Summary
Porphyromonas gingivalis is a prominent periodontal, and emerging systemic, pathogen that redirects host cell signalling pathways and modulates innate immune responses. In this study, we show that P. gingivalis infection induces the dephosphorylation and activation of forkhead box-O (FOXO)1, 3 and 4 in gingival epithelial cells. In addition, immunofluorescence showed that FOXO1 accumulated in the nucleus of P. gingivalis-infected cells. Quantitative reverse transcription PCR demonstrated that transcription of genes involved in protection against oxidative stress (Cat, Sod2, Prdx3), inflammatory responses (IL1β) and anti-apoptosis (Bcl-6) was induced by P. gingivalis, while small-interfering RNA (siRNA)-mediated knockdown of FOXO1 suppressed the transcriptional activation of these genes. P. gingivalis-induced secretion of interleukin (IL)-1β and inhibition of apoptosis were also impeded by FOXO1 knockdown. Neutralization of reactive oxygen species (ROS) by N-acetyl-l-cysteine blocked the activation of FOXO1 by P. gingivalis and concomitantly suppressed the activation of oxidative stress responses, anti-apoptosis programmes and IL-β production. Inhibition of c-Jun-N-terminal kinase (JNK) either pharmacologically or by siRNA, reduced FOXO1 activation and downstream FOXO1-dependent gene regulation in response to P. gingivalis. The results indicate that P. gingivalis-induced ROS activate FOXO transcription factors through JNK signalling, and that FOXO1 controls oxidative stress responses, inflammatory cytokine production and cell survival. These data position FOXO as an important signalling node in the epithelial cell–P. gingivalis interaction, with particular relevance to cell fate and dysbiotic host responses.
Introduction
Infections of the periodontium afflict over half of the population in the United States and are estimated to be the sixth most common disease condition worldwide (Eke et al., 2012; Kassebaum et al., 2014). Periodontal diseases, and periodontal bacteria, are also increasingly recognized as associated with serious systemic conditions such as coronary artery disease, preterm delivery of low birthweight infants and cancers including colorectal and oral squamous cell carcinoma (Han and Wang, 2013; Kumar, 2013; Maddi and Scannapieco, 2013; Whitmore and Lamont, 2014). The initiation and progression of periodontal diseases ensue from the action of a dysbiotic bacterial community that colonizes the hard and soft tissues of the periodontium and is recalcitrant to elimination by immune responses (Darveau, 2010; Hajishengallis and Lamont, 2012). Porphyromonas gingivalis, a Gram-negative anaerobe, is a keystone constituent of pathogenic communities in periodontal disease (Hajishengallis et al., 2011), which can both disrupt tissue homeostasis and elevate the virulence of the polymicrobial community as a whole (Lamont and Jenkinson, 1998; Curtis et al., 2001; Yongqing et al., 2011; Hajishengallis and Lamont, 2012; 2014; Hajishengallis, 2015).
The epithelial cells that line the gingival crevice constitute both a physical barrier to microbial intrusion and an interactive interface that communicates bacterial presence to the underlying immune cells. The encounter between colonizing bacteria and gingival epithelial cells thus sculpts the early stages of both innate and acquired immunity. P. gingivalis actively invades gingival epithelial cells, wherein it can survive for extended periods and spread intercellularly (Lamont et al., 1995; Belton et al., 1999; Yilmaz et al., 2006). P. gingivalis rapidly reprogrammes host cell signal transduction pathways and restructures the transcriptional landscape (Handfield et al., 2005; Moffatt et al., 2012; Takeuchi et al., 2013), one outcome of which is selective modulation of innate immunity (Bostanci and Belibasakis, 2012). For example, production of the neutrophil chemokine CXCL8 [interleukin (IL)-8] is inhibited by the action of a secreted serine phosphatase, SerB, which specifically dephosphorylates S536 on nuclear factor-κB (NF-κB) p65 and prevents translocation of NF-κB to the nucleus (Takeuchi et al., 2013). Conversely, P. gingivalis stimulates production and secretion of the proinflammatory cytokines IL-1β and IL-6. Increased IL-1β and IL-6 production is dependent, at least partially, on induction of reactive oxygen species (ROS), which induce the phosphorylation of Janus kinase (JAK)2 and activation of the transcriptional regulator c-Jun (Wang et al., 2014a). Additionally, secretion of IL-1β involves stimulation of ROS production by adenosine triphosphate (ATP), through a complex consisting of P2X4, P2X7 and pannexin-1 that activates the NLRP3 inflammasome (Hung et al., 2013). Indeed, P. gingivalis engages in multiple strategies to selectively disable critical components of innate immunity, even in the presence of otherwise stimulatory bacteria, while maintaining an overall proinflammatory response. In this manner, the asaccharyolytic and heme-requiring P. gingivalis ensures a supply of inflammatory-derived nutritional substrates (Hajishengallis, 2014).
Infection of gingival epithelial cells by P. gingivalis does not stimulate necrotic or apoptotic cell death, and moreover P. gingivalis can suppress chemically induced apoptosis (Nakhjiri et al., 2001; Mao et al., 2007; Yilmaz et al., 2008; Yao et al., 2010). P. gingivalis can suppress apoptosis in epithelial cells by several mechanisms. The organism activates prosurvival JAK1/Akt/signal transducer and activator of transcription 3 (STAT3) signalling (Yilmaz et al., 2004; Mao et al., 2007), and up-regulation of miR-203 leads to inhibition of the negative regulator SOCS3 and subsequent suppression of apoptosis (Moffatt and Lamont, 2011). At the mitochondrial membrane, proapoptotic Bad is inhibited, and the Bcl2 : Bax ratio increases, consequently curtailing the release of the apoptosis effector, cytochrome c (Mao et al., 2007; Yao et al., 2010). P. gingivalis also secretes a nucleoside diphosphate kinase, which can function as an ATPase and prevent ATP-dependent apoptosis mediated through the purinergic receptor P2X7 (Yilmaz et al., 2008). Although host epithelial cell survival along with an inflammatory environment are important for P. gingivalis persistence, and individual regulatory pathways have been uncovered, it is unknown how P. gingivalis integrates and controls these disparate activities.
In recent years, a critical role has emerged for the forkhead box-O (FOXO) family of transcriptional regulators in a wide range of host cell processes (Eijkelenboom and Burgering, 2013; Wang et al., 2014b). Activated FOXO is involved in protection from oxidative stress through the induction of enzymes that degrade ROS (Storz, 2011). Additionally, FOXO transcription factors can regulate diverse gene expression programmes controlling a number of critical host cell processes including cell cycle progression, innate immune responses and apoptosis (Eijkelenboom and Burgering, 2013; Wang et al., 2014b). FOXO proteins have thus emerged as critical signalling integrators for the maintenance of homeostasis. FOXO activity is controlled by a complex array of post-translational modifications, including phosphorylation that regulates FOXO shuttling in and out of the nucleus (Eijkelenboom and Burgering, 2013). In many cell types, elevated intracellular ROS increases nuclear localization and thus activity of FOXO, predominantly through the action of c-Jun-N-terminal kinase (JNK) (Storz, 2011; Wang et al., 2014b). The FOXO family has four members, FOXO 1, 3, 4 and 6, of which FOXO1 is the best studied. P. gingivalis has been shown to transcriptionally up-regulate FOXO1 and FOXO3 in gingival epithelial cells, and FOXO activity is associated with TLR expression, differentiation and barrier function (Li et al., 2013). However, little else is known regarding the activation or role of FOXO proteins in epithelial responses to P. gingivalis. In the present study, we investigated the activation of FOXO by P. gingivalis and the involvement of FOXO proteins in the gingival epithelial cell responses to P. gingivalis, including oxidative stress, apoptosis and cytokine production. The results define a new and pivotal role for FOXO as a signalling node in the P. gingivalis–gingival epithelial cell interface.
Results
P. gingivalis enhances the activity of FOXO transcription factors
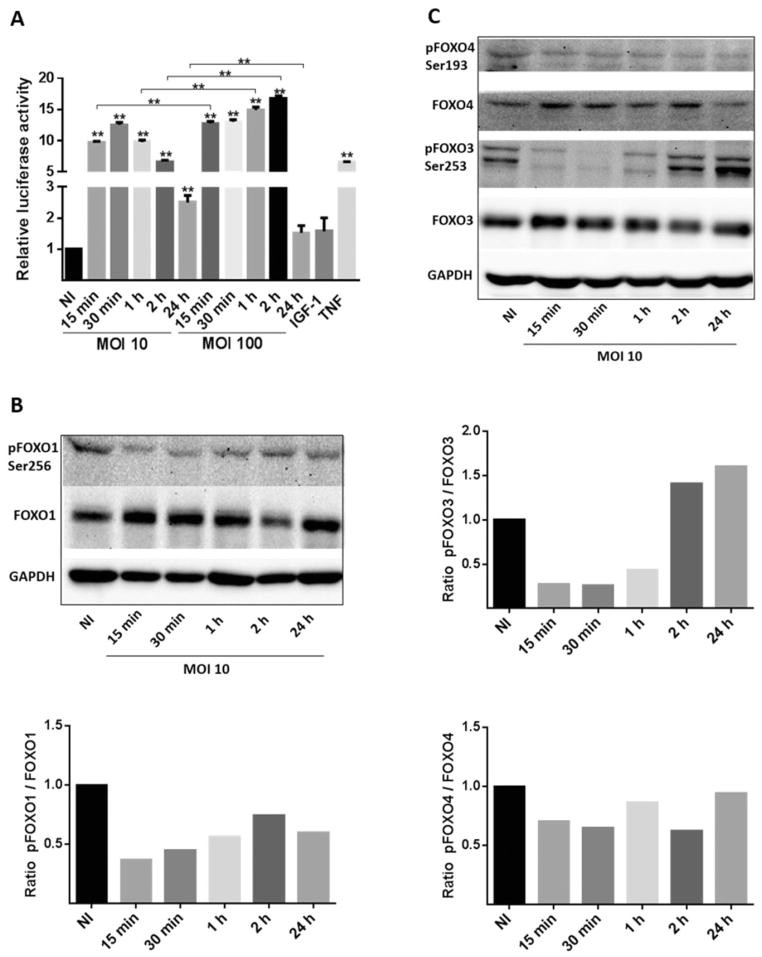
The activity of FOXO in response to P. gingivalis strain 33277 was determined by a luciferase reporter assay, in which the gene for luciferase is under the control of multimers of the FOXO-responsive element. Telomerase-inactivated gingival keratinocytes (TIGKs) transfected with this construct and infected with P. gingivalis at a multiplicity of infection (MOI) of 10 showed a significant increase in FOXO activity after 15 min, which reached maximal amounts after 30 min (Fig. 1A). Thereafter, FOXO activity gradually declined through 24 h, while remaining significantly higher than resting cell levels. Infection with P. gingivalis at MOI 100 also increased FOXO activity after 15 min with higher levels compared with MOI 10 through 2 h. However, by 24 h after infection at MOI 100 FOXO activation returned to background levels, likely as a result of host cell homeostatic control of FOXO. There was no decrease in TIGK viability at MOI 10 or 100 over 24 h (not shown).
Fig. 1. Porphyromonas gingivalis infection dephosphorylates and activates FOXO family members.
A. TIGKs were transiently transfected with the FOXO promoter–luciferase reporter plasmid, or a constitutively expressing Renilla luciferase reporter, and infected with P. gingivalis 33277 for the indicated MOI and time. Control cells were non-infected (NI). FOXO luciferase activity was normalized to the level of Renilla luciferase. Tumour necrosis factor (TNF, 5 ng ml−1) was a positive control for FOXO activation and insulin-like growth factor I (IGF-1, 50 ng ml−1) was a negative control. Results are means ± standard deviation; n = 3; **P < 0.01.
B and C. Immunoblots (upper panels) with scanning densitometry (lower panels) of lysates of TIGKs infected with P. gingivalis 33277 at MOI 10 for the time shown and probed with the antibodies indicated. Control cells were NI. GAPDH was used as a loading control.
The FOXO promoter–reporter assay is constructed with the FOXO3 binding element, which is also responsive to FOXO1 and FOXO4 (Essaghir et al., 2009). To confirm the specificity of the luciferase assay and to examine the activation of individual FOXO family proteins, Western blotting was performed (Fig. 1B and C). Phosphorylation of serine residues in the C-terminal nuclear export domain increases FOXO transport out of the nucleus and into the cytoplasm where it is inactive. P. gingivalis infection at MOI 10 caused a decrease in phosphorylation of FOXO1 at serine 256 after 15 min through 24 h. P. gingivalis also induced dephosphorylation of the equivalent residues of FOXO3 over 15–60 min. Dephosphorylation of FOXO4 was less pronounced than that of FOXO1 or FOXO3, and spanned 15 min to 24 h. These data indicate that P. gingivalis can activate FOXO1, 3 and 4 albeit with differing kinetics.
To verify that FOXO proteins accumulated in the nuclear region following P. gingivalis infection, immunofluorescence confocal microscopy with quantitative colocalization and correlation analyses was performed with FOXO1 antibody staining of TIGK cells. As shown in Fig. 2A–C, in uninfected cells FOXO1 was distributed in the cytoplasm, whereas P. gingivalis challenge at MOI 10 or 100 caused a dose-dependent increase in FOXO amounts in the nucleus where it is functionally active.
Fig. 2. Porphyromonas gingivalis induces nuclear accumulation of FOXO1.
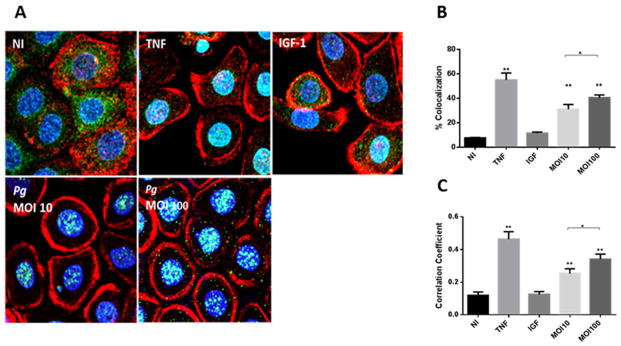
A. Fluorescent confocal microscopy of TIGK cells infected with P. gingivalis 33277 (Pg) at MOI 10 or 100 for 2 h. Control cells were non-infected (NI). Cells were fixed and probed with FOXO1 antibodies (green). Actin (red) was stained with Texas Red phalloidin, and nuclei (blue) stained with 4′,6-diamidino-2-phenylindole (DAPI). Tumour necrosis factor (TNF, 5 ng ml−1) was a positive control for FOXO activation and insulin-like growth factor (IGF-1, 50 ng ml−1) was a negative control. Cells were imaged at magnification ×63 and shown are representative merged images of projections of z-stacks (10 slices/z stack) obtained with Volocity software.
B and C. Nuclear localization of FOXO1 calculated by colocalization of FOXO1 staining with nuclei (B), and Pearson’s correlation coefficient (C) from images in A (n = 200 cells) using Volocity software. Results are means ± standard deviation; *P < 0.05; **P < 0.01.
To ensure that the ability to activate FOXO is not restricted to the 33277 lineage, an additional strain was tested. Strain A7436 induces a spreading lesion in the mouse subcutaneous model of infection whereas inoculation with 33277 results in a localized infection (Genco et al., 1992; Hu et al., 2006). Despite these differing in vivo characteristics, both strains caused a significant increase in FOXO promoter activity and dephosphorylation of FOXO1 (Fig. 3A and B). In contrast, the common oral organism Streptococcus gordonii neither increased FOXO promoter activity nor caused dephosphorylation of FOXO1, indicating specificity of these responses for P. gingivalis.
Fig. 3. Different P. gingivalis strains, but not S. gordonii, activate FOXO.
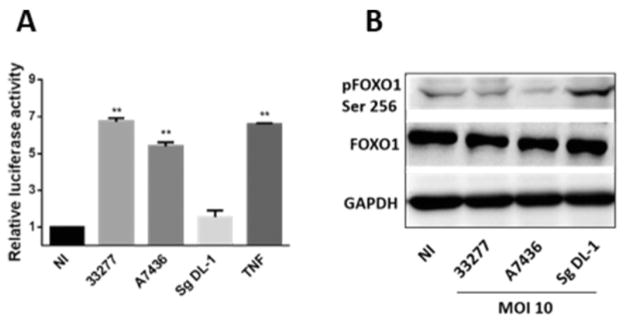
A. Luciferase assay for FOXO activity in TIGKs challenged with different P. gingivalis strains or with S. gordonii at MOI 10 for 2 h. Control cells were non-infected (NI). FOXO luciferase activity was normalized to the level of Renilla luciferase. Tumour necrosis factor (TNF, 5 ng ml−1) was a positive control for FOXO activation. Results are means ± standard deviation; n = 3; **P < 0.01.
B. Immunoblot of lysates of TIGKs infected with P. gingivalis strains or S. gordonii at MOI 10 for 2 h and probed with the antibodies indicated. Control cells were NI. GAPDH was used as a loading control.
Collectively, these results establish that P. gingivalis strains are capable of inducing the rapid dephosphorylation of serine residues in the nuclear export region of FOXO1, 3 and 4. Further, although the kinetics and degree of activation vary according to MOI or strain, dephosphorylation is associated with an increase in FOXO nuclear accumulation and activity in gingival epithelial cells infected with P. gingivalis. FOXO1 is the best characterized of the FOXO family and hence our subsequent experiments focused primarily on FOXO1.
FOXO-dependent epithelial cell responses to P. gingivalis
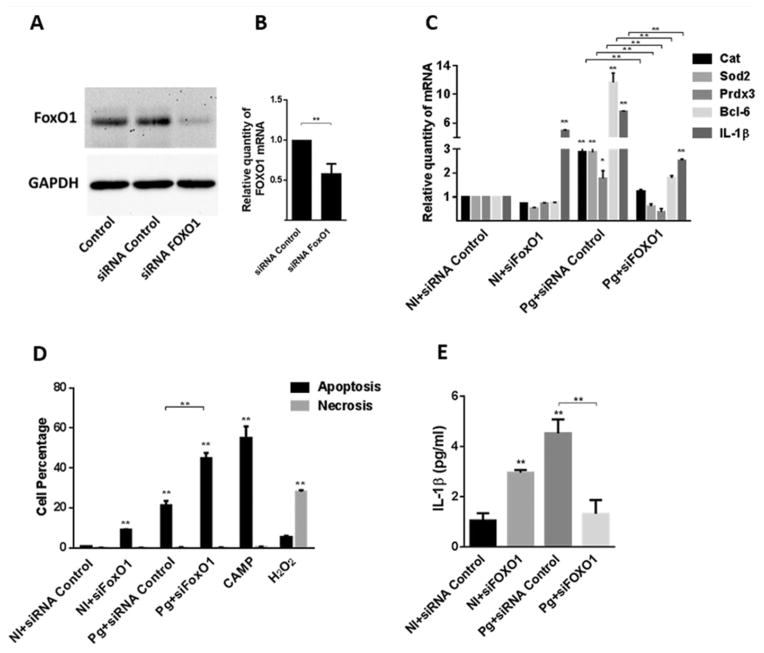
As mentioned, P. gingivalis has a number of effects on gingival epithelial cells including up-regulation of antioxidant responses, proinflammatory cytokine production and suppression of intrinsic apoptotic pathways (Yilmaz et al., 2004; 2008; Mao et al., 2007; Yao et al., 2010; Choi et al., 2013; Hung et al., 2013; Wang et al., 2014a), and all of these processes can be controlled by FOXO1. We used quantitative reverse transcription polymerase chain reaction (qRT-PCR) to examine the expression of genes associated with these events in epithelial cells infected with P. gingivalis. Genes encoding the antioxidant components catalase (Cat), superoxide dismutase (Sod2) and peroxiredoxin 3 (Prdx3), the anti-apoptotic Bcl-6 and the proinflammatory cytokine interleukin (IL)-1β were all up-regulated by P. gingivalis at MOI 10 after 2 h (Fig. 4). Gene expression declined by 24 h but remained significantly higher than the control condition of uninfected cells. The contribution of FOXO1 to this transcriptional pattern was next assessed using small-interfering RNA (siRNA) knockdown. Western blotting (Fig. 5A) and qRT-PCR (Fig. 5B) confirmed that transfection with siRNA specific for FOXO1 reduced expression at both the protein and mRNA levels. Although the transfection conditions caused an overall reduction in transcriptional activity in response to P. gingivalis, Fig. 5C shows that siRNA knockdown of FOXO1 abrogated the ability of P. gingivalis to increase expression of genes for Cat, Sod2, Prdx3, Bcl-6 and IL-1β. The relevance of FOXO1 to control of apoptosis was confirmed by flow cytometry of TIGKs with Annexin V and Sytox Green staining for apoptosis or necrosis respectively. In the control condition with scrambled siRNA, less than 20% of the cells were apoptotic, whereas FOXO1 silencing increased the number of apoptotic cells following P. gingivalis infection to over 50% (Fig. 5D). Similarly, enzyme-linked immunosorbent assay (ELISA) corroborated a corresponding reduction of IL-1β cytokine secretion (Fig. 5E). Interestingly, knockdown of FOXO1 in the absence of P. gingivalis challenge elicited an increase in IL-1β mRNA and protein, indicating that FOXO1 participates in a complex network of regulatory control of IL-1β production, the outcome of which depends on the nature of the stimulation. These results establish FOXO1 as playing a major role in the signalling pathways that control epithelial cell transcriptional responses to P. gingivalis related to oxidative stress, cell fate and proinflammatory cytokine production.
Fig. 4.
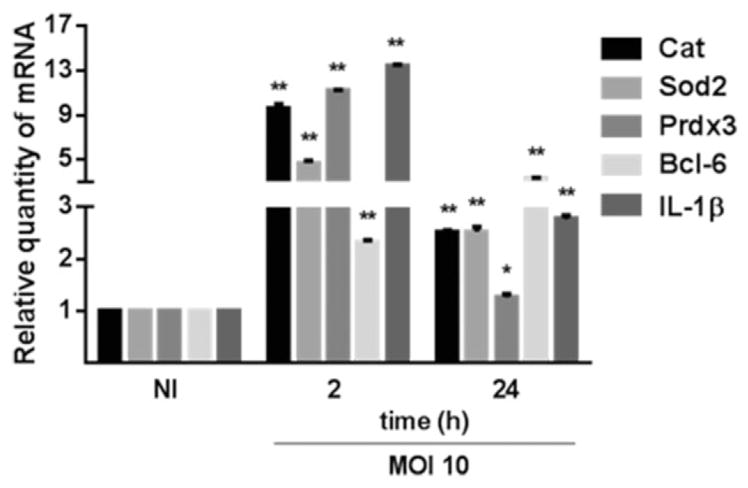
Porphyromonas gingivalis infection up-regulates the expression of FOXO target genes. TIGK cells were infected with P. gingivalis 33277 MOI 10 for the time indicated. mRNA levels for Cat, Sod2 Prdx3, Bcl-6 and IL-1β were measured by qRT-PCR. Data were normalized to GAPDH mRNA and are expressed relative to non-infected controls. Results are means ± standard deviation; n = 3; *P < 0.05; **P < 0.01.
Fig. 5. FOXO1 knockdown suppresses TIGK responses to P. gingivalis.
A. TIGK cells were transfected with siRNA to FOXO1 or scrambled siRNA (siRNA control). Control cells were non-transfected. Cell lysates were immunoblotted with FOXO1 antibodies or GAPDH antibodies as a loading control.
B. FOXO1 mRNA levels in transfected TIGK cells (as in A) were measured by qRT-PCR. Data were normalized to GAPDH mRNA and are expressed relative to the siRNA control. Results are means ± standard deviation (SD); n = 3; **P < 0.01.
C. Transfected TIGK cells were infected with P. gingivalis 33277 (Pg) MOI 10 for 2 h and Cat, Sod2 Prdx3, Bcl-6 and IL-1β mRNA levels were measured by qRT-PCR. Data were normalized to GAPDH mRNA and are expressed relative to non-infected (NI) siRNA controls. Results are mean ± SD; n = 3; *P < 0.05 *; **P < 0.01.
D. Transfected TIGK cells were infected with P. gingivalis 33277 (Pg) MOI 10 for 2 h or left uninfected (NI) and the level of apoptosis and necrosis determined by staining with AnnexinV and SytoxGreen, respectively, followed by flow cytometry. Campthothecin (CAMP, 10 μM) or H2O2 (0.3%) were positive controls for apoptosis or necrosis respectively. Results are mean ± SD; n = 3; **P < 0.01.
E. Transfected TIGK cells were infected with P. gingivalis 33277 (Pg) MOI 10 for 2 h, or left uninfected (NI), and IL-1β levels in culture supernatants determined by ELISA. Results are mean ± SD; n = 3; **P < 0.01.
P. gingivalis-induced FOXO activity relies on ROS production
In addition to controlling antioxidant responses, FOXO transcription factors act as sensors of oxidative stress as their activity is regulated by H2O2 (Essers et al., 2004; Storz, 2011). P. gingivalis stimulates the rapid production of ROS in gingival epithelial cells (Choi et al., 2013; Wang et al., 2014a), and hence we next sought to determine the role of ROS in the activation of FOXO1 by P. gingivalis. The antioxidant N-acetylcysteine (NAC) was used to neutralize ROS, and FOXO responses to P. gingivalis were then examined. Figure 6A shows that NAC treatment of TIGKs reduced FOXO activity in the luciferase reporter assay over a range of P. gingivalis infection levels. Suppression of ROS by NAC also diminished the ability of P. gingivalis to induce transcription of the Cat, Sod2, Prdx3, Bcl-6 and IL1β genes (Fig. 6B). These results suggest that P. gingivalis-induced ROS production plays a major role in the stimulation of FOXO activity and the subsequent increase in FOXO target gene transcription.
Fig. 6. Activation of FOXO by P. gingivalis requires ROS.
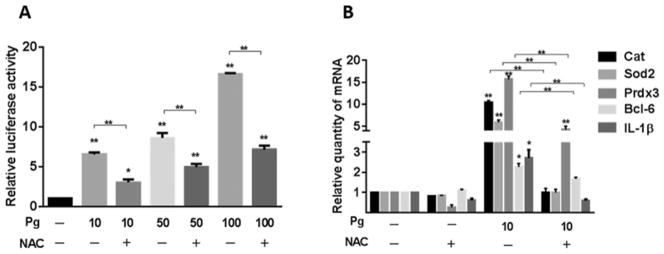
A. TIGK cells were transiently transfected with the FOXO promoter–luciferase reporter plasmid, or a constitutively expressing Renilla luciferase reporter. Transfected cells were infected with P. gingivalis 33277 (Pg) at MOI 10, 50 or 100 for 2 h, or left uninfected (−), in the presence or absence of NAC (20 mM). FOXO luciferase activity was normalized to the level of Renilla luciferase. Results are mean ± standard deviation (SD); n = 3; *P < 0.05; **P < 0.01.
B. TIGK cells were infected with P. gingivalis 33277 (Pg) MOI 10 for 2 h, or left uninfected (−) in the presence or absence of NAC (20 mM). Cat, Sod2 Prdx3, Bcl-6 and IL-1β mRNA levels were measured by qRT-PCR. Data were normalized to GAPDH mRNA and are expressed relative to uninfected controls without NAC. Results are mean ± SD; n = 3; *P < 0.05; **P < 0.01.
P. gingivalis-induced FOXO activity is JNK dependent
JNK is activated by oxidative stress, and JNK signalling is thought to comprise one pathway by which information on the redox status of the cell is relayed to FOXO (McCubrey et al., 2006; van der Horst and Burgering, 2007). Further, JNK has been shown to be activated in gingival epithelial cells infected with P. gingivalis (Watanabe et al., 2001). To assess the involvement of JNK in P. gingivalis-induced FOXO activity, JNK was inhibited pharmacologically and by siRNA. Treatment of TIGK cells with SP600125, a chemical inhibitor of JNK kinase activity, reduced the activation of FOXO by P. gingivalis in the luciferase reporter assay (Fig. 7A). Consistent with these data, inhibition of JNK prevented the up-regulation of the Cat, Sod2, Prdx3, Bcl-6 and IL1β by P. gingivalis (Fig. 7B) and similarly reduced the amount of IL-1β secreted by the TIGKs (Fig. 7C). To further verify the involvement of JNK in FOXO activation, siRNA knockdown was performed. A reduction of JNK protein following transfection was confirmed by Western blotting (Fig. 8A). Similar to the results with the chemical inhibitor, knockdown of JNK prevented P. gingivalis-induced increases in mRNA levels of the Cat, Sod2, Prdx3, IL1β and Bcl-6 genes (Fig. 8B), and diminished secretion of IL-1β (Fig. 8C). Hence, JNK signalling plays a key role in the pathway linking P. gingivalis-induced ROS production to FOXO activation and subsequent transcription of genes important in oxidative stress responses, regulation of apoptotic cell death and inflammatory responses.
Fig. 7. Inhibition of JAK suppresses FOXO-mediated responses to P. gingivalis.
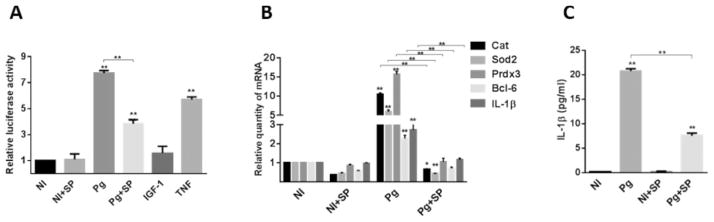
A. TIGK cells were transiently transfected with the FOXO promoter–luciferase reporter plasmid, or a constitutively expressing Renilla luciferase reporter. Transfected cells were pretreated with the JNK inhibitor SP600125 (SP) or vehicle alone, and infected with P. gingivalis 33277 (Pg) MOI 10 for 2 h or left uninfected (NI). Tumour necrosis factor (TNF, 5 ng ml−1) was a positive control for FOXO activation. FOXO luciferase activity was normalized to the level of Renilla luciferase. Results are mean ± standard deviation (SD); n = 3; **P < 0.01.
B. TIGK cells were pretreated with the JNK inhibitor SP600125 (SP) or vehicle alone, and infected with P. gingivalis 33277 (Pg) MOI 10 for 2 h or left uninfected (NI). Cat, Sod2 Prdx3, Bcl-6 and IL-1β mRNA levels were measured by qRT-PCR. Data were normalized to GAPDH mRNA and are expressed relative to NI controls. Results are mean ± SD; n = 3; *P < 0.05; **P < 0.01.
C. TIGK cells were pretreated with the JNK inhibitor SP600125 (SP) or vehicle alone, and infected with P. gingivalis 33277 (Pg) MOI 10 for 2 h or left uninfected (NI). IL-1β levels in culture supernatants were determined by ELISA. Results are mean ± SD; n = 3; **P < 0.01.
Fig. 8. Knockdown of JNK suppresses FOXO-mediated responses to P. gingivalis.
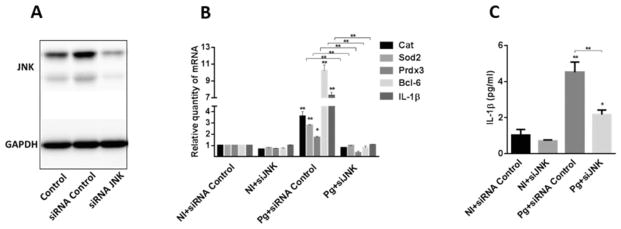
A. TIGK cells were transfected with siRNA to JNK or scrambled siRNA (siRNA Control). Control cells were non-transfected. Cell lysates were immunoblotted with JNK antibodies or GAPDH antibodies as a loading control.
B. Transfected TIGK cells were infected with P. gingivalis 33277 MOI 10 (Pg) for 2 h and Cat, Sod2 Prdx3, Bcl-6 and IL-1β mRNA levels were measured by qRT-PCR. Data were normalized to GAPDH mRNA and are expressed relative to non-infected (NI) siRNA controls. Results are means ± standard deviation (SD); n = 3; *P < 0.05; **P < 0.01.
C. Transfected TIGK cells were infected with P. gingivalis 33277 MOI 10 (Pg) for 2 h, or left uninfected (NI), and IL-1β levels in culture supernatants were determined by ELISA. Results are mean ± SD; n = 3; *P < 0.05; **P < 0.01.
Discussion
The epithelial cells that line the gingival crevice are among the first cell types encountered by colonizing bacteria, and the outcome of the interaction makes a significant contribution to overall tissue homeostasis. P. gingivalis is a keystone pathogen in periodontal disease and engages epithelial cells in an intricate cellular dialog involving several host signalling pathways (Lamont and Jenkinson, 1998; Bostanci and Belibasakis, 2012; Hajishengallis and Lamont, 2012). However, the master control switches that allow P. gingivalis to manipulate multiple phenotypic outputs are poorly understood. FOXO transcription factors are important signalling mediators in the maintenance of homeostasis, including processes involving oxidative stress responses, inflammation and cell fate decisions (van der Horst and Burgering, 2007; Eijkelenboom and Burgering, 2013). In this study, we report the activation of FOXO1, FOXO3 and FOXO4 by P. gingivalis and find that FOXO1, activated by ROS through JNK, is required for the induction of antioxidant responses and the production and secretion of IL-1β and anti-apoptotic activity.
The location and activity of FOXO proteins are regulated by several classes of post-translation modification, including phosphorylation, acetylation and methylation (Eijkelenboom and Burgering, 2013). Phosphorylation of serine residues in the DNA-binding domain introduces a negative charge that disrupts binding to DNA (Brent et al., 2008) and effectuates binding of 14-3-3 proteins resulting in nuclear export and sequestration in the cytoplasm (Obsil and Obsilova, 2011). These residues (serine 256 in FOXO1, serine 253 in FOXO3 and serine 193 in FOXO4) were dephosphorylated following P. gingivalis infection, indicating activation of FOXO1, FOXO3 and FOXO4, albeit with differing kinetics, consistent with individual FOXO factors exhibiting specialized functions and being distal to overlapping, but distinct, signalling pathways (van der Horst and Burgering, 2007; van den Berg and Burgering, 2011; Eijkelenboom and Burgering, 2013; Wang et al., 2014b). Activation of FOXO proteins was also established in a functional promoter–reporter assay. Thus, P. gingivalis can both increase the transcription of FOXO genes (Li et al., 2013) and elevate FOXO activity. Study of the regulation of FOXO by bacterial pathogens is still emerging. Mycobacteria have been shown to activate FOXO3 in infected macrophages (Haoues et al., 2014), and Haemophilus influenzae can activate FOXO in a bronchial epithelial cell line (Seiler et al., 2013). In contrast, Helicobacter pylori inhibits FOXO1 and FOXO3 in gastric epithelial cells (Tabassam et al., 2012). FOXO transcription factors may play an important role in host cell responses to a variety of bacterial pathogens, and functional outputs may depend on the nature of the activating pathway and post-translational modifications.
As a host-adapted pathogen, P. gingivalis is a genetically diverse species with a non-clonal population structure (Tribble et al., 2013). Differences in fimbrial, protease and capsule genes have been reported, and these relate to distinct properties in animal models of infection (Pathirana et al., 2007; Wang et al., 2009; ***Singh et al., 2011; Tribble et al., 2013). Thus for activation of FOXO to have potential in vivo relevance, the effect should not be restricted to the type strain 33277. Strain A7436 that is more disseminating than 33277 in animal lesions (Genco et al., 1992; Hu et al., 2006) induced FOXO activity to a similar degree, indicating that this property may be widespread among P. gingivalis strains. The ability to induce FOXO activity is not universal among oral bacteria, however, as S. gordonii, which is widespread in the oral cavity, did not show this effect. P. gingivalis possesses a number of effector molecules for interaction with epithelial cells, such as the FimA fimbriae and the serine phosphatase SerB (Lamont and Hajishengallis, 2015), and study to investigate the role of these in FOXO activation is underway. Overall, these results begin to establish in vivo significance for the FOXO response to P. gingivalis, although future animal and human studies will be required for a definitive determination.
The genes and pathways controlled by FOXO are manifold and by practical necessity we focused on processes that are well defined and potentially relevant to P. gingivalis infection. P. gingivalis induces ROS production in gingival epithelial cells (Choi et al., 2013; Wang et al., 2014a), and to counteract the damaging effects of ROS, the host cell mobilizes a number of antioxidant mechanisms. These include the production of catalase, superoxide dismutase and peroxiredoxins, all of which are under the control of FOXO (Storz, 2011; Eijkelenboom and Burgering, 2013). Following P. gingivalis infection, gene expression for all of these antioxidants was up-regulated in a FOXO1-dependent manner, consistent with restoration of intracellular redox balance and prevention of long-term oxidative damage. The inhibition of toxic responses in epithelial cells following P. gingivalis infection also aligns with the observed extended viability of epithelial cells containing P. gingivalis (Kuboniwa et al., 2008). In addition, as ROS are used by host cells to inhibit intracellular bacteria, FOXO1 may also contribute to the persistence of intracellular P. gingivalis that continue to replicate and translocate to adjacent cells (Yilmaz et al., 2006). Despite its intrinsic ability to adapt to oxidative stress (Yanamandra et al., 2012; Anaya-Bergman et al., 2015), the importance of strategies to further inhibit ROS for P. gingivalis–epithelial cell cohabitation is suggested by the activation of several other ROS detoxifying mechanisms. Previous studies have shown that P. gingivalis-infected epithelial cells increase the antioxidant glutathione response, suppress eATP-induced cytosolic and mitochondrial ROS generated through the P2X7/NADPH–oxidase interactome, and instigate an increase in anti-oxidative mitochondrial UCP2 levels (Choi et al., 2013). All of these processes contribute to intracellular persistence (Choi et al., 2013). Furthermore, in vivo studies have shown that oral epithelial cells harbour intracellular polymicrobial communities of organisms (Rudney et al., 2005), and thus enhanced anti-oxidative responses elicited by P. gingivalis may contribute to the survival of the intracellular microbial community as a whole and maintain a reservoir for recrudescence of infection.
In addition to enhancing epithelial cell survival by elevation of antioxidant responses, P. gingivalis also contributes to host cell survival through direct suppression of multiple pro-apoptotic pathways (Yilmaz et al., 2004; Mao et al., 2007; Yilmaz, 2008; Whitmore and Lamont, 2014). The role of the FOXO factors in apoptosis vacillates and is context dependent. The FOXO-dependent induction of antioxidant genes will result in decreased apoptosis (Ambrogini et al., 2010); however, a switch in signalling leads to the transcription of proapoptotic genes such as Bim and FasL (Dijkers et al., 2000). The mechanistic basis of the transition of FOXO from prosurvival to apoptotic signalling is not well defined, but may depend on the pattern of PTMs and the degree of cell differentiation (Storz, 2011). We found here that P. gingivalis-induced FOXO activation was anti-apoptotic and knockdown of FOXO1 increased the level of apoptosis following P. gingivalis infection. Although the diminished apoptosis may ensue from antioxidant responses, P. gingivalis also elicited expression of the Bcl-6 encoding gene. Bcl-6 can suppress the transcription of genes involved in apoptosis and also participates in B-cell proliferative responses (Swaminathan et al., 2014). In addition, expression of Bcl-6 is increased in the malignant transitional cell epithelium of bladder cancer (Lin et al., 2003). Although in other contexts Bcl-6 can be pro-apoptotic (Tang et al., 2002), nevertheless overexpression of Bcl-6 through FOXO1 activity may be an additional mechanism by which P. gingivalis can diminish apoptotic responses in epithelial cells.
Gingival epithelial cells produce and secrete IL-1β in response to P. gingivalis infection. The pathway involves ROS-mediated phosphorylation of JAK2 and the subsequent activation of the transcriptional regulator c-Jun (Wang et al., 2014a). However, the IL1β promoter contains a FOXO1 response element and FOXO1 can enhance IL1β transcription through amplification of NF-κB activity (Su et al., 2009). The importance of FOXO1 for P. gingivalis-induced increases in IL-1β mRNA was established in this study by knockdown of FOXO1. Thus, ROS can act through both c-Jun and FOXO1 to increase IL-1β production in response to P. gingivalis. Tight control over proinflammatory cytokine production is important for periodontal health, as dysbiotic cytokine responses contribute to tissue destruction (Hajishengallis, 2015). IL-1β can up-regulate osteoclast activity via RANK/RANKL/OPG pathways and can also induce alveolar bone loss and tissue damage through RANK-independent pathways including those involving matrix metalloproteinases (Graves, 2008; Preshaw and Taylor, 2011). Manipulation of FOXO activity could therefore present an avenue for limiting IL-1β production and associated tissue damage.
In addition to controlling the responses to oxidative stress, FOXO proteins also function as sensors of redox potential and their activity is regulated by ROS through various PTMs (Eijkelenboom and Burgering, 2013). This function was important for P. gingivalis responses, as neutralization of ROS with NAC prevented P. gingivalis-induced FOXO activity. Moreover, inhibition of JNK in turn blocked P. gingivalis-induced FOXO1 responses. JNK, which is activated by oxidative stress, can directly phosphorylate FOXO4 on C-terminal threonine residue and enhance nuclear translocation (Essers et al., 2004). Although the consensus sites for JNK phosphorylation in FOXO4 are not conserved among the other FOXOs (van den Berg and Burgering, 2011), JNK can also phosphorylate the FOXO-binding 14-3-3 proteins resulting in translocation of FOXO to the nucleus (Sunayama et al., 2005). As 14-3-3 interacts with all FOXO family members, this may account for the JNK-dependent regulation of FOXO1 observed in the current study. Although ROS can also affect acetylation of FOXO through p300/CBP (Dansen et al., 2009) and inhibition of JNK may have multiple effects, including suppression of c-Jun activity, JNK activation of FOXO is likely the major means by which the ROS signalling function induced by P. gingivalis is relayed to FOXO1.
The interaction between P. gingivalis and gingival epithelial cells is dynamic and multithreaded. An overarching theme is the tendency of P. gingivalis to invoke a response that prolongs the survival of the epithelial cells in which they reside. Additionally, P. gingivalis selectively targets components of the immune system to maintain that part of the inflammatory response that provides nutrients, while suppressing aspects that could kill and eliminate the organism (Lamont and Hajishengallis, 2015). Our results suggest an overall model whereby ROS production, which occurs rapidly in response to P. gingivalis, is sensed by FOXO through the action of JNK. FOXO activity and nuclear translocation are further enhanced by dephosphorylation of C-terminal serine residues, the mechanistic basis for which requires further study. Activated FOXO proteins, in particular FOXO1, mediate anti-oxidant responses, which along with Bcl-6 contribute to the suppression of apoptotic cell death. FOXO1 also regulates the production and secretion of IL-1β, an important proinflammatory cytokine. FOXO proteins thus constitute a major signalling hub that is manipulated by P. gingivalis for persistence at the epithelial cell interface. Given the importance of FOXO proteins in diseases such as diabetes and cancer, disruption of FOXO signalling could also provide a molecular basis for the association of P. gingivalis with these diseases.
Experimental procedures
Bacterial strains, eukaryotic cells and growth conditions
P. gingivalis ATCC 33277 and A7436 were cultured in trypticase soy broth supplemented with yeast extract (1 mg ml−1), hemin (5 μg ml−1) and menadione (1 μg ml−1). S. gordonii strain DL1 was grown in Todd–Hewitt broth. Both species were cultured anaerobically as described previously (Wright et al., 2014). TIGKs, telomerase immortalized gingival epithelial cells, derived from a primary gingival epithelial cell line, were maintained in Lifeline DermaLife Keratinocyte Medium with supplements (Lifeline Cell Technology) as described (Moffatt-Jauregui et al., 2013). Cells between passages 10 and 20 were cultured to 80% confluence and infected with P. gingivalis or S. gordonii.
Antibodies and reagents
Rabbit monoclonal anti-FOXO1, rabbit monoclonal anti-phospho-FOXO1 (Ser256), rabbit monoclonal anti-phospho-FOXO4 (Ser193), rabbit monoclonal anti-FOXO4, rabbit monoclonal anti-FOXO3, rabbit monoclonal anti-phospho-FOXO3a (Ser253), rabbit monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH), rabbit monoclonal anti-JNK, rabbit monoclonal anti-phospho-JNK (Thr183/Tyr185) and Signal Silence FOXO1 siRNA II were from Cell Signaling Technology. JNK siRNA was from Sigma-Aldrich. Cell lysis buffer was from Cell Signaling. Alexa Fluor 488-conjugated anti-rabbit secondary antibodies, Texas Red phalloidin and Lipofectamine 2000 transfection agent were from Life Technologies. ProLong Gold was from Invitrogen. Horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-mouse IgG and goat anti-rabbit IgG) were from Cell Signaling. ECL Western Blotting detection reagents were from Thermo Pierce. Tumour necrosis factor (PeproTech), NAC (Sigma-Aldrich), SP600125 (Cell Signaling) and insulin-like growth factor I (Sigma-Aldrich) were used for cell stimulation.
Immunoblotting
TIGKs were lysed with cold cell lysis buffer containing PhosSTOP phosphatase inhibitor and protease inhibitor (Roche) and 20 ng of protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto a polyvinylidene fluoride membrane and blocked with 5% bovine serum albumin in TBS with 0.1% Tween 20. Blots were reacted for 16 h with primary antibody at 4°C followed by 1 h with HRP-conjugated secondary antibody at room temperature. The membrane was developed using ECL detection, and densitometric analyses conducted using a ChemiDoc XRS Plus (Bio-Rad).
Luciferase assay
TIGK cells were transfected with the FOXO reporter and negative control reporter (BPS Bioscience) using FuGENE 6 transfection reagent (Promega). At 48 h post-transfection, cells were infected with P. gingivalis or mock treated. Luciferase activity was measured with the Dual-Glo luciferase assay system (Promega) and normalized to the internal control.
Immunofluorescence and confocal laser scanning microscopy
TIGK cells were grown on glass coverslips, washed twice in phosphate-buffered saline and fixed with 4% paraformaldehyde for 10 min. Permeabilization was with 0.2% Triton X-100 for 10 min at room temperature prior to blocking in 10% goat serum for 20 min. FOXO1 was detected by reacting with antibody (1:100) overnight at 4°C, followed by Alexa Fluor 488-conjugated secondary antibodies at 1:200 for 3 h in the dark. Following a 20 min blocking in 0.1% goat serum, actin was labelled with Texas Red phalloidin for 2 h at room temperature in the dark. Coverslips were mounted on glass slides using ProLong Gold with 4′,6-diamidino-2-phenylindole (DAPI) mounting medium prior to imaging with a Leica SP8 confocal microscope. Images were analyzed using Volocity 6.3 software (PerkinElmer).
siRNA transfection
For knockdown experiments, TIGK cells were transfected with FOXO1 siRNA (100 nM), JNK siRNA (100 nM) or scrambled control siRNA (100 nM) utilizing Lipofectamine 2000 according to the manufacturer’s suggested protocol. At 48 h post-transfection, cells were infected with P. gingivalis or mock treated. Knockdown was confirmed by qRT-PCR or Western blot.
qRT-PCR
Total RNA was isolated from P. gingivalis infected or uninfected TIGKs with the Perfect Pure RNA Cell Kit (5Prime) and reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems). TaqMan primers were obtained commercially (Applied Biosystems) and qRT-PCR performed on an Applied Biosystems StepOne plus. mRNA levels were normalized with those of GAPDH mRNA and quantified using the ΔΔCt method (Hirano et al., 2013).
Statistical analysis
Statistical analyses were conducted using the GraphPad Prism software. Data were evaluated by analysis of variance with Tukey’s multiple comparison test. All data shown are representative of at least three biological replicates.
Acknowledgments
We thank the NIH/NIDCR for support through DE011111 and DE017921 (RJL), DE017680 (DAS) and DE023633 (HW).
Footnotes
Conflicts of interest
The authors have no conflict of interest to declare.
References
- Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11:136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaya-Bergman C, Rosato A, Lewis JP. Iron- and hemin-dependent gene expression of Porphyromonas gingivalis. Mol Oral Microbiol. 2015;30:39–61. doi: 10.1111/omi.12066. [DOI] [PubMed] [Google Scholar]
- Belton CM, Izutsu KT, Goodwin PC, Park Y, Lamont RJ. Fluorescence image analysis of the association between Porphyromonas gingivalis and gingival epithelial cells. Cell Microbiol. 1999;1:215–223. doi: 10.1046/j.1462-5822.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- van den Berg MC, Burgering BM. Integrating opposing signals toward Forkhead box O. Antioxid Redox Signal. 2011;14:607–621. doi: 10.1089/ars.2010.3415. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1–9. doi: 10.1111/j.1574-6968.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- Brent MM, Anand R, Marmorstein R. Structural basis for DNA recognition by FoxO1 and its regulation by posttranslational modification. Structure. 2008;16:1407–1416. doi: 10.1016/j.str.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Spooner R, DeGuzman J, Koutouzis T, Ojcius DM, Yilmaz O. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cell Microbiol. 2013;15:961–976. doi: 10.1111/cmi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Aduse-Opoku J, Rangarajan M. Cysteine proteases of Porphyromonas gingivalis. Crit Rev Oral Biol Med. 2001;12:192–216. doi: 10.1177/10454411010120030101. [DOI] [PubMed] [Google Scholar]
- Dansen TB, Smits LM, van Triest MH, de Keizer PL, van Leenen D, Koerkamp MG, et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5:664–672. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009;284:10334–10342. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco CA, Kapczynski DR, Cutler CW, Arko RJ, Arnold RR. Influence of immunization on Porphyromonas gingivalis colonization and invasion in the mouse chamber model. Infect Immun. 1992;60:1447–1454. doi: 10.1128/iai.60.4.1447-1454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44:328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handfield M, Mans JJ, Zheng G, Lopez MC, Mao S, Progulske-Fox A, et al. Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell Microbiol. 2005;7:811–823. doi: 10.1111/j.1462-5822.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- Haoues M, Refai A, Mallavialle A, Barbouche MR, Laabidi N, Deckert M, Essafi M. Forkhead box O3 (FOXO3) transcription factor mediates apoptosis in BCG-infected macrophages. Cell Microbiol. 2014;16:1378–1390. doi: 10.1111/cmi.12298. [DOI] [PubMed] [Google Scholar]
- Hirano T, Beck DA, Wright CJ, Demuth DR, Hackett M, Lamont RJ. Regulon controlled by the GppX hybrid two component system in Porphyromonas gingivalis. Mol Oral Microbiol. 2013;28:70–81. doi: 10.1111/omi.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Hu SW, Huang CH, Huang HC, Lai YY, Lin YY. Transvascular dissemination of Porphyromonas gingivalis from a sequestered site is dependent upon activation of the kallikrein/kinin pathway. J Periodontal Res. 2006;41:200–207. doi: 10.1111/j.1600-0765.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, et al. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS ONE. 2013;8:e70210. doi: 10.1371/journal.pone.0070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa M, Hasegawa Y, Mao S, Shizukuishi S, Amano A, Lamont RJ, Yilmaz O. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008;10:122–128. doi: 10.1016/j.micinf.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS. Oral microbiota and systemic disease. Anaerobe. 2013;24:90–93. doi: 10.1016/j.anaerobe.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Dong G, Moschidis A, Ortiz J, Benakanakere MR, Kinane DF, Graves DT. P. gingivalis modulates keratinocytes through FOXO transcription factors. PLoS ONE. 2013;8:e78541. doi: 10.1371/journal.pone.0078541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Kim H, Park H, Kim Y, Cheon J, Kim I. The expression of Bcl-2 and Bcl-6 protein in normal and malignant transitional epithelium. Urol Res. 2003;31:272–275. doi: 10.1007/s00240-003-0324-3. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Maddi A, Scannapieco FA. Oral biofilms, oral and periodontal infections, and systemic disease. Am J Dent. 2013;26:249–254. [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt CE, Lamont RJ. Porphyromonas gingivalis induction of microRNA-203 expression controls suppressor of cytokine signaling 3 in gingival epithelial cells. Infect Immun. 2011;79:2632–2637. doi: 10.1128/IAI.00082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt CE, Inaba H, Hirano T, Lamont RJ. Porphyromonas gingivalis SerB-mediated dephosphorylation of host cell cofilin modulates invasion efficiency. Cell Microbiol. 2012;14:577–588. doi: 10.1111/j.1462-5822.2011.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt-Jauregui CE, Robinson B, de Moya AV, Brockman RD, Roman AV, Cash MN, et al. Establishment and characterization of a telomerase immortalized human gingival epithelial cell line. J Periodontal Res. 2013;48:713–721. doi: 10.1111/jre.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, Chung WO, Watanabe K, El-Sabaeny A, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Obsil T, Obsilova V. Structural basis for DNA recognition by FOXO proteins. Biochim Biophys Acta. 2011;1813:1946–1953. doi: 10.1016/j.bbamcr.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Pathirana RD, O’Brien-Simpson NM, Brammar GC, Slakeski N, Reynolds EC. Kgp and RgpB, but not RgpA, are important for Porphyromonas gingivalis virulence in the murine periodontitis model. Infect Immun. 2007;75:1436–1442. doi: 10.1128/IAI.01627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011;38(Suppl 11):60–84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- Rudney JD, Chen R, Sedgewick GJ. Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythensis are components of a polymicrobial intracellular flora within human buccal cells. J Dent Res. 2005;84:59–63. doi: 10.1177/154405910508400110. [DOI] [PubMed] [Google Scholar]
- Seiler F, Hellberg J, Lepper PM, Kamyschnikow A, Herr C, Bischoff M, et al. FOXO transcription factors regulate innate immune mechanisms in respiratory epithelial cells. J Immunol. 2013;190:1603–1613. doi: 10.4049/jimmunol.1200596. [DOI] [PubMed] [Google Scholar]
- Singh A, Wyant T, Anaya-Bergman C, Aduse-Opoku J, Brunner J, Laine ML, et al. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect Immun. 2011;79:4533–4542. doi: 10.1128/IAI.05016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14:593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Coudriet GM, Hyun Kim D, Lu Y, Perdomo G, Qu S, et al. FoxO1 links insulin resistance to proinflammatory cytokine IL-1beta production in macrophages. Diabetes. 2009;58:2624–2633. doi: 10.2337/db09-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Duy C, Muschen M. BACH2-BCL6 balance regulates selection at the pre-B cell receptor checkpoint. Trends Immunol. 2014;35:131–137. doi: 10.1016/j.it.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassam FH, Graham DY, Yamaoka Y. Helicobacter pylori-associated regulation of forkhead transcription factors FoxO1/3a in human gastric cells. Helicobacter. 2012;17:193–202. doi: 10.1111/j.1523-5378.2012.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Hirano T, Whitmore SE, Morisaki I, Amano A, Lamont RJ. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-kappaB RelA/p65. PLoS Pathog. 2013;9:e1003326. doi: 10.1371/journal.ppat.1003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem. 2002;277:14255–14265. doi: 10.1074/jbc.M110901200. [DOI] [PubMed] [Google Scholar]
- Tribble GD, Kerr JE, Wang BY. Genetic diversity in the oral pathogen Porphyromonas gingivalis: molecular mechanisms and biological consequences. Future Microbiol. 2013;8:607–620. doi: 10.2217/fmb.13.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou H, Duan X, Jotwani R, Vuddaraju H, Liang S, et al. Porphyromonas gingivalis-induced reactive oxygen species activate JAK2 and regulate production of inflammatory cytokines through c-Jun. Infect Immun. 2014a;82:4118–4126. doi: 10.1128/IAI.02000-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liang S, Hosur KB, Domon H, Yoshimura F, Amano A, Hajishengallis G. Differential virulence and innate immune interactions of type I and II fimbrial genotypes of Porphyromonas gingivalis. Oral Microbiol Immunol. 2009;24:478–484. doi: 10.1111/j.1399-302X.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int. 2014b;2014:925350. doi: 10.1155/2014/925350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Yilmaz O, Nakhjiri SF, Belton CM, Lamont RJ. Association of mitogen-activated protein kinase pathways with gingival epithelial cell responses to Porphyromonas gingivalis infection. Infect Immun. 2001;69:6731–6737. doi: 10.1128/IAI.69.11.6731-6737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Wu H, Melander RJ, Melander C, Lamont RJ. Disruption of heterotypic community development by Porphyromonas gingivalis with small molecule inhibitors. Mol Oral Microbiol. 2014;29:185–193. doi: 10.1111/omi.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra SS, Sarrafee SS, Anaya-Bergman C, Jones K, Lewis JP. Role of the Porphyromonas gingivalis extracytoplasmic function sigma factor, SigH. Mol Oral Microbiol. 2012;27:202–219. doi: 10.1111/j.2041-1014.2012.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Jermanus C, Barbetta B, Choi C, Verbeke P, Ojcius DM, Yilmaz O. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun. 2006;74:703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, et al. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongqing T, Potempa J, Pike RN, Wijeyewickrema LC. The lysine-specific gingipain of Porphyromonas gingivalis: importance to pathogenicity and potential strategies for inhibition. Adv Exp Med Biol. 2011;712:15–29. doi: 10.1007/978-1-4419-8414-2_2. [DOI] [PubMed] [Google Scholar]