Therefore investigation of UE and E PUFA provides a different window of investigation than of PUFA within RBC membrane, into the role of PUFA metabolism in BD.
Changes at follow-up in n-3 PUFA metabolism
We engaged participants during an active mood episode, and monitored symptoms during recovery, asking them to return when they no longer met threshold criteria for mania and depression. Treatment was not altered from usual care during this time. During this follow-up period, we lost approximately half of our participants due to inability to contact them. Therefore, our results in investigative comparisons between baseline and follow-up are exploratory and only suggestive due to the small sample size. We found no difference in plasma levels of PUFA when comparing mean baseline concentrations to concentrations at follow-up, however ratios of some plasma n-3 PUFA concentrations were significantly different at follow-up from baseline in BD subjects. There was no change in self-reported n-3 PUFA intake at follow-up. The ratio changes indicate decreases in UE DHA relative to UE ALA, in UE EPA relative to UE ALA, and in E DHA relative to E ALA. Since ALA once ingested can be elongated and desaturated, particularly in the liver, first to EPA and then to DHA (60), these results suggest some limitation to these metabolic conversions. One possibility is drug effects on the liver (61). Though we cannot comment on mechanism from our data, the ratio data suggest abnormal metabolic relations among the three measured n-3 PUFAs.
Symptom and clinical correlations with fatty acid levels and ratios
Symptom severity has been correlated with circulating PUFA biomarkers in the Sublette et al.(17) and Evans et al. (20) studies, but not in the Chiu et al. (18) and McNamara et al. (19) studies. Sublette et al. found a positive correlation between manic symptoms and the UE AA:EPA ratio. In our study, however, the UE AA:EPA ratio was not correlated with mania, but was positively correlated with psychosis. Two factors that could affect this ratio differed in our trial from the Sublette trial, including the symptomatic state of our subjects (we had few subjects with pure mania in our study), and including patients currently on psychiatric medication. We found the UE:E ratio of EPA was positively correlated with mania, which would not be expected given that a lower UE:E EPA was found in BD compared to HC. This will bear further investigation.
In our study, subjects with panic attacks as part of the current mood episode showed lower n-3 and higher n-6 concentrations, and a higher n-6 to n-3 ratio. This pattern suggests activation of an inflammatory pathway or reduction of an anti-inflammatory pathway. The noradrenergic surge seen in panic attacks has been hypothesized to be related to the dopaminergic surge seen in mania in BD, and perhaps panic identifies a subset of mania or is a severity marker of psychiatric illness (62–65). Together, ours and others’ findings suggest an altered PUFA metabolism in BD, in accord with pre-clinical data.
We found a positive correlation between UE saturated fatty acids and panic, and a negative association between E saturated fatty acids and psychosis. The differentiating factor may be the esterification status, as the UE form preferentially enters brain. UE palmitic acid was shown to increase anxiety-like behaviors and immunosuppression in rodent models (66, 67). UE fatty acids may be associated with clinical phenotype through an immunological link.
Medication status
While both Sublette et al. (17) and McNamara et al. (19) studied PUFA in un-medicated patients, the Chiu et al. study (18) found no difference between un-medicated and medicated patients. Our approach differed, as we studied relations between effective treatment and biological markers. We included patients who were ill though medicated, and those with comorbid psychiatric illness, specifically looking for differences before and after treatment. We had no un-medicated subjects in our sample, but we did not find that concentrations or ratios of PUFA were correlated with antidepressant, antipsychotic, or mood stabilizers as medication groups.
Dietary report
We found no correlations between self-report of ALA, EPA, and DHA intake and plasma fatty acid levels, which could occur for several reasons. The subjects in this study may have been unable to accurately fill out the questionnaire given the severity of mood symptoms. A more controlled dietary regimen would be useful in this regard (68). Additionally, individual metabolic differences may mask correlations with reported intake. The memory difficulty inherent in mood episodes may have interfered with the ability to report food intake accurately. Further study with this instrument would be beneficial to understand barriers to its use.
Limitations
Limitations include the use of patients who are taking medication in a study of biomarkers. While the heterogeneity of the subjects with BD with regard to medication use at baseline, in follow-up and comorbid psychiatric illness may limit the precision with which the findings of PUFA differences can be related to bipolar neuropathology, this study design maximizes the generalizability of the findings to a real-world clinical population.
If EPA metabolism differs in patients who are symptomatic and is altered with successful treatment, regardless of the medication class, this suggests that effective medications in practice have a similar biological impact on the n-3/n-6 metabolic balance, mirroring pre-clinical findings (12). Additionally, our results at follow-up are only suggestive, as (i) treatment was not delivered as a controlled intervention, and (ii) less than 50% of the sample completed the follow-up. While baseline factors did not differ between the groups that did or did not follow-up, we could not measure treatment response in those who did not complete the study. The patients had a high rate of unstable housing and contact information, which may have been a factor in their inability to complete the study. Other limitations include self-report of dietary n-3 input only. Baseline dietary intake of PUFA may indeed influence response of the system to intervention. In addition, this study was not large enough to look for subgroups of subjects who may have PUFA alterations, while others may not.
Conclusions
We found (i) a lower plasma UE:E concentration ratio of EPA, (ii) a trend toward lower U EPA in the symptomatic BD state, and (iii) altered n-3 PUFA ratios upon follow-up in a subset of the BD sample. Altered plasma circulating n-3 PUFA concentrations may influence vulnerability to a mood state, and altered n-3 PUFA ratios could indicate changes in PUFA metabolism concurrent with symptom improvement. Panic attacks, which may be a marker of clinical severity, were correlated with lower n-3 and higher n-6 PUFA concentrations and a higher n-6 to n-3 ratio. Our findings are consistent with preclinical and postmortem data and suggest testing interventions that increase n-3 and decrease n-6 dietary PUFA intake.
Implications
While initial n-3 PUFA supplementation studies in BD demonstrated efficacy in open trials (69–72), and subsequent randomized controlled trials (73, 74) failed to replicate these findings (75–79), preclinical and post-mortem data implicate PUFA in pathophysiology of BD (12, 13). Interpretation of the varied responses seen in randomized, controlled trials is confounded by a number of factors, including heterogeneity of diagnosis, design of trials, compliance to study drug, and composition and dose of supplements (36, 80–84).
One reason that supplement studies may have not shown effect may be that in the past century, the n-6 PUFA precursor LA has increased in the average US diet, resulting in decreasing tissue concentrations of n-3 EPA and DHA and increasing concentrations of AA and LA and their metabolites (85–87). Addition of an n-3 supplement without concurrent reduction in dietary n-6 LA may not produce clinically meaningful benefit. A recent randomized trial investigated PUFA dietary intervention in chronic migraine headache, a condition with considerable overlap with BD in pathophysiology, comorbidity, and treatment (34, 88–101). Clinical efficacy and biochemical effects of a high n-3 EPA + DHA plus low n-6 LA (H3-L6) diet were compared to a low n-6 PUFA diet (L6). The H3-L6 group experienced a significantly greater reduction in headache hours per day, headache days per month, headache-related quality-of-life and psychological distress than the L6 group, though both groups experienced improvement from the run-in phase (68). Interestingly, both the L6 and the H3-L6 groups had increases in UE EPA, specifically, among PUFA (102). Based on the clinical and neuroinflammatory links between BD and migraine, concurrent lowering of dietary n-6 in BD may also be necessary for effective treatment of bipolar disorder with n-3 PUFA supplementation (103). Future studies of the specific signaling pathways and lipid mediators linking n-3 and n-6 PUFA to BD pathogenesis may lead to development of targeted dietary and medication strategies for treating BD.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the participants in the study who donated time and effort to research. The project described was supported by the National Center for Research Resources, Grant KL2 RR033180 (EFHS), and is now at the National Center for Advancing Translational Sciences, Grant KL2 TR000126. We thank Mr. Kaizong Ma at the Brain Physiology and Metabolism Section, Laboratory of Neurosciences, National Institute on Aging, National Institutes of Health (NIH) for performing the plasma fatty acid analyses. The contributions of Kaizong Ma and SIR were supported entirely by the Intramural Program of the National Institute on Aging, NIH, who has no conflict of interest with regard to this paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsors of this research did not have direct influence over the collection, analysis or interpretation of data.
Footnotes
Disclosures
EFHS has been a consultant for Projects in Knowledge, CME. AJG has received investigator-initiated grant support from Pfizer Pharmaceutical; has been a consultant to Zynx, Allergan, Forest, and ZARS Pharma; and is a stock holder in Healthcare Technology Systems, Inc. AR, GS, and SIR do not have any conflicts of interest to report.
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archiv Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craddock N, Sklar P. Genetics of bipolar disorder. Lancet. 2013;381:1654–1662. doi: 10.1016/S0140-6736(13)60855-7. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. New York: Oxford University Press; 2007. p. 1262. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5. Washington, DC: American Psychiatric Association; 2013. DSM-5 Task Force; p. 947. [Google Scholar]
- 5.Ghaemi SN, Bauer M, Cassidy F, et al. Diagnostic guidelines for bipolar disorder: a summary of the International Society for Bipolar Disorders Diagnostic Guidelines Task Force Report. Bipolar Disord. 2008;10:117–128. doi: 10.1111/j.1399-5618.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 6.Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11:453–473. doi: 10.1111/j.1399-5618.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 7.Vieta E, Suppes T. Bipolar II disorder: arguments for and against a distinct diagnostic entity. Bipolar Disord. 2008;10:163–178. doi: 10.1111/j.1399-5618.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 8.Hibbeln JR, Palmer JW, Davis JM. Are disturbances in lipid-protein interactions by phospholipase-A2 a predisposing factor in affective illness? Biol Psychiatry. 1989;25:945–961. doi: 10.1016/0006-3223(89)90274-6. [DOI] [PubMed] [Google Scholar]
- 9.Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nature. 1971;233:267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- 10.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9:570–579. [PubMed] [Google Scholar]
- 11.Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport SI. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem Neurosci. 2014;5:459–467. doi: 10.1021/cn500058v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry. 2002;59:592–596. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- 15.Freeman MP. Omega-3 fatty acids and perinatal depression: a review of the literature and recommendations for future research. Prostaglandins Leukot Essent Fatty Acids. 2006;75:291–297. doi: 10.1016/j.plefa.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 17.Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu CC, Huang SY, Su KP, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 19.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans SJ, Kamali M, Prossin AR, et al. Association of plasma omega-3 and omega-6 lipids with burden of disease measures in bipolar subjects. J Psychiatr Res. 2012;46:1435–1441. doi: 10.1016/j.jpsychires.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans SJ, Prossin AR, Harrington GJ, et al. Fats and factors: lipid profiles associate with personality factors and suicidal history in bipolar subjects. PloS One. 2012;7:e29297. doi: 10.1371/journal.pone.0029297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J Lipid Res. 1997;38:526–530. [PubMed] [Google Scholar]
- 23.Ouellet M, Emond V, Chen CT, et al. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: an in situ cerebral perfusion study. Neurochem Int. 2009;55:476–482. doi: 10.1016/j.neuint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 25.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- 27.Sublette ME, Segal-Isaacson CJ, Cooper TB, et al. Validation of a food frequency questionnaire to assess intake of n-3 polyunsaturated fatty acids in subjects with and without major depressive disorder. J Am Diet Ass. 2011;111:117–123. e1–2. doi: 10.1016/j.jada.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NIAAA Alcohol Facts and Statistics [webpage] National Institute on Alcohol Abuse and Alcoholism; 2015. updated 3/2015; cited 2015 7/7/2015. [Google Scholar]
- 29.Folch J, Lees M, Sloan Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 30.Breckenridge WC, Kuksis A. Specific distribution of short-chain fatty acids in molecular distillates of bovine milk fat. J Lipid Res. 1968;9:388–393. [PubMed] [Google Scholar]
- 31.Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutrition. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- 32.DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 33.Serhan CN. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol Clin. 2006;24:341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Ramsden CE, Mann JD, Faurot KR, et al. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. 2011;12:97. doi: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 38.Evans SJ, Ringrose RN, Harrington GJ, Mancuso P, Burant CF, McInnis MG. Dietary intake and plasma metabolomic analysis of polyunsaturated fatty acids in bipolar subjects reveal dysregulation of linoleic acid metabolism. J Psychiatr Res. 2014;57:58–64. doi: 10.1016/j.jpsychires.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2008;283:12043–12055. doi: 10.1074/jbc.M800122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeng JY, Lee WH, Tsai YH, Chen CY, Chao SY, Hsieh RH. Functional modulation of mitochondria by eicosapentaenoic acid provides protection against ceramide toxicity to C6 glioma cells. J Agric Food Chem. 2009;57:11455–11462. doi: 10.1021/jf902021h. [DOI] [PubMed] [Google Scholar]
- 43.Leroy C, Tricot S, Lacour B, Grynberg A. Protective effect of eicosapentaenoic acid on palmitate-induced apoptosis in neonatal cardiomyocytes. Biochim Biophys Acta. 2008;1781:685–693. doi: 10.1016/j.bbalip.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Yamagata K, Tagami M, Takenaga F, Yamori Y, Nara Y, Itoh S. Polyunsaturated fatty acids induce tight junctions to form in brain capillary endothelial cells. Neurosci. 2003;116:649–656. doi: 10.1016/s0306-4522(02)00715-7. [DOI] [PubMed] [Google Scholar]
- 45.Omura M, Kobayashi S, Mizukami Y, et al. Eicosapentaenoic acid (EPA) induces Ca(2+)-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Letters. 2001;487:361–366. doi: 10.1016/s0014-5793(00)02351-6. [DOI] [PubMed] [Google Scholar]
- 46.Igarashi M, Chang L, Ma K, Rapoport SI. Kinetics of eicosapentaenoic acid in brain, heart and liver of conscious rats fed a high n-3 PUFA containing diet. Prostaglandins Leukot Essent Fatty Acids. 2013;89:403–412. doi: 10.1016/j.plefa.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oldendorf WH, Brown WJ. Greater number of capillary endothelial cell mitochondria in brain than in muscle. Proc Soc Exp Biol Med. 1975;149:736–738. doi: 10.3181/00379727-149-38889. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein BI, Young LT. Toward clinically applicable biomarkers in bipolar disorder: focus on BDNF, inflammatory markers, and endothelial function. Curr Psychiatry Rep. 2013;15:425. doi: 10.1007/s11920-013-0425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pomponi M, Janiri L, La Torre G, et al. Plasma levels of n-3 fatty acids in bipolar patients: deficit restricted to DHA. J Psychiatr Res. 2013;47:337–342. doi: 10.1016/j.jpsychires.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Smith QR, Nagura H. Fatty acid uptake and incorporation in brain: studies with the perfusion model. J Mol Neurosci. 2001;16:167–172. doi: 10.1385/JMN:16:2-3:167. [DOI] [PubMed] [Google Scholar]
- 51.Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 52.Dawson E, Gill M, Curtis D, et al. Genetic association between alleles of pancreatic phospholipase A2 gene and bipolar affective disorder. Psychiatr Genet. 1995;5:177–180. doi: 10.1097/00041444-199524000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Ikenaga EH, Talib LL, Ferreira AS, Machado-Vieira R, Forlenza OV, Gattaz WF. Reduced activities of phospholipases A2 in platelets of drug-naïve bipolar disorder patients. Bipolar Disord. 2015;17:97–101. doi: 10.1111/bdi.12229. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen NJ, Franks EK, Owen MJ, Craddock NJ. Mutational analysis of phospholipase A2A: a positional candidate susceptibility gene for bipolar disorder. Mol Psychiatry. 1999;4:274–279. doi: 10.1038/sj.mp.4000476. [DOI] [PubMed] [Google Scholar]
- 55.Meira-Lima I, Jardim D, Junqueira R, Ikenaga E, Vallada H. Allelic association study between phospholipase A2 genes and bipolar affective disorder. Bipolar Disord. 2003;5:295–299. doi: 10.1034/j.1399-5618.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 56.Noponen M, Sanfilipo M, Samanich K, et al. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol Psychiatry. 1993;34:641–649. doi: 10.1016/0006-3223(93)90157-9. [DOI] [PubMed] [Google Scholar]
- 57.Ross BM, Hughes B, Kish SJ, Warsh JJ. Serum calcium-independent phospholipase A2 activity in bipolar affective disorder. Bipolar Disord. 2006;8:265–270. doi: 10.1111/j.1399-5618.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. 2011;16:419–428. doi: 10.1038/mp.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tremblay BL, Cormier H, Rudkowska I, Lemieux S, Couture P, Vohl MC. Association between polymorphisms in phospholipase A2 genes and the plasma triglyceride response to an n-3 PUFA supplementation: a clinical trial. Lipids Health Dis. 2015;14:12. doi: 10.1186/s12944-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Upregulated liver conversion of alpha-linolenic acid to docosahexaenoic acid in rats on a 15 week n-3 PUFA-deficient diet. J Lipid Res. 2007;48:152–164. doi: 10.1194/jlr.M600396-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Differential effects of antipsychotic medications on polyunsaturated fatty acid biosynthesis in rats: relationship with liver delta6-desaturase expression. Schizophr Res. 2011;129:57–65. doi: 10.1016/j.schres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacKinnon DF, Zamoiski R. Panic comorbidity with bipolar disorder: what is the manic–panic connection? Bipolar Disord. 2006;8:648–664. doi: 10.1111/j.1399-5618.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 63.Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ. Does the brain noradrenaline network mediate the effects of the CO2 challenge? J Psychopharmacol. 2003;17:252–259. doi: 10.1177/02698811030173002. [DOI] [PubMed] [Google Scholar]
- 64.Bunney WE, Jr, Goodwin FK, Murphy DL, House KM, Gordon EK. The “switch process” in manic-depressive illness. II. Relationship to catecholamines, REM sleep, and drugs. Arch Gen Psychiatry. 1972;27:304–309. doi: 10.1001/archpsyc.1972.01750270014002. [DOI] [PubMed] [Google Scholar]
- 65.Joyce PR, Fergusson DM, Woollard G, Abbott RM, Horwood LJ, Upton J. Urinary catecholamines and plasma hormones predict mood state in rapid cycling bipolar affective disorder. J Affect Disord. 1995;33:233–243. doi: 10.1016/0165-0327(94)00094-p. [DOI] [PubMed] [Google Scholar]
- 66.Joesting JJ, Moon ML, Gainey SJ, Tisza BL, Blevins NA, Freund GG. Fasting Induces IL-1 Resistance and Free-Fatty Acid-Mediated Up-Regulation of IL-1R2 and IL-1RA. Frontiers Immunol. 2014;5:315. doi: 10.3389/fimmu.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moon ML, Joesting JJ, Lawson MA, et al. The saturated fatty acid, palmitic acid, induces anxiety-like behavior in mice. Metabolism. 2014;63:1131–1140. doi: 10.1016/j.metabol.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsden CE, Faurot KR, Zamora D, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154:2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatry. 2005;66:726–729. doi: 10.4088/jcp.v66n0608. [DOI] [PubMed] [Google Scholar]
- 70.Sagduyu K, Dokucu ME, Eddy BA, Craigen G, Baldassano CF, Yildiz A. Omega-3 fatty acids decreased irritability of patients with bipolar disorder in an add-on, open label study. Nutrition J. 2005;4:6. doi: 10.1186/1475-2891-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wozniak J, Biederman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Europ J Clin Nutrition. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 73.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 74.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 75.Chiu CC, Huang SY, Chen CC, Su KP. Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J Clin Psychiatry. 2005;66:1613–1614. doi: 10.4088/jcp.v66n1219b. [DOI] [PubMed] [Google Scholar]
- 76.Keck PE, Jr, Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60:1020–1022. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 77.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21:435–439. doi: 10.1177/0269881106067787. [DOI] [PubMed] [Google Scholar]
- 78.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12:142–154. doi: 10.1111/j.1399-5618.2010.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy BL, Stoll AL, Harris PQ, et al. Omega-3 fatty acid treatment, with or without cytidine, fails to show therapeutic properties in bipolar disorder: a double-blind, randomized add-on clinical trial. J Clin Psychopharmacol. 2012;32:699–703. doi: 10.1097/JCP.0b013e318266854c. [DOI] [PubMed] [Google Scholar]
- 80.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21. doi: 10.1186/1476-511X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutrition. 2009;28:525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 82.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144–1149. doi: 10.1038/mp.2012.25. [DOI] [PubMed] [Google Scholar]
- 83.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 84.Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012;17:1161–1163. doi: 10.1038/mp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramsden CE, Ringel A, Feldstein AE, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87:135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baptista T, Uzcategui E, Arape Y, et al. Migraine life-time prevalence in mental disorders: concurrent comparisons with first-degree relatives and the general population. Investigacion Clinica. 2012;53:38–51. [PubMed] [Google Scholar]
- 89.Ortiz A, Cervantes P, Zlotnik G, et al. Cross-prevalence of migraine and bipolar disorder. Bipolar Disord. 2010;12:397–403. doi: 10.1111/j.1399-5618.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 90.Dilsaver SC, Benazzi F, Oedegaard KJ, Fasmer OB, Akiskal HS. Is a family history of bipolar disorder a risk factor for migraine among affectively ill patients? Psychopathology. 2009;42:119–123. doi: 10.1159/000204762. [DOI] [PubMed] [Google Scholar]
- 91.McIntyre RS, Konarski JZ, Wilkins K, Bouffard B, Soczynska JK, Kennedy SH. The prevalence and impact of migraine headache in bipolar disorder: results from the Canadian Community Health Survey. Headache. 2006;46:973–982. doi: 10.1111/j.1526-4610.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 92.McIntyre RS, Konarski JZ, Soczynska JK, et al. Medical comorbidity in bipolar disorder: implications for functional outcomes and health service utilization. Psychiatr Serv. 2006;57:1140–1144. doi: 10.1176/ps.2006.57.8.1140. [DOI] [PubMed] [Google Scholar]
- 93.Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001;21:894–899. doi: 10.1046/j.1468-2982.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 94.Fasmer OB, Oedegaard KJ. Clinical characteristics of patients with major affective disorders and comorbid migraine. World J Biol Psychiatry. 2001;2:149–155. doi: 10.3109/15622970109026801. [DOI] [PubMed] [Google Scholar]
- 95.Saunders EF, Nazir R, Kamali M, et al. Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine. J Clin Psychiatry. 2014;75:512–519. doi: 10.4088/JCP.13m08623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia. 2012;32:822–833. doi: 10.1177/0333102412451360. [DOI] [PubMed] [Google Scholar]
- 97.Moskowitz MA, Buzzi MG. Migraine general aspects. Handbook Clin Neurol. 2010;97:253–266. doi: 10.1016/S0072-9752(10)97021-8. [DOI] [PubMed] [Google Scholar]
- 98.Buzzi MG, Moskowitz MA. The pathophysiology of migraine: year 2005. J Headache Pain. 2005;6:105–111. doi: 10.1007/s10194-005-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buzzi MG, Moskowitz MA. The trigemino-vascular system and migraine. Pathologie-biologie. 1992;40:313–317. [PubMed] [Google Scholar]
- 100.BALANCE Investigators and collaborators. Geddes JR, Goodwin GM, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375:385–395. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- 101.Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;6:CD010611. doi: 10.1002/14651858.CD010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taha AY, Cheon Y, Faurot KF, et al. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot Essent Fatty Acids. 2014;90:151–157. doi: 10.1016/j.plefa.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.MacIntosh BA, Ramsden CE, Faurot KR, et al. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br J Nutrition. 2013;110:559–568. doi: 10.1017/S0007114512005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.