Abstract
Objective
We sought to examine the joint contributions of self-reported adverse childhood experiences (ACEs) and recent life events (RLEs) to inflammation at midlife, by testing three competing theoretical models: stress generation, stress accumulation, and early-life stress sensitization. We also aimed to identify potential mediators between adversity and inflammation.
Methods
Participants were 1180 middle-aged and older adults from the MIDUS Biomarker Project (M age = 57.3 years, SD = 11.5; 56% female). A composite measure of inflammation was derived from five biomarkers, including serum levels of C-reactive protein, interleukin-6, fibrinogen, E-selectin, and ICAM-1. Participants provided self-report data regarding ACEs, RLEs, current lifestyle indices (cigarette smoking, alcohol consumption, physical exercise, waist circumference), current depressive symptoms, and demographic/biomedical characteristics. We also used indices of hypothalamic-pituitary-adrenocortical outflow (12-hour urinary cortisol) and sympathetic nervous system output (12-hour urinary norepinephrine and epinephrine).
Results
Analyses indicated that ACEs and RLEs were independently associated with higher levels of inflammation, controlling for each other’s effects. Their interaction was not significant. The results were consistent with the hypothesis that associations between ACEs and inflammation were mediated through higher urinary norepinephrine output, greater waist circumference, smoking, and lower levels of exercise, whereas higher waist circumference and more smoking partially mediated the association between RLEs and inflammation.
Conclusions
In support of the stress accumulation model, ACEs and RLEs had unique and additive contributions to inflammation at midlife, with no evidence of synergistic effects. Results also suggested that norepinephrine output and lifestyle indices may help explain how prior stressors foster inflammation at midlife.
Keywords: childhood adversity, stress, sympathetic nervous system, depression, inflammation
Experiencing severe, chronic stress during childhood or adulthood has been linked to higher rates of morbidity and mortality from chronic diseases of aging, including coronary heart disease (CHD), type 2 diabetes, and some forms of cancer (Cohen, Janicki-Deverts, & Miller, 2007; Lutgendorf & Sood, 2011; Miller, Chen, & Parker, 2011; Pouwer, Kupper, & Adriaanse, 2010; Steptoe & Kivimäki, 2013). However, childhood and adult adversities have mostly been studied separately with regards to health outcomes. What has often been overlooked is how the interplay between childhood and adult stressors contributes to later health. Furthermore, the biobehavioral mechanisms explaining these associations have yet to be fully characterized.
Many researchers have posited that systemic low-grade inflammation is a pathway linking adversity with morbidity and mortality. Biomarkers of low-grade inflammation, such as CRP and IL-6, are elevated in both youth and adults who are experiencing chronic psychological stress (Hänsel, Hong, Cámara, & von Känel, 2010; Nazmi & Victora, 2007; Rohleder, 2014; Slopen, Koenen, & Kubzansky, 2012). In long-term prospective studies, these same biomarkers predict the development and progression of chronic diseases associated with aging, like CHD, type 2 diabetes, cancer, and preclinical research has implicated inflammation in the pathogenesis of these conditions (Black, 2003; Hansson & Hermansson, 2011; Libby, 2012; Powell, Tarr, & Sheridan, 2013). Thus, the goal of the present study was to test three competing theoretical models for how adversity experienced in childhood and adulthood may jointly relate to low-grade inflammation during middle age. The first possible explanation would fall under a stress generation model, whereby childhood adversity appears as a risk factor for later inflammation simply because it correlates with or generates adult stress. Alternatively, a stress accumulation model would suggest that childhood and adult stressors have independent and additive associations with inflammation later in life. Finally, the early-life stress sensitization model would predict synergistic effects between early and later stressors such that individuals exposed to both have worse outcomes than would be predicted from a purely additive model. We sought to test these competing theoretical models using data from the large national Midlife in the United States (MIDUS) study.
Stress Generation Model
Stress can be defined as a “real or interpreted threat to the physiological or psychological integrity of an individual that results in physiological and/or behavioral responses” (McEwen, 2000, p. 508). Stressful experiences in adulthood, especially when they are severe and chronic (e.g., job strain, social isolation, low income), are associated with poorer overall health status and higher prevalence of some conditions such as CHD or diabetes (Braveman, Cubbin, Egerter, Williams, & Pamuk, 2010; Holt-Lunstad, Smith, & Layton, 2010; Pejtersen, Burr, Hannerz, Fishta, & Eller, 2014; Pollitt, Rose, & Kaufman, 2005; Steptoe & Kivimäki, 2013). What is increasingly recognized is that severe and chronic adversity during childhood (e.g., experiencing maltreatment, family dysfunction, impoverished socioeconomic circumstances) is also linked to poorer health outcomes in adulthood (Felitti et al., 1998; Galobardes, Lynch, & Smith, 2004, 2008; Lawlor, Sterne, Tynelius, Davey Smith, & Rasmussen, 2006; Wegman & Stetler, 2009). However, more research is needed to uncover the mechanisms through which early-life stress might become “biologically embedded” and exert such long-term effects on human health (Hertzman & Boyce, 2010; Miller et al., 2011). One possibility is suggested by a stress generation model –i.e., early-life stress predisposes individuals to experience greater stress in adulthood and this later exposure largely explains any variance associated with childhood adversity (e.g., Hammen, 1991). This could occur in a number of ways. First, environmental continuity is the norm rather than the exception for most individuals –for instance, reviews of existing epidemiological studies suggest that low early-life socioeconomic status (SES) is associated with lower adult income, educational attainment, and occupational prestige. These markers of adult SES mediate some or most of the association between childhood conditions and mortality, depending on the study (Galobardes et al., 2008). Secondly, adverse childhood experiences such as maltreatment, family chaos and poverty can instill cognitive biases towards threat such that even ambiguous stimuli are interpreted as dangerous (Chen, Cohen, & Miller, 2010) and threatening stimuli are allocated more attentional resources (Shackman, Shackman, & Pollak, 2007), compounding levels of anxiety and stress over the lifespan. Early-life stress has similarly been linked to poorer self-regulation skills (Blair & Raver, 2012), as well as lower access to support from close relationships in adulthood (Fagundes, Bennett, Derry, & Kiecolt-Glaser, 2011), which may leave individuals more vulnerable to experience stress that taxes their coping capacity. Moreover, in the realm of trauma, we know that individuals who experience trauma in childhood have a higher than average likelihood of being re-exposed to traumatic events in adolescence or adulthood, with some of these incidents facilitated by continued exposure to violent environments or by the survivors’ depressive or anxious behavioral, emotional and cognitive patterns (Widom, Czaja, & Dutton, 2008). Together, all these influences increase the odds that childhood adversity can directly or indirectly generate adult stress, supporting the main assumption of this theoretical model.
Stress Accumulation Model
Even if the stress generation account is accurate, there are likely to be additional pathways linking early adversity and later health. Indeed, there is mounting evidence that, even after controlling for adult stress, childhood exposures to adversity explain unique portions of variability in health outcomes, particularly in low-grade inflammation (Miller et al., 2011). For instance, this has been shown with research on child maltreatment (Wegman & Stetler, 2009) and epidemiological studies on low early-life SES (Galobardes et al., 2004, 2008; Kittleson et al., 2006). Such patterns suggest that early and later-life stressors may have independent and additive contributions. A parsimonious explanation of these findings would be the stress accumulation model, which views stressors as having additive influences on later health (Evans & Kim, 2010; Evans, Li, & Whipple, 2013). The accumulation of adversity over time would then explain gradients in health based on total stress exposure. In support of this notion, a review by Pollitt et al. (2005) reported that previous studies examining life course SES as a predictor of adult cardiovascular outcomes most consistently supported the accumulation model such that longer duration of total exposure to low SES and negative experiences across any life stages was related to poorer outcomes and was a stronger predictor than either adult or childhood SES. Some argue that the reason low SES is detrimental to human development and health is precisely because it subsumes an additive exposure to multiple risk factors (Evans et al., 2013), both over time and across types of stressors. This cumulative exposure to stress for those experiencing low SES is believed to tax many physiological systems, leading to cumulative “wear and tear” on the organism, accelerated aging and ultimately to heterogeneous disease processes (Seeman, Epel, Gruenewald, Karlamangla, & McEwen, 2010).
A key assumption of the stress accumulation model is that stressors combine additively – i.e., they don’t have interactive or multiplicative effects. There is some evidence supporting this assumption. For instance, one study found a linear dose-response relationship between the number of adversities experienced before age 18 and the prevalence of health-relevant conditions such as obesity or number of comorbid mental health conditions in adulthood (Anda et al., 2006; Felitti et al., 1998). However, this linear and additive pattern was not found with respect to the prevalence of some other health conditions (e.g., stroke, cancer; Felitti et al., 1998). More research is needed to investigate these patterns based on adversity experienced beyond age 18 and across the lifespan, given the possibility of interactive effects between childhood and adult stressors, which are suggested by the next model we discuss.
Early-life Stress Sensitization Model
In contrast to the stress generation model, which proposes that some and perhaps most of the effects of early experience operate through adult exposure to stress, the early-life sensitization model posits an independent and privileged role for early development as a period when the organism is more susceptible to adverse events. As exemplars of this theoretical perspective, the fetal origins hypothesis, which linked early nutritional deprivation and reduced fetal growth to heightened risk of adult diseases such as CHD and type 2 diabetes independently of adult risk factors (Barker, 1998) and the more general developmental origins of health and disease (DOHaD) hypothesis (Gluckman, Hanson, Cooper, & Thornburg, 2008; Wadhwa, Buss, Entringer, & Swanson, 2009) both argue that early environmental inputs shape later risk for disease because they act during periods of heightened plasticity and structural or functional maturation in many organs and systems. This type of biological “programming” is thought to carry forward by permanently shaping the organism’s physiology in a way that magnifies vulnerability to later disease.
When applied in the present context, the early-life stress sensitization model would predict that adverse childhood experiences would not only have independent and long-lasting effects on the functioning of the stress and immune systems, but they would also amplify reactions to stressors encountered later –i.e., there would be synergistic effects between early and later adversity. There is some emerging evidence in humans that early-life stress may shape brain circuits and peripheral physiology in ways that are associated with alterations in the functioning of the hypothalamic-pituitary-adrenocortical (HPA) axis and the sympathetic-adreno-medullary system in adulthood (Gunnar & Quevedo, 2007; Lupien, McEwen, Gunnar, & Heim, 2009). Similarly, there is mounting evidence that early-life stress may shape the functioning of certain immune functions, specifically by promoting exaggerated pro-inflammatory tendencies in monocytes and macrophages (Miller et al., 2011). However, it remains unclear (a) whether these associations are independent of adult stress exposure, and (b) whether early and late-life stressors have independent versus overlapping and/or interactive influences. Furthermore, the biological and behavioral pathways mediating the connections between early-life stress and inflammation late in life proposed in theoretical models (Miller et al., 2011) need to be clarified as little is known about them. We also know little about whether early and later chronic stressors are linked to inflammation through similar or different pathways. The present study aimed to address these gaps in the literature.
Linking Stressors to Inflammation: Mediational Scenarios
Both acute and chronic psychological stressors are associated with changes in various functions of the immune system (Segerstrom & Miller, 2004). Inflammation is an adaptive response by innate immune cells to injuries and infections. However, if this response becomes sustained and disseminated, either because the evoking stimulus remains or the system is dysregulated, a low-grade, chronic inflammation can develop. This “nonresolving inflammation” (Nathan & Ding, 2010) has been linked to morbidity and mortality from a variety of chronic illnesses, including CHD, type 2 diabetes, metabolic syndrome, and some cancers (Black, 2003; Hansson & Hermansson, 2011; Libby, 2012; Powell et al., 2013). There is also a growing body of evidence linking exposure to various adversities with inflammation. In childhood, chronic stressors such as maltreatment or low SES have been linked to biomarkers thought to reflect non-resolving inflammation, such as CRP and IL-6 (Coelho, Viola, Walss-Bass, Brietzke, & Grassi-Oliveira, 2014; Fagundes, Glaser, & Kiecolt-Glaser, 2013; Nazmi & Victora, 2007). Adults confronting chronic stressors (e.g., low SES, familial caregiving, job burnout, loneliness) also display higher levels of these biomarkers (Hänsel et al., 2010; Nazmi & Victora, 2007).
However, the mediational pathways through which these adversities predispose individuals to low-grade inflammation have not been comprehensively tested in humans. It is thought that the primary mediators linking stress to inflammation are dysregulation of the HPA axis and sympathetic nervous system (SNS) (Glaser & Kiecolt-Glaser, 2005; Hänsel et al., 2010; Irwin & Cole, 2011), and health-compromising behaviors that are occasioned or exacerbated by stress (e.g., Kiecolt-Glaser & Glaser, 1988; Raposa, Bower, Hammen, Najman, & Brennan, 2014). Cortisol, the primary hormonal product of the HPA axis, is known at high doses to counter the pro-inflammatory activity of monocytes and macrophages (Irwin & Cole, 2011). There is evidence that dysregulated cortisol levels, whether abnormally low or chronically high, can impair control of inflammatory responses (Raison & Miller, 2003; Sapolsky, Romero, & Munck, 2000). Secondly, SNS fibers innervate lymphoid organs, primarily releasing norepinephrine onto resident cells, which have adrenergic receptors. Norepinephrine then accentuates inflammation by changing patterns of cell trafficking and cytokine release (Elenkov, Wilder, Chrousos, & Vizi, 2000; Irwin & Cole, 2011; Nance & Sanders, 2007). The sympathetic-adreno-medullary system also releases epinephrine and, in a small proportion, norepinephrine, into the general circulation, providing a systemic route for regulating immune cells through adrenergic receptors (Elenkov et al., 2000). Secondly, lifestyle indices such as smoking, alcohol use, physical exercise or adiposity have also been shown to mediate some of the associations between stress exposure and heightened inflammation (Hagger-Johnson, Mõttus, Craig, Starr, & Deary, 2012; Kershaw, Mezuk, Abdou, Rafferty, & Jackson, 2010; Matthews, Chang, Thurston, & Bromberger, 2014; Raposa et al., 2014). In addition to these hypothesized pathways, there is an expanding evidence base documenting the bidirectional connections between depressive symptoms and inflammatory states (Messay, Lim, & Marsland, 2012; Raison & Miller, 2012; Slavich & Irwin, 2014). There are also well-known associations amongst stressful experiences, lifestyle indices, HPA and SNS activity, and depressive phenotypes (Kessler, 1997; Lang & Borgwardt, 2013; Miller & Cole, 2012; Pariante & Lightman, 2008). These observations suggest a possible mediating role of depressive symptoms for the association between adversity and inflammation. Despite these emerging findings, more empirical evidence is needed to test the unique and independent contributions of HPA output, SNS indices, lifestyle indices, and depressive symptoms to inflammation, which would provide clearer targets for prevention and intervention efforts.
Aims of the Present Study
The primary goals of this investigation were to (a) jointly examine the roles of childhood and recent stressors in inflammation at midlife, a time when many chronic diseases of aging begin to manifest clinically; and (b) to shed light on some of the biobehavioral mechanisms that could plausibly serve as candidate mediators for these associations. This report is based on data from the Biomarker Project of the longitudinal MIDUS study, a national survey focused on uncovering the role of behavioral and psychosocial factors in shaping age-related differences in physical and mental health. Inflammation was assessed via a panel of five biomarkers derived from fasting blood samples (C-reactive protein, interleukin-6, fibrinogen, E-Selectin, and ICAM-1), as detailed in the next sections.
Methods
Participants
Participants were drawn from the nationally representative MIDUS study, which began between 1995 and 1996 with 7,108 non-institutionalized adults selected via random-digit phone dialing from the 48 contiguous states. To allow genetically informed analyses, MIDUS included 957 pairs of twins and 950 non-twin siblings. An average of 9 years later, 75% of surviving respondents participated in a follow-up study, known as MIDUS II. Biological data were collected from a subset of participants (this is known as the Biomarker Project), who traveled to a General Clinical Research Center (GCRC) for a two-day, overnight visit. Participants from the West Coast, East Coast, and Midwestern United States were invited to attend a GCRC close to their location (UCLA, Georgetown University, or University of Wisconsin). As a refinement to MIDUS II, an African American subsample was also recruited from the Milwaukee, Wisconsin area, and they were invited to complete all measures from MIDUS I and MIDUS II, as well as the biological sample collection. When including the Milwaukee sample, the total number of participants in the Biomarker Project was N = 1255. These individuals had higher educational attainment than the overall MIDUS II sample but were comparable on other demographic factors (age, sex, race, income) and biomedical characteristics (subjective health, chronic conditions, health behaviors; Dienberg Love, Seeman, Weinstein, & Ryff, 2010). The mean age of the sample was 46.40 years and 56.80 years at the MIDUS I and II assessments, respectively.
For the analyses reported here, we included 1180 MIDUS II participants from the Biomarker Project that had available data for childhood adversity, recent life events, inflammation composite, and demographic or biomedical covariates. Participants included in this analysis were on average 57.3 years old (SD = 11.5), 56% female and exhibited some diversity in terms of racial/ethnic background: 74.9% Non-Hispanic White, 17.9% African American, 3.2% Hispanic, and 4% other. The average total household income in this sample was $69,145 (SD = $57,516, range $0 - $300,000; for other participant characteristics, please see Table 1). The 1180 participants included here did not differ from the full Biomarker sample comprised of 1255 adults with respect to age, gender, race, educational level, history of heart disease or diabetes, or any of the major study variables such as childhood adverse events, recent life events, and the inflammation composite (p’s > .51). There were 153 sibling sets in the Biomarker sample and 142 among participants included in this report (see Results section for details on how they were treated in our analyses).
Table 1.
Participant characteristics (N = 1180).
| Characteristic | Mean (SD) or Number (%) |
|---|---|
| Age (years) | 57.3 (11.5) |
| Sex (female) | 661 (56%) |
| Race/ethnicity: White (non-Hispanic) | 884 (74.9%) |
| African American | 211 (17.9%) |
| Hispanic | 38 (3.2%) |
| Other | 47 (4%) |
| Educational level* | 7.97 (2.57) |
| History of heart disease | 137 (11.6%) |
| History of diabetes | 145 (12.3%) |
| Taking anti-hypertensive medications | 430 (36.4%) |
| Taking cholesterol-lowering medications | 328 (27.8%) |
| Taking corticosteroid medications | 55 (4.7%) |
| Taking daily NSAID medications** | 319 (27%) |
Notes:
Highest educational level completed by self or spouse was used and coded as follows: 1 = no school/some grade school; 2 = eighth grade; 3 = some high school; 4 = GED; 5 = high school degree; 6 = one or two years of college; 7 = 3 or more years of college; 8 = degree from 2-year college, vocational school or Associate’s degree; 9 = college degree; 10 = some graduate school; 11 = Master’s degree; 12 = Doctoral degree.
NSAID = non-steroidal anti-inflammatory medications included aspirin, ibuprofen, and naproxen.
Procedure
Participants arrived to one of the three GCRCs and were checked in for their two-day overnight stay. On Day 1, they were assisted by medical staff in completing their medical history, a physical exam, and a bone densitometry scan. They were also provided with a packet of self-administered questionnaires and were given instructions for a 12-hour, overnight urine sample collection (7:00 pm to 7:00 am). Nursing staff collected the urine specimens the following morning, when they also collected fasting blood samples from which the inflammatory biomarker concentrations were later derived. After breakfast, a cognitive challenge protocol was conducted (results not included here).
Measures
Inflammation composite
Five serum markers of low-grade inflammation derived from fasting blood samples were considered: C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, E-Selectin, and Intercellular Adhesion Molecule-1 (ICAM-1). CRP was measured using a particle enhanced immunonepholometric assay (BNII nephelometer, Dade Behring Inc., Deerfield, IL). Serum IL6 was assessed using the Quantikine® High-sensitivity ELISA kit #HS600B according to manufacturer guidelines (R & D Systems, Minneapolis, MN). Fibrinogen antigen was measured using the BNII nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc., Deerfield, IL). Soluble E-selectin, also known as endothelial leukocyte adhesion molecule-1 (ELAM-1) and CD62E, was measured using a high sensitivity ELISA assay (Parameter Human sE-Selectin Immunoassay; R&D Systems, Minneapolis, MN). Soluble ICAM-1 was measured with an ELISA assay (Parameter Human sICAM-1 Immunoassay; R&D Systems, Minneapolis, MN). The laboratory intra- and inter-assay coefficients of variance (CV) for all protein assays were in acceptable ranges (< 10%).
The inflammation indicators were all significantly correlated with each other (all p’s < .002; mean r = .26, range r = .09 –.54). A maximum likelihood factor analysis suggested a one-factor solution (with CRP, IL-6, Fibrinogen, E-Selectin, and ICAM-1 having loadings of .78, .68, .67, .24, and .24, respectively, on a single factor). Thus, the five indices were standardized and combined to yield one composite measure of low-grade inflammation (note: 99.1% of participants included in this analysis had all five measures available; 11 participants had only two or three of the indices available; for them, these measures were standardized and combined in the same way to obtain an imputed mean value; results were unchanged when excluding these participants, thus analyses are reported on the full sample). A measure of serum soluble IL-6 receptor was also collected in the Biomarker Project, but it did not load onto the common factor (loading = .07) and it did not correlate significantly (mean r = .04, range r = .02 –.06, p’s > .05) with four of the five other inflammatory indices (only had a significant but small association with ICAM-1). Thus this sixth measure was excluded from the Inflammation composite and from present analyses.
Cortisol output
A cumulative cortisol measure was obtained from 12-hour overnight urinary samples. Cortisol concentrations were assessed using an Enzymatic Colorimetric Assay and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Data from participants with renal failure or severe renal decline according to glomerular filtration rate were set to “missing”. Values were adjusted for urinary creatinine, which was obtained from the same samples using an Enzymatic Colorimetric Assay performed at the Mayo Medical Laboratory (Rochester, MN). The inter-assay CV was 5.23%.
Sympathetic nervous system
Two indices of SNS outflow were used in this analysis: 12-hour urinary norepinephrine and epinephrine output. To obtain norepinephrine and epinephrine concentrations, High-Performance Liquid Chromatography (HPLC) was used for Urinary Free Catecholamine Fractionation at the Mayo Medical Laboratory (Rochester, MN). Similar to urinary cortisol procedures described above, values were adjusted for urinary creatinine and participants with renal failure or severe renal decline according to glomerular filtration rate had their catecholamine urinary concentrations set to “missing”. The inter- and intra-assay CVs for urinary norepinephrine and epinephrine were between 6.7% and 8%.
Adverse childhood events (ACEs)
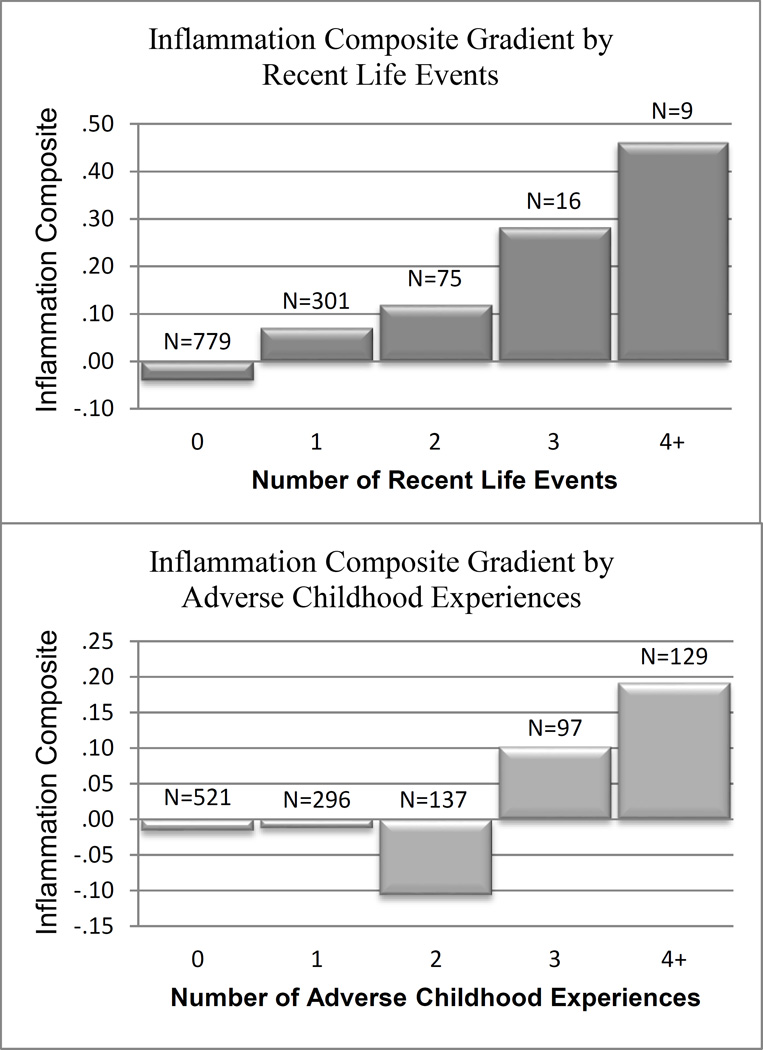
We used the ACE Study Questionnaire (Felitti et al., 1998) as a template to construct a summary measure of adverse events experienced before age 18. In other large-scale epidemiologic studies, scores on this questionnaire have predicted a wide range of health-related outcomes (Anda et al., 2006; Bellis et al., 2014; Felitti et al., 1998). The questionnaire includes dichotomous items (yes/no) asking participants whether they experienced any of ten adverse experiences before age 18. The MIDUS study did not collect information on two of these experiences (witnessing violence against their mother while growing up and having either of their parents incarcerated at any point), but participants did complete questions inquiring about the other eight experiences (physical abuse, emotional abuse, sexual abuse, emotional neglect, physical neglect, parental divorce, any parent abusing alcohol or drugs, and parental depression). Items covering the first five adverse experiences were derived from the Childhood Trauma Questionnaire (CTQ, Bernstein et al., 2003) completed by participants at the biomarker collection. The CTQ is a widely-used measure of childhood adversity and has high external validity, such that self-reports on the CTQ questionnaire are consistent with information derived from clinical interviews and objective sources of information –e.g., Child Protective Services records (Bernstein et al., 2003). Information regarding the other three adverse events was gleaned from MIDUS II questionnaires. A score of “1” was assigned for each experience endorsed, leading to an overall possible maximum score of 8 on this scale. Given the low frequencies for scores of 5 to 8, we created a single category for experiencing 4 or more stressors such that the final range for this index was 0–4 (see Figure 1 for final sample size in each category).
Figure 1.
Mean levels on Inflammation composite by number of RLEs and ACEs.
Recent life events (RLEs)
Participants completed the MIDUS Stressful Life Event Inventory created for the purposes of this study. The inventory was based on standard life stress measures (Turner & Wheaton, 1995) and included a comprehensive list of 20 possible events that could be experienced during adulthood (e.g., being a victim of physical or sexual assault, death of their child, loss of home, being fired from a job, jail detention, experiencing combat). For each item, subjects were asked if that event occurred at any point in their life and the age when it happened. We added a score of “1” for every event experienced in the previous 5 years and summed them to create a cumulative index of recent life events experienced. Given the low frequencies for scores greater than 4, we grouped all participants experiencing 4 or more events together and coded their score as a 4 (see Figure 1 for frequencies in each category). Results were similar when using a scale where each life event was weighted by the participants’ subjective appraisal of the event’s short-term and long-term negative impact on their lives.
Lifestyle indices
At the biomarker assessment, information regarding cigarette smoking, alcohol consumption, physical exercise and waist circumference (measured in centimeters and standardized within each gender) was collected. Because the distributions of smoking, alcohol use, and exercise variables were extremely skewed and could not be corrected with transformations, they were recoded into ordinal variables. For smoking, the new variable was coded as 0 = never smoker, 1 = former smoker, and 2 = current smoker. For alcohol, it was 0 = zero drinks per week, 1 = less than 10 drinks per week, and 2 = 10 or more drinks per week. For physical exercise, number of minutes of weekly strenuous activity were coded as 0 = none, 1= less than 500 minutes per week, 2 = 500–1000 minutes per week, and 3 = more than 1000 minutes per week. These categories were chosen based on a previous MIDUS report, which significantly linked the exercise variable coded in this fashion to inflammatory outcomes (Strohacker, Wing, & McCaffery, 2013).
Depressive symptoms
The 20-item Center for Epidemiologic Studies Depression (CES-D) Inventory was used at the time of biomarker collection to assess depressive symptoms in the prior week. In prior studies the measure has shown high internal consistency and test-retest reliability, as well as adequate validity assessed via correlations with other self-report measures and clinical ratings (Radloff, 1977). In this sample the measure also had high internal consistency (Cronbach’s alpha = .89).
Covariates
Basic sociodemographic, medical history, and medication usage information was obtained during the biomarker collection and MIDUS II assessments (see summary data for all of these characteristics in Table 1). Age, sex, and educational level were included in our models. Additionally, race/ethnicity was dummy-coded for analyses, with the most numerous group –non-Hispanic Whites- serving as the reference and African American, Hispanic, or Other race/ethnicity being coded a “1”. Medical diagnoses and medications with potential associations with inflammation were also selected for inclusion –namely, history of heart disease or diabetes; use of anti-hypertensive, cholesterol-lowering, corticosteroid, or non-steroidal anti-inflammatory medications. As measures of potential reporting biases, the CTQ Minimization/Denial Scale and the Neuroticism scale from the Midlife Development Inventory-Personality Scales were tested as covariates to assess the role of under-reporting or over-reporting childhood adversity, respectively.
Data Analysis Plan
Data preparation
Variables were examined for outliers and for their approximation of the normal distribution before analyses. Values that exceeded four standard deviations from the mean were Winsorized and replaced with the value at the 99.9th percentile (CRP: n = 17; IL-6: n = 22; fibrinogen: n = 4; E-Selectin: n = 5; I-CAM1: n = 9; Inflammation composite: n = 1; urinary cortisol: n = 4; urinary norepinephrine: n = 7; urinary epinephrine: n = 10; waist circumference: n = 2; CES-D scores: n = 5). A logarithmic transformation was also applied to normalize the distributions of skewed variables (CRP, IL-6, urinary cortisol, norepinephrine, epinephrine, and CES-D scores; all had a right skew prior to log transformation). Continuous variables were mean-centered before calculating interaction terms and before use in multiple regression analyses.
Statistical analyses
We first used multiple regression analyses to examine the independent as well as interactive effects of ACEs and RLEs in predicting the Inflammation Composite. We initially tested the unadjusted associations, then repeated the analyses controlling for demographic, SES, and medical history covariates. To pit the three competing stress models against each other, the results were interpreted as follows: a significant bivariate association between ACEs and Inflammation combined with a non-significant role of ACEs after controlling for RLEs or current lifestyle indices were considered supportive of the stress generation model. Significant main effects for both ACEs and RLEs without a significant interaction term were deemed to support the stress accumulation model, whereas a significant interaction such that individuals scoring high on both ACEs and RLEs would exhibit greater inflammation than all other groups, in addition to or in the absence of main effects, were considered evidence for the early-life stress sensitization model.
In the next stage of the analysis, we used Structural Equation Modeling (SEM) implemented using the Mplus Software (version 6.12, Muthén & Muthén, 2011) to estimate the viability of indirect pathways involving adrenocortical and SNS biomarkers (urinary cortisol output, urinary norepinephrine, and urinary epinephrine), lifestyle indices (cigarette smoking, alcohol consumption, physical exercise, and waist circumference), and depressive symptoms. We first tested each of these candidate mediators in a separate model, to examine whether they constituted indirect pathways from ACEs and RLEs (included together to control for each other’s effects) to the Inflammation composite. Then, we conducted a more stringent analysis and modeled all these mediators simultaneously, after also parsing out variance due to demographics, SES, and medical history variables from the Inflammation composite. The latter was accomplished by including these possibly confounding factors in a multiple regression analysis as predictors of the Inflammation composite and saving the unstandardized regression residual. In effect, this residual represents the Inflammation composite after parsing out variance due to covariates, and was thus used as an outcome in subsequent SEM analyses.
Missing data
Given a rate of missing data on the dependent variable of only 6% (N = 75 out of the 1255 participants in the Biomarker Project), multiple imputation was not deemed necessary as estimates are not likely to become biased when the rate of missingness is less than 10% (Bennett, 2001). Furthermore, results were very similar when SEM analyses were conducted on the full sample of 1255 participants using the MLR estimator (maximum likelihood with robust standard errors), thus results are reported for the sample of 1180 participants to ensure that multiple regression models using different covariates are describing the same participants.
Results
Bivariate correlations and descriptive statistics for the main study variables are shown in Table 2.
Table 2.
Bivariate correlations and descriptive statistics for primary study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Inflammation | - | .09** | .12** | −.11** | .22** | −.07* | .46** | .17** | −.07* | −.19** | .15** | .10** | .10** | −.20** |
| 2. ACEs | - | .10** | −.09** | .06* | −.06* | .08** | .17** | .05 | −.10** | .29** | −.16** | .16** | −.10** | |
| 3. RLEs | - | −.07* | −.03 | −.08** | .12** | .10** | .02 | −.01 | .11** | −.18** | .00 | −.10** | ||
| 4. Log cortisol | - | .09** | .21** | −.19** | −.09** | −.01 | .09** | −.10** | .09** | .10** | .05 | |||
| 5. Log norepinephrine | - | .49** | .14** | .06* | −.06* | −.10** | −.01 | .24** | .22** | −.08** | ||||
| 6. Log epinephrine | - | −.19** | −.05 | −.06 | .04 | −.08** | .15** | .02 | .04 | |||||
| 7. Waist circumference± | - | .03 | −.09** | −.19** | .11** | .06* | .01 | −.15** | ||||||
| 8. Smoking cigarettes regularly | - | .14** | .00 | .18** | −.04 | −.09** | −.27** | |||||||
| 9. Alcohol consumption | - | .06* | .02 | −.05 | −.19** | .02 | ||||||||
| 10. Physical exercise | - | −.08** | −.03 | −.12** | −.01 | |||||||||
| 11. Log CES-D Depressive Symptoms | - | −.16** | .04 | −.17** | ||||||||||
| 12. Age | - | −.04 | −.00 | |||||||||||
| 13. Sex (1 = female) | - | −.05 | ||||||||||||
| 14. Educational level | - | |||||||||||||
| Means | .01 | 1.17 | .45 | 1.05 | 1.39 | .22 | −.006 | .63 | .76 | 1.04 | .83 | 57.33 | .56 | 7.97 |
| SDs | .64 | 1.36 | .74 | .35 | .19 | .26 | .96 | .73 | .64 | .82 | .40 | 11.5 | .50 | 2.57 |
Waist circumference was standardized within gender to account for significant gender differences.
p < .05;
p < .01.
Do Early-life and Recent Stressors Have Independent or Interactive Roles in Predicting Inflammation?
Multiple regression results indicated that both ACEs and RLEs were independently and uniquely associated with higher levels of inflammation (β = .07, t = 2.51, p = .01 and β = .11, t = 3.75, p < .001, respectively), after controlling for each other’s effects (second model in Table 3). The bivariate association between ACEs and inflammation (first model in Table 3: β = .09, t = 2.91, p <.004) decreased slightly when RLEs were added in the model, but remained significant. Their interaction, however, was not significant (β = .001, t = .05, p = .96). The additive roles of ACEs and RLEs, as well as their non-significant interaction, were also evident in models after controlling for a variety of demographic, SES, and medical history characteristics (third model in Table 3)1. Figure 1 shows the positive associations of RLEs and ACEs with the Inflammation composite2. Results of these analyses were consistent with the stress accumulation model, partly consistent with stress generation, and not supportive of the early-life stress sensitization model.
Table 3.
Multiple regression results predicting the Inflammation composite from ACEs (Model 1), ACEs, RLEs, and their interaction (Model 2) and controlling for possible confounds (Model 3). All analyses were conducted with N = 1180 who had available data for all Model 3 variables.
| Unstandardized Coefficients | Standardized Coefficients |
|||||
|---|---|---|---|---|---|---|
| Model | B | Std. Error | Beta | t | Sig. | |
| 1 | Constant | .01 | .02 | .36 | .72 | |
| ACEs | .04 | .01 | .09 | 2.91 | .004** | |
| 2 | Constant | .01 | .02 | .36 | .72 | |
| ACEs | .03 | .01 | .07 | 2.51 | .01* | |
| RLEs | .10 | .03 | .11 | 3.75 | <.001*** | |
| ACEs × RLEs | .001 | .02 | .00 | .05 | .96 | |
| 3 | Constant | −.23 | .12 | −1.94 | .052 | |
| ACEs | .03 | .01 | .07 | 2.29 | .02* | |
| RLEs | .09 | .02 | .10 | 3.69 | <.001*** | |
| ACEs × RLEs | .00 | .02 | .00 | −.11 | .91 | |
| Age | .01 | .00 | .08 | 2.66 | <.01** | |
| Gender (1=female) | .09 | .04 | .07 | 2.55 | .01* | |
| African American | .25 | .05 | .15 | 4.96 | <.001*** | |
| Hispanic | .07 | .11 | .02 | .63 | .53 | |
| Other | .07 | .10 | .02 | .69 | .49 | |
| Educational level | −.03 | .01 | −.11 | −3.90 | <.001*** | |
| History of heart disease | .23 | .06 | .12 | 3.86 | <.001*** | |
| History of diabetes | .21 | .06 | .11 | 3.74 | <.001*** | |
| Anti-hypertensive medications | .17 | .04 | .13 | 4.03 | <.001*** | |
| Cholesterol-lowering medications | −.04 | .04 | −.03 | −.88 | .38 | |
| Corticosteroids | .05 | .08 | .02 | .55 | .58 | |
| NSAID medications | −.04 | .04 | −.03 | −.98 | .33 | |
p < .05;
p < .01;
p < .001.
Sensitivity analyses revealed that results were identical when excluding all participants who had a medical history of heart disease or diabetes or who were taking any of the medications considered as covariates in the previous analysis (remaining sample size: N = 486). Namely, in this healthy subsample, both ACEs and RLEs remained significant predictors of Inflammation (β = .11, t = 2.36, p = .019; and β = .12, t = 2.67, p = .008), and their interaction continued to be non-significant (p = .55). This finding was also robust regardless of inclusion of demographic covariates (age, gender, race, educational level). Furthermore, our results were identical when controlling for the two measures of potential reporting biases, the CTQ Denial/Minimization Scale and the Neuroticism scale, as well as when excluding the top 5% highest-scoring participants on these measures. Lastly, neither ACEs nor RLEs interacted significantly with age, gender, or race to predict inflammation (p’s range = .21 - .96) thus moderation was not considered further.
As mentioned, MIDUS recruited some twin and non-twin sibling sets and some of these participants were included in the Biomarker project. Because their data are likely to be correlated and violate the assumption of independent and identically distributed observations, we conducted the analyses above again but including only one sibling from each family (each sibling was selected using a random number generator). We found the same pattern of results in this analysis (N = 1038; ACEs: β = .06, t = 1.97, p = .049; RLEs: β = .13, t = 4.05, p < .001; interaction: p = .64).
Testing Candidate Mediators: HPA, SNS Indices, Lifestyle Factors, and Depressive Symptoms
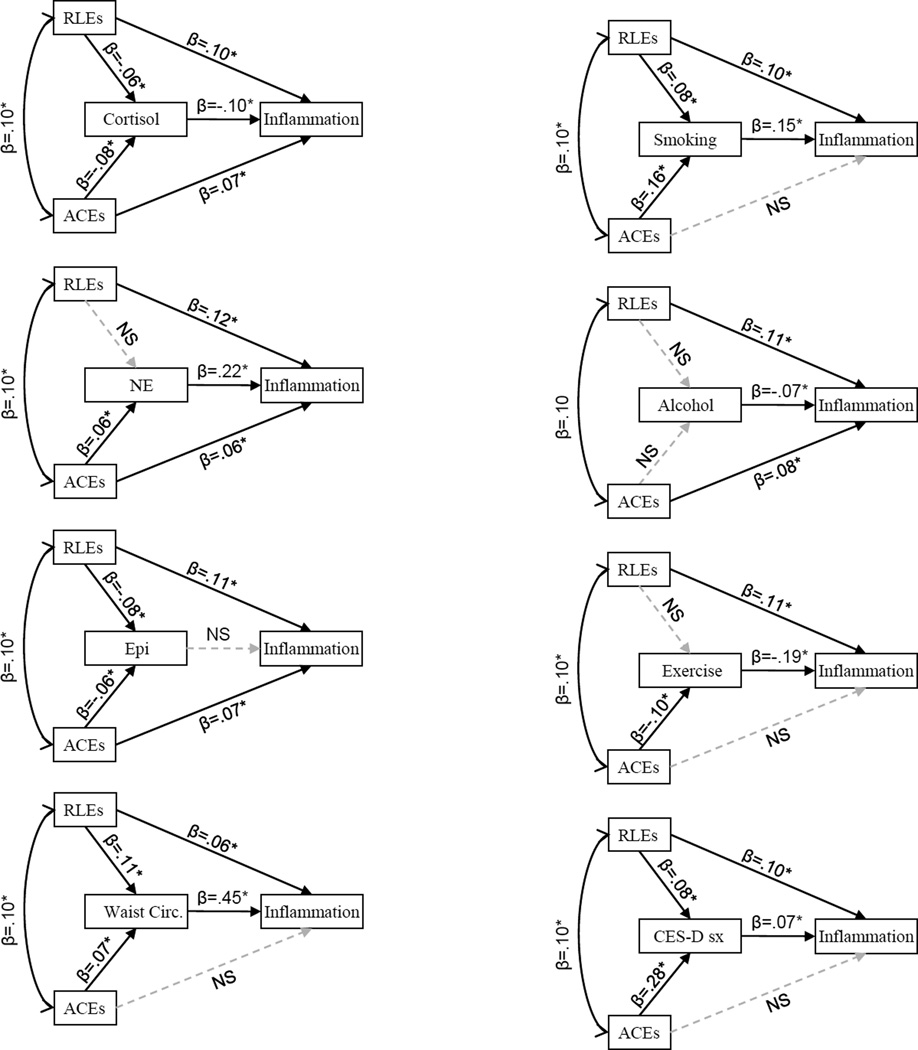
Given the significant associations of both ACEs and RLEs with Inflammation, in the next step we proceeded to test indirect pathways that may help explain these associations. Table 4 and Figure 2 shows the results of separate SEM analyses, which tested each of hypothesized mediators3 as indirect pathways linking ACEs and RLEs with the Inflammation composite. In the case of ACEs, multiple significant indirect pathways emerged, suggesting that early adversity was linked to current inflammation via lower urinary cortisol output, higher urinary norepinephrine output, greater waist circumference, more smoking, lower levels of exercise, and more frequent depressive symptoms. These models included RLEs, so the indirect pathways reflect unique associations of ACEs with the candidate mediator and the Inflammation composite. For RLEs, there were significant indirect pathways linking recent events with current inflammation via more smoking and larger waist circumference. (Again, these models included ACEs, so reflect the distinct associations of RLEs.)
Table 4.
Results of SEM mediation tests (unadjusted for covariates). Each mediator was tested separately, including both ACEs and RLEs in each model and estimating the significance of the indirect, mediated path after controlling for the direct paths from ACEs and RLEs to Inflammation.
| Model | Indirect Paths | β | SE | p-value |
|---|---|---|---|---|
| 1 | ACEs → ↓ Urinary cortisol output → Inflammation | .008 | .004 | .03* |
| RLEs → Urinary cortisol output → Inflammation | .006 | .003 | .08 | |
| 2 | ACEs → ↑Urinary NE output → Inflammation | .014 | .006 | .03* |
| RLEs → Urinary NE output → Inflammation | −.009 | .006 | .16 | |
| 3 | ACEs → Urinary EPI output → Inflammation | .003 | .002 | .16 |
| RLEs → Urinary EPI output → Inflammation | .004 | .003 | .13 | |
| 4 | ACEs → ↑Waist circumference → Inflammation | .032 | .014 | .02* |
| RLEs → ↑Waist circumference → Inflammation | .049 | .014 | .001** | |
| 5 | ACEs → ↑ Smoking → Inflammation | .011 | .003 | <.001*** |
| RLEs → ↑ Smoking → Inflammation | .011 | .004 | .02* | |
| 6 | ACEs → Alcohol use → Inflammation | −.003 | .002 | .18 |
| RLEs → Alcohol use → Inflammation | −.001 | .002 | .60 | |
| 7 | ACEs → ↓ Exercise → Inflammation | .018 | .006 | .001** |
| RLEs → Exercise → Inflammation | .000 | .006 | .95 | |
| 8 | ACEs → CES-D Symptoms → Inflammation | .02 | .008 | .015* |
| RLEs → CES-D Symptoms → Inflammation | .006 | .003 | .065 | |
NE = norepinephrine; EPI = epinephrine.
p < .05;
p < .01;
p < .01.
Figure 2.
Standardized path coefficients for models in Table 4. Significant paths shown in solid black lines, non-significant paths in gray dashed lines. Bivariate associations before the addition of mediators were β =.07* for ACEs and Inflammation and β =.11* for RLEs and Inflammation.
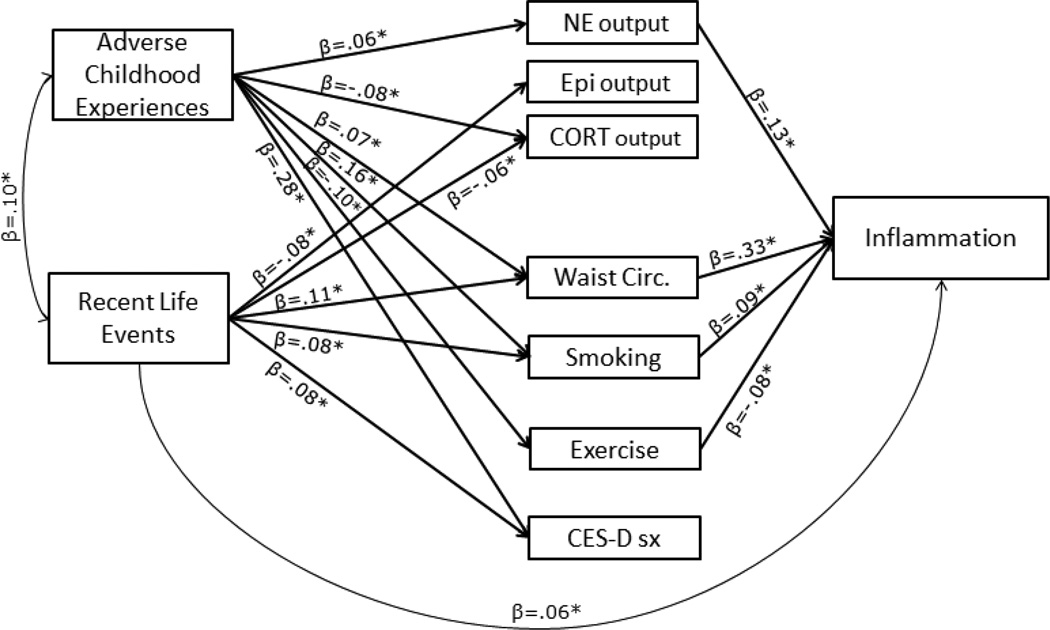
Results changed very little when testing all these mediators simultaneously and co-varying out the effects of demographic and medical confounds from the Inflammation composite (Table 5 and Figure 3). For ACEs, the indirect pathways involving norepinephrine, waist circumference, smoking, and exercise retained an explanatory role. The indirect pathway involving cortisol dropped to non-significance (p = .37) when covariates were included, likely because lower urinary cortisol was significantly associated with being African American, male gender, taking anti-hypertensive medications, cholesterol-lowering medications, corticosteroids, or having a history of diabetes (p’s ranging from <.001 to .049). The indirect pathway involving depressive symptoms also became non-significant (p = .28). When norepinephrine, waist circumference, smoking, and exercise were modeled together, the direct pathway from ACEs to Inflammation was no longer significant (β = −.001, SE = .03, p = .98), suggesting full statistical mediation. For RLEs, including the panel of covariates, smoking, and waist circumference in the SEM together did not change the pattern of results. Here, there was evidence suggestive of partial mediation; when these variables were in the model, the direct pathway from RLEs to the Inflammation composite remained significant (β = .06, SE = .03, p = .03).
Table 5.
Model with all candidate mediators entered simultaneously and with the Inflammation composite adjusted for demographic and biomedical history covariates.
| Model | Indirect Paths | β | SE | p-value |
|---|---|---|---|---|
| 9 | ACEs → Urinary cortisol output → Inflammation | −.002 | .003 | .37 |
| RLEs → Urinary cortisol output → Inflammation | −.002 | .002 | .38 | |
| ACEs → ↑Urinary NE output → Inflammation | .008 | .004 | .06Δ | |
| RLEs → Urinary NE output → Inflammation | −.005 | .004 | .18 | |
| ACEs → Urinary EPI output → Inflammation | .003 | .002 | .23 | |
| RLEs → Urinary EPI output → Inflammation | .004 | .003 | .20 | |
| ACEs →↑Waist circumference → Inflammation | .024 | .01 | .02* | |
| RLEs → ↑Waist circumference → Inflammation | .037 | .011 | .001** | |
| ACEs → ↑Smoking → Inflammation | .015 | .005 | .006* | |
| RLEs → ↑Smoking → Inflammation | .007 | .004 | .036* | |
| ACEs → Alcohol use → Inflammation | .000 | .001 | .93 | |
| RLEs → Alcohol use → Inflammation | .000 | .000 | .93 | |
| ACEs → ↓ Exercise → Inflammation | .007 | .003 | .026* | |
| RLEs → Exercise → Inflammation | .000 | .002 | .95 | |
| ACEs → Depressive symptoms → Inflammation | .008 | .008 | .28 | |
| RLEs → Depressive symptoms → Inflammation | .002 | .002 | .31 |
NE = norepinephrine; EPI = epinephrine.
p < .10;
p < .05;
p < .01.
Figure 3.
Model 9 includes all mediators entered simultaneously and the Inflammation composite adjusted for demographic and biomedical history covariates. Only the significant standardized paths are shown in the figure (please see Table 5 for tests of significance for all indirect paths tested).
Discussion
Despite much interest in inflammation as a mediator of stress-disease connections (Glaser & Kiecolt-Glaser, 2005; Hänsel et al., 2010; Rohleder, 2014), some basic questions in this area remain unanswered. In particular, little is known about how stressors at different points in the lifespan relate, combine, or interact, or which biobehavioral mechanisms they set into motion to accentuate disease risks. The present study leveraged data from the large national MIDUS study to address these gaps and test three competing models for how childhood and recent major adverse events might jointly contribute to inflammation in middle-aged adults. The data provided the most support for the stress accumulation model, such that childhood adversity and recent stressors had independent and additive roles in association with inflammation, after accounting for their shared variance and any demographic or biomedical confounds, with no evidence of synergistic effects. Furthermore, we considered indices of HPA and SNS outflow, as well as lifestyle variables and depressive symptoms as candidate mediators linking these stressors with inflammation. SEM analyses identified several significant indirect pathways. First, adverse childhood experiences were associated with inflammation via greater SNS activity (indexed by 12-hour urinary norepinephrine output) and unhealthy lifestyle indices at midlife (greater abdominal adiposity, cigarette smoking and low levels of physical exercise). Secondly, recent life events were linked to inflammation via smoking and greater abdominal adiposity. These results advance our knowledge of lifespan pathways leading to risk of diseases with inflammatory underpinnings and open new avenues of research into some of the underlying mechanisms.
The stress accumulation model posits that the effects of stressful events accrue linearly across the lifespan and across stressor types (Evans & Kim, 2010; Evans et al., 2013) and exert cumulative damage that eventually results in disease, as hypothesized for instance by the allostatic load model (McEwen, 2008; Seeman et al., 2010). As discussed above, results were consistent with this hypothesis. An implication of this finding is that childhood experiences might have long-lasting consequences for adult health, in addition to the significant and independent explanatory contribution of recent life events.
There was also partial support for the stress generation model. Empirically, we found that childhood adversity was significantly correlated with experiencing major stressors during midlife. We also found that childhood adversity’s association with inflammation was somewhat attenuated when recent stressors were entered simultaneously. These findings are not surprising, given that individuals often experience environmental continuity across the lifespan (e.g., low SES, Galobardes et al., 2008). Furthermore, chronic stress during childhood is associated with psychological and behavioral proclivities that may leave individuals more vulnerable to experiencing or exacerbating stress later in life (Chen et al., 2010; Fagundes et al., 2011; Miller et al., 2011; Shackman et al., 2007; Widom et al., 2008). With that said, ACEs continued to predict inflammation when RLEs were added to the regression model. These findings suggest that stress generation is part, but not all, of the pathway through which childhood adversity relates to midlife inflammation.
Lastly, the non-significant interaction effect between ACEs and RLEs contradicted the prediction derived from the early-life stress sensitization model. Nevertheless, the significant associations of ACEs with both lower urinary cortisol output and higher norepinephrine output in middle-aged and older adults, even after controlling for the effects of RLEs, might be construed as supportive of the early programming hypothesis embedded within this model. Moreover, we cannot definitively rule out this model given that our composite measures of self-reported adverse events experienced before age 18 may not have the temporal specificity required to detect critical periods for the sensitization of stress and immune systems. Animal models suggest specific and fairly narrow time windows of plasticity for obtaining long-lasting effects of early experience on adult adrenocortical responsivity (e.g., first week of life in rodents, Meaney & Aitken, 1985). Human studies have also hinted at the existence of such sensitive periods –e.g., school-aged children who had experienced physical or sexual abuse before age 5 and exhibited depressive symptoms showed flattened diurnal cortisol slopes, whereas later maltreatment was not associated with this pattern (Cicchetti, Rogosch, Gunnar, & Toth, 2010). Future research should incorporate measures of the exact timing and duration of adverse childhood experiences, to better characterize potentially sensitive periods for the programming of the stress and immune systems in humans and more fully test the early sensitization model. Additionally, isolating which types of stressors matter at which developmental stages will be particularly informative, given recent evidence from the MIDUS study that some childhood stressors such as physical abuse and socioeconomic disadvantage are the strongest predictors of biological risk in middle-age (Friedman et al. 2015).
The second aim of the study was to investigate whether the data would be consistent with a mediating role for hormonal outflow from the HPA axis and SNS, lifestyle factors, and depressive symptoms. The results were consistent with the hypothesis that associations between ACEs and Inflammation were mediated through higher urinary norepinephrine output, greater waist circumference, smoking, and lower levels of exercise, even after including all these purported mediators simultaneously in a model (i.e., estimating their contributions independently of the others) and parsing out effects due to RLEs, demographic and biomedical confounds. The explanatory role of norepinephrine is consistent with studies that have linked exposure to chronically stressful circumstances in childhood (e.g., conflictual or neglectful family environments) with heightened sympathetic reactivity (for a review, see Repetti, Taylor, & Seeman, 2002). Furthermore, children exposed to these adverse environments also show emotion-processing and self-regulation deficits that might continue to facilitate exacerbated SNS reactions to stressors later into adulthood (Repetti et al., 2002). In turn, activation of sympathetic fibers releasing norephinephrine onto lymphoid organs is known to potentiate inflammation by changing patterns of cell trafficking and cytokine release (Elenkov et al., 2000; Irwin & Cole, 2011; Nance & Sanders, 2007). Showing that norepinephrine output partially explained the associations between ACEs and inflammation in humans is a novel contribution to the literature. Of course, with the study’s cross-sectional, observational design, firm conclusions about mediational scenarios are not appropriate. More definitive inferences about norepinephrine’s role in these processes will have to be gleaned from prospective, multi-wave studies with humans and corresponding experiments in animal models.
When tested by itself in a separate model and before adjustment for covariates, lower cortisol output also constituted a significant indirect path from ACEs to inflammation. This finding is consistent with meta-analytic reviews showing that chronic stress is associated with relatively lower cortisol output (Miller, Chen, & Zhou, 2007). Furthermore, lower cortisol levels were also associated with greater inflammation in this study, which would be compatible with the anti-inflammatory properties of cortisol (Irwin & Cole, 2011; Sapolsky et al., 2000). However, this indirect path no longer played an explanatory role when pitted against other mediators and parsing out variance due to demographic or biomedical confounds. This result could mean that lower cortisol levels were an artifact for those with higher ACEs (i.e., perhaps a transient effect caused by taking cholesterol-lowering medications, anti-hypertensive medications, or corticosteroids, and not intrinsically linked to higher exposure to ACEs). An alternative possibility is that of overadjustment for covariates in our analysis, which can occur when controlling for factors that could be part of the causal pathway between a hypothesized predictor and the outcome (Schisterman, Cole, & Platt, 2009). In this instance, cortisol might be one part of a common causal pathway from ACEs to inflammation, disease, and medication use, and its role obscured when these covariates were included in the regression equations. More longitudinal research will be needed to tease apart these possibilities.
The association of lifestyle factors such as smoking, excessive alcohol consumption, physical inactivity and related adiposity with both inflammation and childhood adversity has been previously noted (Hagger-Johnson et al., 2012; Matthews et al., 2014; Raposa et al., 2014). Our findings revealed that after adjustment for recent life events and demographic or biomedical covariates, only waist circumference, smoking, and lower levels of exercise were involved in significant indirect paths from ACEs to inflammation. These indirect pathways could be explained by previous research showing that adverse early-life events are associated with self-control depletion, which are in turn linked to greater adiposity, more smoking and lower levels of physical exercise (Hostinar, Ross, Chen, & Miller, 2014).
Smoking and waist circumference were the only candidate mediators tested that formed significant indirect paths from RLEs to inflammation, and they only partially explained this association. These patterns suggest that RLEs give rise to inflammation via pathways not considered here. One candidate is diet. A previous report from MIDUS showed that self-reported stress eating was significantly related to higher waist circumference (Tsenkova, Boylan, & Ryff, 2013) and it is known that visceral adiposity, particularly in the abdomen, is a major source of inflammatory mediators such as IL-6 (Hotamisligil, 2006). Stress-evoked eating can stimulate endogenous opioid release and thereby improve mood (Adam & Epel, 2007). This may weaken the pathway from RLEs to HPA/SNS dysregulation and to inflammation, while still promoting inflammation via increases in abdominal adiposity. Another possibility is that RLEs might operate through the same pathways as ACEs, but more time would have needed to elapse for these lifestyle and hormonal effects on inflammation to unfold.
Alcohol consumption did not significantly mediate any associations between either childhood or recent adverse events and inflammation and it was negatively correlated with inflammation in this sample. It could be that participants with serious health problems or greater medication use, which were abundant in this sample because of its focus on middle-aged and older adults, had to reduce or eliminate their alcohol use for health reasons. These speculations are difficult to verify with cross-sectional data, thus longitudinal tracking of stressors, alcohol intake, and inflammation will be critical for clarifying the nature of these links.
Lastly, depressive symptoms were significantly associated with ACEs, RLEs, and inflammation, and appeared to act as mediators between ACEs and inflammation in initial, unadjusted analyses. However, when all mediators were included simultaneously and biomedical and demographic confounds were parsed out, this indirect pathway no longer served a significant explanatory role. This suggests that depressive symptoms might be part of the causal pathway from adversity to inflammation, but might act through and share variance with lifestyle and hormonal pathways that are more proximal predictors of immune function. This would be consistent with other studies of depression and immunity showing that their association is, at least in part, explained by other mediators such as physical activity (Miller, Cohen, & Herbert, 1999). The cross-sectional design does not allow an effective test of the order and directionality of effects in such multi-step mediational pathways, but prospective longitudinal research will hopefully examine these underlying processes more closely.
The present study had several notable strengths, including the recruitment of a relatively large sample in biomarker research, with diverse participation across the United States. The availability of multiple inflammatory indices and of physiological measures assessing both HPA and SNS function was a unique opportunity afforded by the design. The analyses presented here also had a number of limitations. The design is correlational, and the concurrent assessment of stress, presumed mediators, and inflammatory outcomes precludes any definitive conclusions about mediation or causal pathways. Furthermore, the use of self-reported and retrospective measures of childhood adversity and recent stressors may be a source of measurement error due to memory problems or other sources of response bias. Nevertheless, our analyses revealed associations with childhood adversity even when controlling for recent stressors that might shape participants’ mindsets, and for reporting bias tendencies captured by the CTQ Minimization/Denial scale and the Neuroticism scale. Furthermore, the fact that participants’ self-reports are associated with objective indices of inflammatory processes suggests that more research is needed to reveal what mediates these associations. The considerable sample size can also diminish measurement error associated with using self-report instruments. Finally, even though the prospective and objective documentation of stressors experienced from birth into middle age would indeed be preferable for addressing the questions posed here, this approach has its own challenges in terms of cost and feasibility.
Despite the limitations noted, studies like MIDUS are an important first step before conducting more extensive longitudinal investigations across the human lifespan. These examinations may inform clinical efforts to reduce the burden of stress-related illness across the lifespan. For instance, it has been argued that research on ACEs should inform public health policies and connect them more closely to social work and intervention/prevention programs designed to reduce childhood adversity (Larkin, Felitti, & Anda, 2014). Additionally, in light of ongoing national debates regarding the utility of introducing questions about ACEs in routine physical examinations (Starecheski, 2015), there is an acute need for research that moves us closer to specifying the concrete pathways through which childhood adversity shapes later health.
Acknowledgements
Data used for this research was provided by the longitudinal study titled “Midlife in the United States” (MIDUS) managed by the Institute on Aging, University of Wisconsin, and supported by a grant from the National Institute on Aging (P01-AG020166). The authors’ efforts on this manuscript were supported by grants from the National Institute of Child Health and Human Development (F32HD078048 and R01 HD058502), the National Institute on Aging (R01 AG018436) and the National Institute on Drug Abuse (P30 DA027827).
Footnotes
We obtained similar results when creating a single measure of cumulative early and recent stress, coded as follows: 0 = no ACEs or RLEs; 1 = either ACEs or RLEs experienced; 2 = both ACEs and RLEs reported. There was a linear dose-response relation between this ordinal variable and inflammation (β = .10, t = 3.08, p = .002), however this summary measure explained less variability than ACEs and RLEs, thus they were modeled separately.
Regression analyses using orthogonal polynomial coding revealed that RLEs related to inflammation in a linear fashion (linear term: t = 2.66, p = .008), whereas the quadratic, cubic, and quartic terms were not significant (p > .60). When using orthogonal polynomials to characterize the association between ACEs and Inflammation, there was some evidence of curvilinearity (both linear and quadratic terms were significant, p < .009, with no other significant terms). However, follow-up GLM analyses comparing inflammation levels for the five ACE categories showed that groups reporting 0, 1, and 2 adverse events did not differ significantly from each other (p’s > .57), thus the variable was best modeled as linear in subsequent analyses.
We use the term “mediator” only in the statistical sense, as it was not possible to meet all conditions for true mediation given the lack of temporal separation between the measurement of predictors, mediators, and outcomes.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiology & Behavior. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. http://doi.org/10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. http://doi.org/10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of chronic disease. Clinical Science. 1998;95(2):115–128. [PubMed] [Google Scholar]
- Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: A national survey. Journal of Public Health. 2014 doi: 10.1093/pubmed/fdu065. http://doi.org/10.1093/pubmed/fdu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA. How can I deal with missing data in my study? Australian and New Zealand Journal of Public Health. 2001;25(5):464–469. [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. http://doi.org/10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, Behavior, and Immunity. 2003;17(5):350–364. doi: 10.1016/s0889-1591(03)00048-5. http://doi.org/10.1016/S0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity. American Psychologist. 2012;67(4):309–318. doi: 10.1037/a0027493. http://doi.org/10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health. 2010;100(Suppl):S186–S196. doi: 10.2105/AJPH.2009.166082. http://doi.org/10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science. 2010;21(31) doi: 10.1177/0956797609355566. http://doi.org/10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch Fa, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81(1):252–269. doi: 10.1111/j.1467-8624.2009.01393.x. http://doi.org/10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: A systematic review. Acta Psychiatrica Scandinavica. 2014;129(3):180–192. doi: 10.1111/acps.12217. http://doi.org/10.1111/acps.12217. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22(8):1059–1080. doi: 10.1177/0898264310374355. http://doi.org/10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve -an integrative interface between two supersystems: The brain and the immune system. Pharmacological Reviews. 2000;52(4):595–638. [PubMed] [Google Scholar]
- Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Annals of the New York Academy of Sciences. 2010;1186:174–189. doi: 10.1111/j.1749-6632.2009.05336.x. http://doi.org/10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychological Bulletin. 2013;139(6):1342–1396. doi: 10.1037/a0031808. http://doi.org/10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Bennett JM, Derry HM, Kiecolt-Glaser JK. Relationships and inflammation across the lifespan: Social developmental pathways to disease. Social and Personality Psychology Compass. 2011;5(11):891–903. doi: 10.1111/j.1751-9004.2011.00392.x. http://doi.org/10.1111/j.1751-9004.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain, Behavior, and Immunity. 2013;27(1):8–12. doi: 10.1016/j.bbi.2012.06.014. http://doi.org/10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Karlamangla AS, Gruenewald TL, Koretz B, Seeman TE. Early life adversity and adult biological risk profiles. Psychosomatic Medicine. 2015;77(2):176–185. doi: 10.1097/PSY.0000000000000147. http://doi.org/10.1097/PSY.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: Systematic review and interpretation. Epidemiologic Reviews. 2004;26:7–21. doi: 10.1093/epirev/mxh008. http://doi.org/10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. http://doi.org/10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews. Immunology. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. The New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. http://doi.org/10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. http://doi.org/10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hagger-Johnson G, Mõttus R, Craig LCA, Starr JM, Deary IJ. Pathways from childhood intelligence and socioeconomic status to late-life cardiovascular disease risk. Health Psychology. 2012;31(4):403–412. doi: 10.1037/a0026775. http://doi.org/10.1037/a0026775. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100(4):555–561. doi: 10.1037//0021-843x.100.4.555. http://doi.org/10.1037//0021-843X.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hänsel A, Hong S, Cámara RJA, von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neuroscience and Biobehavioral Reviews. 2010;35(1):115–121. doi: 10.1016/j.neubiorev.2009.12.012. http://doi.org/10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nature Immunology. 2011;12(3):204–212. doi: 10.1038/ni.2001. http://doi.org/10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. http://doi.org/10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Medicine. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. http://doi.org/10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chen E, Miller GE. Modeling the association between lifecourse socioeconomic disadvantage and systemic inflammation in healthy adults: The role of self-control. Health Psychology. 2014 doi: 10.1037/hea0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. http://doi.org/10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Reviews. Immunology. 2011;11(9):625–632. doi: 10.1038/nri3042. http://doi.org/10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw KN, Mezuk B, Abdou CM, Rafferty JA, Jackson JS. Socioeconomic position, health behaviors, and C-reactive protein: A moderated-mediation analysis. Health Psychology. 2010;29(3):307–316. doi: 10.1037/a0019286. http://doi.org/10.1037/a0019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. http://doi.org/10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain, Behavior, and Immunity. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Kittleson MM, Meoni LA, Wang N, Chu AY, Ford DE, Klag MJ. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Archives of Internal Medicine. 2006;166:2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- Lang UE, Borgwardt S. Molecular mechanisms of depression: Perspectives on new treatment strategies. Cellular Physiology and Biochemistry. 2013;31(6):761–777. doi: 10.1159/000350094. http://doi.org/10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- Larkin H, Felitti VJ, Anda RF. Social work and adverse childhood experiences research: Implications for practice and health policy. Social Work in Public Health. 2014;29(1):1–16. doi: 10.1080/19371918.2011.619433. http://doi.org/10.1080/19371918.2011.619433. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Sterne JAC, Tynelius P, Davey Smith G, Rasmussen F. Association of childhood socioeconomic position with cause-specific mortality in a prospective record linkage study of 1,839,384 individuals. American Journal of Epidemiology. 2006;164(9):907–915. doi: 10.1093/aje/kwj319. http://doi.org/10.1093/aje/kwj319. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(9):2045–2051. doi: 10.1161/ATVBAHA.108.179705. http://doi.org/10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. http://doi.org/10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: Physiological pathways and mechanisms. Psychosomatic Medicine. 2011;73(9):724–730. doi: 10.1097/PSY.0b013e318235be76. http://doi.org/10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Chang Y-F, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: Role of obesity. Brain, Behavior, and Immunity. 2014;36:29–34. doi: 10.1016/j.bbi.2013.09.013. http://doi.org/10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Stress, definition and concepts of. In: Fink G, editor. Encyclopedia of stress. Vol. 3. San Diego, CA: Academic Press; 2000. pp. 508–509. [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583(2-3):174–185. doi: 10.1016/j.ejphar.2007.11.071. http://doi.org/10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: Temporal parameters. Developmental Brain Research. 1985;22(2):301–304. doi: 10.1016/0165-3806(85)90183-x. http://doi.org/10.1016/0165-3806(85)90183-X. [DOI] [PubMed] [Google Scholar]
- Messay B, Lim A, Marsland AL. Current understanding of the bi-directional relationship of major depression with inflammation. Biology of Mood & Anxiety Disorders. 2012;2(1):4. doi: 10.1186/2045-5380-2-4. http://doi.org/10.1186/PREACCEPT-1461493759628561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959–997. doi: 10.1037/a0024768. http://doi.org/10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. http://doi.org/10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Herbert T. Pathways linking major depression and immunity in ambulatory female patients. Psychosomatic Medicine. 1999;860:850–860. doi: 10.1097/00006842-199911000-00021. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. http://doi.org/10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6th ed. Los Angeles, CA: Muthén & Muthén; 2011. [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, Behavior, and Immunity. 2007;21(6):736–745. doi: 10.1016/j.bbi.2007.03.008. http://doi.org/10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. http://doi.org/10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Nazmi A, Victora CG. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: a systematic review of population-based studies. BMC Public Health. 2007;7:212. doi: 10.1186/1471-2458-7-212. http://doi.org/10.1186/1471-2458-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NPR. What shapes health. 2015 Retrieved from http://www.npr.org/series/389312217/what-shapes-health.
- Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. http://doi.org/10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pejtersen JH, Burr H, Hannerz H, Fishta A, Eller NH. Update on work-related psychosocial factors and the development of ischemic heart disease. A systematic review. Cardiology in Review. 2014 doi: 10.1097/CRD.0000000000000033. http://doi.org/10.1097/CRD.0000000000000033. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: A systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. http://doi.org/10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwer F, Kupper N, Adriaanse MC. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discovery Medicine. 2010;9(45):112–118. [PubMed] [Google Scholar]
- Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain, Behavior, and Immunity. 2013;30(Suppl):S41–S47. doi: 10.1016/j.bbi.2012.06.015. http://doi.org/10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003 Sep;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Current Psychiatry Reports. 2012;13(6):467–475. doi: 10.1007/s11920-011-0232-0. http://doi.org/10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa EB, Bower JE, Hammen CL, Najman JM, Brennan PA. A developmental pathway from early life stress to inflammation: The role of negative health behaviors. Psychological Science. 2014;25(6):1268–1274. doi: 10.1177/0956797614530570. http://doi.org/10.1177/0956797614530570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. http://doi.org/10.1037//0033-2909.128.2.330. [PubMed] [Google Scholar]
- Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosomatic Medicine. 2014;76(3):181–189. doi: 10.1097/PSY.0000000000000049. http://doi.org/10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. http://doi.org/10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. http://doi.org/10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. http://doi.org/10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman JE, Shackman AJ, Pollak SD. Physical abuse amplifies attention to threat and increases anxiety in children. Emotion. 2007;7(4):838–852. doi: 10.1037/1528-3542.7.4.838. http://doi.org/10.1037/1528-3542.7.4.838. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140(3):774–815. doi: 10.1037/a0035302. http://doi.org/10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain, Behavior, and Immunity. 2012;26(2):239–250. doi: 10.1016/j.bbi.2011.11.003. http://doi.org/10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Starecheski L. 10 Questions some doctors are afraid to ask. 2015 Mar 3; Retrieved from: http://www.npr.org/sections/health-shots/2015/03/03/377569539/even-some-doctors-fear-these-10-questions.
- Steptoe A, Kivimäki M. Stress and cardiovascular disease: An update on current knowledge. Annual Review of Public Health. 2013;34:337–354. doi: 10.1146/annurev-publhealth-031912-114452. http://doi.org/10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- Strohacker K, Wing RR, McCaffery JM. Contributions of body mass index and exercise habits on inflammatory markers: A cohort study of middle-aged adults living in the USA. BMJ Open. 2013;3(5):1–8. doi: 10.1136/bmjopen-2013-002623. http://doi.org/10.1136/bmjopen-2013-002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsenkova V, Boylan JM, Ryff C. Stress eating and health. Findings from MIDUS, a national study of US adults. Appetite. 2013;69:151–155. doi: 10.1016/j.appet.2013.05.020. http://doi.org/10.1016/j.appet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B. Checklist measurement of stressful life events. In: Cohen S, Kessler R, Underwood Gordon L, editors. Measuring stress. A guide for health and social scientists. New York: Oxford University Press; 1995. pp. 29–58. [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Seminars in Reproductive Medicine. 2009;27(5):358–368. doi: 10.1055/s-0029-1237424. http://doi.org/10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine. 2009;71(8):805–812. doi: 10.1097/PSY.0b013e3181bb2b46. http://doi.org/10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- Widom CS, Czaja SJ, Dutton MA. Childhood victimization and lifetime revictimization. Child Abuse & Neglect. 2008;32(8):785–796. doi: 10.1016/j.chiabu.2007.12.006. http://doi.org/10.1016/j.chiabu.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]