Abstract
Purpose
To identify the frequency of human papilloma virus (HPV) in ocular surface squamous neoplasia (OSSN), and evaluate differences in clinical features and treatment response of HPV positive versus negative tumors.
Design
Retrospective case series.
Participants
27 patients with OSSN
Methods
OSSN specimens were analyzed for the presence of HPV. Clinical features and response to interferon were retrospectively determined and linked to the presence (versus absence) of HPV.
Main Outcome Measures
Clinical characteristics of OSSN by HPV status.
Results
Twenty one of 27 tumors (78%) were HPV positive. HPV genotypes identified included HPV 16 in 10 (48%), HPV 31 in 5, HPV 33 in 1, HPV 35 in 2, HPV 51 in 2, and a novel HPV in 3 (total 23 as one tumor had 3 genotypes identified). Tumors found in the superior limbus were more likely to be HPV positive (48% vs 0%, Fisher exact P = 0.06). HPV 16 positive tumors were larger (68mm2 vs 34 mm2, Mann Whitney U P = 0.08) and were more likely to have a papillomatous morphology (50% vs 12%, Fisher exact P = 0.07) compared to HPV 16 negative tumors. HPV status was not found to associate with response to interferon therapy (Fisher exact P = 1.0). Metrics found to associate with a non-favorable response to interferon were male gender and tumors located in the superior conjunctivae.
Conclusions
HPV presence in OSSN appears more common in lesions located in the non-exposed, superior limbus. HPV presence does not seem to be required for a favorable response to interferon therapy.
Introduction
Ocular surface squamous neoplasia (OSSN) represents a spectrum of disease, ranging from mild dysplasia to invasive squamous cell carcinoma (SCC), and is the most common non-pigmented tumor of the ocular surface.1 While human papillomavirus has been implicated in the pathogenesis of a variety of cancers, most notably cervical cancer2, its association with OSSN has been a subject of much debate. Our group previously found HPV DNA and mRNA in 10 out of 10 OSSN specimens (five HPV 16 and five HPV 18); neither HPV was detected in control specimens or in any clinically uninvolved conjunctival specimens from OSSN eyes.3 Other groups, however, have not replicated these findings with some studies reporting no HPV and others reporting lower rates of HPV detection in OSSN (Table 1).4-20 It is not clear why certain areas seem to have a very low frequency of HPV in OSSN tumors (e.g. India, Germany, Taiwan)4-6, while others (e.g. Miami)3 have a much higher frequency. The effect of latitude and sexual activity on HPV positivity in OSSN is worth further study.
Table 1. Review of published epidemiology on human papillomavirus (HPV) and ocular surface squamous neoplasia (OSSN).
| Author | Location | Population | Technique | HPV frequency |
|---|---|---|---|---|
| Auw-Haedrich9 | Freiburg, Germany | 12 OSSN/pts 15 controls |
PCR | 16.7% CIN (HPV-16) 0% controls |
| Chauhan10 | New Delhi, India | 64 OSSN/pts 15 controls |
PCR | 11% OSSN (HPV-16) 0% control |
| Guthoff5 | Wuerzburg, Germany | 31 OSSN/pts 11 pterygia 5 controls |
IHC, PCR | 0% OSSN 0% pterygia 0% controls |
| Lauer11 | Louisiana, USA | 5 OSSN/pts | PCR | 80% OSSN (2 HPV-16, 2 HPV-16/18) |
| Manderwad12 | Hyderabad, India | 57 OSSN/48 pts | ISH, PCR | 0% OSSN |
| McDonnell13 | Los Angeles, USA | 48 OSSN/44 pts 6 pterygium |
PCR | 88% OSSN (HPV-16) 0% controls |
| Mochizuki14 | Gifu, Japan | 1 OSSN | ISH, PCR | 100% OSSN (HPV-16) |
| Moubayed15 | Dar es Salaam, Tanzania | 14 OSSN | ISH | 93% OSSN (12 HPV-6/11/16/18, 1 HPV-18) |
| Nakamura7 | Japan | 8 OSSN 9 papillomas |
IHC, ISH, PCR | 50% OSSN (2 HPV-16, 2 HPV-18) 44% papillomas (HPV-6) |
| Saegusa16 | Kitasato, Japan | 3 OSSN 16 papillomas |
ISH, PCR | 38% OSSN (HPV-16) 75% papillomas (HPV-16) |
| Scott3 | Miami, USA | 10 OSSN 5 controls |
ISH, PCR | 100% OSSN (5 HPV-16, 5 HPV-18) 0% controls |
| Sen17 | New Delhi, India | 30 OSSN 35 papillomas 30 controls |
IHC | 0% OSSN 17% papillomas 0% controls |
| Simbiri18 | Gaborone, Botswana | 28 OSSN 8 pterygia |
IHC, ISH, PCR | 75% OSSN (6 HPV-6, 13 HPV-11, 17 HPV-16, 15 HPV-18, 7 HPV 31, 1 HPV-33) 75% pterygia (5 HPV 11, 6 HPV 16, 5 HPV 18) |
| Tuppurainen19 | Kuopio, Finland | 4 OSSN | ISH, PCR | 0% OSSN |
| Woods20 | Sydney, Australia | 46 OSSN 42 pterygia 69 controls |
IHC, PCR, sequencing | 7% OSSN (HPV-16) 0% pterygia 0% controls |
| Yu8 | Uganda and Kenya | 38 OSSN | PCR | 61% OSSN (17 HPV-18, 6 HPV-16/18) |
Pts=patients; PCR=polymerase chain reaction; IHC=immunohistochemistry; ISH=in situ hybridization
Understanding the epidemiology of HPV in OSSN is important since in non-ocular HPV associated malignancies, prognosis and treatment may be altered based on viral presence.2 For example, some HPV positive tumors are treated with interferon (IFN) in isolation or as part of combination therapy.2,21 While its exact mechanism is unknown, interferon is known to have both anti-viral and anti-neoplastic properties.22 For OSSN, interferon has become a popular treatment modality and has been reported successful in 80–90% of OSSN tumors.23,24 Conversely, 10-20% of tumors do not respond to therapy. It is currently unknown what tumor factors such as clinical features or viral presence might predict response or lack of efficacy to IFN. This information is important as it can help individualize therapy based on tumor characteristics. For example, physicians may proceed directly to surgery, or use a different agent, in patients in whom interferon is unlikely to be effective. The aim of this study was to evaluate the frequency of HPV in our more recent OSSN specimens, and to examine whether clinical characteristics, including response to interferon, were different based on HPV status.
Materials and Methods
Samples
Approval was obtained from the University of Miami Institutional Review Board and the methods adhered to the tenets of the Declaration of Helsinki and were HIPAA-compliant. Twenty-eight OSSN specimens, collected from 27 patients between 03/18/1997 and 2/14/2013, underwent testing for HPV presence. Patient records were retrospectively reviewed for information on demographics and prior OSSN history. Clinical features were also collected by chart review and photographs, when available. Clinical features studied included lesion location, clinical appearance (papillomatous, leukoplakic, gelatinous, flat/nodular); and size. Furthermore, tumors were staged based on the American Joint Committee on Cancer (AJCC) clinical staging system.25
HPV testing
In situ hybridization was used to evaluate for HPV in OSSN tissue.26,27 In brief, multiple four micron sections were placed on sequentially labeled silane coated slides. The tissue was deparaffinized, proteased (30 minutes in 2mg/mL of pepsin), washed in sterile water, then 100% ethanol, and air dried. The probe cocktail containing the biotin-labeled genomic probe and the tissue DNA were co-denatured at 95°C for 5 minutes, hybridized for 15 hours at 37°C, then washed at either low (Tm 30°C, for identification of HPV DNA) or high stringency (Tm 58°C, for identification of the specific HPV type). Streptavidin conjugated alkaline phosphatase then reacted with the chromogen nitroblue tertrazolium and bromochloroindolyl phosphate (NBT/BCIP) to localize the probe/target complex. Nuclear fast red served as the counterstain. All samples were tested for HPVs 2, 6, 11, 13, 16, 18, 26, 27, 30, 31, 32, 33, 35, 39, 40, 41, 42, 43, 44, 45, 51,52, 56, 59, 68, 70 as well as other “novel” types (an HPV detected that is related to but distinct from those included in the probe cocktail) as previously described.26,27 An HPV 16 positive cervical intra-epithelial neoplasia lesion and an HPV 6/11 positive genital condyloma lesion were utilized as positive controls. The negative controls were cervical and ocular squamous cell lesions that were histologically and molecularly negative for HPV infection.
p53 detection
The immunohistochemistry detection of p53 was done using our previously published protocol.26 In brief, the optimal conditions for detection of p53 using the antibody from Enzo Life Sciences (Farmingdale, NY, USA) included antigen retrieval for 30 minutes and a dilution of 1:500. Omission of the primary antibody served as the negative control and a cervical intraepithelial neoplasm known to express p53 was the positive control. The testing was done using the automated Leica Bond Max (Buffalo Grove, IL, USA) instrument.
Statistical analysis
Student t-test, Mann-Whitney U, Chi square and Fisher exact analyses were used, as appropriate, to evaluate for differences between HPV positive and negative tumors and to evaluate factors which associated with a non-favorable response to interferon. P values < 0.05 were considered significant.
Results
Demographics of the population and HPV status
Patient age ranged from 31 to 87 years (mean 65 years), and males (n = 18) outnumbered females (n = 9). Twenty-two subjects self-identified as white (85%), and 10 as Hispanic (37%). Five had a previous history of OSSN (19%). Of the 27 tumor samples, 21 (78%) were HPV positive. Twenty patients had only one type of HPV genotype identified. This included HPV 16 in 9, HPV 31 in 4, HPV 33 in 1, HPV 35 in 2, HPV 51 in 1, and a novel HPV in 3. One patient underwent testing on an incisional biopsy specimen prior to starting interferon treatment and was found to have HPV 31 and 51 in the specimen. He failed interferon treatment and his excisional biopsy specimen from the same eye was also analyzed and found to contain HPV 16. Analyzing tumors by HPV status (positive or negative) and by HPV-16 status (positive or negative), no demographic data was associated with the frequency of HPV in a tumor (Table 2).
Table 2. Demographic information in patients with ocular surface squamous neoplasia (OSSN) by human papillomavirus (HPV) status.
| Number of eyes/patients | HPV positive | HPV negative | HPV 16 positive | HPV 16 negative | P value/ P value |
|---|---|---|---|---|---|
| Age (years), mean [SD] | 64 (16) | 70 (11) | 68 (6.4) | 64 (18) | 0.45/0.44 |
| Gender, female % (n) | 29% (6) | 50% (3) | 20% (2) | 41% (7) | 0.37/0.41 |
| Race, white % (n) | 80% (16) | 100% (6) | 78% (7) | 88% (15) | 0.54/0.59 |
| Ethnicity, Hispanic % (n) | 29% (6) | 67% (4) | 20% (2) | 47% (8) | 0.15/0.23 |
| Involved eye, right % (n) | 33% (7) | 50% (3) | 30% (93) | 41% (7) | 0.64/0.69 |
| History of OSSN % (n) | 19% (4) | 17% (1) | 20% (2) | 18% (3) | 1.0/1.0 |
n = number of individuals in group; SD = standard deviation
Clinical features and HPV status
There was a trend for tumors found in the superior limbus to be HPV positive more frequently than tumors in the temporal, nasal, or inferior limbus (Fisher exact P = 0.06). (Table 3). HPV 16-positive tumors, on average, were larger and more likely to have a papillomatous morphology compared to HPV 16-negative tumors (Mann Whitney U P = 0.08 and 0.07, respectively), although again not to a statistically significant level.
Table 3. Clinical information in patients with ocular surface squamous neoplasia (OSSN) by human papillomavirus (HPV) status.
| HPV positive | HPV negative | HPV 16 positive | HPV 16 negative | p-value/ p-value | |
|---|---|---|---|---|---|
| OSSN Area mm2 | 53 (47) | 25 (22) | 68 (58) | 34 (28) | 0.18/0.08 |
| AJCC Classification | |||||
| T1 % (n) | 10% (2) | 17% (1) | 0% (0) | 18% (3) | 0.32/0.35 |
| T2 % (n) | 19% (4) | 0% (0) | 20% (2) | 12% (2) | |
| T3 % (n) | 71% (15) | 83% (5) | 80% (8) | 71% (12) | |
| Location* | |||||
| Temporal % (n) | 52% (11) | 33% (2) | 50% (5) | 47% (8) | 0.65/0.88 |
| Inferior % (n) | 43% (9) | 17% (1) | 60% (6) | 24% (4) | 0.36/0.10 |
| Superior % (n) | 48% (10) | 0% (0) | 50% (5) | 29% (5) | 0.06/0.29 |
| Nasal % (n) | 38% (8) | 50% (3) | 50% (5) | 35% (6) | 0.66/0.45 |
| Cornea % (n) | 85% (17) | 100% (6) | 90% (9) | 89% (14) | 1.0/1.0 |
| Multifocal % (n) | 10% (2) | 0% (0) | 20% (2) | 0% (0) | 1.0/0.13 |
| Appearance | |||||
| Papillomatous % (n) | 29% (6) | 17% (1) | 50% (5) | 12% (2) | 1.0/0.07 |
| Nodular % (n) | 38% (8) | 50% (3) | 40% (4) | 41% (7) | 0.66/1.0 |
| Leukoplakia % (n) | 43% (9) | 67% (4) | 50% (5) | 47% (8) | 0.39/0.88 |
| Gelatinous % (n) | 33% (7) | 33% (2) | 40% (4) | 29% (5) | 1.0/0.68 |
| Pathological grade | |||||
| CIN 1 % (n) | 10%(2) | 17% (1) | 0% (0) | 18% (3) | 0.84/0.21 |
| CIN 2 % (n) | 76 % (16) | 67% (4) | 80% (8) | 71% (12) | |
| CIN 3 % (n) | 14% (3) | 17% (1) | 20% (2) | 12% (2) | |
| Interferon failure % (n) | 30% (6) | 17% (1) | 33% (3) | 24% (4) | 1.0/0.66 |
| P53+% (n) | 57% (10) | 80% (4) | 56% (5) | 82% (9) | 1.0/0.34 |
Tumors could involve more than one quadrant, e.g. a tumor that involved the temporal and superior bulbar conjunctivae would be represented both in the temporal and the superior location category.
CIN=conjunctival intraepithelial neoplasia; CIS= carcinoma in situ; SCC=squamous cell carcinoma; AJCC= American Joint Committee on Cancer
p53 and response to interferon by HPV status
Twenty of the 27 specimens underwent concomitant testing for p53 expression (indicative of a mutated protein). No differences in p53 expression were noted by HPV status. Of 26 patients treated with interferon, 24 underwent incisional biopsy prior to treatment and 2 underwent excisional biopsies after failing interferon. Nineteen patients responded to interferon treatment alone and 7 went on to receive other treatments including mitomycin C (MMC), 5-fluorouracil (5-FU), and/or surgical resection. HPV status was not found to correlate with response to interferon treatment (Table 3).
Other metrics and response to interferon
There was a trend for females to have a more favorable response to interferon over men as all females (n = 9) responded to therapy while only 10 of 17 males (59%) responded (Fisher exact P = 0.06). There was likewise a trend for lesions in the superior bulbar conjunctivae to respond less well to interferon, with 50% of superior lesions (n = 5) failing therapy compared to 13% (n = 2) of non-superior lesions (Fisher exact P = 0.07). None of the other factors examined in Table 3 associated with interferon response with a p < 0.1.
Recurrences
Only 1 patient of 27 recurred during our follow up time, which ranged from 0 to 13.6 years after lesion resolution (mean 2.2 years). This patient was the one who was not treated with interferon during the course of his tumor.
Discussion
Similar to our previous report3, we found a high frequency of HPV in our OSSN tumors with approximately half the genotypes being HPV 16. None of our samples harbored HPV 6 or HPV 11, which is consistent with other studies showing that these genotypes associate with benign ocular lesions.28,29 Furthermore, we found that certain clinical features (superior location for all HPV subtypes and a papillomatous morphology for HPV 16) were more common in tumors harboring virus, although with our limited sample size, none of these reached statistical significance. Interestingly, although we hypothesized that HPV positive tumors would have a more favorable response to interferon therapy, we found no difference in response to therapy based on viral status.
HPV associated carcinogenesis has been attributed to its ability to interact with host cellular proteins. Two of its proteins (E6 and E7) can alter the function of critical cellular proteins, such as p53 (enhancing degradation of normal protein).30 In fact, p53 expression (a finding suggestive of a mutated protein) has previously been reported in OSSN.31 This is not surprising, however, as sun exposure, another OSSN risk factor,32 is a known inducer of p53 mutations.33 We found abnormal p53 expression in the majority (70%) of our specimens; however the frequency of p53 expression did not segregate with HPV status. This lack of association is similar to a report by Toth et al. that found no significant relationship between p53 gene expression (detected in 78% of 23 OSSN) and HPV (detected in 17% of cases).34
HPV has also been shown to affect the epidermal growth factor receptor (EGFR) signaling pathway, a pathway with demonstrated abnormalities in OSSN, and one associated with tumor invasiveness.8 Invasive OSSN tumors were more likely to stain positive for proteins involved in the EGFR signaling pathway including cytoplasmic p-MAPK and p-Akt, and nuclear p-EGFR.34 Our study, however, with limited power, did not find a relationship between AJCC clinical grade or pathologic stage and HPV presence. It is also interesting that in one OSSN study, the presence of HPV (detected in 11% of tumors) was associated with significantly improved disease-free survival in 48 OSSN patients all treated with surgical excision.9 Unfortunately, with only one local recurrence in our group, we do not have data to answer this question in our patient sample.
As with all studies, our findings need to be considered in light of our study limitations, which included a small sample size with limited follow up and a minimal number of recurrences after treatment. In the future, we hope to further study our findings and the role of HPV in OSSN. Despite these limitations, we found that viral presence was not required for a favorable response to interferon therapy. This is interesting because since interferon has antiviral properties, one might hypothesize that the HPV lesions might in fact be more responsive to IFN.
To the best of our knowledge, this is the first report that attempts to correlate viral presence with response to interferon therapy. The importance of this study is that we found that the presence of HPV had no effect on the response to treatment with interferon. These findings highlight the need for more research on tumor and patient factors that predict tumor course and response to treatment. These data are essential so as to individualize OSSN treatment and select the therapy that will lead to an optimal clinical outcome, while minimizing patient morbidity, time, and costs.
Figure 1.
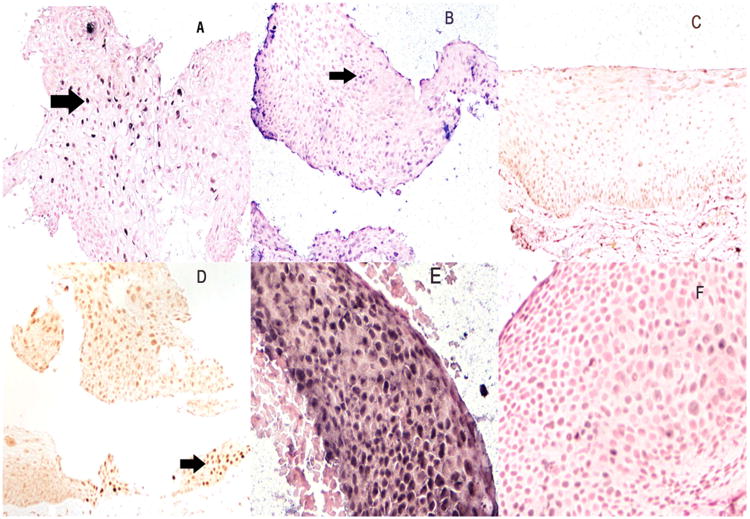
Correlation of HPV DNA detection and p53 expression in conjunctival lesions. Panel A shows the positive control for the detection of HPV 16 DNA in a cervical intraepithelial neoplasia grade 1. Note the strong nuclear based signal (arrow). Panel B shows a high grade conjunctival dysplasia also positive for HPV 16 DNA by in situ hybridization (arrow). Note that the normal epithelia adjacent to the dysplasia is HPV negative (panel C) which thus serves as an internal negative control. Panel D is a serial section to panel B where the p53 immunohistochemistry shows rare positive cells (arrow) typical of productive HPV infection. Panels E and F are serial sections of a different conjunctival high grade dysplasia. Note the intense signal for HPV 31 in panel E whereas the serial section tested for HPV 16 by in situ hybridization shows a weak signal (panel F) reflecting the weak cross homology between HPVs 16 and 31. Panels A-D are at 200×; panels E and F are at 600×.
Human papilloma viral presence in ocular surface squamous neoplasia was not necessary for response to interferon therapy. This is essential in developing future individualized therapy for patients with ocular surface squamous neoplasia.
Acknowledgments
Funding: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development's Career Development Award CDA-2-024-10S (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD- Grant#W81XWH-09-1-0675 and Grant# W81XWH-13-1-0048 ONOVA) (institutional), The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant, Grant and Diana Thornbrough Grant, The Gordon Charitable Trust, and the Richard Azar Family Grant(institutional grants).
Footnotes
Propriety Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shields CL, Demirci H, Karatza E, Shields JA. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004;111:1747–54. doi: 10.1016/j.ophtha.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World J Clin Oncol. 2014;5:1002–19. doi: 10.5306/wjco.v5.i5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology. 2002;109:542–7. doi: 10.1016/s0161-6420(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 4.Eng HL, Lin TM, Chen SY, et al. Failure to detect human papillomavirus DNA in malignant epithelial neoplasms of conjunctiva by polymerase chain reaction. Am J Clin Pathol. 2002;117:429–36. doi: 10.1309/RVUP-QMU3-5X6W-3CQ1. [DOI] [PubMed] [Google Scholar]
- 5.Guthoff R, Marx A, Stroebel P. No evidence for a pathogenic role of human papillomavirus infection in ocular surface squamous neoplasia in Germany. Curr Eye Res. 2009;34:666–71. doi: 10.1080/02713680903007162. [DOI] [PubMed] [Google Scholar]
- 6.Tulvatana W, Bhattarakosol P, Sansopha L, et al. Risk factors for conjunctival squamous cell neoplasia: a matched case-control study. Br J Ophthalmol. 2003;87:396–8. doi: 10.1136/bjo.87.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y, Mashima Y, Kameyama K, et al. Detection of human papillomavirus infection in squamous tumours of the conjunctiva and lacrimal sac by immunohistochemistry, in situ hybridisation, and polymerase chain reaction. Br J Ophthalmol. 1997;81:308–13. doi: 10.1136/bjo.81.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JJ, Fu P, Pink JJ, et al. HPV infection and EGFR activation/alteration in HIV-infected East African patients with conjunctival carcinoma. PLoS One. 2010;5:e10477. doi: 10.1371/journal.pone.0010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auw-Haedrich C, Martin G, Spelsberg H, et al. Expression of p16 in conjunctival intraepithelial neoplasia does not correlate with HPV infection. Open Ophthalmol J. 2008;2:48–56. doi: 10.2174/1874364100802010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan S, Sen S, Sharma A, et al. Human papillomavirus: a predictor of better survival in ocular surface squamous neoplasia patients. Br J Ophthalmol. 2012;96:1517–21. doi: 10.1136/bjophthalmol-2012-301907. [DOI] [PubMed] [Google Scholar]
- 11.Lauer SA, Malter JS, Meier JR. Human papillomavirus type 18 in conjunctival intraepithelial neoplasia. Am J Ophthalmol. 1990;110:23–7. doi: 10.1016/s0002-9394(14)76932-6. [DOI] [PubMed] [Google Scholar]
- 12.Manderwad GP, Kannabiran C, Honavar SG, Vemuganti GK. Lack of association of high-risk human papillomavirus in ocular surface squamous neoplasia in India. Arch Pathol Lab Med. 2009;133:1246–50. doi: 10.5858/133.8.1246. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell JM, McDonnell PJ, Sun YY. Human papillomavirus DNA in tissues and ocular surface swabs of patients with conjunctival epithelial neoplasia. Invest Ophthalmol Vis Sci. 1992;33:184–9. [PubMed] [Google Scholar]
- 14.Mochizuki K, Takahashi T, Furusawa Y, et al. Detection of human papillomavirus type 16 in tear fluid before conjunctival neoplasia excision. Jpn J Ophthalmol. 2005;49:176–7. doi: 10.1007/s10384-004-0170-z. [DOI] [PubMed] [Google Scholar]
- 15.Moubayed P, Mwakyoma H, Schneider DT. High frequency of human papillomavirus 6/11, 16, and 18 infections in precancerous lesions and squamous cell carcinoma of the conjunctiva in subtropical Tanzania. Am J Clin Pathol. 2004;122:938–43. doi: 10.1309/T189-UWWV-B71M-9VRC. [DOI] [PubMed] [Google Scholar]
- 16.Saegusa M, Takano Y, Hashimura M, et al. HPV type 16 in conjunctival and junctional papilloma, dysplasia, and squamous cell carcinoma. J Clin Pathol. 1995;48:1106–10. doi: 10.1136/jcp.48.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen S, Sharma A, Panda A. Immunohistochemical localization of human papilloma virus in conjunctival neoplasias: a retrospective study. Indian J Ophthalmol. 2007;55:361–3. doi: 10.4103/0301-4738.33822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simbiri KO, Murakami M, Feldman M, et al. Multiple oncogenic viruses identified in ocular surface squamous neoplasia in HIV-1 patients. Infect Agent Cancer. 2010;5:6. doi: 10.1186/1750-9378-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuppurainen K, Raninen A, Kosunen O, et al. Squamous cell carcinoma of the conjunctiva. Failure to demonstrate HPV DNA by in situ hybridization and polymerase chain reaction. Acta Ophthalmol (Copenh) 1992;70:248–54. doi: 10.1111/j.1755-3768.1992.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 20.Woods M, Chow S, Heng B, et al. Detecting human papillomavirus in ocular surface diseases. Invest Ophthalmol Vis Sci. 2013;54:8069–78. doi: 10.1167/iovs.13-13140. [DOI] [PubMed] [Google Scholar]
- 21.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 22.Baron S, Tyring SK, Fleischmann WR, Jr, et al. The interferons. Mechanisms of action and clinical applications. JAMA. 1991;266:1375–83. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 23.Galor A, Karp CL, Chhabra S, Barnes S, Alfonso EC. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94:551–4. doi: 10.1136/bjo.2008.153197. [DOI] [PubMed] [Google Scholar]
- 24.Karp CL, Galor A, Chhabra S, et al. Subconjunctival/perilesional recombinant interferon alpha2b for ocular surface squamous neoplasia: a 10-year review. Ophthalmology. 2010;117:2241–6. doi: 10.1016/j.ophtha.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 25.The AJCC Ophthalmic Oncology Task Force. Carcinoma of the conjunctiva. In: Edge S, Byrd D, Carducci M, Compton C, editors. AJCC Cancer Staging Manual. 7th. New York: Springer; 2009. pp. 531–3. [Google Scholar]
- 26.Nuovo GJ. In situ detection of human papillomavirus DNA after PCR-amplification. Methods Mol Biol. 2011;688:35–46. doi: 10.1007/978-1-60761-947-5_4. [DOI] [PubMed] [Google Scholar]
- 27.Stierman S, Chen S, Nuovo G, Thomas J. Detection of human papillomavirus infection in trichilemmomas and verrucae using in situ hybridization. J Cutan Pathol. 2010;37:75–80. doi: 10.1111/j.1600-0560.2009.01348.x. [DOI] [PubMed] [Google Scholar]
- 28.Minchiotti S, Masucci L, Serapiao Dos Santos M, et al. Conjunctival papilloma and human papillomavirus: identification of HPV types by PCR. Eur J Ophthalmol. 2006;16:473–7. doi: 10.1177/112067210601600320. [DOI] [PubMed] [Google Scholar]
- 29.Sjo NC, Heegaard S, Prause JU, et al. Human papillomavirus in conjunctival papilloma. Br J Ophthalmol. 2001;85:785–7. doi: 10.1136/bjo.85.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammas IN, Sourvinos G, Giannoudis A, Spandidos DA. Human papilloma virus (HPV) and host cellular interactions. Pathol Oncol Res. 2008;14:345–54. doi: 10.1007/s12253-008-9056-6. [DOI] [PubMed] [Google Scholar]
- 31.Mahomed A, Chetty R. Human immunodeficiency virus infection, Bcl-2, p53 protein, and Ki-67 analysis in ocular surface squamous neoplasia. Arch Ophthalmol. 2002;120:554–8. doi: 10.1001/archopht.120.5.554. [DOI] [PubMed] [Google Scholar]
- 32.McClellan AJ, McClellan AL, Pezon CF, et al. Epidemiology of ocular surface squamous neoplasia in a veterans affairs population. Cornea. 2013 doi: 10.1097/ICO.0b013e31829e3c80. 10.1097/ICO.0b013e31829e3c80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichrath J, Rass K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: an update. Adv Exp Med Biol. 2014;810:208–33. doi: 10.1007/978-1-4939-0437-2_12. [DOI] [PubMed] [Google Scholar]
- 34.Toth J, Karcioglu ZA, Moshfeghi AA, Issa TM, Al-Ma'ani JR, Patel KV. The relationship between human papillomavirus and p53 gene in conjunctival squamous cell carcinoma. Cornea. 2000;19:159–62. doi: 10.1097/00003226-200003000-00007. [DOI] [PubMed] [Google Scholar]


