Abstract
Cells rely on multiple intracellular trafficking pathways to capture antigens for proteolysis. The resulting peptides bind to MHC class II molecules to promote CD4+ T cell recognition. Endocytosis enhances the capture of extracellular and cell surface bound antigens for processing and presentation, while autophagy pathways shunt cytoplasmic and nuclear antigens for presentation in the context of MHC class II molecules. Understanding how physiological changes and cellular stress alter antigen trafficking and the repertoire of peptides presented by class II molecules remains challenging, yet important in devising novel approaches to boost immune responses to pathogens and tumors. An abundant, constitutively expressed cytoplasmic chaperone, HSC70 plays a central role in modulating antigen transport within cells to control MHC class II presentation during nutrient stress. HSC70 may serve as a molecular switch to modulate endocytic and autophagy pathways, impacting the source of antigens delivered for MHC class II presentation during cellular stress.
Keywords: HSC70, Antigen Presentation, Cell Stress, MHC class II Molecules, Autophagy
MHC class II presentation and HSC70
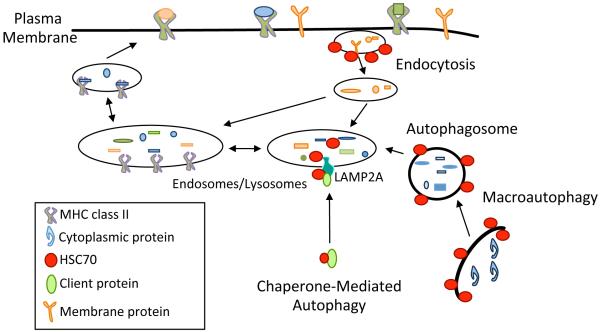
MHC class II molecules are detected on the surface of professional Ag presenting cells (APCs): dendritic cells, B cells, and macrophages as well as some endothelial, epithelial and tumor cells (Crotzer and Blum, 2008). The heterodimeric MHC class II molecules fold in the endoplasmic reticulum (ER) and are trafficked to the protease rich-endosomal/lysosomal network where they bind to small peptides derived from proteolytically processed protein antigens (Ags). These peptide-loaded MHC class II molecules are then shuttled to the cell surface for display to CD4+ T cells (Figure 1). T cell receptors and accessory molecules on CD4+ T cells recognize specific MHC class II:peptide complexes along with co-stimulatory molecules on these APCs. Upon this recognition, CD4+ T cells are activated, secreting cytokines and initiating an immune response.
Figure 1. The role of HSC70 in MHC class II antigen trafficking and presentation.
Exogenous Ags are internalized via clathrin-mediated endocytosis and delivered to the endosomal/lysosomal network. Endogenous Ags can be delivered by autophagy pathways such as macroautophagy (MA) and chaperone-mediated autophagy (CMA). Once delivered into the endosomal/lysosomal network, acidic proteases within these compartments process the Ag into small peptides. These peptides then bind MHC class II molecules. MHC class II:peptide complexes are shuttled to the surface to be displayed for recognition by CD4+ T cells. HSC70 is required for clathrin-mediated endocytosis, as the ATPase activity of HSC70 is required for coating and uncoating of vesicles. HSC70 is a key component of CMA, binding to client proteins and guiding them to the lysosomal membrane for interactions with LAMP2A. Furthermore, HSC70 in the lumen of the lysosome is required for translocation of these peptides. While association of HSC70 with autophagsome membranes has been detected, the function of this has not been established though it may be related to selective-autophagy. During selective-autophagy, HSC70 and its co-chaperone BAG3 guide client proteins to p62 and LC3, leading to their degradation via MA.
MHC class II molecules display thousands of peptide ligands on the surface of a cell, yet how this spectrum of peptides is shaped by cell stress or metabolic changes is unclear. Classically MHC class II molecules were thought to only present epitopes derived from membrane and exogenous Ags that are internalized via endocytosis. Yet, epitopes derived from cytoplasmic and nuclear sources are also detected bound to these MHC molecules (Crotzer and Blum, 2008; Zhou et al., 2005). Peptide elution from MHC class II molecules on the surface of human B cells revealed greater than 85% of peptides were derived from endogenous sources, including peptides derived from cytoplasmic, nuclear and membrane proteins (Chicz et al., 1993). These studies suggest Ags may be delivered by several pathways to encounter MHC class II molecules including endocytosis, macroautophagy and chaperone-mediated autophagy (CMA) (Crotzer and Blum, 2008).
HSC70, sometimes referred to as HSC73, is a constitutively expressed cytosolic member of the heat shock protein 70 family. This abundantly expressed protein constitutes approximately 1% of the total protein within a cell. While primarily localized in the cytoplasm, HSC70 has also been reported to be associated with lysosomes, endosomes and exosomes (Stricher et al., 2013). As a chaperone, HSC70 binds hydrophobic and basic amino acids with a KFERQ-like motif to prevent the unfolding and aggregation of proteins, thereby facilitating numerous cellular processes (Stricher et al., 2013). Furthermore, studies have implicated this versatile chaperone in several protein trafficking pathways including CMA (Stricher et al., 2013). Taking into consideration its abundance and involvement in intracellular protein folding and trafficking, it is not surprising that HSC70 has been implicated in immune recognition. Studies from our laboratory have revealed MHC class II presentation of cytoplasmic autoantigens via CMA is dependent on HSC70 (Zhou et al., 2005). Furthermore, within the lumen of the lysosome, HSC70 protects peptides from degradation by binding and delivery to MHC class II molecules for presentation (Auger et al., 1996). These studies point to a role for HSC70 in selectively binding Ags or antigenic epitopes, to impact MHC class II molecule function in APCs. Yet, recent work by our laboratory and others suggest HSC70 plays an equally important role in intracellular Ag sorting and responses to cell stress.
Nutrient deprivation and Ag presentation
Malnutrition remains a global problem, affecting 1 in 4 individuals worldwide, adversely affecting the neuroendocrine, cardiac and hematopoietic systems. Although poorly understood, malnutrition severely disrupts host immunity and responses to infection. Studies have shown malnourished children have reduced Ab responses as well as reduced B cell expansion in response to infection (Cripps et al., 2008; Najera et al., 2004). Amino acid starvation of human B lymphoblasts affects cathepsin activity and MHC class II epitope selection, raising questions as to how severe nutrient stress may impact Ag presentation (Dengjel et al., 2005). Induction of high affinity, long-lasting humoral immunity is dependent upon interactions between B and T lymphocytes. Taken together these studies suggest that the nutrient status of the host may affect MHC class II Ag presentation.
In response to serum deprivation, B lymphocytes altered cellular autophagy pathways leading to enhanced MHC class II presentation of Ags delivered into autophagosomes. Unexpectedly, under these same conditions, MHC class II presentation of Ags delivered by CMA and conventional clatherin-mediated endocytosis were perturbed (Deffit and Blum, 2015). These stress-induced differences in MHC class II presentation of Ags via macroautophagy and CMA were most surprising. While the proteolysis of Ags in autophagosomes increased with B cell stress, the proteolysis of Ags which are targeted for degradation by CMA and endocytosis was greatly diminished. Yet, cellular proteasome and acidic cathepsin activity increased several fold with serum nutrient deprivation, suggesting alterations in Ag trafficking, processing and presentation. Perhaps the most dramatic change during nutrient stress was the diminished endocytosis of the B cell receptor (BCR). BCR levels on the cell surface increased while endocytosis and turnover of this receptor dropped significantly in serum deprived B cells. Similar results were observed using human B lymphoblasts and peripheral blood B cells (Deffit and Blum, 2015). BCR-derived peptides are typically abundant among MHC class II ligands, yet MHC class II presentation of these endogenous ligands was severely diminished with nutrient stress. To understand this rapid shift in MHC class II presentation with nutrient deprivation, we looked for commonalities in the pathways which target Ags for processing and presentation.
Endocytosis and HSC70
Clathrin-mediated endocytosis is the process by which cell surface receptor-ligand complexes are internalized into cells. During clathrin-mediated endocytosis, adaptor proteins, including AP2, associate with membrane signaling molecules at the plasma membrane. These adaptor proteins then associate with clathrin, which forms the main coat directing membrane invagination to give rise to clathrin-coated vesicles containing receptors such as the BCR, mannose receptor, transferrin receptor, and to a lesser extent MHC molecules. More than 60 proteins are involved in clathrin-mediated endocytosis, including HSC70 (Merrifield and Kaksonen, 2014). HSC70 is involved in chaperoning clathrin, not only to prime vesicle formation but also uncoating clathrin from vesicles as these mature into early endosomes (Jiang et al., 2000). A requirement for the ATPase activity of HSC70 was established for clathrin-mediated endocytosis (Figure 1) (Stricher et al., 2013).
HSC70’s role in chaperoning client proteins for proteolytic processing
In conjunction with other cytoplasmic chaperones, HSC70 selectively guides proteins to the proteasome for degradation or alternatively to lysosomes for translocation via CMA. This role of HSC70 in determining client protein fate, appears widely conserved as shown in neural and immune cells. In CMA, HSC70 binds to a pentapeptide (KFERQ-like) motif within proteins, transiting proteins and peptides to the lysosomal membrane (Majeski and Dice, 2004). Chaperones, such as HSP90 also are required for CMA and MHC class II presentation of Ags via this pathway (Houlihan et al., 2009). On the lysosomal membrane a transmembrane protein, LAMP2A, associates with HSC70 and its client protein. Disruption of LAMP2A expression impacts MHC class II presentation of Ags via CMA (Zhou et al., 2005). During CMA the client protein is unfolded and translocated into the lysosome for degradation (Salvador et al., 2000). While cytoplasmic HSC70 has been implicated in the targeting of proteins to the lysosome for CMA, lumenal HSC70 may function in ratcheting or pulling proteins across the lysosomal membrane through a pore formed by LAMP2A (Figure 1) (Cuervo et al., 1995). HSC70 has also been reported to facilitate microautophagy, a process where the endosomal membrane invaginates to deliver cytoplasmic proteins into these vesicles in dendritic cells (Stricher et al., 2013). Whether microautophagy contributes epitopes for Ag presentation remains unclear.
Macroautophagy and the capture of protein aggregates via HSC70
During macroautophagy, a crescent shaped membrane is formed in the cytoplasm, which can engulf some organelles, large portions of the cytoplasm, or intracellular pathogens during infection. The autophagosome then matures as it fuses with lysosomes and endosomes in a process which can also deliver nuclear and cytoplasmic Ags to MHC class II molecules (Crotzer and Blum, 2008). Several protein complexes are required for macroautophagy including Ulk1/2 complex, Beclin complex, ATG12/5 conjugation system and the LC3 conjugation system (Mizushima et al., 2011). Conjugation of Ags to LC3 can promote their delivery into autophagosomes and presentation by MHC class II molecules (Schmid et al., 2007). While macroautophagy is generally thought of as a bulk form of degradation, specific targeting of cytoplasmic proteins to autophagosomes in a form of selective autophagy has been demonstrated. The macroautophagy receptor p62 has been implicated in this process as it binds ubquitinated targets and links them to the LC3 protein on autophagosome membranes (Ichimura et al., 2008). Interestingly, HSC70 is known to associate with protein aggregates, inducing ubiquitination of these substrates and targeting them for recognition by p62 (Arndt et al., 2010). Furthermore, HSC70 associates with autophagosome membranes (Figure 1) (Kettern et al., 2011). The mechanism triggering HSC70 binding to autophagosomes remains poorly defined.
Cellular stress and HSC70
Intracellular competition for HSC70 during cell stress has been observed, as induction of macroautophagy by protein aggregates depleted chaperone reserves impairing clathrin-mediated endocytosis (Yu et al., 2014). Furthermore, studies from our laboratory indicate during nutrient stress in B lymphocytes, endocytosis and CMA were impaired while macroautophagy was up-regulated (Deffit and Blum, 2015). These trafficking pathways may compete for the conserved chaperone HSC70 to impact MHC class II presentation; with upregulation of macroautophagy and proteasome activity at the expense of endocytosis and CMA. To address this question, we examined whether ectopic overexpression of HSC70 in B lymphocytes could overcome the effects of nutrient deprivation on antigen trafficking and presentation. Expression of ectopic HSC70 in B cells restored BCR endocytosis and Ag trafficking as well as MHC class II presentation. These studies suggest under conditions of limiting nutrients, HSC70 may be diverted to promote macroautophagy at the expense of endocytosis and CMA pathways. These studies also reveal a critical role for cellular stress in shifting the repertoire of epitopes captured and presented by MHC class II molecules. HSC70 plays a key role in this process by selectively regulating endocytosis and autophagy during nutritional stress, to impact MHC class II Ag presentation and B-T cell interactions.
Interestingly, in our study cells ectopically expressing HSC70 failed to induce macroautophagy during nutrient deprivation in contrast with parental cells with lower levels of this chaperone (Deffit and Blum, 2015). While studies have linked HSC70 to autophagosomes and selective autophagy, ours is the first to suggest HSC70 may directly regulate macroautophagy in the absence of protein misfolding or aggregation.
Future Perspectives
Together these studies depict the extensive role of HSC70 in maintaining cellular homeostasis and protein trafficking. As HSC70 regulates several protein trafficking pathways, it is not surprising to find that it plays a vital role in immune recognition including the trafficking of the BCR and Ag presentation. As studies reveal mechanisms that regulate the activity of HSC70, manipulation of specific functions of this protein may become possible, opening the door to development of therapeutics that can regulate immune recognition and disease. For example, whether aggregated antigens captured by macroautophagy are presented by MHC class II molecules remains untested. If so, manipulating cellular HSC70 function could impact this pathway. This pathway could be important in modulating the clearance of protein aggregates to prevent neuroinflammation or autoimmunity. Conversely, modulating HSC70 activity in cells could be exploited to promote improved antigen presentation in the context of infection and malnutrition. While it has been known for decades that protein malnourished individuals have a reduced capacity to mount immune responses and are therefore more susceptible to infection, the exact mechanisms that govern this immune deficiency are still unclear (Scrimshaw et al., 1968). Multiple mechanisms likely contribute to compromised adaptive immune responses in malnourished individuals (Najera et al., 2004). For example, zinc deficiency is linked to protein malnourishment and can impair T cell responses, suggesting a role for this micronutrient in regulating host immunity (Fraker and King, 2004). In B lymphocytes, protein malnourishment directly impacts MHC class II Ag presentation (Deffit and Blum, 2015; Dengjel et al., 2005). The observed effect of exogenous macronutrient levels on Ag trafficking in B cells offers a previously unrecognized explanation for reduced Ab responses and impaired T cell mediated immunity observed in protein malnourished individuals.
Highlights.
Classically MHC class II present peptides derived from endocytosed antigens
Several nonclassical pathways including CMA and macroautophagy process antigens
Trafficking of proteins through these pathways is altered during cell stress
HSC70 availability regulates antigen processing through these pathways
HSC70 may serve a molecular switch to modulate the MHC class II pathway
Acknowledgement
The authors have no financial conflict of interest. Supported by the National Institutes of Health grants T32DK007519 and RO1AI079065.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Furst DO, Saftig P, Saint R, Fleischmann BK, et al. Chaperone-assisted selective autophagy is essential for muscle maintenance. Current biology : CB. 2010;20:143–148. doi: 10.1016/j.cub.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Auger I, Escola JM, Gorvel JP, Roudier J. HLA-DR4 and HLA-DR10 motifs that carry susceptibility to rheumatoid arthritis bind 70-kD heat shock proteins. Nature medicine. 1996;2:306–310. doi: 10.1038/nm0396-306. [DOI] [PubMed] [Google Scholar]
- Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. The Journal of experimental medicine. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps AW, Otczyk DC, Barker J, Lehmann D, Alpers MP. The relationship between undernutrition and humoral immune status in children with pneumonia in Papua New Guinea. Papua and New Guinea medical journal. 2008;51:120–130. [PubMed] [Google Scholar]
- Crotzer VL, Blum JS. Cytosol to lysosome transport of intracellular antigens during immune surveillance. Traffic. 2008;9:10–16. doi: 10.1111/j.1600-0854.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Knecht E, Terlecky SR, Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. The American journal of physiology. 1995;269:C1200–1208. doi: 10.1152/ajpcell.1995.269.5.C1200. [DOI] [PubMed] [Google Scholar]
- Deffit SN, Blum JS. Macronutrient Deprivation Modulates Antigen Trafficking and Immune Recognition through HSC70 Accessibility. Journal of immunology. 2015;194:1446–1453. doi: 10.4049/jimmunol.1402472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annual review of nutrition. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- Houlihan JL, Metzler JJ, Blum JS. HSP90alpha and HSP90beta isoforms selectively modulate MHC class II antigen presentation in B cells. J Immunol. 2009;182:7451–7458. doi: 10.4049/jimmunol.0804296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E, Yamane T, Tanaka K, Komatsu M. Structural basis for sorting mechanism of p62 in selective autophagy. The Journal of biological chemistry. 2008;283:22847–22857. doi: 10.1074/jbc.M802182200. [DOI] [PubMed] [Google Scholar]
- Jiang R, Gao B, Prasad K, Greene LE, Eisenberg E. Hsc70 chaperones clathrin and primes it to interact with vesicle membranes. The Journal of biological chemistry. 2000;275:8439–8447. doi: 10.1074/jbc.275.12.8439. [DOI] [PubMed] [Google Scholar]
- Kettern N, Rogon C, Limmer A, Schild H, Hohfeld J. The Hsc/Hsp70 co-chaperone network controls antigen aggregation and presentation during maturation of professional antigen presenting cells. PloS one. 2011;6:e16398. doi: 10.1371/journal.pone.0016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeski AE, Dice JF. Mechanisms of chaperone-mediated autophagy. The international journal of biochemistry & cell biology. 2004;36:2435–2444. doi: 10.1016/j.biocel.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Kaksonen M. Endocytic Accessory Factors and Regulation of Clathrin-Mediated Endocytosis. Cold Spring Harbor perspectives in biology. 2014 doi: 10.1101/cshperspect.a016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annual review of cell and developmental biology. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Najera O, Gonzalez C, Toledo G, Lopez L, Ortiz R. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clinical and diagnostic laboratory immunology. 2004;11:577–580. doi: 10.1128/CDLI.11.3.577-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador N, Aguado C, Horst M, Knecht E. Import of a cytosolic protein into lysosomes by chaperone-mediated autophagy depends on its folding state. The Journal of biological chemistry. 2000;275:27447–27456. doi: 10.1074/jbc.M001394200. [DOI] [PubMed] [Google Scholar]
- Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw NS, Taylor CE, Gordon JE. Monograph series. Vol. 57. World Health Organization; 1968. Interactions of nutrition and infection; pp. 3–329. [PubMed] [Google Scholar]
- Stricher F, Macri C, Ruff M, Muller S. HSPA8/HSC70 chaperone protein: structure, function, and chemical targeting. Autophagy. 2013;9:1937–1954. doi: 10.4161/auto.26448. [DOI] [PubMed] [Google Scholar]
- Yu A, Shibata Y, Shah B, Calamini B, Lo DC, Morimoto RI. Protein aggregation can inhibit clathrin-mediated endocytosis by chaperone competition. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1481–1490. doi: 10.1073/pnas.1321811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]