Abstract
Background
The ceramide metabolite, sphingosine-1-phosphate (S1P), regulates multiple cellular functions in keratinocytes (KC). We recently discovered that production of a key innate immune element, cathelicidin antimicrobial peptide (CAMP), is stimulated via a NF-κB-dependent mechanism that is activated by S1P when S1P is generated by sphingosine kinase (SPHK) 1.
Objective
We investigated whether pharmacological modulation of SPHK1 activity, using a novel synthetic SPHK1 activator, (S)-Methyl 2-(hexanamide)-3-(4-hydroxyphenyl) propanoate (MHP), stimulates CAMP expression.
Methods
MHP-mediated changes in both S1P and CAMP downstream mediators were analyzed in normal cultured human KC by qRT-PCR, Western immunoblot, ELISA, confocal microscopy for immunohistochemistry, HPLC and ESI-LC/MS/MS, and microbial pathogen invasion/colonization in a human epidermal organotypic model.
Results
Treatment with MHP directly activated SPHK1 and increased cellular S1P content in normal cultured human KC. Because MHP did not inhibit S1P lyase activity, which hydrolyses S1P, augumented S1P levels could be attributed to increased synthesis rather than blockade of S1P degradation. Next, we found that exogenous MHP significantly stimulated CAMP mRNA and protein production in KC, increases that were significantly suppressed by siRNA directed against SPHK1, but not by a scrambled control siRNA. NF-κB activation, assessed by nuclear translocation of NF-κB, occurred in cells following incubation with MHP. Conversely, pretreatment with a specific inhibitor of SPHK1 decreased MHP-induced nuclear translocation of NF-κB, and significantly attenuated the MHP-mediated increase in CAMP production. Finally, topical MHP significantly suppressed invasion of the virulent Staphylococcus aureus into murine skin explants.
Conclusion
MHP activation of SPHK1, a target enzyme of CAMP production, can stimulate innate immunity.
Keywords: antimicrobial defense, cathelicidin antimicrobial peptide, sphingosine kinase 1, small molecule
INTRODUCTION
Epidermis deploys multiple diverse barriers, including permeability, oxidative stress, ultraviolet irradiation (UV), and antimicrobial barriers [1]. Antimicrobial peptides (AMPs), which show broad-spectrum antimicrobial activities, are critical components of the antimicrobial barrier and epidermal innate immunity [2], as well as contributors to the permeability barrier [3]. Cathelicidin antimicrobial peptide (CAMP) is a major epidermal AMP [4] that increases following not only microbial pathogen colonization and/or invasion, but also external perturbations, such as permeability barrier disruption, UVB exposure or oxidative stress [5, 6]. Our recent studies demonstrate that different, unrelated types of external perturbations, if subtoxic, induce endoplasmic reticulum (ER) stress that increases production of ceramide and levels of one of its metabolites, sphingosine-1-phosphate (S1P). The latter in turn stimulates CAMP production via NF-κB activation, independent of the well-known, vitamin D receptor (VDR)-dependent transcriptional regulation of CAMP [7, 8], which is suppressed under ER-stressed conditions. In addition to activating CAMP, S1P signaling modulates cell proliferation, migration, differentiation, and apoptosis [9, 10].
Ceramide is hydrolyzed by ceramidase to sphingosine, which then is phosphorylated by sphingosine kinase (SPHK) to S1P [11]. We previously synthesized a SPHK1 activator, N-(1,3-dihydroxyisopropyl)-2-hexyl-3-oxo-decanamide (K6PC-5), based on molecular design technology [12]. K6PC-5 improves epidermal barrier function through stimulation of keratinocyte differentiation, while also stimulating dermal fibroblast proliferation and collagen synthesis [12, 13]. Because K6PC-5 exhibits poor solubility in water/DMSO binary mixtures and organic solvents, we recently synthesized (S)-Methyl 2-(hexanamide)-3-(4-hydroxyphenyl) propanoate (MHP), a derivative of K6PC-5 that markedly improved solubility in hydrophilic solvents. Here, we demonstrate that MHP activates SPHK1, which also stimulates CAMP production and enhances epidermal antimicrobial defense via the recently identified S1P-dependent mechanism. Consistent with our prior studies [8], these studies further indicate that SPHK1 activity is a key determinant in the regulation of CAMP production.
MATERIALS AND METHODS
Cell culture
Primary cultured human keratinocytes (KC) isolated from human neonatal foreskins were grown, as described previously [8] under an Institutional Review Board-approval protocol (University of California San Francisco; NeoPharm Co., Ltd). Cell viability and cytotoxicity were determined using MTT assay kit in accordance with the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using 30 ng of cDNA prepared from total RNA fraction of cell lysates, as described previously [7]. The following primer sets were used: CAMP, 5′-CACAGCAGTCACCAGAGGATTG-3′ and 5′-GGCCTGGTTGAGGGTCACT-3′; human glyceraldehyde3′-phosphate dehydrogenase (GAPDH), 5′-GGAGTCAACGGATTTGGTCGTA-3′ and 5′-GCAACAATATCCACTTTACCAGAGTTAA-3. mRNA expression was normalized to levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Western immunoblot analysis
Western immunoblot analysis was performed, as described previously [7]. Briefly, cell lysates (25 μg), prepared in RIPA buffer, were resolved by electrophoresis on 4-12% Bis-Tris Gel (Invitrogen, Carlsbad, CA). Resultant bands blotted onto nitrocellulose membranes were probed with anti-CAMP (LifeSpan BioSciences, Seattle, WA), anti-human β-actin (Sigma-Aldrich, St. Louis, MO), and detected using enhanced chemiluminescence (Thermo Scientific, Waltham, MA).
ELISA for CAMP quantifications
CAMP content of cell lysates of KC previously incubated with (S)-Methyl 2-(hexanamide)-3-(4-hydroxyphenyl) propanoate (MHP) is determined by ELISA kit (Hycult Biotech, Plymouth Meeting, PA) in accordance with the manufacturer’s instructions.
Immunofluorescence
Immunofluorescence was performed, as described previously, NF-κB [14] and murine CAMP (mCAMP) [15]. KC were treated with MHP or vehicle for 30 min. NF-κB distribution was assessed using anti- NF-κB p65 (Santa Cruz Biotechnology, Dallas, TX) and anti-rabbit IgG conjugated with fluorescein isothiocyanate (Invitrogen). Cells were counterstained with the nuclear marker histone H4 (Vector Laboratories, Burlingame, CA). Sections fixed with formalin (5 μm) were incubated for 1 hour in blocking buffer (4% BSA, 0.5% cold water fish gelatin in PBS), and then incubated with anti-mCAMP (LifeSpan Biosciences) overnight at 4°C. The sections were then incubated for 1 hour at room temperature with goat anti-rabbit Alexa Fluor 488 (Invitrogen) followed by counterstaining with propidium iodide. Images were viewed under a fluorescence microscope (Carl Zeiss, Thornwood, NY).
siRNA and transfections
KC were transfected with 20 nM siRNA for SPHK1 or non-targeted, control siRNA (Dharmacon, Lafayette, CO), using siLentFect (Bio-Rad, Hercules, CA), as previously described [7, 8].
Measurement of intracellular levels of sphingosine-1-phosphate
To assess the levels of cellular S1P, KC were incubated with MHP and washed with phosphate-buffered saline followed by extraction of total S1P, as we reported previously [16]. S1P was derivatized with o-phthalaldehyde (OPA) reagent and then quantitated using an HPLC system equipped with a fluorometrical detector system (JASCO, Tokyo, Japan), as described previously [8, 17]. S1P levels were expressed as pmol per mg protein.
Enzyme activity assays for sphingosine kinase 1
SPHK1 activity was assessed as described previously [18, 19]. Briefly, recombinant SPHK1 (Sigma-Aldrich, St. Louis, MO), (2 μg) was incubated with sample reaction buffer (10 mM ATP, 200 mM MgCl2, 200 μM C17-Sphingosine, 5 mM NaF, Na3VO4, 5% Triton X-100) for 30 min. The reaction was terminated by the addition of CHCl3/MeOH/HCl (8:4:3, v/v/v) with 100 pmol C17-sphinganine-1-phosphate as an internal standard. The organic phage separated by addition of CHCl3 was dried using a vacuum system (Vision, Seoul, Korea). The dried residue was re-dissolved in MeOH and then injected into the LC-ESI-MS/MS system (ABCIEX, Toronto, Canada). The HPLC column effluent was introduced onto an API 3200 Triple quadruple mass (ABCIEX) and analyzed using electrospray ionization in positive mode with multiple reaction monitoring (MRM) to select both parent and characteristic daughter ions specific to each analyte simultaneously from a single injection. The MS/MS transitions (m/z) of 366 → 250 for C17-S1P and 368 → 270 for C17-Sa1P were used as quantifier for the MRM with a dwell time of 100 ms. Data were acquired using Analyst 1.4.2 software (Life Technologies, Grand Island, NY) and the activity of SPHK1 is expressed as S1P pmol per mg protein per min.
Enzyme activity assays for sphingosine-1-phosphate lyase
To determine the activity of S1P lyase, KC were treated with MHP for 24 h followed by washing with phosphate-buffered saline. Cell lysates (50 μg) were incubated with 10 nmol C17- sphinganine-1-phosphate for 20 min. Lipid extraction was performed by the addition of 100 pmol of (2E)-d5-hexadecenal as the internal standard. Total lipid extracts were derivatized with 5 mM semicarbazide hydrochloride in methanol containing 5% formic acid at 40 °C for 2 h and analyzed by LC-ESI-MS/MS (ABCIEX), as described previously [20]. The activity of S1P lyase is expressed as pentadecanal pmol per mg protein per min.
Staphylococcus aureus invasion assay
Staphylococcus (S.) aureus invasion assay was performed, as we described previously [8]. Briefly, full-thickness pieces of murine skin treated with MHP or vehicle were harvested from hairless mice (10-week-old female, hr/hr, n=5) under an Institutional Animal Care and Use Committee-approved protocol. Epidermal permeability barrier was attenuated by topical application of oxazolone (0.1%) once every other day five times. Mice were treated with MHP two times daily for the last 3 days, during the oxazolone treatment, before harvesting skin. Skin was placed on a filter paper, dermis side down, and maintained at the air–medium interface in KC growth medium. S. aureus in PBS or PBS was epicutaneously applied (20 μl/cm−2), followed by incubation for 24 hours at 37 °C in 5% CO2. Gram staining was performed to assess S. aureus invasion [8].
Statistical Analyses
The differences among treatments were determined by either the unpaired Student t Test or the one-way ANOVA coupled with Duncan’s multiple comparison test. The p values were <0.01 in all cases.
RESULTS
MHP increases CAMP expression in cultured human keratinocytes (KC)
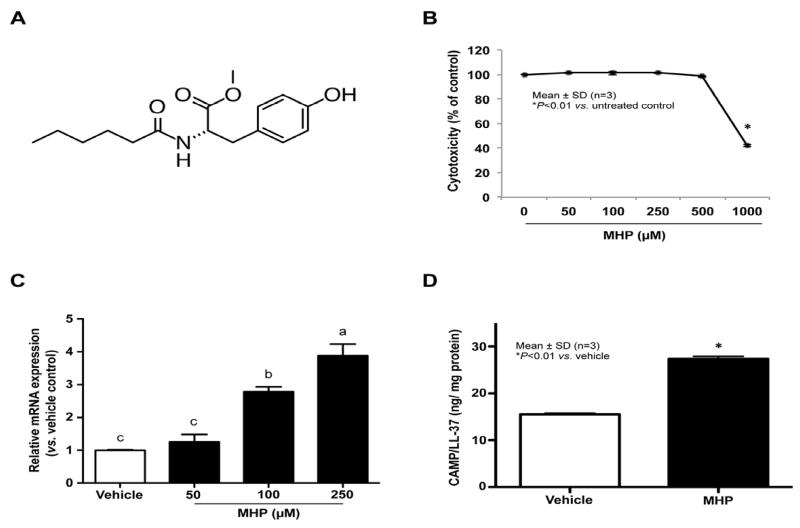
We first investigated whether expression of two major epidermal antimicrobial peptides, CAMP and hBD3, is altered by treatment of cultured human KC with MHP (Fig. 1A). qRT-PCR analyses revealed a significant dose-dependent increase in CAMP mRNA expression in human KC after incubation with MHP (50 – 250 μM) (Fig. 1C). ELISA and Western immunoblot analyses showed that CAMP protein levels in cell lysates also increased following treatment with 100 μM of MHP (Fig. 1D and 2B). However, MHP treatment did not alter hBD3 mRNA and protein levels (not shown). Since MHP at concentrations < 500 μM did not significantly alter cell viability (Fig. 1B), we employed MHP at concentrations of 100 μM in subsequent studies.
Fig. 1. MHP increases CAMP mRNA and protein expression.
The chemical structure of MHP (A). Primary cultured keratinocytes (KC) were incubated with the indicated concentrations of MHP for 24 h. Cell toxicity was assessed by MTT assay (B). CAMP mRNA (C) and protein expression (D) was determined by qRT-PCR and ELISA, respectively. Similar results were obtained when the experiment was repeated (more than twice) using different cell preparations. Values are means ± SD (n=3). Means with different letters differ at p < 0.01 by one-way ANOVA coupled with Duncan’s multiple comparison test (C).
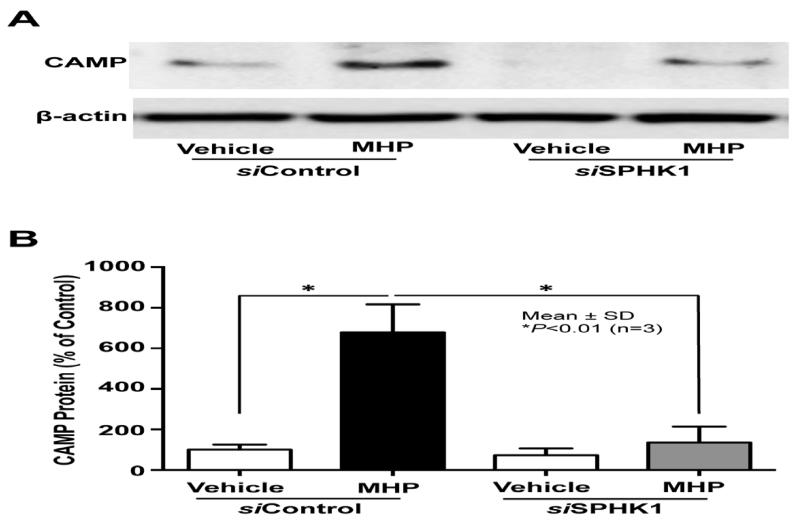
Fig. 2. SPHK1 activity is required for MHP-induced increase in CAMP expression.
KC pretreated with or without siRNA against SPHK1 were treated with MHP for 24 h. CAMP protein levels were assessed by Western immunoblot analysis (A). Intensity of individual bands was quantified using a LAS-3000 chemiluminescent image analyzer (Fujifilm). The ratio of CAMP band to that of β-actin in the scrambled siRNA (siControl)-transfected, vehicle-treated cells was set to 100%, to which the corresponding ratios in the other samples were normalized (B). Similar results were obtained when the experiment was repeated (more than twice) using different cell preparations.
MHP increases cellular S1P levels by activation of SPHK1
Prior studies demonstrated that an increase in cellular S1P synthesis by a chemically-related SPHK1 activator stimulates CAMP production [8]. Here, we investigated whether the next generation of exogenous SPHK1 activator, MHP, also increases cellular S1P levels. Lipid quantification demonstrated that MHP significantly increased S1P levels in human KC (Table 1). Since cellular levels of S1P are tightly regulated not only by SPHK, but also by the S1P degrading enzyme, S1P lyase, we next assessed whether MHP altered activities of one or both these enzymes. Enzyme activity assays showed that MHP treatment significantly increased SPHK1 activity, but not S1P lyase activity (Table 2). Moreover, we further assessed the role of SPHK1 on S1P-mediated CAMP stimulation by transfecting cells with SPHK1 siRNA. Treatment with MHP increased CAMP protein expression, whereas, CAMP expression was significantly attenuated in cells in which SPHK1 levels were knocked down by siRNA (Fig. 2). Together, these results suggest that MHP activates SPHK1, increasing cellular levels of S1P leading to stimulation of CAMP production.
Table 1.
Sphingosine-1-Phosphate contents in human KC exposed to MHP
| Treatment | Sphingosine-1-Phosphate (pmol/mg protein ± SD) |
|---|---|
| Vehicle | 12.34 ± 0.81 |
| MHP | 18.39 ± 1.26* |
All values are mean ± SD (n=3).
P <0.01 vs. Vehicle control.
Table 2.
MHP activates SPHK1, but not S1P lyase
| Treatment | SPHK1 activity (S1P pmol/mg protein/min ± SD) |
S1P lyase activity (pentadecanal pmol/mg protein/min ± SD) |
|---|---|---|
| Vehicle | 759.23 ± 29.84 | 14.17 ± 1.25 |
| MHP | 970.36 ± 57.81* | 13.42 ± 1.13 |
All values are mean ± SD (n=3).
P <0.01 vs. Vehicle control.
Mechanisms responsible for MHP-mediated stimulation of CAMP expression
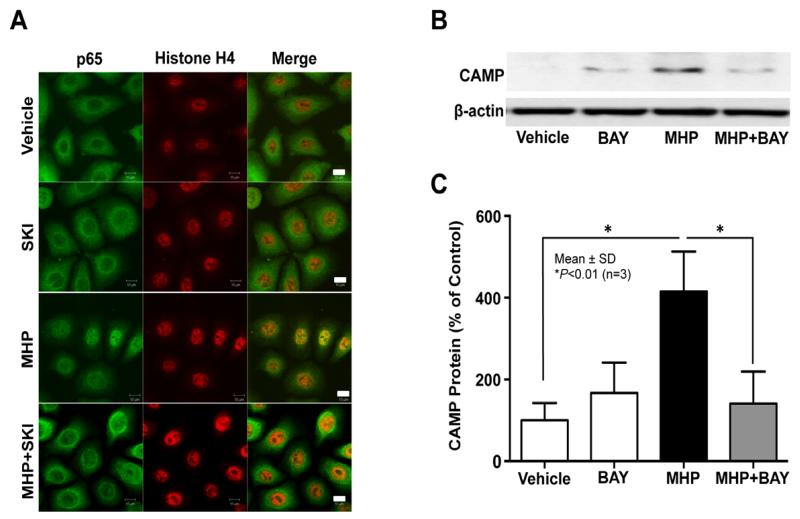
Because our recent studies revealed that NF-κB activation is a key downstream signal of S1P-dependent stimulation of CAMP expression [8], we next assessed whether MHP treatment induces nuclear translocation of NF-κB. Immunohistochemical studies showed that nuclear translocation of NF-κB (p65) occurred in KC after treatment with MHP, while conversely blockade of the conversion of sphingosine to S1P by pretreatment with a specific inhibitor of SPHK1, SKI, significantly reduced MHP-mediated nuclear translocation of NF-κB (Fig. 3A). Moreover, inhibition of NF-κB using a specific inhibitor treatment, BAY11-7082, diminished the MHP-induced upregulation of CAMP protein expression, while inhibitor treatment alone did not alter CAMP protein levels (Fig. 3B and C). Together, these results indicate that S1P-dependent NF-κB activation accounts for the MHP-induced stimulation of CAMP production.
Fig. 3. NF-κB activation is required for MHP-induced upregulation of CAMP production.
KC were pretreated with or without either SPHK1 inhibitor, SKI (1 μM, 30 min) or NF-κB inhibitor, BAY11-7082 (1 μM, 30 min), were incubated with MHP for 30 min or 24 h. MHP-induced nuclear translocation of NF-κB was determined by immunofluorescence (A). CAMP protein expression was determined by western immunoblot analysis (B). The ratio of CAMP band to that of β-actin in the vehicle-treated cells was set to 100%, to which the corresponding ratios in the other samples were normalized (C). Similar results were obtained when the experiment was repeated (in triplicate) using different cell preparations.
Topical MHP inhibits S. aureus invasion into human epidermal equivalent
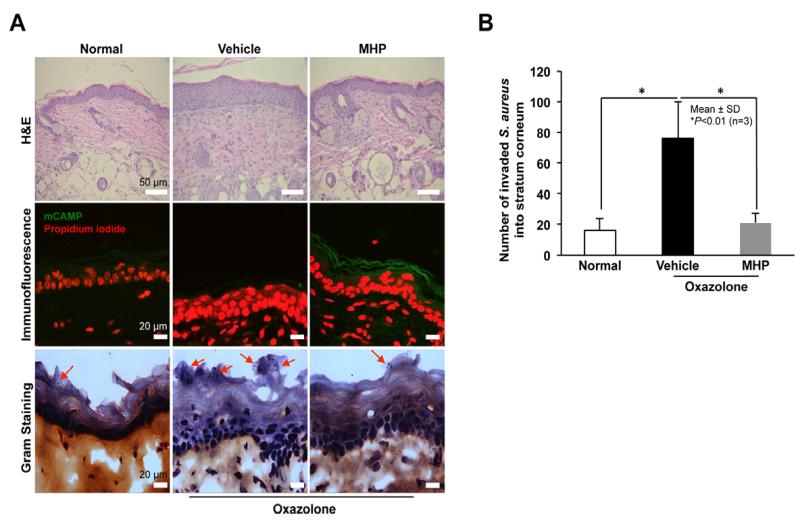
Finally, we assessed whether MHP stimulates antimicrobial defense against microbial pathogen colonization and invasion. Since S. aureus infection often complicates and aggravates atopic dermatitis, we utilized atopic dermatitis model mouse skin established by repeated topical challenges with oxazolone [21]. Again, topical MHP significantly increased murine CAMP (mCAMP) expression (Fig. 4A). Gram staining revealed that S. aureus readily invaded vehicle-treated atopic skin, but not normal mice, while in contrast invasion significantly declined in skin pretreated with topical MHP (Fig. 4A and B). These results suggest that MHP-induced stimulation of mCAMP production interdicts colonization and invasion of murine atopic dermatitis skin by a highly virulent microbial pathogen.
Fig. 4. Inhibition of S. aureus invasion into murine skin.
S. aureus was applied epicutaneouly to full-thickness pieces of murine skin (n=3) treated with or without MHP, followed by incubation for 6 h at 37°C. Hematoxylin and eosin (H&E) staining (A, top panel). MHP-induced mCAMP expression was determined by immunofluorescence (A, middle panel). Bacterial invasion in stratum corneum was assessed by Gram staining (A, bottom panel). The number of invasive bacteria was counted in stratum corneum (B). Data are means ± SD (n=3). Arrows indicate invaded bacteria.
DISCUSSION
Sphingosine-1-phosphate (S1P) is a signaling sphingolipid that modulates multiple cellular functions, depending upon cell/tissue types [9, 11, 22]. S1P displays beneficial roles; i.e., anti-apoptotic functions as well as stimulation of wound healing, but also deleterious effects; i.e., stimulation of inflammation, depending upon cell or tissue type [23-25]. S1P also modulates an additional beneficial function; i.e., cutaneous innate immunity [8]. Accordingly, we recently demonstrated that low levels of endoplasmic reticulum (ER) stress increase cellular S1P levels, directly stimulating production of a key innate immune element, CAMP [8]. We also demonstrated that stimulation of CAMP production could also be enhanced through provision of the S1P precursor, ceramide, or suppression of S1P lyase activity, a S1P degrading enzyme, using naturally-occurring small molecules [14, 26]. For example, we recently demonstrated that the naturally-occurring stilbenoid, resveratrol, and the isoflavone genistein stimulate S1P-signaled CAMP production [14, 26]. While the former stimulates production of ceramide, the latter inhibits S1P lyase activity, as well as also increasing acidic- and neutral ceramidase activity production, resulting in elevated cellular S1P levels. As shown here, MHP increases S1P levels through SPHK1 activation without changing either ceramide production or S1P hydrolysis by S1P lyase. Therefore, the current and prior studies indicate multiple target points whereby pharmacological agents can modulate cellular S1P levels and S1P-dependent signaling of CAMP production. Specifically, activation of sphingosine kinase (SPHK) 1 provides another target that can modulate CAMP production directly by increasing S1P levels. In order to improve bioavailability, we synthesized a new SPHK1 activator, MHP, a derivative of K6PC-5 [12], which shows excellent solubility in aqueous solutions, and demonstrated here that this new SPHK1 activator increases cellular S1P levels (Table 1).
We further investigated whether the MHP-induced increase in S1P activates the S1P → NF-κB activation → C/EBP activation → CAMP mechanism, which we recently identified [8], and showed that MHP stimulates increased CAMP production through this S1P-dependent, downstream signaling pathway. Specifically, we demonstrate that SPHK1 activation also efficiently enhances cutaneous antimicrobial defense through stimulation of CAMP production in KC.
To assess the clinical relevance of MHP, we then showed that topical MHP enhances antimicrobial defense in murine skin exposed to virulent S. aureus. Since MHP does not increase human β-defensin production, these studies show that CAMP plays an important role in suppression of S. aureus invasion and colonization. Activation of S1P production could also be valuable in several other clinical settings; i.e., not only to correct CAMP deficiency in atopic dermatitis [10], but also to enhance wound healing [27], or to boost innate immunity in chronically ill or immune compromised patients.
Three small molecules, stilbenoid, isoflavone, and MHP, are useful chemicals to enhance innate immunity and antimicrobial defense. Hence, we name MHP as “Defensamide”.
Highlights.
-
▶
MHP directly increases SPHK1 activity
-
▶
MHP significantly increases cellular S1P levels in human keratinocytes
-
▶
MHP-mediated increase in S1P stimulates CAMP production via NF-κB activation
-
▶
SPHK1 responsible for S1P synthesis is a key enzyme of CAMP production.
-
▶
MHP activation of SPHK1 can enhance antimicrobial defense and innate immunity
ACKNOWLEDGMENTS
The authors thank Ms. Joan Wakefield for superb editorial assistance (Northern California Institute for Research and Education).
Funding: The Ministry of Knowledge Economy (MKE) and Korea Institute for Advancement of Technology (KIAT) through the Research and Development for Regional Industry, National Institutes of Health Grant AR062025 (YU). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AMP
antimicrobial peptide
- CAMP
cathelicidin antimicrobial peptide
- ER
endoplasmic reticulum
- KC
keratinocytes
- K6PC-5
N-(1,3-dihydroxyisopropyl)-2-hexyl-3-oxo-decanamide
- MHP
(S)-Methyl 2-(hexanamide)-3-(4-hydroxyphenyl) propanoate
- SPHK
sphingosine kinase
- S1P
sphingosine-1-phosphate
- Staphylococcus aureus
S. aureus
- VDR
vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflict of Interest: SK Jeong, SH Lee, JE Jeon, and BW Kim are current employees of NeoPharm Co., Ltd. Other authors have no conflict of interest to declare.
References
- [1].Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- [2].Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2009;124:R13–8. doi: 10.1016/j.jaci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- [3].Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–25. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mendez-Samperio P. The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–8. doi: 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- [5].Schroder JM. The role of keratinocytes in defense against infection. Curr Opin Infect Dis. 2010;23:106–10. doi: 10.1097/QCO.0b013e328335b004. [DOI] [PubMed] [Google Scholar]
- [6].Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, et al. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008;128:2880–7. doi: 10.1038/jid.2008.169. [DOI] [PubMed] [Google Scholar]
- [7].Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of Cathelicidin Antimicrobial Peptide Expression by an Endoplasmic Reticulum (ER) Stress Signaling, Vitamin D Receptor-independent Pathway. J Biol Chem. 2011;286:34121–30. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013;33:752–62. doi: 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–55. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Park K, Lee S, Lee YM. Sphingolipids and antimicrobial peptides: function and roles in atopic dermatitis. Biomol Ther (Seoul) 2013;21:251–7. doi: 10.4062/biomolther.2013.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Uchida Y. Ceramide signaling in mammalian epidermis. Biochim Biophys Acta. 2014;1841:453–62. doi: 10.1016/j.bbalip.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hong JH, Youm JK, Kwon MJ, Park BD, Lee YM, Lee SI, et al. K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J Invest Dermatol. 2008;128:2166–78. doi: 10.1038/jid.2008.66. [DOI] [PubMed] [Google Scholar]
- [13].Youm JK, Jo H, Hong JH, Shin DM, Kwon MJ, Jeong SK, et al. K6PC-5, a sphingosine kinase activator, induces anti-aging effects in intrinsically aged skin through intracellular Ca2+ signaling. J Dermatol Sci. 2008;51:89–102. doi: 10.1016/j.jdermsci.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [14].Park K, Elias PM, Hupe M, Borkowski AW, Gallo RL, Shin KO, et al. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol. 2013;133:1942–9. doi: 10.1038/jid.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Martin-Ezquerra G, Man MQ, Hupe M, Rodriguez-Martin M, Youm JK, Trullas C, et al. Psychological stress regulates antimicrobial peptide expression by both glucocorticoid and beta-adrenergic mechanisms. Eur J Dermatol. 2011;21(Suppl 2):48–51. doi: 10.1684/ejd.2011.1273. [DOI] [PubMed] [Google Scholar]
- [16].Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–80. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- [17].Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem. 2002;303:167–75. doi: 10.1006/abio.2002.5579. [DOI] [PubMed] [Google Scholar]
- [18].Shin KO, Seo CH, Cho HH, Oh S, Hong SP, Yoo HS, et al. Ginsenoside compound K inhibits angiogenesis via regulation of sphingosine kinase-1 in human umbilical vein endothelial cells. Arch Pharm Res. 2014;37:1183–92. doi: 10.1007/s12272-014-0340-6. [DOI] [PubMed] [Google Scholar]
- [19].Jin YX, Yoo HS, Kihara A, Choi CH, Oh S, Moon DC, et al. Sphingosine kinase assay system with fluorescent detection in high performance liquid chromatography. Arch Pharm Res. 2006;29:1049–54. doi: 10.1007/BF02969290. [DOI] [PubMed] [Google Scholar]
- [20].Berdyshev EV, Goya J, Gorshkova I, Prestwich GD, Byun HS, Bittman R, et al. Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal. Anal Biochem. 2011;408:12–8. doi: 10.1016/j.ab.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hisano Y, Nishi T, Kawahara A. The functional roles of S1P in immunity. J Biochem. 2012;152:305–11. doi: 10.1093/jb/mvs090. [DOI] [PubMed] [Google Scholar]
- [23].Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–73. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pyne S, Lee SC, Long J, Pyne NJ. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell Signal. 2009;21:14–21. doi: 10.1016/j.cellsig.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [26].Park K, Kim YI, Shin KO, Seo HS, Kim JY, Mann T, et al. The dietary ingredient, genistein, stimulates cathelicidin antimicrobial peptide expression through a novel S1xP-dependent mechanism. J Nutr Biochem. 2014;25:734–40. doi: 10.1016/j.jnutbio.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Afshar M, Kotol P, Miller J, Gallo R, Hata T. The effect of pimecrolimus on innate immunity in atopic dermatitis subjects: a double-blind, randomized, vehicle-controlled study. Br J Dermatol. 2013;168:426–8. doi: 10.1111/j.1365-2133.2012.11052.x. [DOI] [PubMed] [Google Scholar]