Abstract
Background
An extensive analysis of white matter plaques in a large sample of MS autopsies provides insights into the dynamic nature of MS pathology.
Methods
120 MS cases (1220 tissue blocks) were included. Plaque types were classified according to demyelinating activity based on stringent criteria. Early-active, late-active, smoldering, inactive, and shadow plaques were distinguished. 2476 MS white matter plaques were identified. Plaque type distribution was analyzed in relation to clinical data.
Findings
Active plaques were most often found in early disease, whereas at later stages, smoldering, inactive and shadow plaques predominated. The presence of early-active plaques rapidly declined with disease duration. Plaque type distribution differed significantly by clinical course. The majority of plaques in acute-monophasic and RRMS were active. Among SPMS cases with attacks, all plaque types could be distinguished including active plaques, in contrast to SPMS without attacks in whom inactive plaques predominated. Smoldering plaques were frequently and almost exclusively found in progressive MS. At 47-years of age, an equilibrium was observed between active and inactive plaques, whereas smoldering plaques began to peak. Men displayed a higher proportion of smoldering plaques.
Interpretation
Disease duration, clinical course, age and gender contribute to the dynamic nature of white matter MS pathology. Active MS plaques predominate in acute and early RRMS and are the likely substrate of clinical attacks. Progressive MS transitions to an accumulation of smoldering plaques characterized by microglial activation and slow expansion of pre-existing plaques. Whether current MS therapeutics impact this pathological driver of disease progression remains uncertain.
Introduction
Multiple Sclerosis (MS) is the most common cause of non-traumatic disability in young adults. 1 MS classically has been described as a chronic, inflammatory, demyelinating disease of the CNS with the focal white matter lesion, the “plaque”, as its hallmark pathology. 2–4 There is considerable heterogeneity in the clinical, radiographic and pathological features of MS. Relapses and progression are the two basic clinical phenomena. Relapses are considered to be the clinical expression of focal acute inflammatory demyelinating plaques disseminated in the CNS. 2–4 More recently, gray matter pathology and diffuse white matter injury have been linked to neurological disability and disease progression, and distinct interindividual patterns of demyelination and tissue injury have been described. 2,3,5 Still, the clinical correlate of active demyelination in the pathogenesis of progressive MS remains controversial. 4,6 Moreover, mechanisms that drive disease progression from the relapsing-remitting to the progressive stage and mechanisms that further drive disease progression in that stage are not fully elucidated. 3 Although the fundamental features of the MS plaque have been characterized, criteria for the classification of subtypes are inconsistent and different schemes have been applied, particularly with respect to clinical correlation and underlying pathogenic mechanisms of disease. 2,4 Consequently, there remains a significant ongoing need to systematically and rigorously analyze white matter MS plaques from different stages and phases of the disease.
In this study, we present an extensive analysis of white matter MS plaques in a large sample of human archival autopsied tissues. We evaluate plaque type distribution in relation to age, gender, clinical course and disease duration and thus gain new insights into the dynamic nature of the MS plaque.
Patients and methods
Sample retrieval and inclusion criteria
Study approval was granted by the ethics commission of the Medical University Vienna and by the Institutional Review Board of the Mayo Clinic Rochester. Studies were performed on paraffin-embedded archival autopsy tissues of the Centre for Brain Research, Medical University Vienna, Austria, and the Department of Neurology, Mayo Clinic, Rochester, USA. MS cases with sufficient material for histopathological work-up were identified. For each case included in the study, the total available archived material was analysed. Pathological evidence consistent with MS was confirmed by a certified neuropathologist. Clinical diagnosis was determined according to McDonald or Poser criteria. 7,8 Clinical course was defined by a certified neurologist and categorized as acute-monophasic MS, relapsing-remitting (RR), secondary progressive with attacks (SP+) or without attacks (SP-), primary progressive (PP), asymptomatic or uncertain. 9 Patients with clinical and/or pathologic features of acute disseminated encephalomyelitis or neuromyelitis optica and patients with confounding CNS pathologies such as whole brain radiation or large infarcts were excluded from this study. 10–12
Neuropathological techniques and immunohistochemistry
For classification of white matter plaques, sections were stained with hematoxylin-eosin (HE), luxol fast blue (LFB), Bielschowsky silver and by immunohistochemistry for CD68/KiM1P, proteolipid protein (PLP) and glial fibrillary acidic protein (GFAP). Active plaques were further classified by immunohistochemistry for myelin oligodendrocyte glycoprotein (MOG), cyclic nucleotide phosphodiesterase (CNPase) and myelin-associated glycoprotein (MAG). Immunohistochemistry was performed as described previously (Table 1). 13
Table 1.
Primary antibodies used in this study
| Primary Antibodies | ||||||
|---|---|---|---|---|---|---|
| # | Antigen | Pre- treatment |
Dilution | Antibody type |
Target | Source |
| 1 | PLP | ST, E | 1:1000 | mAb; mouse | proteolipid protein myelin | Serotec, Oxford, UK |
| 2 | CD68 | ST, E | 1:100 | mAb; mouse | sialomucin microglia, macrophages | Dako, Glostrup, Denmark |
| 3 | KiM1P | ST, C | 1: 5000 | mAb; mouse | pan-microglia-macrophages | W.Brück Göttingen,Germany |
| 4 | MAG | ST, E | 1: 1000 | mAb; mouse | myelin associated glycoprotein | Abcam, Cambridge, UK |
| 5 | MOG | ST, C | 1:1000 | mAb; mouse | myelin oligodendrocyte glycoprotein | Abcam, Cambridge, UK |
| 6 | CNPase | ST, E | 1: 2000 | mAb; mouse | 2', 3'-cyclic nucleotide 3'-phosphodiesterase | Sternberger Monoclonals Inc., Lutherville, USA |
| 7 | NF 150kD | ST, E | 1:2000 | Ab; rabbit | phosphorylated neurofilament chain M | Chemicon, Temecula, CA, USA |
| 8 | GFAP | ST, E | 1: 3000 | Ab; rabbit | glial fibrillary acidic protein | Dako, Glostrup, Denmark |
Ab = polyclonal antibody; C = Citrate buffer (10mM, pH 6.0); E = EDTA (EDTA 1mM, TRIS 10mM), pH 9.0; mAb = monoclonal antibody; ST = Steamer
Plaque type classification
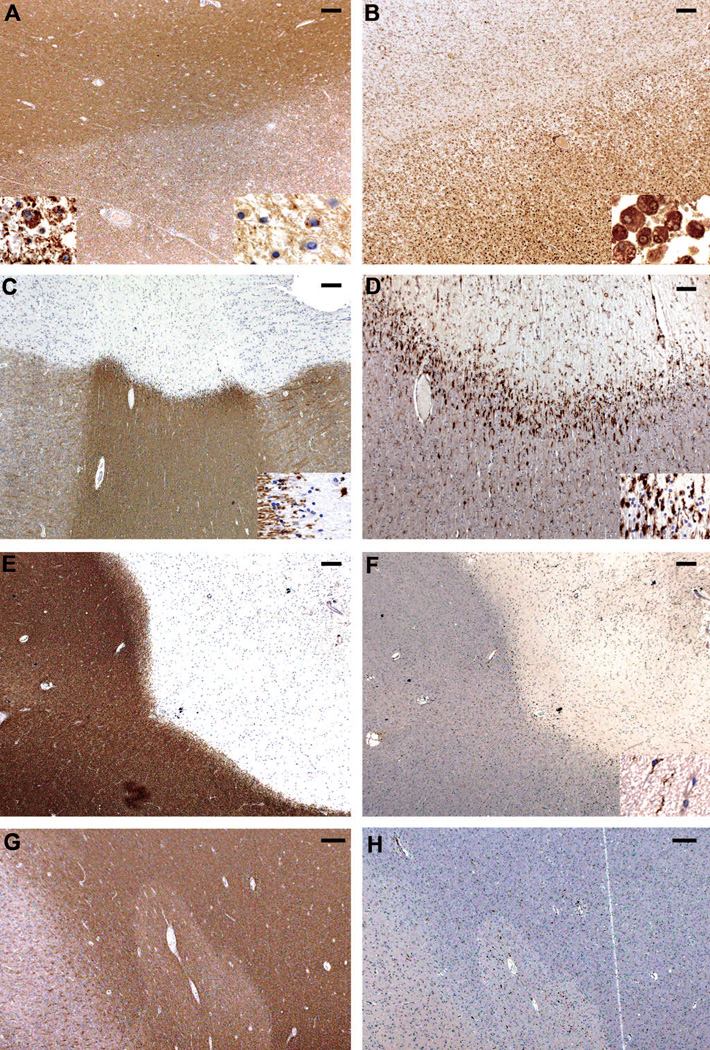
White matter plaques were classified as described previously. 13,14 White matter active plaques were characterized by dense infiltrates of macrophages containing LFB-positive and PLP-reactive myelin degradation products (Figure 1A and left insert, Figure 1B). Among active plaques, early-active and late-active plaques were distinguished. Early-active plaques were further defined by macrophages containing both minor myelin proteins (CNPase, MOG, MAG; Figure1A and right insert) as well as major myelin proteins (MBP, PLP). Macrophages in late-active plaques contained only major myelin proteins. 14 White matter smoldering (slowly expanding) plaques typically showed an inactive center with no or few macrophages, surrounded by a rim of activated microglia (Figure 1C, 1D). 13,15 White matter inactive plaques revealed a sharp plaque border without or only few macrophages or activated microglia (Figure 1E, 1F). 13,14 Completely remyelinated white matter plaques were classified as shadow plaques (Figure 1G, 1H). 16,17
Figure 1. Characterization of plaque types.
Figure 1 gives an overview of plaque types. Left pannel (A, C, E, G) depicts PLP staining. Right pannel (B, D, F, H) shows staining for KiM1P positive microglia / macrophages. Characterization of plaque types reflects the demyelinating activity of each plaque. Bar in A-C, E-H= 200µm, Bar in D=100 µm.
(A, B) Early active plaques (EAL) were defined by macrophages immunoreactive for minor myelin proteins (MOG positive macrophages right insert in A) as well as major myelin proteins (PLP positive macrophages left insert in A).
(C, D) Smoldering plaques (also called slowly expanding plaques) typically showed a rather inactive centre with no or few macrophages, surrounded by a rim of activated microglia. Only few of these macrophages or microglia cells contained early myelin degradation products. Inserts depict plaque edge.
(E, F) Inactive plaques revealed a sharp plaque border without or only few macrophages or activated microglia (insert).
(G, H) Completely remyelinated plaques typically containing few macrophages without early myelin degradation products were classified as shadow plaques. Shadow plaques presented with a sharp plaque edge and were associated with fibrillary gliosis.
Statistical analysis
Statistical analyses were performed by R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria). Plaque type distributions were grouped with patient characteristic using bar plots. Multinomial regression was performed to model plaque type proportions as a function of one of the following predictors: disease duration, clinical course or age at death. 18 Plaque type was treated as a five-level or four-level categorical outcome or response variable and modeled with multinomial regression, an extension of binary logistic regression to the case where the outcome or response has more than two categories. 18 These regression models were used to provide estimates of the probability of each plaque type at each level of a covariate. These probabilities can be multiplied by 100 and interpreted as estimated percentages or relative frequencies. Multiple plaques from the same patient cannot be assumed to be statistically independent. A cluster bootstrap procedure was used to calculate 95% confidence intervals that account for intra-patient correlation among multiple plaques from the same individual. By taking this into account no undue weight is given to patients with more versus fewer plaques. This approach does not make assumptions about causes and effects but rather allows us to estimate and contrast the distribution of plaque type at different ages, among different clinical courses, or at different disease durations. In terms of the composition of the sample, a flexible restricted cubic spline modeling approach was used so that plaque type distribution estimates at longer disease durations were largely unaffected by how many subjects with shorter disease duration were included. Thus, fulminant MS cases have little to no influence on our estimates for disease durations beyond about 2 years.
To complement this plaque-level analysis, a similar but distinct patient-level analysis was performed. For each plaque type, patients were classified as having or not having at least one plaque of that type. We then modeled this binary outcome using logistic regression. Thus, the plaque-level and patient-level analyses provided complimentary information.
Results
Patient characterization
A total of 120 subjects (76 (63%) women; 44 (37 %) men) were studied (Table 2). Median age at death was 56 years (range, 17 – 86). Median disease duration in the total cohort was 12 years (range, 0 – 48) but differed (p < 0.0001) among disease courses. The longest disease duration occurred among SPMS patients without attacks, followed by SPMS patients with attacks. Consequently, age at death also differed significantly (p < 0.0001) by disease course. Patients with acute-monophasic disease died at an earlier age whereas especially SPMS patients without attacks were significantly older (Table 2).
Table 2.
Sample characterization
| Acute-Mono n=21 |
RR n=10 |
SP+ n=25 |
SP- n=29 |
PP n=20 |
|
|---|---|---|---|---|---|
| Age at death, y | |||||
| Median (IQR) | 45 (28, 48) | 54 (42, 58) | 50 (45, 60) | 65 (58, 72) | 58 (51, 75) |
| Range | 17 to 78 | 19 to 69 | 28 to 76 | 31 to 84 | 34 to 86 |
| Gender | |||||
| Female | 11 (52%) | 6 (60%) | 17 (68%) | 18 (62%) | 13 (65%) |
| Male | 10 (48%) | 4 (40%) | 8 (32%) | 11 (38%) | 7 (35%) |
| Disease duration, y | |||||
| Median (IQR) | 0.2 (0, 0.3) | 10 (3, 20) | 16 (9, 28) | 22 (16, 34) | 14 (6, 27) |
| Range | 0 to 1 | 0 to 43 | 1 to 48 | 10 to 45 | 2 to 47 |
| Cause of death | |||||
| Directly MS related | 21 (100%) | 2 (20%) | 2 (8%) | 0 (0%) | 0 (0%) |
| Cardiovascular failure | 0 (0%) | 4 (40%) | 8 (32%) | 14 (49%) | 9 (45%) |
| Respiratory failure | 0 (0%) | 1 (10%) | 6 (24%) | 7 (24%) | 5 (25%) |
| Other | 0 (0%) | 0 (0%) | 5 (20%) | 5 (17%) | 1 (5%) |
| Not known | 0 (0%) | 3 (30%) | 4 (16%) | 3 (10%) | 5 (25%) |
Clinical course is categorized as acute-monophasic (n=21, 18%), relapsing-remitting (RR n=10, 8%), secondary progressive with attacks (SP+ n=25, 21%), secondary progressive without attacks (SP- n=29, 24%) and primary progressive (PP n=20, 17%). An acute-monophasic disease course leads to death under 1 year. A small group of 7 (6%) patients are classified as asymptomatic MS (not shown). In 8 (6%) patients the disease course could not be sufficiently evaluated by retrospective chart review (not shown). Cause of death distinguishes between directly related to MS, cardiovascular failure (due to peripheral septic conditions, myocardial infarction or congestive heart failure), respiratory failure (due to pulmonary embolism or pneumonia) and other causes (trauma, renal failure or malignancies other than CNS malignancies). In 15 (12%) patients cause of death is unavailable.
Plaque type distribution
Overall, 1220 blocks (median: 9 blocks per patient, range: 1–46) were analyzed including single hemispheric (n=19, 2%), double hemispheric (n=71, 6%), large (n=60, 5%) and routine blocks (n=1070, 87%). The majority of routine blocks (n=705, 58%) were taken from supratentorial locations, while additional blocks were from brainstem (n=179, 15%), cerebellum (n=95, 8%), spinal cord (n=112, 9%) or optic nerve (n=39, 3%). Double/single hemispheric blocks were cut either as coronal or horizontal sections at the thalamic level (n=37, 3%) or at a frontal or occipital level (n=24, 2%). Additional double hemispheric sections included cerebellum and brainstem (n=29, 2%).
In total, 2476 plaques were identified, including 869 (35%) active plaques, 374 (15%) smoldering plaques, 875 (35%) inactive plaques and 358 (14%) shadow plaques. Among active plaques, 432/869 (50%) plaques were early-active. Median plaque count per patient was 11 (range: 1–123).
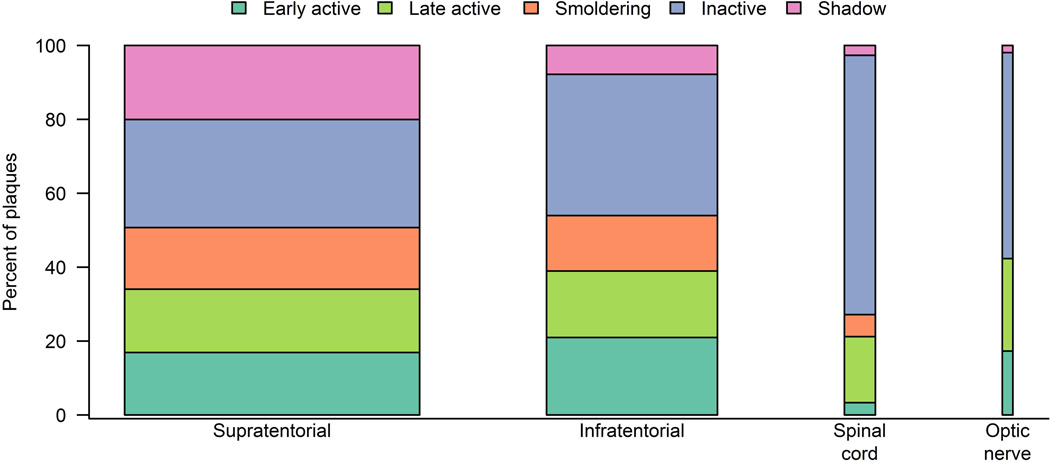
In general, plaque type distribution was rather similar across block locations between supratentorial and infratentorial white matter (Figure 2). Especially active plaques were equally distributed across all block locations. Differences were observed only among smoldering and inactive lesions: Lesions in the spinal cord were more likely to be inactive. In contrast, no/ few smoldering plaques were found in the spinal cord or optic nerve. However, smoldering and inactive plaques were also both equally distributed between the supratentorial and the infratentorial white matter (Figure 2).
Figure 2. Distribution of plaque location.
Figure 2 shows the distribution of plaque location. Bar widths indicate relative sample size. The majority of plaques (n=1439, 58%) were located supratentorial. A fair amount of plaques were found infratentorial (n=834, 34%). Additional plaques were found in the spinal cord (n=151, 6%) and in the optic nerve (n=52, 2%). Plaque type distribution was rather similar across block locations between supratentorial and infratentorial white matter. When using separate logistic regression models adjusted for age and disease duration, no regional differences were found for active plaques including specifically testing for early active or late active plaques. Differences were only observed among smoldering and inactive lesions: Lesions in the spinal cord were more likely to be inactive (p < 0.001, p=0.002) compared to supratentorial and infratentorial lesions. Lesions in the spinal cord were less likely to be smoldering (p=0.02) compared to supratentorial lesions. However, smoldering and inactive plaques were also both equally distributed between the supratentorial and the infratentorial white matter.
Plaque type in relation to disease duration
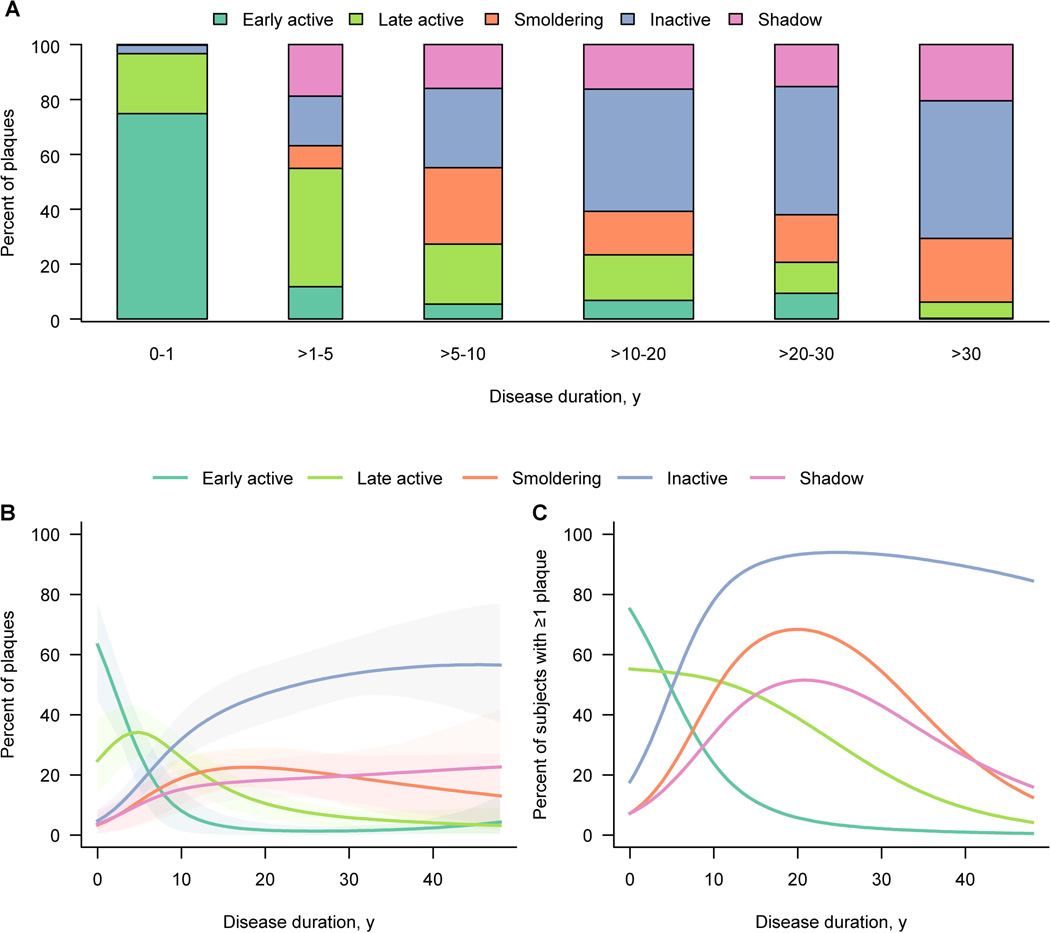
Overall, the percentage of an individual patient’s plaques that were active declined with disease duration (Spearman rank correlation rs= −0.8, p < 0.0001). Most strikingly, the percentage of early-active plaques declined with disease duration (Spearman rank correlation rs= −0.7, p < 0.0001). In patients with disease duration up to 1 year, the majority of plaques (318/425, 75%) were early-active, while in patients with disease duration of over 30 years, the percentage of early-active plaques (1/440, 0%) was nearly zero (Figure 3A). After 30 years of disease duration, inactive (221/440, 50%), smoldering (102/440, 23%) and shadow plaques (90/440, 20%) predominated.
Figure 3. Distribution of plaque types by disease duration.
(A) The bar plot shows the actual plaque distribution in relation to disease duration. The percentages shown for each subgroup or bar are calculated among all plaques for those subjects within the subgroup. Bar width is related to subgroup size. On multinomial modeling plaque type distribution differs significantly (p < 0.0001) among disease duration subgroups.
(B) Graph B provides estimates of the percentage of plaques by type expressed as a function of the patient’s disease duration using multinomial modeling accounting for clustering of plaques within patient. Percentages across the curves add to 100 percent at a given duration. Shaded regions represent 95% cluster bootstrap confidence intervals. Significant differences are observed (p < 0.0001) based on multinomial modeling.
(C) In graph C the estimated proportion of patients with at least one of each plaque type as a function of disease duration is depicted. Estimates are based on separate logistic regression models at the patient level. Percentages across the curves add to more than 100 percent since subjects could have at least one of several types of plaques.
A multinomial regression model was fit at the plaque-level (Figure 3B). The probability that an observed plaque was early-active rapidly declined with disease duration. Conversely, the corresponding curve for inactive and shadow plaques increased with inactive plaques clearly predominating from about 15 years onward. Smoldering plaques were seen mainly in patients with disease duration longer than 10 years (Figure 3B). Next, a logistic regression model was fit at the patient-level estimating the probability a patient had at least one of each type of plaque as a function of disease duration (Figure 3C). The probability of early- or late- active plaques in a patient again declined with disease duration, whereas the probability of inactive plaques inversely rose (Figure 3C). At disease onset, most patients had an early- active plaque. At 25 years of disease duration, the probability a patient harbouring at least one early-active plaque fell as low as 3%, while nearly all patients (94%) had an inactive plaque (Figure 3C). At the same stage, the probability of at least one smoldering plaque or one shadow plaque was 64% or 49%, respectively. Smoldering plaques peaked at approximately 20 years of disease duration (Figure 3C).
Plaque type in relation to clinical course
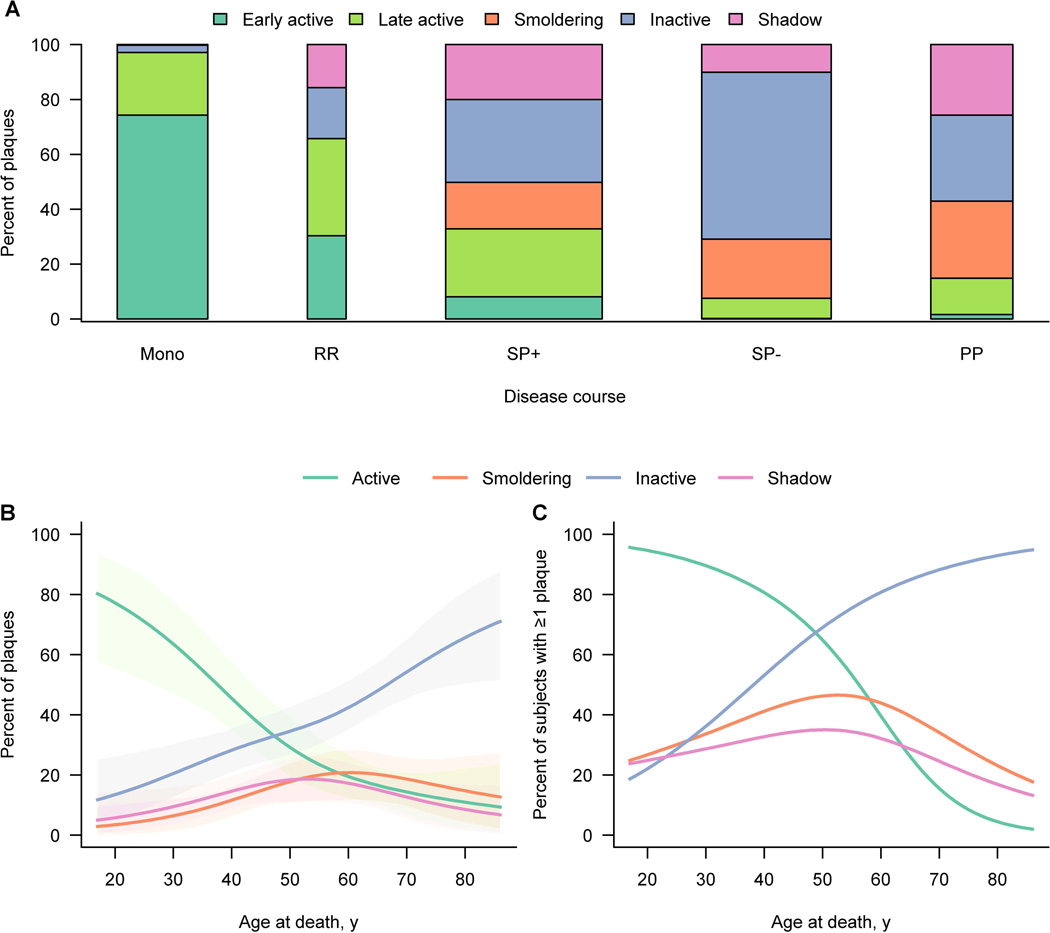
Plaque type frequencies differed significantly by clinical course (p < 0·0001, Figure 4A), even after adjusting for age or disease duration. Early-active plaques (309/416, 74%) predominated in acute-monophasic MS. A striking two thirds of all plaques were active in RRMS compared to just one third of all plaques in SPMS with attacks. Moreover, an even distribution of early-active and late-active plaques was found in RRMS. In SPMS patients with attacks, early-active plaques still contributed a fair, albeit smaller, proportion of all active plaques (Figure 4A). Generally, a rather even distribution of active plaques (236/719, 33%), smoldering plaques (122/719, 17%), inactive (217/719, 30%) and shadow plaques (144/719, 20%) was found in SPMS patients with attacks. Among SPMS patients without attacks, inactive (362/595, 61%) and smoldering plaques (128/595, 22%) predominated, and active plaques in general (45/595, 8%) were rarely seen. Among PPMS patients, a high number of smoldering plaques (106/377, 28%) but also inactive (118/377, 31%) and shadow plaques (97/377, 26%) were evident. Active plaques (56/377, 15%) were less frequent in PPMS. Interestingly, smoldering plaques occurred almost exclusively in patients with progressive MS. The dominant plaque type among incidental/asymptomatic MS patients was inactive.
Figure 4. Distribution of plaque types by disease course and age.
(A) The bar plot shows the actual plaque distribution in relation to clinical course. The percentages shown for each subgroup or bar are calculated among all plaques for those subjects within the subgroup. Bar width is related to subgroup size. On multinomial modeling plaque type distribution differs significantly (p < 0.0001) among disease courses.
(B) Graph B provides estimates of the percentage of plaques by type expressed as a function of the patient’s age at death using multinomial modeling accounting for clustering of plaques within patient. Percentages across the curves add to 100 percent at a given age. Shaded regions represent 95% cluster bootstrap confidence intervals. Early active and late active plaques are pooled as active plaques. Significant differences are observed (p < 0.0001) based on multinomial modeling.
(C) In graph C the estimated proportion of patients with at least one of each plaque type as a function of age is depicted. Estimates are based on separate logistic regression models at the patient-level. Percentages across the curves add to more than 100 percent since subjects could have at least one of several types of plaques. Early active and late active plaques are pooled as active plaques.
Plaque type in relation to age and gender
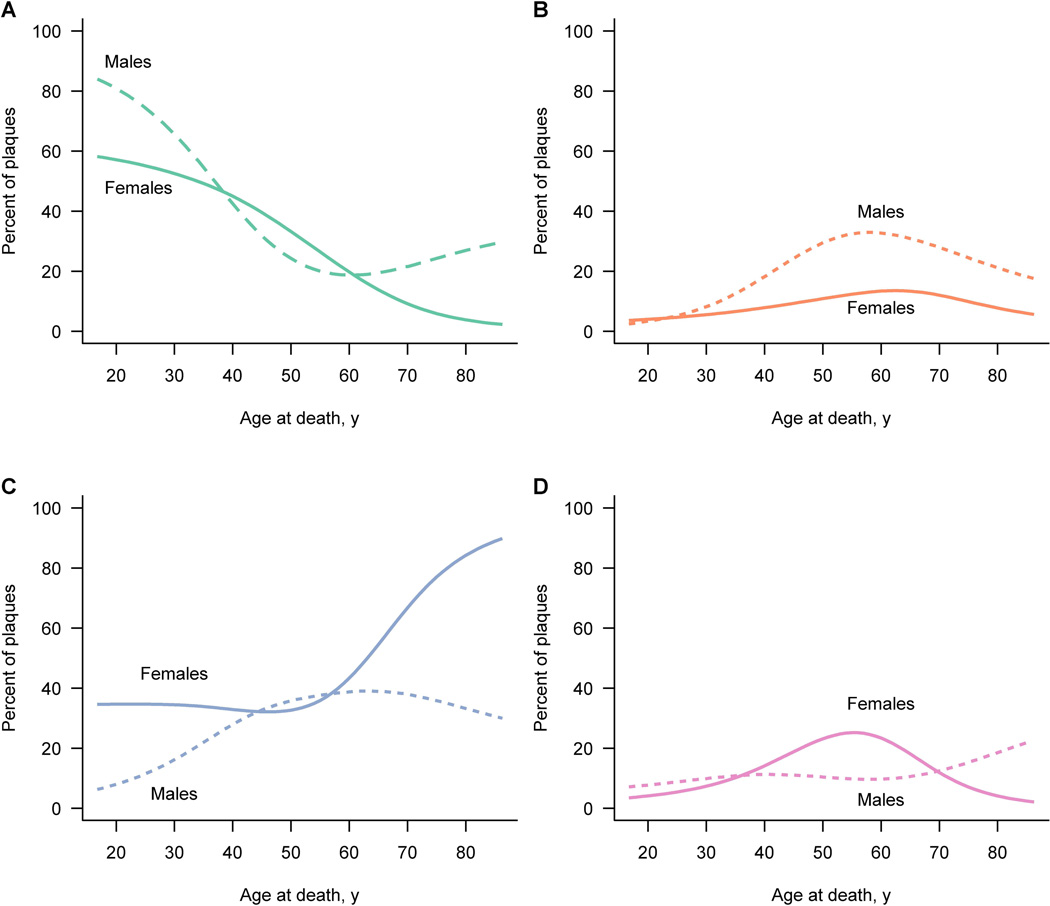
At age 47-years, percentages of active and inactive plaques were equal (see crossing point of two curves in Figure 4B). This age also marked the beginning of the peak of smoldering and shadow plaques. When estimating the probability that a patient had at least one of each plaque type as a function of age, similar findings as described above were observed (Figure 4C). Again, the probability curves for active and inactive plaques were inversed, intersecting at 49-years of age. Both smoldering and shadow plaques peaked around 50-years of age (Figure 4C). The probability of a specific plaque type as a function of age-at-death was analyzed separately for male and female patients (Figure 5). Active plaque frequencies declined similarly in men and women (Figure 5A). However, a clear difference between the sexes was seen with smoldering plaques. Between ages 45- and 55-years, men harboured a significant higher percentage of smoldering plaques than women (p=0.046, Figure 5B). At age 60 years and older, the probability of inactive plaques was lower for men than women (Figure 5C). In contrast, shadow plaques were rather equally distributed between the genders (Figure 5D).
Figure 5. Gender differences in plaque type distribution.
Figure 5 depicts the estimated percent of each plaque type by age at death for males and females separately based on a multinomial regression model accounting for clustering of plaques within patient. Estimates for women are denoted by a solid line while estimates for men are denoted by a dotted line.
Discussion
Classification criteria for the white matter MS plaque are controversial and data on different courses and time points are lacking. Our analysis has focused on clinical and pathological features of an extensive collection of archival MS autopsy cases, representing a comprehensive cross section of different disease courses and time points of age and disease duration. 6 Blocks from the supra-and infratentorial white matter as well as the spinal cord and the optic nerve are included. In addition, double/single hemispheric sections provide an even more expansive sampling. Plaques types are rather equally distributed among block locations, showing that especially active plaques may occur anywhere in the CNS white matter. Clinical data are similar to previously published studies. 1,6 In comparison to their epidemiological occurrence, there is an overrepresentation of fulminant MS cases in this study. 19 However, this focus on the acute MS stage has been conducted to be able to fully evaluate and appreciate the differences between early and chronic MS. In addition, the statistical analysis directly modelled disease duration and age effects in a flexible way that fulminant MS cases have little to no influence on our estimates for disease durations beyond about 2 years. Moreover, our sample also represents the clinical RRMS-SPMS continuum. Further, SPMS patients with attacks compared to SPMS patients without attacks represent earlier and later stages in disease progression.
Our data indicate that features of white matter MS pathology vary with time. Active plaques predominate in early MS, while inactive plaques are most frequently found in chronic MS with long standing disease duration. The probability of a patient harbouring an active plaque declines with time. More importantly, the probability of early-active plaques declines even more strikingly with disease duration. Thus, while early-active plaques are frequently found in early MS stages, they rarely occur among patients with long standing disease. Early-active plaques are a prerequisite for immunopathological pattern classification. 20 Therefore, our published data describing interindividual immunopathological heterogeneity in early active MS, in contrast to reported findings of immunopathological homogeneity among chronic MS patients may simply reflect the rarity of classifiable early active plaques in later stages of the disease. 20,21
Active plaques typically are associated with gadolinium enhancement on MRI and most likely represent the pathological substrate of attacks. 22,23 In support of these data, active and early-active plaques represent the vast majority of plaques found in acute-monophasic and about two-thirds of those in relapsing-remitting MS. Active plaques are also common in SPMS patients with attacks, although late-active plaques predominate over early-active plaques. However, among SPMS patients without attacks, active plaques are rarely found whereas inactive plaques predominate. Interestingly, smoldering (slowly expanding) plaques are almost exclusively seen among progressive MS patients as well as in SPMS patients with and without attacks. Especially among PPMS patients, smoldering plaques are frequently found while active plaques contribute a smaller percentage of plaques.
Smoldering plaques have been associated with microglial activation, ongoing axonal injury and neurodegeneration. 13,15 Consequently, this chronic plaque type confirms slow but active demyelination among progressive MS patients and may also explain why worsening of pre-existing symptoms is a typical feature of progressive MS. 24,25 However, there are also lesions, which have a clear rim of activated microglia, but do not show evidence for ongoing demyelination and neurodegeneration, defined by the presence of early myelin degradation products in macrophages. It has been proposed that the adaptive and innate immune systems are involved in the immunopathogenesis of MS to different extents during the course of the disease. 26 The early, relapsing-remitting stage is associated with the antigen-specific T and B cell-mediated adaptive immune responses whereas the progressive phase is associated with innate immune responses characterized by chronic inflammation and microglial activation. 26 Thus, smoldering plaques provide further pathological evidence that the active demyelinating process seems to decelerate as the disease transforms into the progressive stage and the immune response may shift from adaptive to innate. 26
In light of these pathological data, it is important to determine whether smoldering MS plaques can be distinguished on MRI. The high prevalence of smoldering, slowly expanding plaques with ongoing neurodegeneration in progressive MS may imply that a subset of pre-existing plaques increases in size. The presence of enlarging lesions has been demonstrated in several MRI studies. 27,28 Poor inter-rater correlation is a major problem that limits the use of enlarging lesions in clinical practice. 29 This may be overcome by the use of 3D acquisition techniques and subtraction MRI, where registered volumes representing an earlier time point are subtracted from a later time point, enabling more objective visualization of enlarging lesions. 30 Gadolinium enhancement on MRI is considered absent among enlarging smoldering plaques. 22 USPIO, a negative contrast material may be able to visualize macrophages at the edges of smoldering plaques, although conclusive correlative pathological studies are lacking. 31 In 2010, an International CMSC Consensus Conference suggested that in addition to gadolinium enhancing lesions or new T2 lesions, enlarging T2 lesions should also be considered a marker that distinguishes relapsing from progressive forms of MS. 32 However, generalized brain atrophy is also considered an important correlate of MS disease progression. 5,33 The presence of tissue atrophy secondary to ongoing axonal degeneration and loss within the inactive plaque center may, with time, counterbalance plaque expansion at the smoldering plaque edge. 34 Therefore, in advanced stages of MS, plaque size by MRI might not, per se, increase, and the overall volumetric lesion load may paradoxically decrease.
In contrast to other plaque types, shadow plaque frequencies are similar across different disease courses, with the exception of the acute-monophasic stage, in which they are rarely found. Completely remyelinated shadow plaques as described in our study represent the late stage of remyelination. 17,23 In contrast, early remyelination has been described in early MS and active plaques. 17 However, remyelinated areas seem to be more susceptible to a second-hit inflammatory demyelinating attack than the normal-appearing white matter. 35 Since chronic MS has been associated with compartmentalized inflammation and a decrease in inflammation over time, it seems surprising that shadow plaques are not more common among progressive MS patients with longstanding disease. 3,13 As previously described, this failure to remyelinate axons might be caused by an age-dependent loss of trophic support from microglia or the local environment in demyelinated plaques. 3,36 However, we cannot know when the remyelination took place and how long such shadow plaques with reduced myelin density persist. Thus, the results could also mean that those shadow plaques, are lesions which have been formed in early disease stages and show persistent remyelination through the subsequent course of the disease. 3,13,17,36
Observations from different studies have led to the hypothesis that the accumulation of disability in MS is mainly an age-related process, seemingly independent of the clinical subtype of MS. 24 In our cohort, the occurrence of active plaques declines with age, whereas inactive plaques increase. Around 47 years of age, an apparent equilibrium between active and inactive plaques is reached. In contrast, smoldering plaques begin to peak at that age. These observations are consistent with a recent study of over 900 MS cases which reported that the onset of progression is an age dependent milestone in MS, occurring at around 46 years of age in both SPMS and PPMS cases. 37 A conclusion from these data is that MS pathology transforms from new and active white matter plaques to slow expansion of pre-existing plaques in progressive MS. Consequently, a rather even distribution of the different white matter plaque types is seen at the beginning of the progressive stage. Previously published data show that there is a poor correlation between focal white matter lesions with cortical lesions and diffuse white matter injury. 41 Taking already existing data on diffuse white matter injury and cortical demyelination into account, this transition of white matter pathology seen in our study also appears to be associated with extensive cortical demyelination and diffuse white matter injury in progressive MS. 3,5 Thus, the dynamic nature of MS pathology is reflected in the gray as well as in the white matter. 3,5
In our pathological cohort, male patients show a higher fraction of smoldering plaques than female patients, especially at an age of 45 years and above. Several gender related differences in the natural history of MS have been described. Although MS is mainly reported in women, epidemiological studies show that women reach disability milestones later than men. 1,38,39 Men demonstrate a more progressive disease course, associated with a shorter time to and a younger age of conversion to secondary progressive MS. 38,39 Interestingly, a recent study reported that the expression of sex steroidogenic enzymes and hormone receptors in MS plaques and white matter demonstrated gender specific differences and further suggested a higher capacity for females to increase progesterone synthesis and signaling. 40 Progesterone and its metabolite DHP have been shown to ameliorate the clinical signs of EAE and decrease inflammatory activity of microglia. 40 These data may help explain the differences we observed in plaque types between genders.
Conclusion
Our findings show that features of white matter MS pathology vary over time. Active plaques are the pathological substrate of attacks and predominate in acute or relapsing MS. Smoldering, slowly expanding plaques are almost exclusively found in progressive MS and contribute to disease progression. Gender and age specific differences in plaque development are evident. In chronic MS, the underlying pathology transforms from new and active white matter plaques to slow expansion of pre-existing plaques and inactive plaques. Our pathological data support the concept that as MS transitions into the progressive stage, immune responses may shift from adaptive to innate. Whether current MS therapeutics impact this pathological driver of disease progression remains uncertain.
Acknowledgement and Funding
We would like to thank Marianne Leisser, Ulrike Koeck, Angela Kury and Patricia Ziemer for expert technical assistance. This work was supported by grants from NIH R01 NS 49577-6 (CFL), Novartis CFTY720DUS37T (CFL) and partly from the Austrian Science Fund, FWF Project P24245-B19 (HL) and FWF Project J3508-B24 (JMF). WB and IM were supported by grants from the German Ministry for Education and Research (BMBF, “German Competence Network Multiple Sclerosis” (KKNMS), Pattern MS/NMO) during the conduct of the study.
Footnotes
Author’s Contributions
Study design: CFL, HL, WB. Data collection: JMF, NK, SB, YG, IM, JEP, HL, CFL. Data analysis: SW, JM, JMF. Data interpretation: JMF, HL, WB, CFL. Figures: JMF, YG, SW, JM. Drafting of the manuscript: JMF, CFL, HL, SW, JEP, IP. Critical revision of the manuscript: All authors.
Potential Competing Interests Statement
In addition to the reported funding JMF, YG, SW, JM, SB, NK, and JEP have nothing to disclose. IP reports grants from Novartis, outside the submitted work. HL reports personal fees from TEVA, Novartis, Baxter, and AMGEN outside the submitted work. WB reports grants and personal fees from Teva Pharma, Biogen Idec, Novartis and personal fees from Merck-Serono, Bayer Vital, Genzyme outside the submitted work. IM reports personal fees from Biogen Idec, Bayer Healthcare, TEVA, Serono, Novartis, outside the submitted work. CFL shares in royalties from marketing of kits for detecting AQP4 autoantibody and reports research grants from Biogen Idec, Novartis and Alexion Pharmaceuticals outside the submitted work.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17(2):210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 4.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annu Rev Pathol. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- 5.Kutzelnigg A, Lassmann H. Cortical lesions and brain atrophy in MS. J Neurol Sci. 2005;233(1–2):55–59. doi: 10.1016/j.jns.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129(Pt 3):606–616. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- 7.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 8.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 9.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 10.Hart MN, Earle KM. Haemorrhagic and perivenous encephalitis: a clinical-pathological review of 38 cases. J Neurol Neurosurg Psychiatry. 1975;38(6):585–591. doi: 10.1136/jnnp.38.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 12.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53(5):1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 13.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruck W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38(5):788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- 15.Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50(5):646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- 16.Barkhof F, Bruck W, De Groot CJ, et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol. 2003;60(8):1073–1081. doi: 10.1001/archneur.60.8.1073. [DOI] [PubMed] [Google Scholar]
- 17.Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(Pt 12):3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 18.Agresti A. Categorial Data Analysis. 2nd, ed. Hoboken, NJ: Wiley-Interscience; 2002. [Google Scholar]
- 19.Capello E, Mancardi GL. Marburg type and Balo's concentric sclerosis: rare and acute variants of multiple sclerosis. Neurol Sci. 2004;25(Suppl 4):S361–S363. doi: 10.1007/s10072-004-0341-1. [DOI] [PubMed] [Google Scholar]
- 20.Metz I, Weigand SD, Popescu BF, et al. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Ann Neurol. 2014;75(5):728–738. doi: 10.1002/ana.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breij EC, Brink BP, Veerhuis R, et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol. 2008;63(1):16–25. doi: 10.1002/ana.21311. [DOI] [PubMed] [Google Scholar]
- 22.Filippi M, Rocca MA, Barkhof F, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11(4):349–360. doi: 10.1016/S1474-4422(12)70003-0. [DOI] [PubMed] [Google Scholar]
- 23.Popescu BF, Pirko I, Lucchinetti CF. Pathology of multiple sclerosis: where do we stand? Continuum (Minneap Minn) 2013;19(4):901–921. doi: 10.1212/01.CON.0000433291.23091.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Confavreux C, Vukusic S. The clinical course of multiple sclerosis. Handb Clin Neurol. 2014;122:343–369. doi: 10.1016/B978-0-444-52001-2.00014-5. [DOI] [PubMed] [Google Scholar]
- 25.Cottrell DA, Kremenchutzky M, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain. 1999;122(Pt 4):625–639. doi: 10.1093/brain/122.4.625. [DOI] [PubMed] [Google Scholar]
- 26.Weiner HL. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol. 2008;255(Suppl 1):3–11. doi: 10.1007/s00415-008-1002-8. [DOI] [PubMed] [Google Scholar]
- 27.Rovaris M, Barkhof F, Bastianello S, et al. Multiple sclerosis: interobserver agreement in reporting active lesions on serial brain MRI using conventional spin echo, fast spin echo, fast fluid-attenuated inversion recovery and post-contrast T1-weighted images. J Neurol. 1999;246(10):920–925. doi: 10.1007/s004150050483. [DOI] [PubMed] [Google Scholar]
- 28.Tan IL, van Schijndel RA, Fazekas F, et al. Image registration and subtraction to detect active T(2) lesions in MS: an interobserver study. J Neurol. 2002;249(6):767–773. doi: 10.1007/s00415-002-0712-6. [DOI] [PubMed] [Google Scholar]
- 29.Erbayat Altay E, Fisher E, Jones SE, Hara-Cleaver C, Lee JC, Rudick RA. Reliability of classifying multiple sclerosis disease activity using magnetic resonance imaging in a multiple sclerosis clinic. JAMA Neurol. 2013;70(3):338–344. doi: 10.1001/2013.jamaneurol.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liguori M, Meier DS, Hildenbrand P, et al. One year activity on subtraction MRI predicts subsequent 4 year activity and progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(10):1125–1131. doi: 10.1136/jnnp.2011.242115. [DOI] [PubMed] [Google Scholar]
- 31.Vellinga MM, Oude Engberink RD, Seewann A, et al. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain. 2008;131(Pt 3):800–807. doi: 10.1093/brain/awn009. [DOI] [PubMed] [Google Scholar]
- 32.Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of Magnetic Resonance Imaging as Well as Clinical Disease Activity in the Clinical Classification of Multiple Sclerosis and Assessment of Its Course: A Report from an International CMSC Consensus Conference, March 5–7, 2010. Int J MS Care. 2012;14(3):105–114. doi: 10.7224/1537-2073-14.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones BC, Nair G, Shea CD, Crainiceanu CM, Cortese IC, Reich DS. Quantification of multiple-sclerosis-related brain atrophy in two heterogeneous MRI datasets using mixed-effects modeling. Neuroimage Clin. 2013;3:171–179. doi: 10.1016/j.nicl.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornek B, Storch MK, Weissert R, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157(1):267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramow S, Frischer JM, Lassmann H, et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133(10):2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- 36.Rist JM, Franklin RJ. Taking ageing into account in remyelination-based therapies for multiple sclerosis. J Neurol Sci. 2008;274(1–2):64–67. doi: 10.1016/j.jns.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 37.Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188–198. doi: 10.1177/1352458512451510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- 39.Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. 2012;8(5):255–263. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luchetti S, van Eden CG, Schuurman K, van Strien ME, Swaab DF, Huitinga I. Gender differences in multiple sclerosis: induction of estrogen signaling in male and progesterone signaling in female lesions. J Neuropathol Exp Neurol. 2014;73(2):123–135. doi: 10.1097/NEN.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 41.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(11):2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]