Abstract
Articular cartilage has obvious and fundamental roles in joint function and body movement. Much is known about its organization, extracellular matrix and phenotypic properties of its cells, but less is known about its developmental biology. Incipient articular cartilage in late embryos and neonates is a thin tissue with scanty matrix and small cells, while adult tissue is thick and zonal and contains large cells and abundant matrix. What remains unclear is not only how incipient articular cartilage forms, but how it then grows and matures into a functional, complex and multifaceted structure. This review focuses on recent and exciting discoveries on the developmental biology and growth of articular cartilage, frames them within the context of classic studies, and points to lingering questions and research goals. Advances in this research area will have significant relevance to basic science, and also considerable translational value to design superior cartilage repair and regeneration strategies.
Keywords: articular cartilage, joint formation, lineage tracing, progenitor cells, extracellular matrix, joint disease
Introduction
Articular cartilage is a unique and multifaceted tissue, well adapted to bearing compressive loads and significant shear forces throughout a synovial joint’s range of motion. In adult joints, the tissue displays a complex multi-zonal organization consisting of surface, medial and deep zones, an intricate and abundant extracellular matrix made of numerous molecules and macromolecules, and a subchondral junction important for its physical stability and link to underlying bone [1,2]. The main matrix components are collagen II organized in fibrils providing tensile strength, and aggrecan organized in multimeric superstructures providing elasticity. Together, these macromolecules establish the basic and fundamental biomechanical property of articular cartilage: resilience. In addition, it has long been known that articular cartilage is susceptible to malfunction following acute injury or chronic diseases such as osteoarthritis (OA), mainly because the tissue has very poor intrinsic repair and regenerative capacity [3]. This is particularly perplexing given that the tissue, even in adults, has been found to contain a considerable number of progenitors [4–6] that, however, do not seem to be able to protect or regenerate the tissue when needed. Thus, much effort has been devoted to finding ways by which articular cartilage repair could be induced and enhanced. Surgical drilling techniques and other bioengineered treatment options developed over recent decades have led to several clinical treatment modalities for acutely injured or osteoarthritic joints [7]. While these treatments may improve joint function and reduce pain, they have been found to be only partially effective on the long run -- mainly because they fail to elicit regeneration of native articular cartilage with its distinct nature, architecture and multifaceted function [8–10].
One way to improve therapeutic efficacy would thus be to create novel strategies based on the developmental biology of articular cartilage, incorporating and exploiting the embryonic mechanisms that produce the tissue to begin with. This is a quite reasonable and very attractive goal, but remains elusive for now mainly because of current poor understanding of the developmental biology of articular cartilage. Thus, it is unclear how articular cartilage formation initiates in the embryo and how it is brought to completion and maturity postnatally [11]. Just prior to birth and at early neonatal stages, incipient articular cartilage is a compact, highly cellular and matrix-poor tissue with an isotropic distribution of cells [12]. With further postnatal time, articular cartilage grows significantly in thickness, the chondrocytes enlarge in size, the matrix accumulates and becomes abundant, and the tissue eventually acquires its zonal anisotropic organization. The surface zone is made of flat cells oriented along the main axis of movement and producing lubricating molecules, and the medial and deep zone are made of large round chondrocytes surrounded by typical cartilage matrix and arranged in vertical rows. These structural and organizational changes are thought to be required for articular cartilage to exert its mature, functional, biomechanical and long lasting roles in joint motion and lubrication through life [13–15]. How do all these intricate, sequential and highly orchestrated processes and steps come about and how are they regulated? What triggers the early postnatal explosive growth in incipient articular cartilage, how are the zones created and their distinct architecture formed? How does articular cartilage acquire its permanent status and last through life? These and many other questions do not have definitive answers at the moment, but progress has been made toward addressing at least some of them. This review then focuses on such recent advances and provides a roadmap for future goals and questions of interest in this critical biomedical research area. It is certain that further consideration and understanding of mechanisms of articular cartilage development and growth will provide valuable insight into successful repair and regeneration strategies.
Origin of articular cartilage
Classic studies showed decades ago that in the early embryonic limbs, the skeletal template is made of a continuous and uninterrupted cartilaginous anlaga lacking joints [16,17]. The first overt sign of onset of joint formation was found to be the appearance of compacted and flat mesenchymal cells at each prospective joint site. Because the cells interrupted the cartilaginous anlagen at each joint site, they were named the “interzone,” and classic embryological studies found that their microsurgical removal from developing chick embryo prospective elbow sites resulted in failure of joint formation over time [16]. Since cells within the interzone emerge at sites previously occupied by chondrocytes, it was proposed that they are direct descendants of de-differentiated chondrocytes [18,19]. More recently, Hyde and collaborators performed genetic cell lineage tracing studies using Col2a1Cre;R26R reporter mice and found that joint site-associated cells within the anlagen did cease expression of the cartilage marker Col2a1 as they gave rise to the mesenchymal interzone, which in turn gave rise to articular cartilage [20]. Such chondrocyte-to-interzone cell relationship and lineage continuity have been substantiated by other genetic cell lineage tracing studies on Sox9 and Dcx cell progenies [21,22].
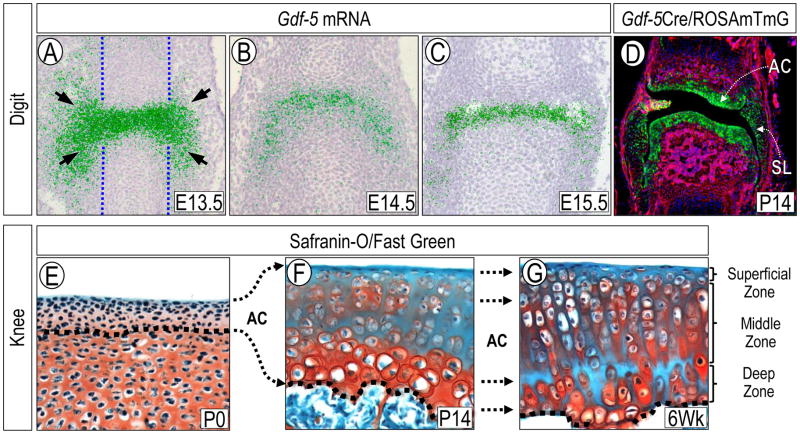
One of the earliest gene markers of interzone cells is growth and differentiation factor-5 (Gdf-5), whose expression becomes strong at each presumptive synovial joint limb site [23]. Interestingly, its transcripts are present not only on the histologically recognizable interzone (Fig. 1A) within the confines of the cartilaginous anlaga (Fig. 1A, blue dashed lines), but also in cells immediately surrounding the joint site (Fig 1A, arrows). To determine if the Gdf5-expressing cells participate in development of joint tissues, we and our collaborators performed genetic cell lineage tracing experiments using compound Gdf5-Cre;R26R (LacZ) reporter mice [24,25]. These studies showed that joint progenitor cells with a Gdf-5 lineage, including those within and surrounding the histological interzone, gave rise to multiple joint tissues over time, including the articular cartilage, synovial lining and intrajoint ligaments (Fig. 1D) that persisted in adults. Because Gdf-5 expression characterizes the histological interzone and surrounding cells, and because Gdf5-lineage cells give rise to diverse tissues, there have been long-standing questions as to whether the cells share a common ancestry and whether they are developmentally homogenous or contain sub-populations. To address the first issue, we carried out Gdf5-Cre;R26R cell lineage tracing experiments in Indian hedgehog (Ihh)-null mice. Ihh is produced by prehypertrophic chondrocytes in growth plate and interestingly, its ablation not only causes limb skeletal growth retardation, but also prevents formation of joints [26]. Because the mutants have no joints, they should have no Gdf5-lineage cells at prospective joint sites. Surprisingly, limb cartilaginous anlagen in mutant Gdf5-Cre;R26R;Ihh−/ − embryos did display Gdf-5-lineage cells [27]. The cells were located outside of, and closely flanked, each prospective joint site and expressed other typical interzone markers including Erg [28]. Therefore, it appears that Gdf-5-lineage cells at the joint site comprise de-differentiated chondrocytes from within the cartilaginous anlaga plus a seemingly independent population of progenitors flanking the joint site and recruited into the Gdf-5-lineage. At this regard, Spagnoli and collaborators recently used TGFβ type II receptor (Tgfbr2) expression and lineage tracing strategies to characterize origin and fate of interzone and interzone-associated cells [29]. By monitoring Tgfbr2-expressing cells in developing digit joints over time, they found that Tgfbr2-βGal-positive cells were initially limited to dorsal and ventral regions of E13.5 joints and were not detected within the central/histological interzone. With time, these cells were maintained within those localized niches, and later gave rise to the synovial lining, meniscal surface, outer ligaments and groove of Ranvier. These data lead to the tantalizing possibility that these progenitors represent a subset of joint site-associated cells with specific developmental roles and fate, and could be related to the joint site-flanking progenitors we observed in the Ihh-null limbs. These ideas are in line with other studies indicating that cells from outside of the original Col2a1 positive anlagen may give rise to joint tissues such as the meniscus [20].
Figure 1.
Prenatal and postnatal analyses of developing and growing mouse limb joints. (A–C) In situ hybridization showing that at E13.5 Gdf-5 is expressed in interzone cells as well as cells immediately surrounding the joint site and outside the cartilaginous anlagen (dashed vertical lines) (A), and becomes more restricted to the developing joint by E14.5 and E15.5 (B–C). Hybridization signal is pseudo-colored in green. (D) Genetic cell lineage tracing with Gdf5Cre/ROSAmTmG mice reveals that Gdf-5-lineage cells (green) give rise to multiple joint tissues, including the articular cartilage (AC), synovial lining (SL) and capsule tissue. It should be noted that majority of cells within these tissues are reporter-positive. (E–G) Images of proximal tibia articular cartilage sections from neonatal to juvenile mice stained with Safranin O-Fast green. (E) At P0, the incipient articular cartilage (AC) is thin, matrix-poor and made of small flat cells. At this stage, the tissue is molecularly defined by expression of genes including Prg4 and tenascin-C and lack of expression of matrillin-1 and Ucma and is made entirely of Gdf5-lineage cells (see text). (F) By P14, articular cartilage is much thicker and contains abundant matrix and large cells many of which are scattered isotropically through the tissue. (G) By 6 weeks of age, the tissue exhibits a mature organization with distinct zones and a columnar organization of the chondrocytes.
In the knee, the mesenchymal interzone initially appears homogenous and compacted, but soon after, the joint site becomes occupied by, and is histologically distinguishable into, a dense “intermediate” compartment and two flanking “outer” compartments with more loosely arranged cells [20]. Jenner and collaborators recently asked whether cells in those compartments have distinct phenotypes and fates, and performed transcriptional profiling of the cells in mouse embryo knee joints just prior to cavitation [30]. They found that genes associated with joint formation were more evident in cells from the intermediate compartment, while genes associated with cartilage maturation and hypertrophy were over-represented in outer compartment cells. They concluded that intermediate compartment cells gave to articular cartilage –thus likely representing initial interzone cells-, while cells in outer compartments were destined for endochondral ossification and thus part of the secondary ossification center formation. Recently, Ray and collaborators analyzed the phenotype of interzone cells and those flanking them proximally and distally in mouse and chick embryo limbs [31]. They found that the interzone cells were mitotically quiescent whereas the flanking cells were mitotically active, leading them to suggest that the interzone cells are not sufficient to sustain embryonic articular cartilage formation and that the underlying proliferative cells would be recruited into the process. Because no long-term genetic tracing approaches were used, the conclusions need further testing. Taken together, these and other studies [32,33] have provided new insights into the origin and fate of joint progenitor cells, pointing to the possibility that joint formation involves subsets of progenitor cells with distinct origin, developmental fate and roles.
Classic studies showed long ago that joint formation is hampered when the limbs are paralyzed pharmacologically or microsurgically and may even be prevented altogether [34,35]. It has remained an open question of how muscle activity and movement in embryogenesis influence joint formation. Studies on the joint lubricating molecule hyaluronan (HA) have indicated that expression of its synthesizing enzymes and cell surface receptors negatively influenced by limb paralysis [36,37], indicating that a key role of movement in joint formation would be to sustain cavitation and creation of synovial fluid. This issue has been further examined in recent studies in which muscle development and function were blocked genetically [38]. The authors found that ablation of such muscle master genes as Myf5 and MyoD in mice not only prevent limb muscle development, but also disrupted joint formation. Closer analysis indicated that the joint defects arose from substandard activation of β-catenin that is required for interzone and joint formation [39], suggested that muscle-dependent movement would act at a stage earlier than cavitation. Interestingly, several limb joints were affected in the muscle-less mutants but some were not, indicating that not all joints obey and depend on the same set of systemic influences and mechanisms.
Postnatal articular cartilage growth and expansion
Postnatally, the skeleton undergoes tremendous growth, modeling and remodeling. For example, the long bones increase in length and their epiphyses expand and enlarge markedly to accommodate the joints and provide adequate biomechanical function for the growing body [11]. Likewise, incipient articular cartilage at birth is highly cellular and as pointed out above, is made of scanty matrix and small cells, but becomes thick and matrix rich over postnatal time and expands laterally to cover the expanding and growing epiphyses (Fig. 1E–G). How does articular cartilage undergo such growth and modeling changes? Mankin was among the first to suggest that a region of proliferating cells “subjacent to the gliding surface of the joint” was responsible for interstitial growth of articular cartilage and increasing thickness of the articular surface [40]. At later stages of postnatal growth, Mankin and collaborators found that proliferation had ceased within the sub-superficial zone, but persisted within a deeper region adjacent to the calcified cartilage. Studies evaluating tritiated thymidine incorporation into articular cartilage of immature rabbits confirmed the existence of these two proliferative cell regions [40,41]. Archer and collaborators did not identify a proliferative region within the deeper zones, but confirmed the presence of proliferative cells in the superficial zone, and suggested that this region was primarily responsible for growth and thickening of postnatal articular cartilage by an appositional process [42,43]. Later, Hunziker and collaborators used bromodeoxyuridine (BrdU) labeling and identified a population of slow-cycle cells in the superficial zone; using concurrent administration of 3H-thymidine, they identified rapidly proliferating chondrocytes in the deeper zones [11]. These authors hypothesized that proliferation of cells within the superficial zone led to lateral expansion of articular surface, also giving rise to daughter cells in a more rapidly proliferating cell population in the deeper zones presumably responsible for vertical tissue growth.
A recent genetic cell lineage study has tackled the issue of postnatal articular cartilage growth by focusing on the proteoglycan 4 gene [44]. Prg4 encodes diverse products including lubricin/superficial zone protein (SZP) that is secreted by articular cartilage surface zone cells and synovial cells and is thought to be an essential joint lubricant [45]. Prg4 expression conspicuously starts in the developing joints in late embryogenesis and continues postnatally [24]. The authors created a new inducible Prg4CreER knock-in mouse line and evaluated phenotype and fate of Prg4-LacZ lineage cells over time [44]. They found that tamoxifen injection in Prg4CreER/LacZ mice at E17.5 resulted in reporter expression in a single layer of cells present at the very surface of incipient articular cartilage at P0. By one month of age, the labeled cells and/or their progeny were found throughout the thickness of articular cartilage, leading the authors to conclude that the early Prg4-expressing superficial cell population served as progenitors of the entire articular cartilage and did so by appositional growth. Though potentially interesting, these data are somewhat unexpected. Previous studies had shown that endogenous Prg4 mRNA expression characterizes the entire incipient articular cartilage at late embryonic and neonatal stages [24,46,47], and so it is surprising that only cells in a single surface layer were labeled by P0. Thus, it is possible that the observed patterns of reporter activation and cell progeny behavior may have been influenced by: insufficient recombination; the Prg4 heterozygous-null status of the knock-in transgenic mice; or other reasons [48]. If the data are verified however, they would lead to the intriguing idea that postnatal articular cartilage is made from scratch by Prg4-lineage cells, leaving open the question of what would have been the fate of embryonic articular cells with a Sox9, Gdf5 and Dcx lineage [21,22,24]. An additional issue not considered in all the studies above is that postnatal articular cartilage growth and thickening do not necessarily need to rely only on cell proliferation and/or apposition, but could include major contributions by the substantial increases in cell size of resident cells and substantial accumulation of matrix. In this regard, it is worth remembering that the main factor in skeletal elongation by the growth plate is not chondrocyte proliferation, but chondrocyte hypertrophy and matrix accumulation [49–52].
Articular cartilage zone organization
As postnatal growth proceeds, articular cartilage will eventually acquire a mature zonal organization thought to be very important for its long lasting and biomechanical function [53] (Fig. 1G). In such mature articular cartilage, the surface zone consists of elongated small cells within a dense extracellular matrix that are oriented along the main axis of motion, produce lubricants and provide the tissue with the important ability of resisting shear stress. In the middle or transitional zone, the chondrocytes are very large and round, and most are organized into column-like stacks perpendicular to the tissue synovial surface. Chondrocytes within this middle zone produce, deposit and maintain all the typical cartilage matrix molecules, including collagen II and aggrecan, and thus confer the tissue with key biomechanical resilience. The chondrocytes in the bottom zone tend to be even larger in size, are also active in matrix production, and are likely to be responsible for interactions and interplays with the underlying subchondral bone. The mechanisms by which articular cartilage is able to elicit its transformation into a mature and organizationally complex tissue over postnatal life remain largely unclear.
To gain insights into these questions, gene array studies were conducted in several species and at different stages. Mienaltowski et al. studied the gene expression patterns in equine articular cartilage and found that neonatal tissue displayed a character consistent with tissue growth and expansion, while mature cartilage’s profile indicated a functional transition to withstanding shear and weight-bearing stresses [15]. Using laser capture microdissection on sections of 1 week-old mouse proximal tibia epiphysis, Lui and collaborators isolated the most superficial 2–3 tissue layers abutting the join cavity, the next adjacent 4–5 layers, and several deeper (but not adjacent) layers [54]. The three samples were termed surface, middle and deep zones of articular cartilage though the boundary between genuine articular cartilage and underlying secondary ossification center was not determined. Comparison of superficial and deep zone samples revealed that nearly 2000 genes were differentially expressed in the two regions. At that stage of postnatal development, the middle zone was found to represent a transitional area between the superficial and deep regions rather than a functionally distinct zone. Interestingly but surprisingly, comparison with spatial gene expression patterns in growth plate cartilage suggested that the superficial zone was similar to the hypertrophic zone of growth plate, while the deep zone was similar to the resting zone of growth plate. In a related study, Amanatullah and collaborators found that gene expression patterns in top, middle and deep zones of adult bovine articular cartilage are functionally distinct, with a dramatic difference in expression of extracellular matrix genes between the superficial and underlying zones, while the middle and deep zones were more similar [55]. These studies have reaffirmed the idea that articular cartilage becomes a phenotypically complex tissue at maturity, including the possibility raised in the latter study that there is a greater rate of matrix turnover within the middle zone and a more stable matrix in the superficial zone. It appears also that changes in gene expression throughout articular cartilage depth become appreciable prior to the appearance of histologically distinct zones.
Articular cartilage permanent status
A defining trait of articular cartilage is that it is a permanent structure and persists and remains functional through life at least under normal healthy circumstances. Thus, it differs from transient cartilage that constitutes the embryonic skeleton and the growth plates in which the chondrocytes undergo proliferation, maturation and hypertrophy, and the tissue is eventually replaced by endochondral bone. It has long remained unclear in what manner permanent and transient chondrocytes differ and how they undertake these divergent developmental and functional paths during embryogenesis and postnatal life [56]. This question is of broader interest and significance because articular chondrocytes can actually acquire growth plate-like traits during OA and most notably a hypertrophic phenotype and expression of associated catabolic mechanisms, thus contributing to articular cartilage demise [57,58]. To tackle this important issue, several studies have focused on what may help articular chondrocytes to resist OA-associated changes and maintain their phenotype and function. For example, ablation of Adamts5 or a reduction in hedgehog signaling were found to render mouse joints more resistant to surgically-induced OA involving joint destabilization, indicating that these mechanisms are pathogenic and could be therapeutic targets [59,60]. In a related fashion, endogenous parathyroid hormone-related protein (PTHrP) expression, systemic treatment with parathyroid hormone or virally-driven over-expression of Prg4 were found to elicit protection against experimental OA [61–63], indicating that these could all be therapeutics. Another step ahead in this field has been made by our own studies on the transcription factor Erg, a member of the large Ets family [64]. As pointed above, Erg is expressed in incipient embryonic limb joints along with Gdf5 and Wnt9a, and expression decreases over time and persists in superficial cells [24,46]. To determine Erg function, we created transgenic mouse embryos overexpressing it throughout the developing skeleton under control of cartilage collagen II regulatory sequences. We found that the entire skeleton remained cartilaginous, the chondrocytes failed to undergo hypertrophy, and the cartilaginous skeleton did not undergo endochondral ossification and replacement by bone, indicating that Erg over-expression had imposed a permanent-like quality onto the entire chondrocyte population [46]. We followed up these findings in a just published study in which we conditionally ablated Erg in developing mouse joints using Gdf5-Cre mice [65]. Limb joints did form in the mutant embryos and appeared normal in juvenile mice as well, likely due to redundancy by Fli-1, a closely-related Ets member also expressed in developing joints. However, juvenile mutant mice were more sensitive to surgically-induced OA than control littermates and also displayed spontaneous onset of OA-like changes starting around 6 to 7 months of age. It appears then that while dispensable from joint formation, Erg is needed for articular cartilage endurance and resistance to experimental and age-related OA.
Conclusions
This brief synopsis makes it clear that much has been learned about articular cartilage development and growth, but much remains to be uncovered as well. The genetic lineage tracing studies have certainly advanced our understanding of interzone cell origin and fate. Taken in aggregate they point to the notion that incipient joint progenitors originate from de-differentiated chondrocytes as well as surrounding cells external to the cartilaginous anlaga and recruited into the Gdf5-lineage. It is possible but not firmly established yet that the fate and function of these various cells may be distinct, with the externally-recruited cells contributing to synovial lining, capsule and groove of Ranvier and with the centrally-located progenitors contributing to articular cartilage and intrajoint ligaments. Confirmation or confutation of such conclusions will need to wait until more specific inducible genetic tools are available to trace and track progenitors at distinct spatio-temporal locations. It is also clear, and has been for a long time, that incipient articular cartilage in neonates is not yet equipped with typical phenotypic and organizational traits of mature functional cartilage, but acquires them between birth and early adulthood, including a characteristic thickness, abundant and resilient matrix, surface lubricants, zonal organization and chondrocyte columnar arrangement. Much work will be needed to understand how this remarkable series of changes is initiated, orchestrated, accomplished and maintained long term. There is a role for cell proliferation in articular cartilage growth, and there is a clear and unequivocal role for the surface zone with its critical production of lubricants. There is also compelling transcriptome evidence that cells in different zones are endowed with significantly distinct phenotypic traits. However, other possible major contributors to cartilage growth and thickening -including increases in cell diameter and matrix amounts- will need to be taken in full consideration and examined. Likewise, the process of chondrocyte columnar organization may depend on appositional growth as several groups have proposed [11,43,44], but this would require considerable cell turnover in postnatal articular cartilage which, however, remains to be demonstrated. The initial discovery of progenitors in adult articular cartilage including slow-cycle progenitors in the surface zone [5] led to much initial interest and hope since the cells could be potential targets of therapeutics to induce or enhance the notoriously poor repair and regenerative capacity of articular cartilage. However, it still remains unclear the extent to which these cells can be mobilized and activated to elicit more effective repair and/or regeneration of tissues with native traits. Hence, there continues to be much interest in finding bioengineering strategies by which progenitors isolated from other sources including marrow and fat could be coaxed into repairing articular cartilage or other joint tissues after transplantation [66,67]. As pointed out in recent reviews, these approaches have achieved some measure of success, but are far from ideal and effective [9,68]. Also, these strategies have not yet relied on genetic reprogramming of progenitors prior to transplantation as it is being done in other areas of translational medicine and by which the progenitors are given tissue specific repair instructions [69,70]. Whether combinations of Erg, Gdf5, Wnt9a, Dcx or other interzone-expressed genes may be able to reprogram generic progenitors in joint-forming cells remains untested. Given the persisting challenges in the cartilage repair field, there continues to be much interest also in finding pharmacologic ways by which the endogenous protective capacity of articular cartilage -and joint cells in general- could be enhanced, making the tissues more resistant to daily mechanical use and abuse or to acute and chronic joint disease. In this vein, recent studies showing that Kartogenin, virally-encoded lubricin or parathyroid hormone given intrajoint can reduce articular cartilage defects in mouse surgery models of OA, bring much hope to the field [71]. Our own study [72] showing that Kartogenin stimulates chondrogenic cell differentiation and expression of joint beneficial genes including Prg4[63] and PthrP [62–63] make this drug particularly attractive as an encompassing and multi-acting agent for maintaining or boosting articular cartilage endurance as well as repair capacity.
Acknowledgments
Work we originally carried out and summarized here was supported by NIH grants AR062908 and AR046000. R.S.D. is the recipient of a postdoctoral training grant (1F32AR064071) from the NIH. We express our gratitude to our several colleagues who contributed to the original studies described here, and apologize for not citing and describing the work of other relevant groups given the succinct format of this mini-review.
Footnotes
Conflict of Interest Statement
The authors of this paper declare they have no conflicts of interest
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
All studies by Drs. Decker, Koyama, Pacifici involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Contributor Information
Rebekah S. Decker, Email: DeckerR@chop.edu, Translational Research Program in Pediatric Orthopaedics, Division of Orthopaedic Surgery, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd, Philadelphia, PA 19104 USA
Eiki Koyama, Translational Research Program in Pediatric Orthopaedics, Division of Orthopaedic Surgery, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd Philadelphia, PA 19104, USA.
Maurizio Pacifici, Translational Research Program in Pediatric Orthopaedics, Division of Orthopaedic Surgery, The Children’s Hospital of Philadelphia, 3401 Civic Center Blvd, Philadelphia, PA 19104, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lyons TJ, McClure SF, Stoddart RW, McClure J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet Disord. 2006;7(1):52. doi: 10.1186/1471-2474-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunziker E, Quinn T, Häuselmann H-J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr Cartil. 2002;10(7):564–572. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- 3.Mankin HJ. The reaction of articular cartilage to injury and osteoarthritis (first of two parts) N Engl J Med. 1974 Dec 12;291(24):1285–1292. doi: 10.1056/NEJM197412122912406. [DOI] [PubMed] [Google Scholar]
- 4.Williams R, Khan IM, Richardson K, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5(10):e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 6.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis & Rheum. 2004;50(5):1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 7.Tetteh ES, Bajaj S, Ghodadra NS, Cole BJ. The Basic Science and Surgical Treatment Options for Articular Cartilage Injuries of the Knee. J Orthop Sports Phys Ther. 2012;42(3):243–253. doi: 10.2519/jospt.2012.3673. [DOI] [PubMed] [Google Scholar]
- 8.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone B, Alini M, Cucchiarini M, et al. Tissue engineering for articular cartilage repair–the state of the art. Eur Cell Mater. 2013;25(248):e67. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell K, Wang J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthr Cartil. 2015;23(3):351–362. doi: 10.1016/j.joca.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr Cartil. 2007;15(4):403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Weiss C, Rosenberg L, Helfet AJ. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg. 1968;50(4):663–674. doi: 10.2106/00004623-196850040-00002. [DOI] [PubMed] [Google Scholar]
- 13.Gannon A, Nagel T, Bell A, Avery N, Kelly D. Postnatal changes to the mechanical properties of articular cartilage are driven by the evolution of its collagen network. Eur Cell Mater. 2014;29:105–123. doi: 10.22203/ecm.v029a09. [DOI] [PubMed] [Google Scholar]
- 14.Helminen HJ, Hyttinen MM, Lammi MJ, et al. Regular joint loading in youth assists in the establishment and strengthening of the collagen network of articular cartilage and contributes to the prevention of osteoarthrosis later in life: a hypothesis. J Bone Miner Metab. 2000;18(5):245–257. doi: 10.1007/pl00010638. [DOI] [PubMed] [Google Scholar]
- 15.Mienaltowski MJ, Huang L, Stromberg AJ, MacLeod JN. Differential gene expression associated with postnatal equine articular cartilage maturation. BMC Musculoskelet Disord. 2008;9:149. doi: 10.1186/1471-2474-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holder N. An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol. 1977 Jun;39:115–127. [PubMed] [Google Scholar]
- 17.Mitrovic D. Development of the diarthrodial joints in the rat embryo. Am J Anat. 1978 Apr;151(4):475–485. doi: 10.1002/aja.1001510403. [DOI] [PubMed] [Google Scholar]
- 18.Craig FM, Bentley G, Archer CW. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987 Mar 1;99(3):383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- 19.Nalin AM, Greenlee TK, Jr, Sandell LJ. Collagen gene expression during development of avian synovial joints: transient expression of types II and XI collagen genes in the joint capsule. Dev Dyn. 1995 Jul;203(3):352–362. doi: 10.1002/aja.1002030307. [DOI] [PubMed] [Google Scholar]
- 20.Hyde G, Boot-Handford RP, Wallis GA. Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J Anat. 2008;213(5):531–538. doi: 10.1111/j.1469-7580.2008.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soeda T, Deng JM, de Crombrugghe B, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis. 2010;48(11):635–644. doi: 10.1002/dvg.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Cigan AD, Marrero L, et al. Expression of doublecortin reveals articular chondrocyte lineage in mouse embryonic limbs. Genesis. 2011;49(2):75–82. doi: 10.1002/dvg.20702. [DOI] [PubMed] [Google Scholar]
- 23.Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996 Dec;122(12):3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- 24.Koyama E, Shibukawa Y, Nagayama M, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316(1):62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rountree RB, Schoor M, Chen H, et al. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004 Nov;2(11):e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes & Dev. 1999;13(16):2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama E, Ochiai T, Rountree RB, et al. Synovial joint formation during mouse limb skeletogenesis: roles of Indian hedgehog signaling. Ann N Y Acad Sci. 2007 Nov;1116:100–112. doi: 10.1196/annals.1402.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decker RS, Koyama E, Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014 Oct;39:5–10. doi: 10.1016/j.matbio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Li T, Longobardi L, Myers TJ, et al. Joint TGF-β Type II Receptor-Expressing Cells: Ontogeny and Characterization as Joint Progenitors. Stem Cells Dev. 2012;22(9):1342–1359. doi: 10.1089/scd.2012.0207. Specialized niches of joint progenitor cells give rise to unique tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Jenner F, IJpma A, Cleary M, et al. Differential Gene Expression of the Intermediate and Outer Interzone layers of developing articular cartilage in murine embryos. 2014 Aug 15;23(16):1883–98. doi: 10.1089/scd.2013.0235. Spatially defined cells within the murine knee interzone have unique roles in joint development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray A, Singh PNP, Sohaskey ML, Harland RM, Bandyopadhyay A. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development. 2015;142(6):1169–1179. doi: 10.1242/dev.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dy P, Wang W, Bhattaram P, et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012 Mar 13;22(3):597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Kan A, Tabin CJ. c-Jun is required for the specification of joint cell fates. Genes & Dev. 2013;27(5):514–524. doi: 10.1101/gad.209239.112. Transcription facter c-Jun works upstream of Wnt9a to determine joint progenitor cell fate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fell HB, Canti R. Experiments on the development in vitro of the avian knee-joint. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1934;116(799):316–351. [Google Scholar]
- 35.Persson M. The role of movements in the development of sutural and diarthrodial joints tested by long-term paralysis of chick embryos. J of Anat. 1983;137(Pt 3):591. [PMC free article] [PubMed] [Google Scholar]
- 36.Dowthwaite GP, Edwards JC, Pitsillides AA. An essential role for the interaction between hyaluronan and hyaluronan binding proteins during joint development. J Histochem Cytochem. 1998 May;46(5):641–651. doi: 10.1177/002215549804600509. [DOI] [PubMed] [Google Scholar]
- 37.Osborne A, Lamb K, Lewthwaite J, Dowthwaite G, Pitsillides A. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J Musculoskelet Neuronal Interact. 2002;2(5):448–456. [PubMed] [Google Scholar]
- 38.Kahn J, Shwartz Y, Blitz E, et al. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell. 2009;16(5):734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/β-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280(19):19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 40.Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. III. Mature Articular Cartilage. J Bone Joint Surg. 1963;45(3):529–540. [Google Scholar]
- 41.Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. II. Repair in Immature Cartilage. J Bone Joint Surg. 1962;44(4):688–698. [Google Scholar]
- 42.Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat. 1994;184(Pt 3):447. [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001 Jun;203(6):469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 44.Kozhemyakina E, Zhang M, Ionescu A, et al. Identification of a prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015 May;67(5):1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90(3–4):291–297. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 46.Iwamoto M, Tamamura Y, Koyama E, et al. Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol. 2007;305(1):40–51. doi: 10.1016/j.ydbio.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(115) 3:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefebvre V, Bhattaram P. Editorial: prg4-expressing cells: articular stem cells or differentiated progeny in the articular chondrocyte lineage? Arthritis Rheumatol. 2015 May;67(5):1151–1154. doi: 10.1002/art.39045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilsman NJ, Leiferman EM, Fry M, Farnum CE, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14(6):927–936. doi: 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- 50.Breur G, VanEnkevort B, Farnum C, Wilsman N. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. Journal of Orthopaedic Research. 1991;9(3):348–359. doi: 10.1002/jor.1100090306. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn JL, Delacey JH, Leenellett EE. Relationship between bone growth rate and hypertrophic chondrocyte volume in New Zealand white rabbits of varying ages. J Orthop Res. 1996;14(5):706–711. doi: 10.1002/jor.1100140505. [DOI] [PubMed] [Google Scholar]
- 52.Cooper KL, Oh S, Sung Y, Dasari RR, Kirschner MW, Tabin CJ. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013;495(7441):375–378. doi: 10.1038/nature11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Z Zellforsch Mikrosk Anat. 1925;2(5):783–862. [Google Scholar]
- 54.Lui JC, Chau M, Chen W, et al. Spatial regulation of gene expression during growth of articular cartilage in juvenile mice. Pediatr Res. 2015;77(3):406–415. doi: 10.1038/pr.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amanatullah DF, Yamane S, Reddi AH. Distinct patterns of gene expression in the superficial, middle and deep zones of bovine articular cartilage. J Tissue Eng Regen Med. 2014 Jul;8(7):505–514. doi: 10.1002/term.1543. [DOI] [PubMed] [Google Scholar]
- 56.Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;75(3):237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- 57.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rhematol. 2005;32(5):876–886. [PubMed] [Google Scholar]
- 58.Loeser RF, Olex AL, McNulty MA, et al. Disease progression and phasic changes in gene expression in a mouse model of osteoarthritis. PloS one. 2013;8(1):e54633. doi: 10.1371/journal.pone.0054633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glasson SS, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 60.Lin AC, Seeto BL, Bartoszko JM, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15(12):1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 61.Macica C, Liang G, Nasiri A, Broadus AE. Genetic evidence of the regulatory role of parathyroid hormone–related protein in articular chondrocyte maintenance in an experimental mouse model. Arthritis Rheum. 2011;63(11):3333–3343. doi: 10.1002/art.30515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sampson ER, Hilton MJ, Tian Y, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Science Trans Med. 2011;3(101):101ra193–101ra193. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Ruan MZ, Erez A, Guse K, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Trans Med. 2013;5(176):176ra134–176ra134. doi: 10.1126/scitranslmed.3005409. Prg4 gene therapy is potentially a novel tool for prevention of post-traumatic osteoarthrits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2(11):827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 65••.Ohta Y, Okabe T, Larmour C, et al. Articular cartilage endurance and resistance to osteoarthritic changes require transcription factor Erg. Arthritis Rheum. 2015 doi: 10.1002/art.39243. Erg is required for maintenance of permanent articular cartilage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290(2):763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 67.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil. 2002;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 68.Musumeci G, Castrogiovanni P, Leonardi R, et al. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J Orthop. 2014;5(2):80. doi: 10.5312/wjo.v5.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirschi KK, Li S, Roy K. Induced pluripotent stem cells for regenerative medicine. Ann Rev Biomed Eng. 2014;16:277. doi: 10.1146/annurev-bioeng-071813-105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet. 2014;15(2):82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71••.Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science. 2012 May 11;336(6082):717–721. doi: 10.1126/science.1215157. The novel small-molecule Kartogenin has chondroprotective effects in mouse OA models. [DOI] [PubMed] [Google Scholar]
- 72.Decker RS, Koyama E, Enomoto-Iwamoto M, et al. Mouse limb skeletal growth and synovial joint development are coordinately enhanced by Kartogenin. Dev Biol. 2014 Nov 15;395(2):255–267. doi: 10.1016/j.ydbio.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]