Abstract
Background
Epidemiologic studies find sex-based differences in incidence, survival and long-term outcomes for children with cancer. The purpose of this study was to determine if male and female patients differ with regard to acute treatment-related toxicities.
Procedure
We reviewed data collected on the Children’s Cancer Group (CCG) high-risk acute lymphoblastic leukemia (ALL-HR) study (CCG-1961), and compared male and female patients’ toxicity incidence and related variables in the first four phases of treatment. Similar analyses were performed with standard-risk ALL (ALL-SR) patients enrolled in CCG-1991.
Results
Among ALL-HR patients, females had significantly more hospital days, delays in therapy, grade 3 or 4 toxicities (e.g., gastro-intestinal, liver) and supportive care interventions (e.g., transfusions, intravenous antibiotics) than males. Females were significantly more likely to have died of treatment-related causes than males (Hazard Ratio=2.8, 95% CI=1.5–5.3, p=0.002). Five months after beginning treatment, the cumulative incidence of treatment-related deaths was 2.6% for females and 1.2% for males. Similar disparities were found among ALL-SR patients with females experiencing significantly more hospital days and treatment-related toxicities than males.
Conclusion
This study complements cancer survivorship studies that also report an increase in treatment-related late effects among females. Risk profiles appear to be different for male and female patients with females having greater risk of developing both acute and long-term treatment-related toxicities. The underlying biological mechanisms for these sex differences are poorly understood and warrant further study in order to determine how sex-based outcome disparities can be addressed in future clinical trials and practice.
Keywords: acute toxicities, pediatric oncology, sex, acute lymphoblastic leukemia, disparities
INTRODUCTION
Epidemiologic studies in pediatric oncology have documented sex-based differences in incidence, survival and long-term outcomes. Females (0–19 years) have lower incidence of cancer[2] and increased 5-year survival than males[3]. Survivorship studies find females are at increased risk of treatment-related late effects, including cardiovascular disease, osteonecrosis and early mortality[4,5]. The underlying mechanisms for these disparities are poorly understood.
The relationship between drugs and clinical response is complex, affected by multiple intrinsic and extrinsic factors. Pharmacokinetic studies in adults have demonstrated male/female differences in hepatic drug metabolizing enzymes, volume distribution and clearance of specific drugs[6–8], with reports of females experiencing significantly more adverse events than adult males[9]. Eight of the ten prescription drugs withdrawn from the market (1997–2001) were due to higher health risks among women[10]. Proposed mechanisms for sex-based differences in drug response include sexual dimorphism in genetics, biology, biochemistry, and physiology that influence drug absorption, distribution, and metabolism[1,4,11]. In a review on how sex affects health and disease, the Institute of Medicine (IOM) highlights sex as “an important basic human variable”[1] that should be included in all levels of biomedical research design, especially when assessing drug dosing and toxicity.
In pediatric oncology sex differences in drug metabolism, including pharmacogenomics, pharmacokinetics, and pharmacodynamics studies, have been largely under-appreciated. Chemotherapy dosing for children/adolescents is based primarily on body surface area (BSA) without regard to patient sex in the belief that BSA standardizes hepatic and renal function (and therefore drug metabolism) among patients [12]. However, several studies find poor correlation between BSA and hepatic function, renal function and volume distribution[12–14]. Although the majority of children with cancer are enrolled in clinical trials that systematically collect toxicity data, comparative analyses based on sex are seldom performed. As a result, there is limited information regarding how treatment-related toxicities during active treatment differ for male and female patients.
The purpose of this study was to test the hypothesis that female patients experience more acute treatment-related toxicities than males. We analyzed toxicity data from high-risk ALL (ALL-HR) patients treated on CCG-1961 and standard-risk ALL (ALL-SR) patients enrolled in CCG-1991 to determine if results were similar for the two study populations. These protocols were selected because samples were large, treatments represent current therapies used in childhood ALL and toxicity data were collected systematically using standardized methods.
METHODS
Patients and Treatment
High Risk Cohort: CCG-1961
Eligibility
Patients were eligible if they were 1–21 years of age and diagnosed with ALL-HR according to the National Cancer Institute (NCI) Rome criteria[15]. Enrollment occurred from 1996–2002.
Summary of Study Design and Treatment
Treatment details have been previously published[16,17]. Briefly, patients enrolled in CCG-1961 received a four-drug Induction, consisting of vincristine, daunorubicin, asparaginase, and prednisone. Based on definitions of bone marrow morphology assessed on day 7 of treatment, patients who demonstrated a rapid early response (RER) were randomized to post-induction therapy of either standard or increased intensity and either standard or increased duration using a 2X2 factorial design. Slow early responsers (SERs) received post-induction therapy increased in both intensity and duration that compared two forms of anthracycline and received preventive whole brain irradiation (Study Schema, Supplemental Table 1).
CCG-1961 analytical file included 2,054 patients with end-of-phase toxicity data for Induction, Consolidation, Interim Maintenance I (IM-I), and Delayed Intensification I (DI-I). Toxicities were graded using the CCG toxicity rating scale (0 [within normal limits (WNL)], 1 [mild], 2 [moderate], 3 [severe], 4 [unacceptable]). End-of-phase reports collected information on phase duration (days), hospital days, intensive care unit (ICU) days, events (failure to respond to treatment, toxicity-related deaths, disease-related deaths, and relapse), toxicity-related therapy delays over one week, significant infections (pneumocystis, varicella/zoster, bacteremia/sepsis, central line related infection, fungal infection and other infections that required hospitalization); and use of supportive care interventions (e.g., parenteral nutrition, blood products).
Standard Risk Cohort: CCG-1991
Eligibility
Patients eligible were 1–9 years of age and diagnosed with ALL-SR according to the NCI Rome criteria[15]. Enrollment occurred from 2000–2005.
Summary of Study Design and Treatment
Treatment details have been previously published[18]. Briefly, patients received a three-drug Induction consisting of vincristine, asparaginase, and dexamethasone. Based on definitions of bone marrow morphology assessed at days 7, 14 and 28 of treatment, patients were classified as RER or SER. RERs were randomized to post-Induction therapy of either oral or escalating intravenous methotrextate during both IM phases and either standard or increased duration of therapy with a second DI phase. SERs received augmented therapy of greater intensity and duration than RER patients (Study Schema, Supplemental Figure 2).
The study’s final analytic file included 3,054 patients. Thirty-eight patients were excluded from analyses due to being ineligible for the study or having no end-of-phase forms. The file included toxicity data collected during Induction, Consolidation, IM-I, DI-I, IM-II, and DI-II. Toxicities were rated using the Common Toxicity Criteria, version 2.0 (0 [WNL], 1 [mild], 2 [moderate], 3 [severe], 4 [life-threatening /disabled]) and 5 (toxic deaths)[19]. The study collected information on phase duration and hospital days but no supportive care data.
Studies were approved by the National Cancer Institute and the Institutional Review Boards (IRB) of participating institutions. This retrospective analytical study qualified for a notice of exemption by the Children’s Hospital Los Angeles IRB.
Statistical Analysis/Methods
Separate analyses were conducted for patients enrolled in CCG-1961 and CCG-1991. Pooling of data was not feasible because studies used different toxicity scales.
For CCG-1961, chi-square and t-tests were used to test for male/female differences in demographic and presenting clinical factors. Differences between males and females in length of treatment phase, number of hospital and ICU days were estimated using linear regression analysis adjusted for age, race, treatment regimen, and BSA, with each treatment phase analyzed separately.
To illustrate the incidence of various toxicities and use of supportive care, the proportion of patients who experienced a grade ≥3 toxicity, significant infection, toxicity-related delay in therapy, and received supportive care interventions in each treatment phase was calculated. In addition, the proportion of patients experiencing a toxicity or using supportive care any time during the first four treatment phases, was estimated using the life table method[20], i.e., patients were counted as events during a course if a toxicity or use of supportive care occurred at any time during that course, with patients who started the course considered at risk.
Differences between males and females in the proportion of patients experiencing toxicities, delay in therapy, significant infection, and supportive care use were based on logistic regression, adjusted for age, race, treatment phase, regimen, and BSA, with data from the four treatment phases analyzed together. Since there were multiple treatment phases and each patient contributed data to one or more treatment phase, this patient effect was treated as a random effect in logistic regression analysis[21].
Competing risk analysis[22] was performed with CCG-1961 data to assess whether there was a significant difference in treatment-related death rates between males and females, with competing risks for treatment-related deaths being failure to respond to treatment, relapse/disease progression or non-treatment-related deaths. Patients who did not experience a relapse, progression or death by the end of their first delayed intensification were censored at the time of completion of DI-I. Multivariate analyses for CCG-1961 were adjusted for age, race, treatment phase, regimen and BSA. Interactions between age and sex were tested using various age cut-points. Additional analyses were performed, adjusting for additional factors, including white cell count, splenomegaly, lymph node involvement and pubertal status at diagnosis. Since pubertal status was not available, we estimated puberty at 12 years for females and 14 years for males[23].
While the analyses performed for CCG-1991 were similar to CCG-1961, there were some differences. Comparative analyses for CCG-1991 were not adjusted for BSA because these data were not included in the analytic file for this study. Since all subjects enrolled in CCG-1991 were diagnosed between the ages of 1–9 years, analyses were not adjusted for age.
All p-values are two sided; level of significance is p<0.05. Statistical computation was performed using Stata 9.2[24].
RESULTS
Patients
CCG-1961: Females were more likely to be Hispanic (Table 1). Males presented with higher white blood cell counts and hemoglobin levels and more extensive extramedullary disease. No significant sex differences were found for age at diagnosis, body mass index (BMI), Down syndrome, treatment arm assignments or early response status.
Table I.
Patient characteristics, females vs. males CCG-1961
| CCG-1961 High-risk ALL protocol |
|||
|---|---|---|---|
| Female N (%) |
Male N (%) |
p-value1 | |
| Total | 830 (100) | 1,224 (100) | |
| Age at diagnosis | |||
| Mean±SD | 10.2±5.1 | 10.5±5.1 | 0.24 |
| 0–9 years | 304 (36.6) | 465 (38.0) | |
| 10–15 years | 436 (52.5) | 587 (48.0) | |
| 16–21 years | 90 (10.9) | 172 (14.0) | |
| Race | |||
| Non-Hispanic white | 492 (59.9) | 881 (72.7) | <0.0001 |
| Hispanic | 225 (27.4) | 188 (15.5) | |
| Other | 104 (12.7) | 142 (11.8) | |
| Unknown | 9 | 13 | |
| White blood cells × 103/mm3 | |||
| < 50 | 440 (53.2) | 530 (43.3) | <0.0001 |
| 50 – 199 | 301 (36.4) | 528 (43.2) | |
| ≥200 | 86 (10.4) | 165 (13.5) | |
| Unknown | 3 | 1 | |
| Spleen | |||
| Normal | 387 (46.9) | 473 (38.7) | 0.001 |
| Moderately enlarged | 349 (42.3) | 597 (48.9) | |
| Markedly enlarged | 90 (10.9) | 151 (12.4) | |
| Unknown | 4 | 3 | |
| Lymph nodes | |||
| Normal | 422 (51.2) | 517 (42.3) | 0.0001 |
| Moderately enlarged | 325 (39.4) | 594 (48.5) | |
| Markedly enlarged | 78 (9.4) | 112 (9.2) | |
| Unknown | 5 | 1 | |
| Mediastinal mass | |||
| Absent | 718 (87.1) | 1,019 (83.4) | 0.020 |
| Present | 106 (12.9) | 203 (16.6) | |
| Unknown | 6 | 2 | |
| Hemoglobin (g/dL) | |||
| 1 – 7.9 | 420 (52.4) | 483 (41.3) | <0.0001 |
| 8.0 – 10.9 | 246 (30.6) | 385 (32.9) | |
| ≥11.0 | 136 (17.0) | 301 (25.8) | |
| Unknown | 28 | 55 | |
| Platelets, × 103/mm3 | |||
| 1 – 49 | 428 (52.0) | 642 (52.7) | 0.85 |
| 50 – 149 | 284 (34.5) | 406 (33.3) | |
| ?150 | 111 (13.5) | 170 (14.0) | |
| Unknown | 7 | 6 | |
| Body Mass Index2 | |||
| Underweight | 38 (4.9) | 75 (6.4) | 0.40 |
| Healthy weight | 515 (66.0) | 753 (64.7) | |
| Overweight | 121 (15.5) | 166 (14.3) | |
| Obese | 106 (13.6) | 170 (14.6) | |
| Unevaluable3 | 50 | 60 | |
| Down Syndrome | |||
| Yes | 21 (2.5) | 30 (2.5) | 0.90 |
| No | 806 (97.5) | 372 (97.5) | |
| Unknown | 3 | 1 | |
| Day 7 Bone Marrow Result | |||
| Rapid early response | 584 (71.4) | 842 (69.4) | 0.33 |
| Slow early response | 234 (28.6) | 372 (30.6) | |
| Unknown | 12 | 10 | |
Abbreviation: ALL = acute lymphoblastic leukemia; SD=standard deviation;
T-test for age; Chi-square tests for all other variables;
Per CDC guidelines for children and adolescents, ages 2–20 years; http://www.cdc.gov/healthyweight/assessing/bmi/;
< 24 months
CCG-1991: Male patients had more lymph node involvement at the time of diagnosis (Table 2). Male and female patients did not differ significantly in regards to Down syndrome and treatment arm assignments.
Table II.
Patient characteristics, females vs. males CCG-1991
| CCG-1991 Standard-risk ALL protocol |
|||
|---|---|---|---|
| Female N (%) |
Male N (%) |
p-value1 | |
| Total | 1,348 (100) | 1,668 (100) | |
| Age at diagnosis | |||
| Mean±SD | 4.5±2.2 | 4.4±2.1 | 0.051 |
| 0–9 years | 1,348 (100) | 1,668 (100) | |
| Race | |||
| Non-Hispanic white | 923 (69.8) | 1,128 (69.8) | 0.63 |
| Hispanic | 258 (19.5) | 301 (18.6) | |
| Other | 141 (10.7) | 188 (11.6) | |
| Unknown | 26 | 51 | |
| White blood cells × 103/mm3 | |||
| < 50 | 1,346 (100) | 1,666 (100) | 0.99 |
| 50 | - | - | |
| Unknown | 2 | 2 | |
| Spleen | |||
| Not enlarged | 759 (56.7) | 914 (54.9) | 0.64 |
| Enlarged, not below umbilicus | 525 (39.1) | 679 (40.8) | |
| Enlarged, below umbilicus | 58 (4.3) | 71 (4.3) | |
| Unknown | 6 | 4 | |
| Lymph nodes | |||
| Normal | 771 (57.4) | 835 (50.2) | 0.0003 |
| Enlarged < 3 cm | 520 (38.7) | 762 (45.8) | |
| Individual node ≥ 3cm diameter, group of nodes > 5cm diameter, or grossly visible nodes | 53 (3.9) | 67 (4) | |
| Unknown | 4 | 4 | |
| Anterior mediastinal mass | |||
| Absent | 1,232 (92.2) | 1,549 (93.1) | 0.61 |
| Mass <1/3 thoracic diameter of TS | 86 (6.4) | 95 (5.7) | |
| Mass ≥1/3 thoracic diameter of TS | 19 (1.4) | 20 (1.2) | |
| Unknown | 11 | 4 | |
| Hemoglobin (g/dL) | |||
| 1 to 7.9 | 809 (61.3) | 956 (58.4) | 0.17 |
| 8.0 to 10.9 | 407 (30.9) | 526 (32.2) | |
| More than 11.0 | 103 (7.8) | 154 (9.4) | |
| Unknown | 29 | 32 | |
| Platelets × 103/mm3 | |||
| 1 to 49 | 640 (47.6) | 735 (44.1) | 0.15 |
| 50 to 149 | 428 (31.8) | 576 (34.6) | |
| More than 150 | 278 (20.7) | 355 (21.3) | |
| Unknown | 2 | 2 | |
| Downs Syndrome | |||
| Yes | 54 (4) | 51 (3) | 0.16 |
| No | 1,292 (96) | 1,615 (97) | |
| Unknown | 2 | 2 | |
| Day 7 Bone Marrow Result | |||
| M1 | 549 (42.1) | 725 (44.6) | 0.33 |
| M2 | 379 (29.1) | 466 (28.7) | |
| M3 | 376 (28.8) | 435 (26.8) | |
| Unknown | 44 | 42 | |
| CNS disease | |||
| Yes | 25 (1.9) | 19 (1.1) | 0.10 |
| No | 1,321 (98.1) | 1,647 (98.9) | |
| Unknown | 2 | 2 | |
Abbreviations: ALL=acute lymphoblastic leukemia; SD=standard deviation, TS= thoracic spine; M1 = <5% blasé, M2 = 5–25% blasts, M3 = > 25% blasts.
T-tests for age; Chi-square tests for all other variables
Hospital Days and Phase Duration
CCG-1961: Females had more hospital days during Induction (mean difference= 1.6, p=0.0002), Consolidation (mean difference=1.3, p=0.006), IM-I (mean difference=1.2, p=0.0001), and DI-I (mean difference=1.4, p=0.0002), after adjusting for age, race, treatment regimen, and BSA (Table 3). No significant differences in treatment duration or ICU days were found between males and females.
Table III.
Length of treatment phase, number of hospital days and ICU days, females vs. males
| CCG-1961 | Female | Male | Females vs. Males1 | |||
|---|---|---|---|---|---|---|
| N | Mean+/− SE |
N | Mean+/− SE |
Difference in Mean (95%CI) |
p-value | |
| Phase Duration (days) | ||||||
| 1. Induction | 830 | 40.0±0.26 | 1,224 | 36.3±0.19 | 0.61 (−0.02, 1.2) | 0.057 |
| 2. Consolidation | 765 | 66.2±0.59 | 1,124 | 65.8±0.50 | −0.23 (−1.4, 0.97) | 0.71 |
| 3. Interim maintenance I | 735 | 59.2±0.32 | 1,077 | 58.8±0.23 | 0.26 (−0.50, 1.03) | 0.50 |
| 4. Delayed intensification I | 699 | 68.5±0.51 | 1,023 | 68.4±0.40 | −0.28 (−1.5, 0.96) | 0.65 |
| Hospital Days (days) | ||||||
| 1. Induction | 830 | 14.1±0.35 | 1,224 | 12.3±0.24 | 1.6 (0.75, 2.4) | 0.0002 |
| 2. Consolidation | 765 | 8.4±0.38 | 1,124 | 6.9±0.29 | 1.3 (0.38, 2.2) | 0.006 |
| 3. Interim maintenance I | 735 | 3.4±0.28 | 1,077 | 2.1±0.15 | 1.2 (0.58, 1.7) | 0.0001 |
| 4. Delayed intensification I | 699 | 6.3±0.33 | 1,023 | 4.6±0.21 | 1.4 (0.67, 2.1) | 0.0002 |
| ICU Days (days) | ||||||
| 1. Induction | 830 | 1.4±0.16 | 1,224 | 1.1±0.11 | 0.24 (−0.15, 0.62) | 0.23 |
| 2. Consolidation | 765 | 0.33±0.09 | 1,124 | 0.23±0.06 | 0.09 (−0.12, 0.30) | 0.38 |
| 3. Interim maintenance I | 735 | 0.13±0.06 | 1,077 | 0.05±0.02 | 0.06 (−0.05, 0.17) | 0.32 |
| 4. Delayed intensification I | 699 | 0.28±0.08 | 1,023 | 0.16±0.04 | 0.10 (−0.06, 0.27) | 0.22 |
| CCG-1991 | ||||||
| Phase Duration (days) | ||||||
| 1. Induction | 1,346 | 29.7±0.19 | 1,668 | 29.5±0.15 | 0.10 (−0.31, 0.51) | 0.62 |
| 2. Consolidation | 1,095 | 34.6±0.47 | 1,367 | 34.5±0.41 | 0.36 (−0.26, 0.97) | 0.25 |
| 3. Interim maintenance I | 1,053 | 59.4±0.24 | 1,322 | 58.7±0.19 | 0.65 (0.05, 1.2) | 0.032 |
| 4. Delayed intensification I | 1,043 | 69.4±0.37 | 1,302 | 69.2±0.31 | 0.16 (−0.76, 1.1) | 0.73 |
| 5. Interim maintenance II | 1,017 | 60.1±0.23 | 1,275 | 59.7±0.19 | 0.37 (−0.21, 0.95) | 0.21 |
| 6. Delayed intensification II | 562 | 72.7±0.55 | 675 | 70.4±0.47 | 2.3 (0.88, 3.7) | 0.001 |
| Hospital Days (days) | ||||||
| 1. Induction | 1,345 | 10.8±0.22 | 1,667 | 10.1±0.19 | 0.71 (0.21, 1.2) | 0.006 |
| 2. Consolidation | 1,095 | 2.8±0.17 | 1,367 | 2.6±0.17 | 0.14 (−0.32, 0.59) | 0.56 |
| 3. Interim maintenance I | 1,053 | 1.3±0.11 | 1,322 | 1.1±0.11 | 0.24 (−0.06, 0.55) | 0.12 |
| 4. Delayed intensification I | 1,043 | 4.3±0.19 | 1,302 | 3.4±0.15 | 0.94 (0.48, 1.4) | 0.0001 |
| 5. Interim maintenance II | 1,016 | 1.2±0.09 | 1,275 | 0.96±0.09 | 0.29 (0.04, 0.55) | 0.026 |
| 6. Delayed intensification II | 562 | 5.1±0.42 | 675 | 3.5±0.22 | 1.6 (0.72, 2.5) | 0.0004 |
Abbreviations: ICU = Intensive care unit; CI = Confidence Interval; SE = Standard Error;
Linear regression analyses controlling for inter-patient correlation, CCG-1961 adjusted for age, race, treatment regimen, and BSA; CCG-1991 adjusted for race and treatment regimen.
CCG-1991: Females had significantly more hospital days during Induction (mean difference=0.71, p=0.006), DI-I (mean difference=0.94, p=0.0001), IM-II (mean difference=0.29, p=0.03) and DI-II (mean difference=1.6, p=0.0004) (Table 3). Interim maintenance I (mean difference=0.65, p=0.03) and DI-II (mean difference=2.3, p=0.001) treatment phases were significantly longer for females.
Grade 3 or Higher Non-hematological Toxicities and Treatment Delay
CCG-1961: Females were significantly more likely to have a grade 3 or 4 toxicity (p<0.0001), delay in therapy (p=0.012) and clinically significant infection (p=0.001) than males (Table 4). The chance of experiencing a grade 3 or grade 4 toxicity was higher (OR>1) for females compared to males for 15 of the 17 toxicity domains, and for five domains the difference was statistically significant. Females experienced significantly more nervous system toxicities (p=0.002), pancreatitis (p<0.0001), gastrointestinal (GI) toxicities (p<0.0001) and mood disturbances (p=0.02). There was no evidence of an interaction between sex and age, phase of treatment or our age-defined puberty variable. Controlling for white blood cell count, spleen and lymph node involvement at diagnosis did not significantly change estimated effect sizes.
Table IV.
Risk of developing toxicities, females vs. males, CCG 1961 and CCG 1991
| CCG-1961 | Toxicity Rate1 (%) | Females vs. Males2 | |||
|---|---|---|---|---|---|
| All patients | OR | 95%CI | p-value | ||
| Any Grade 3 or 4 toxicities | 72.8 | 1.5 | (1.3, 1.8) | <0.0001 | |
| Therapy delayed over a week due to toxicities | 73.6 | 1.2 | (1.04, 1.4) | 0.012 | |
| Significant infection 3 | 58.1 | 1.3 | (1.1, 1.5) | 0.001 | |
| Toxicity Domain | |||||
| Liver | 34.2 | 1.2 | (0.96, 1.5) | 0.11 | |
| Gastrointestinal | 24.7 | 1.6 | (1.3, 2.0) | <0.0001 | |
| Pancreas | 23.5 | 1.7 | (1.3, 2.1) | <0.0001 | |
| Pancreas - glucose | 21.1 | 1.7 | (1.4, 2.3) | <0.0001 | |
| Pancreas - other | 3.8 | 1.4 | (0.85, 2.2) | 0.20 | |
| Nervous System | 15.8 | 1.6 | (1.2, 2.1) | 0.002 | |
| Peripheral nervous system | 9.6 | 1.8 | (1.3, 2.6) | 0.002 | |
| Central nervous system | 8.5 | 1.5 | (1.04, 2.1) | 0.031 | |
| Coagulation | 14.1 | 0.99 | (0.72, 1.4) | 0.97 | |
| Electrolytes | 9.3 | 0.96 | (0.70, 1.3) | 0.81 | |
| Pulmonary | 7.7 | 1.3 | (0.96, 1.9) | 0.082 | |
| Renal/Genitourinary | 6.6 | 1.2 | (0.84, 1.7) | 0.30 | |
| Cardiac | 6.4 | 1.1 | (0.75, 1.6) | 0.64 | |
| Skin | 5.9 | 1.01 | (0.69, 1.5) | 0.93 | |
| Allergy | 3.1 | 1.1 | (0.63, 1.8) | 0.81 | |
| Mood | 2.7 | 2.0 | (1.1, 3.6) | 0.02 | |
| Performance4 | 2.4 | 1.3 | (0.73, 2.4) | 0.37 | |
| Weight Change | 2.1 | 1.5 | (0.79, 2.8) | 0.22 | |
| Fever | 2.0 | 1.5 | (0.79, 2.9) | 0.21 | |
| Local5 | 1.4 | 1.6 | (0.73, 3.5) | 0.24 | |
| Vision | 0.2 | 3.6 | (0.36, 35.7) | 0.24 | |
| CCG-1991 | |||||
|
OS,OD, IS,ID Regimes |
Augmented Regime |
||||
| Any Grade 3, 4 or 5 toxicities | 76.9 | 89.6 | 1.2 | (1.1, 1.4) | 0.0003 |
| Significant Infections 3 | 55.3 | 73.8 | 1.2 | (1.1, 1.4) | 0.001 |
| Toxicity Domain | |||||
| Hepatic | 35.3 | 45.5 | 1.1 | (0.97, 1.3) | 0.11 |
| Gastrointestinal | 25.4 | 37.6 | 1.2 | (1.01, 1.5) | 0.045 |
| Metabolic | 17.1 | 32.4 | 1.5 | (1.2, 1.9) | 0.001 |
| Pain | 9.6 | 14.6 | 1.4 | (1.1, 2.0) | 0.019 |
| Cardiovascular | 8.0 | 15.0 | 1.01 | (0.73, 1.4) | 0.96 |
| Coagulation | 7.7 | 10.7 | 1.3 | (0.84, 1.9) | 0.25 |
| Dermatology | 5.8 | 7.8 | 1.04 | (0.72, 1.5) | 0.81 |
| Pulmonary | 4.1 | 9.7 | 1.2 | (0.78, 1.8) | 0.44 |
| Constitutional symptoms | 3.5 | 4.1 | 1.1 | (0.70, 1.8) | 0.62 |
| Hemorrhage | 2.0 | 11.3 | 0.99 | (0.60, 1.6) | 0.96 |
| Musculoskeletal | 1.5 | 1.1 | 1.1 | (0.55, 2.3) | 0.75 |
| Neurology | 1.5 | 1.6 | 1.8 | (0.79, 4.1) | 0.16 |
| Renal/genitourinary | 1.4 | 1.5 | 1.7 | (0.80, 3.7) | 0.17 |
| Allergy/immunology | 1.2 | 3.7 | 0.25 | (0.09, 0.66) | 0.002 |
| Cardiac – arrhythmias | 0.7 | 3.2 | 1.5 | (0.65, 3.5) | 0.34 |
| Endocrine | 0.1 | 1.5 | 0.14 | (0.008, 2.5) | 0.086 |
| Ocular | 0.3 | 1.3 | 2.8 | (0.54, 14.3) | 0.19 |
Abbreviations: OR= Odds Ratio; CI = Confidence Interval; OS=oral methotrexate in Interim Maintenance, single Delayed Intensification; OD=oral methotrexate in Interim Maintenance, Double Delayed Intensification; IS = IV methotrexate in Interim Maintenance, Single Delayed Intensification; ID=IV methotrexate in Interim Maintenance, Double Delayed Intensification;
Rates estimated using Kaplan-Meier method; CCG-1961 – showing percent experiencing a grade 3 or 4 toxicity at any time during Induction, Consolidation, Interim Maintenance I, Delayed Intensification I. Toxicity ratings based on CCG Toxicity Rating Scale; CCG-1991 – showing percent experiencing a grade ≥3 toxicity at any time during Induction, Consolidation, Interim Maintenance I, Delayed Intensification I, Interim Maintenance II, Delayed Intensification II. Toxicity ratings based on Common Toxicity Criteria, version 2;
Logistic regression; CCG-1961 - adjusted for age, race, phase, treatment regimen, and BSA; CCG-1991 - adjusted for race, phase and treatment regimen;
Significant infections include pneumocystis, varicella/zoster, bacteremia/sepsis, central line related infection, fungal infection and other infections that required hospitalization;
Karnofsky/Lansky scale;
Local: grade 3 =pain/swelling with inflammation; grade 4 =phlebitis, ulceration, plastic surgery indicated
CCG-1991: Females had increased risk of grade 3–5 toxicities (p=0.0003) and ‘significant’ infections (p=0.001) (Table 4). Grade 3 to 5 toxicity rate was higher (OR>1) in females for 14 of the 17 toxicity domains, and for four of these the difference was statistically significant. Females experienced significantly more GI toxicities (p=0.045), metabolic/laboratory toxicities (p=0.001) and pain (p=0.019). In this cohort only, male patients experienced more allergy/immunology toxicities than female patients (p=0.002).
Secondary analyses adjusted for age as a continuous variable provided results similar to the primary analyses presented in this paper. No significant interactions were found between sex and age.
Supportive Care
CCG-1961: In aggregate, the odds of receiving supportive care interventions were significantly higher for females than for males (p=0.0001) (Table V). There was a significant increase in the use of all supportive care interventions among female patients. Females were one and a half to two times more likely than males to have received antifungals, antivirals, parenteral nutrition, blood products and hematopoietic growth factor. No interaction was found between sex and age at diagnosis or treatment phase.
Table V.
Supportive care interventions, CCG-1961
| Supportive Care Interventions | Rate1 | Females vs. Males2 | ||
|---|---|---|---|---|
| OR | 95%CI | p-value | ||
| Any type | 99.8% | 1.5 | (1.2, 1.8) | 0.0001 |
| IV antibiotics | 93.2% | 1.2 | (1.1, 1.4) | 0.003 |
| Antifungals | 25.3% | 1.7 | (1.3, 2.1) | <0.0001 |
| Antivirals | 14.0% | 1.5 | (1.1, 2.1) | 0.008 |
| Parenteral nutrition | 23.1% | 1.9 | (1.5, 2.5) | <0.0001 |
| Blood product | 98.8% | 1.8 | (1.5, 2.1) | <0.0001 |
| Platelet transfusion | 86.4% | 1.6 | (1.4, 1.9) | <0.0001 |
| Red blood cell transfusion | 98.1% | 1.7 | (1.4, 2.0) | <0.0001 |
| Hematopoietic growth factor3 | 16.5% | 2.0 | (1.5, 2.7) | <0.0001 |
| IV analgesic | 47.6% | 1.3 | (1.03, 1.6) | 0.028 |
Abbreviations: OR = odds ratio; CI = confidence interval; IV = intravenous.
Rates estimated using life table method, showing percent of patients who received supportive care interventions at any time during Induction, Consolidation, Interim Maintenance I, Delayed Intensification I;
Logistic regression, adjusted for age, race, phase, treatment regimen, and BSA;
Justification - profound neutropenia and infection.
Treatment-related Deaths
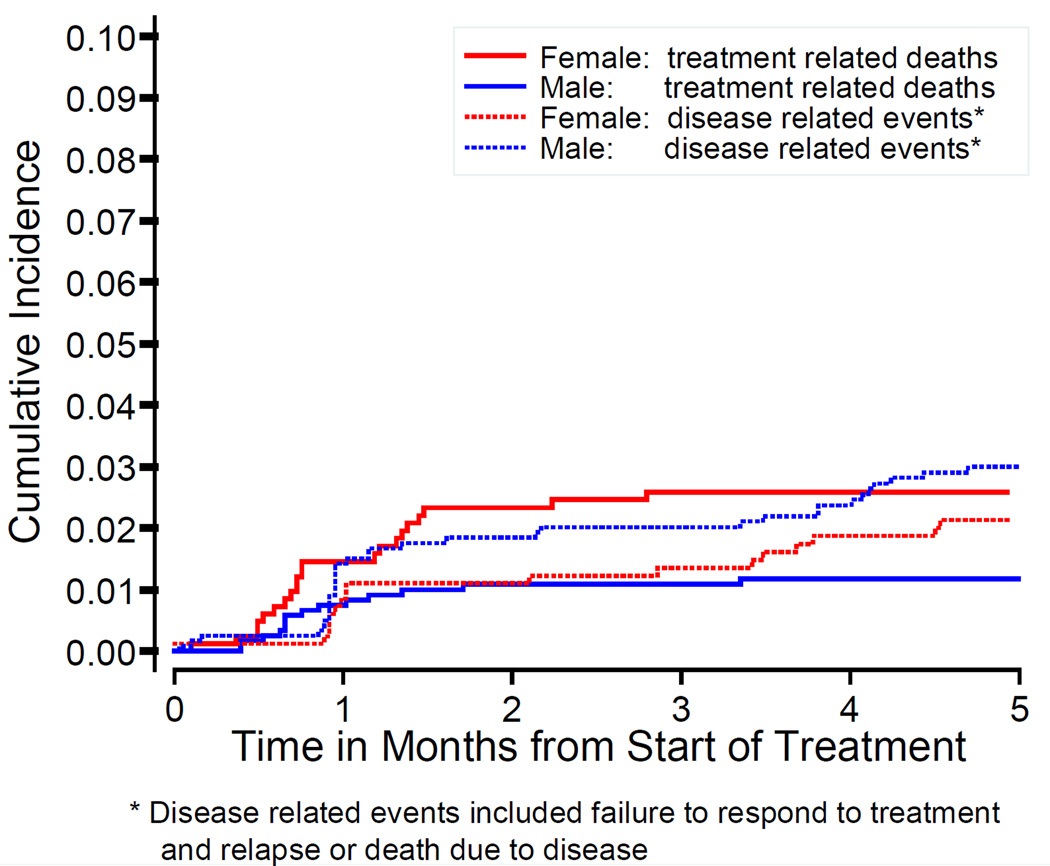
CCG-1961: By the end of DI-I, there were 74 non-treatment-related events (failure to respond to treatment, relapse and death due to disease) and 45 treatment-related deaths (infection, toxicity, hemorrhage, and other causes). Females were significantly more likely to die of treatment-related causes (Hazard Ratio=2.8, 95% CI=1.5–5.3, p=0.002) (Figure 1). Five months after beginning treatment, cumulative incidence of treatment-related deaths was 2.6% for females and 1.2% for males.
Figure 1.
Treatment-related deaths and relapses/death due to disease, females vs. males CCG-1961 during first five months of therapy
CCG-1991: By the end of DI-II, there were 13 non-treatment-related deaths and 27 treatment-related deaths. Females were nominally more likely to die of treatment-related causes, but the difference was not significant (Hazard Ratio=1.3, 95%CI=0.6–2.8, p=0.45). The cumulative incidence of treatment-related deaths by the end of DI-II was 1.1% for females and 0.8% for males.
Supplemental tables provide phase-specific rates for toxicities and support care interventions by sex for patients enrolled in CCG-1961 and CCG-1991.
DISCUSSION
While pharmacodynamic studies have reported sex differences in toxicity for some anti-cancer drugs (e.g., doxorubicin and 5-fluorouracil) [25–27], this study compares toxicities for females and males treated for ALL in the context of two large, phase III cooperative group clinical trials. The toxicities reflect synergistic and additive effects of multiple therapies, including both cancer-directed treatments and supportive care treatments (e.g., antibiotics). Among high-risk and standard-risk ALL patients, females experienced more grade 3 and 4 toxicities than males. In both clinical trials, females demonstrated a higher incidence of serious infections and GI toxicities. Female ALL-HR patients experienced significantly more pancreatic, nervous system, mood toxicities and treatment-related deaths; female ALL-SR patients experienced more pain and metabolic toxicities.
Increased toxicity among females resulted in longer hospitalizations and increased use of supportive care interventions. High-risk females required more blood transfusions, nutritional support, and intravenous (IV) medications (antibiotics, analgesics). These significant sex differences were observed in all four treatment phases (Induction, Consolidation, IM I, DI I) (supplemental data). This study’s detailed analysis of multiple measures and indicators of toxicity by phase of treatment is rare. The consistency of our findings across measures provides strong support for our study hypothesis; our secondary toxicity measures (e.g., supportive care use, hospital days) provide data regarding the clinical significance of higher toxicities among females. For example, HR-risk females were 50% more likely to develop grade 3 or 4 GI toxicities and twice as likely to receive parental nutrition than males. This increase in GI toxicities, present during all four treatment phases (Supplemental Table I), translates into more physical and psychological challenges for females. Furthermore, although we were unable to calculate actual financial costs associated with toxicities and supportive care interventions, the significant increase in supportive care use among females translates into a higher financial burden for female patients [28], an important factor in today’s health care environment. Since we do not understand why females experience more toxicities than males, the analyses conducted in this study are hypothesis generating. For instance, it is possible that GI toxicities may increase the females’ vulnerability to other complications/toxicities, such as infections. This is an example of a toxicity that warrants further study.
With regards to male and female differences, treatment related death is the primary toxicity variable that has been examined in ALL patients. Our finding that females experienced more treatment related deaths during the pre-Maintenance portion of therapy is consistent with these reports [29–31]. In the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL-92 and ALL-2000 clinical trials [29] investigators found females were twice as likely to experience treatment-related deaths. Primary cause of death was infection, with 76% of deaths occurring within 80 days of initiating therapy. Significantly more infection-related deaths during ALL induction therapy were also reported for females enrolled in the Medical Research Council (MRC) UKALL X trial[30]. Authors speculate that poorer outcomes for females were partially due to male/female differences in immunological responses to infection. Interestingly in the general population, it has been documented that female adolescents and adults generate a stronger antibody response to viral, bacterial and fungal infections than males. Sex hormones play a major role in regulating the immune response including production of specific antibodies and pro-inflammatory mediators[1,32]). Females tend to be more resilient [32,33] because female sex hormones (e.g., estrogen) enhance the body’s immune response while the male hormone testosterone suppresses immune function. It is unknown why this relationship is reversed with pediatric and adolescent female ALL patients being more vulnerable to serious infections and infection-related deaths than males. These findings are provocative, clinically significant and warrant further investigation.
A few toxicity studies have been conducted with pediatric solid tumor patients. Some report increased toxicities for females and others report no sex differences. Hodgkin lymphoma studies consistently report more toxicities and better survival for female patients. [34,35]. Interestingly, the German Hodgkin Lymphoma Study Group (HD6 and HD9-trials) found that although survival was better, high-toxicity patients had more toxicity-related dose reductions and received significantly lower drug doses than low-toxicity patients [34]. Among adolescent/young adults with Ewing sarcoma, osteosarcoma and Hodgkin lymphoma, Khamly and colleagues found chemotherapy both more toxic (greater neutropenia and transfusion use) and more effective for female patients [35]. In contrast, Sharib and colleagues [36] found no male/female differences in treatment-related toxicities among Ewing sarcoma patients (n=142). Some of the above studies suggest a positive relationship between toxicities and treatment outcome, a relationship that is intriguing considering the differences in toxicities found for males and females. While our analyses were limited to pre-maintenance treatment phases, it would be of value to examine the associations between sex-related toxicities and disease outcomes and long-term survival.
In the general population, women are 1.5 to 1.7 times more likely than men to have an adverse drug reaction [37]. While the underlying mechanisms are poorly understood, sex differences in metabolizing enzymes, body composition, renal clearance, hormonal environment, and genetic composition have been identified as possible contributing factors. Glomerular filtration rate is 10% lower in females than in males, affecting the excretion of some drugs[37]. Liver clearance for methotrexate and doxorubicin, both drugs used in the treatment of childhood ALL, is lower for adult females than adult males, resulting in higher drug levels and toxicities for females[38,39]. Dobbs et al. found that, among adult patients with normal liver biochemistry, sex was the only factor predicting doxorubicin clearance after adjusting for BSA[25]. These findings are significant since rapid drug clearance (e.g., doxorubicin and methotrexate) is associated with reduced cure rates[25].
A dimorphism in fat patterning among males and females begins at age five and affects the distribution of certain drugs[40]. For example, high body fat and the lipophilic nature of anthracyclines result in sustained anthracycline exposure. It has been suggested that higher incidence of anthracycline-related cardiotoxicity among female pediatric cancer patients[41] may be due to increased body fat among females. For unknow reasons, the benefits of dexrazoxane, a cardio-protectant for patients receiving anthracyclines, also have been found to be sex-specific, offering more protection for female pediatric cancer patients than males[42]. In the general population, males and females differ significantly in regards to cardiac disease, including risk factors, presenting symptoms, disease management and outcome[1]. With childhood cancer survivors, cardiac morbidity (stroke, coronary heart disease, coronary artery disease) and mortality are higher than expected for long-term survivors, with the risk being significantly higher among females[5,43]. Researchers are beginning to examine the role of sex-linked genes as a potential risk factor/mechanism in the development of treatment-related cardiomyopathy.
Significant biological and hormonal changes occur with puberty that influence drug metabolism. These have particular significance in pediatric oncology because a clinical trial often includes children and adolescents. For example, CCG-1961 includes pre- and post-pubertal patients, ages 1–21 years. Recently, it has been discovered that at puberty there is a hormone-related physiological divergence in cardiac function that increases a woman’s risk of drug-induced adverse events[44] (e.g., QTc prolongation, ventricular arrhythmias). These sex- and age-specific cardiac adverse events are life-threatening and have resulted in a number of drugs being removed from the market[45]. Studies have found oral contraceptives reduce clearance of some drugs (diazepam, cyclosporine) and increase clearance of others (morphine, acetaminophen)[37,39,46]; some drug toxicities are related to menstrual cycle [47,48]. While we found no significant interactions between sex and our age-defined puberty variable among high-risk patients, our analyses were limited in our use of age as a proxy estimate for puberty. Collecting information on pubertal status and hormonal supplements is a critical step in evaluating a patient’s hormonal environment. This area that has received little attention and may help explain some male/female differences in outcomes.
The principal limitation to our study is that analyses were based on assigned treatment rather than actual drug doses delivered because these data were not collected in a manner that permitted retrospective analysis in a sample this large. While we are unable to determine if chemotherapy doses differed for male and female patients, dose reductions (lower drug doses) would be expected to be more common for females since they experienced more drug related-toxicities. In CCG, doses are modified for toxicities according to strict guidelines applied equally to both sexes. With cooperative groups, reporting of toxicities varies among clinicians and treatment centers; no data suggest a sex-based bias in clinicians’ reporting of toxicities. However, studies have shown that females tend to report more subjective symptoms(e.g., pain)[49]. While this may explain some of the sex differences, its effect is limited because most toxicities in this study were defined using clinical data. Given the large sample size, some differences between males and females, such as duration of hospital stay, were statistically significant but relatively small (e.g. 0.07 days) and of uncertain clinical relevance. However, in some phases hospital day differences approached two days, which is important not only as a marker of increased morbidity among females, but also as a source of greater treatment burden with increased cost, important in today’s health economy climate.
This study complements childhood cancer survivorship studies[4]. Risk profiles appear to be different for male and female patients with risk of acute and long-term treatment-related toxicities being greater for females. The underlying biological mechanisms for sex disparities are poorly understood and warrant further study. Our challenge is to determine how the study of sex-based disparities can be incorporated into future clinical trials and translated into optimal clinical practice[1,50].
Supplementary Material
Acknowledgements
This study was supported in part by the Concern Foundation for Cancer Research, the Children’s Center for Cancer and Blood Diseases, Children’s Hospital Los Angeles and the Children’s Oncology Group Chair’s Grant (U10 CA098453) from the National Cancer Institute, National Institutes of Health.
Abbreviations
- CCG
Children’s Cancer Group
- ALL-HR
High-risk acute lymphoblastic leukemia
- ALL-SR
Standard-risk acute lymphoblastic leukemia
- IOM
Institute of Medicine
- BSA
Body surface area
- NCI
National Cancer Institute
- RER
Rapid early response
- SER
Slow early response
- IM
Interim Maintenance
- DI
Delayed Intensification
- WNL
Within normal limits
- ICU
Intensive care unit
- IRB
Institutional Review Board
- BMI
Body mass index
- IV
Intravenous
- NOPHO
Nordic Society of Pediatric Hematology and Oncology
- MRC
Medical Research Council
- SD
Standard deviation
- TS
Thoracic spine
- CI
Confidence interval
- SE
Standard error
- OR
Odds ratio
- OS
Oral methotrexate in Interim Maintenance, single Delayed Intensification
- OD
Oral methotrexate in Interim Maintenance, double Delayed Intensification
- IS
IV methotrexate in Interim Maintenance, single Delayed Intensification
- ID
IV methotrexate in Interim Maintenance, double Delayed Intensification
Footnotes
The Institute of Medicine defines sex “as the classification of living things, generally as male or female according to their reproductive organs and functions assigned by the chromosomal complement, and gender as a person’s self-representation as male or female, or how that person is responded to by social institutions on the basis of the individual’s gender presentation.” [1]
Conflict of Interest statement: The authors declare no competing financial interests.
References
- 1.Wizemann TM, Pardue ML. Exploring the biological contributions to human health: Does sex matter? Washington D.C.: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 2004 Incidence and Mortality. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2007. [Google Scholar]
- 3.Reis LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, CLegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 4.Armstrong GT, Sklar CA, Hudson MM, Robison LL, Armstrong GT, Sklar CA, Hudson MM, Robison LL. Long-term health status among survivors of childhood cancer: does sex matter? Journal of Clinical Oncology. 2007;25(28):4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Pan Z, Ness KK, Srivastava D, Robison LL, Armstrong GT, Pan Z, Ness KK, Srivastava D, Robison LL. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. Journal of Clinical Oncology. 2010;28(7):1224–1231. doi: 10.1200/JCO.2009.24.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MA. Gender-based differences in the toxicity of pharmaceuticals--the Food and Drug Administration's perspective. International Journal of Toxicology. 2001;20(3):149–152. doi: 10.1080/109158101317097728. [DOI] [PubMed] [Google Scholar]
- 7.Nicolson TJ, Mellor HR, Roberts RR, Nicolson TJ, Mellor HR, Roberts RRA. Gender differences in drug toxicity. Trends in Pharmacological Sciences. 2010;31(3):108–114. doi: 10.1016/j.tips.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Soldin OP, Mattison DR, Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clinical Pharmacokinetics. 2009;48(3):143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kando JC, Yonkers KA, Cole JO. Gender as a risk factor for adverse events to medications. Drugs. 1995;50(1):1–6. doi: 10.2165/00003495-199550010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Department of Government Accountability Office, Drug Saftey. [Accessed January 2, 2015];Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women. http://www.gao.gov/products/GAO-01-286R2001. Published January 19, 2001.
- 11.Anderson GD. Gender Differences in Pharmacological Response. International review of neurobiology. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- 12.Sawyer M, Ratain MJ. Body surface area as a determinant of pharmacokinetics and drug dosing. Investigational New Drugs. 2001;19(2):171–177. doi: 10.1023/a:1010639201787. [DOI] [PubMed] [Google Scholar]
- 13.Nawaratne S, Brien JE, Seeman E, Fabiny R, Zalcberg J, Cosolo W, Angus P, Morgan DJ. Relationships among liver and kidney volumes, lean body mass and drug clearance. British Journal of Clinical Pharmacology. 1998;46(5):447–452. doi: 10.1046/j.1365-2125.1998.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. American Journal of Physiology. 1984;247(4 Pt 2):F632–F636. doi: 10.1152/ajprenal.1984.247.4.F632. [DOI] [PubMed] [Google Scholar]
- 15.Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, Gelber R, Heerema N, Korn EL, Link M, Murphy S, Pui CH, Pullen J, Reamon G, Sallan SE, Sather H, Shuster J, Simon R, Trigg M, Tubergen D, Uckun F, Ungerleider R. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. Journal of Clinical Oncology. 1996;14(1):18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, Freyer DR, Mattano LA, Jr, Hastings CA, Rubin CM, Bertolone K, Franklin JL, Heerema NA, Mitchell TL, Pyesmany AF, La MK, Edens C, Gaynon PS, Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, Freyer DR, Mattano LA, Jr, Hastings CA, Rubin CM, Bertolone K, Franklin JL, Heerema NA, Mitchell TL, Pyesmany AF, La MK, Edens C, Gaynon PS. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;111(5):2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, Franklin J, Nachman J, Ettinger LJ, La M, Steinherz P, Cohen LJ, Siegel SE, Avramis VI, Children's Cancer Group Study CCG. Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, Franklin J, Nachman J, Ettinger LJ, La M, Steinherz P, Cohen LJ, Siegel SE, Avramis VI. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children's Cancer Group Study CCG-1961. Journal of Pediatric Hematology/Oncology. 2004;26(4):217–226. doi: 10.1097/00043426-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Matloub Y, Bostrom BC, Hunger SP, Stork LC, Angiolillo A, Sather H, La M, Gastier-Foster JM, Heerema NA, Sailer S, Buckley PJ, Thomson B, Cole C, Nachman JB, Reaman G, Winick N, Carroll WL, Devidas M, Gaynon PS, Matloub Y, Bostrom BC, Hunger SP, Stork LC, Angiolillo A, Sather H, La M, Gastier-Foster JM, Heerema NA, Sailer S, Buckley PJ, Thomson B, Cole C, Nachman JB, Reaman G, Winick N, Carroll WL, Devidas M, Gaynon PS. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2012;118(2):243–251. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute, Division of Cancer Prevention. [Accessed January 2, 2015];The Revised Common Toxicity Criteria, Version 2.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv2nom-4-30-99-final3.pdf. Published March 1998.
- 20.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons; 2002. [Google Scholar]
- 21.Stata Longitudinal/Panel Data Reference Manual, Release 9. College Station TX: Stata Press; 2005. [Google Scholar]
- 22.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. British Journal of Cancer. 2004;91(7):1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adolescent Health Care: A Practical Guide. Minneapolis: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 24.Stata Statistical Software, Release 9. College Station, TX: StataCrop LP; 2005. [Google Scholar]
- 25.Dobbs NA, Twelves CJ, Gillies H, James CA, Harper PG, Rubens RD. Gender affects doxorubicin pharmacokinetics in patients with normal liver biochemistry. Cancer Chemotherapy & Pharmacology. 1995;36(6):473–476. doi: 10.1007/BF00685796. [DOI] [PubMed] [Google Scholar]
- 26.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. New England Journal of Medicine. 1995;332(26):1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 27.Milano G, Etienne MC, Cassuto-Viguier E, Thyss A, Santini J, Frenay M, Renee N, Schneider M, Demard F. Influence of sex and age on fluorouracil clearance. Journal of Clinical Oncology. 1992;10(7):1171–1175. doi: 10.1200/JCO.1992.10.7.1171. [DOI] [PubMed] [Google Scholar]
- 28.Russell H, Panchal J, Vonville H, Franzini L, Seint J. Economic evaluation of pediatric cancer treatment: a systematic literature review. Pediatrics. 2013;131(1):e273–e287. doi: 10.1542/peds.2012-0912. [DOI] [PubMed] [Google Scholar]
- 29.Lund B, Asberg A, Heyman M, Kanerva J, Harila-Saari A, Hasle H, Soderhall S, Jonsson OG, Lydersen S, Schmiegelow K, Nordic Society of Paediatric H, Oncology. Lund B, Asberg A, Heyman M, Kanerva J, Harila-Saari A, Hasle H, Soderhall S, Jonsson OG, Lydersen S, Schmiegelow K. Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatric Blood & Cancer. 2011;56(4):551–559. doi: 10.1002/pbc.22719. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler K, Chessells JM, Bailey CC, Richards SM. Treatment related deaths during induction and in first remission in acute lymphoblastic leukaemia: MRC UKALL X. Archives of Disease in Childhood. 1996;74(2):101–107. doi: 10.1136/adc.74.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prucker C, Attarbaschi A, Peters C, Dworzak M, Potschger U, Urban C, Fink F-M, Meister B, Schmitt K, Haas O, Gadner H, Mann G. Incudction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Munster study group. Leukemia. 2009;23:1264–1269. doi: 10.1038/leu.2009.12. [DOI] [PubMed] [Google Scholar]
- 32.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature Reviews Immunology. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. Journal of Autoimmunity. 2012;38(2–3):J282–J291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Brosteanu O, Hasenclever D, Loeffler M, Diehl V German Hodgkin's Lymphoma Study G. Low acute hematological toxicity during chemotherapy predicts reduced disease control in advanced Hodgkin's disease. Annals of Hematology. 2004;83(3):176–182. doi: 10.1007/s00277-003-0727-9. [DOI] [PubMed] [Google Scholar]
- 35.Khamly KK, Thursfield VJ, Fay M, Desai J, Toner GC, Choong PF, Ngan SY, Powell GJ, Thomas DM, Khamly KK, Thursfield VJ, Fay M, Desai J, Toner GC, Choong PFM, Ngan SYK, Powell GJ, Thomas DM. Gender-specific activity of chemotherapy correlates with outcomes in chemosensitive cancers of young adulthood. International Journal of Cancer. 2009;125(2):426–431. doi: 10.1002/ijc.24376. [DOI] [PubMed] [Google Scholar]
- 36.Sharib JM, Cyrus J, Horvai A, Gray Hazard FK, Neuhaus J, Matthay KK, Goldsby R, Marina N, DuBois SG, Sharib JM, Cyrus J, Horvai A, Gray Hazard FK, Neuhaus J, Matthay KK, Goldsby R, Marina N, DuBois SG. Predictors of acute chemotherapy-associated toxicity in patients with Ewing sarcoma. Pediatric Blood & Cancer. 2012;59(4):611–616. doi: 10.1002/pbc.24031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson GD. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. Journal of Women's Health. 2005;14(1):19–29. doi: 10.1089/jwh.2005.14.19. [DOI] [PubMed] [Google Scholar]
- 38.Holmboe L, Andersen AM, Morkrid L, Slordal L, Hall KS, Holmboe L, Andersen AM, Morkrid L, Slordal L, Hall KS. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. British Journal of Clinical Pharmacology. 2012;73(1):106–114. doi: 10.1111/j.1365-2125.2011.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franconi F, Brunelleschi S, Steardo L, Cuomo V, Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacological Research. 2007;55(2):81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Webster-Gandy J, Warren J, Henry CJ, Henry CJK. Sexual dimorphism in fat patterning in a sample of 5 to 7-year-old children in Oxford. International Journal of Food Sciences & Nutrition. 2003;54(6):467–471. doi: 10.1080/09637480310001322323. [DOI] [PubMed] [Google Scholar]
- 41.Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, Lipshultz SE, Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, Lipshultz SE. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatric Cardiology. 2011;32(3):342–353. doi: 10.1007/s00246-010-9878-3. [DOI] [PubMed] [Google Scholar]
- 42.Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, Barry EV, Asselin BL, Athale U, Clavell LA, Larsen E, Moghrabi A, Samson Y, Michon B, Schorin MA, Cohen HJ, Neuberg DS, Orav EJ, Colan SD, Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, Barry EV, Asselin BL, Athale U, Clavell LA, Larsen E, Moghrabi A, Samson Y, Michon B, Schorin MA, Cohen HJ, Neuberg DS, Orav EJ, Colan SD. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncology. 2010;11(10):950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME, Jr, Ruccione K, Smithson WA, Robison LL. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2001;19(13):3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 44.Parekh A, Fadiran EO, Uhl K, Throckmorton DC, Parekh A, Fadiran EO, Uhl K, Throckmorton DC. Adverse effects in women: implications for drug development and regulatory policies. Expert Review of Clinical Pharmacology. 2011;4(4):453–466. doi: 10.1586/ecp.11.29. [DOI] [PubMed] [Google Scholar]
- 45.Office GA, (GAO) Drugs withdrawn from market. Washington D.C.: GAO; 2001. [Google Scholar]
- 46.Anthony M, Berg MJ, Anthony M, Berg MJ. Biologic and molecular mechanisms for sex differences in pharmacokinetics, pharmacodynamics, and pharmacogenetics: Part II. Journal of Womens Health & Gender-Based Medicine. 2002;11(7):617–629. doi: 10.1089/152460902760360568. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher CV, Acosta EP, Strykowski JM. Gender differences in human pharmacokinetics and pharmacodynamics. Journal of Adolescent Health. 1994;15(8):619–629. doi: 10.1016/s1054-139x(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285(10):1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 49.Barsky A, Peekna H, Borus J. Somatic symptom reporting in women and men. Journal of Internal Medicine. 2001;16:266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinn V. Sex Differences and Implications for Translational Neuroscience Research – A Workshop. Institute of Medicine. San Francisco: 2010. Mar, [Accessed January 2, 2015]. Barriers which impede sex differences research and how to overcome them. http://www.iom.edu/~/media/85952038CE03450E9E05B7AA1FE412AB.ashx. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.