Abstract
Purpose
To determine whether progressive retinal nerve fiber layer (RNFL) loss occurs in the contralateral eye of patients with glaucoma showing unilateral progression according to conventional diagnostic methods.
Design
Prospective longitudinal observational cohort study.
Participants
346 eyes of 173 patients (118 eyes with glaucoma and 228 eyes with suspect glaucoma at baseline) recruited from the Diagnostic Innovations Glaucoma Study (DIGS) and followed for an average of 3.5 ± 0.7 years.
Methods
All subjects had standard automated perimetry (SAP, HFA) and spectral domain optical coherence tomography (SDOCT, Spectralis) in both eyes at 6-month intervals, with a minimum of 5 SAP and 5 SDOCT examinations in each eye. Eyes were determined as progressing by conventional methods if there was progression on masked grading of optic disc stereophotographs or SAP guided progression analysis (GPA, “likely progression”). Rates of change in SDOCT average RNFL thickness were obtained by linear mixed effects model.
Rate of global loss was calculated using a random coefficient model and compared for non-progressing patients, progressing eyes and fellow eyes of unilateral progressing patients.
Main Outcomes Measures
Rate of change in global RNFL thickness.
Results
39 subjects had evidence of unilateral progression by GPA and/or disc photographs during follow-up. Mean ± SE rate of RNFL loss in eyes progressing by conventional methods was −0.89 ± 0.22 μm/year; P < 0.001. The contralateral eyes of these subjects also showed significant loss of RNFL over time (−1.00 ± 0.20 μm/year; P < 0.001). 134 subjects did not show progression by conventional methods in either eye. These eyes also had significant decline over time in average RNFL thickness (−0.71 ± 0.09 μm/year; P < 0.001); however, the rate of change in these eyes was slower than the contralateral eye of patients showing unilateral progression (P < 0.001).
Conclusion
RNFL thickness loss was seen in a substantial number of the contralateral eyes of glaucoma patients showing unilateral progression by conventional methods. These findings indicate that assessment of RNFL thickness by SDOCT may show progressive glaucomatous damage that is not detected by visual fields or optic disc stereophotography.
Keywords: RNFL, rate of change, glaucoma, progression, Spectralis SDOCT
Introduction
Detection of progression is the cornerstone of management of patients with glaucoma. Assessment of progression relies on establishing accurate baseline measurements and monitoring for change over time 1. In clinical practice, progressive visual field loss has generally been evaluated using standard automated perimetry (SAP). Although there is currently no consensus with regard to the best method for detecting progressive field loss on SAP, tools such as the Guided Progression Analysis (GPA) have been widely used in clinical practice to assist in management and treatment decisions 2–4.
Despite SAP being the most commonly used method to assess progression, the results of several longitudinal investigations have provided evidence that many patients may show progressive structural damage in the abscence of detectable visual field losses on SAP. Clinical trials such as the Ocular Hypertension Treatment Study (OHTS) have used serial assessment of optic disc stereophotographs in order to detect progressive damage from glaucoma 5. However, interpretation of optic disc stereophotographs is subjective, and this method might be relatively insensitive to detect certain patterns of progression, such as diffuse retinal nerve fiber layer (RNFL) loss 6. Therefore, it is conceivable that progression might occur in some patients, despite going largely undetected by conventional tests such as SAP GPA and optic disc stereophotography.
Over the past decade, several longitudinal investigations have validated the use of imaging technologies for detection of progressive structural damage in glaucoma 7–10. RNFL assessment with spectral domain optical coherence tomography (SDOCT) can provide objective and reproducible measurements of RNFL thickness and quantify rates of structural deterioration in glaucoma. Rates of RNFL thinning as measured by OCT have been shown to be predictive of future functional losses in the disease and to be related to measures of quality of life 11–14.
In spite of the fact that glaucoma is typically a bilateral disease, unilateral progression may be commonly seen in clinical practice. However, it is conceivable that assessment of progression with more sensitive methods may actually reveal deterioration to occur in those eyes that do not show progression when measured by conventional methods. Despite the increasing use of OCT in clinical practice, to the best of our knowledge, there have been no reports evaluating the ability of this technology to detect progression in the fellow eyes of patients who show only unilateral progression by conventional assessment with SAP or optic disc photographs. This is an important topic, as arguably undetected progression in the better eye may be of greater significance for a patient’s quality of life than progression in the worse eye 14, 15.
The purpose of the present study was to determine whether progressive RNFL loss occurs in the contralateral eye of patients with glaucoma showing unilateral progression according to conventional diagnostic methods and compare rates of change in these eyes.
Methods
Patients
This was a longitudinal observational cohort study involving 346 eyes of 173 participants from the Diagnostic Innovations in Glaucoma Study (DIGS), a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma. The study was conducted at the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California San Diego (UCSD). Methodological details have been described previously 16. Written informed consent was obtained from all participants, and the Institutional Review Board and Human Subjects Committee at University of California San Diego prospectively approved all protocols and methods, which adhered to the Declaration of Helsinki. The study was also conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act.
At each visit during follow-up, patients underwent a comprehensive ophthalmologic examination including review of medical history, best corrected visual acuity, slit lamp biomicroscopy, IOP measurement with Goldmann applanation tonometry, gonioscopy, dilated fundoscopic examination with 78-diopter lens, stereoscopic optic disc photography (Kowa WX3D; Kowa OptiMed, Torrance, CA, USA), Spectralis SDOCT (software version 5.4.7.0, Heidelberg Engineering Inc., Carlsbad, CA, USA) and standard automated perimetry (SAP, Humphrey Filed Analyser; Carl Zeiss Meditec, Dublin, CA, USA) using Swedish interactive threshold algorithm (SITA standard 24-2). Only patients with open angles on gonioscopy were included. Subjects were excluded if they presented visual acuity less than 20/40, spherical refraction outside ± 5.0 diopters, and/or cylinder correction outside ± 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
The study included patients diagnosed with glaucoma, as well as those suspected of having the disease, both of whom were determined at baseline visit. Patients were diagnosed with glaucoma if either eye had repeatable (>= 3 consecutive) abnormal SAP tests, defined as pattern standard deviation outside 95% normal confidence limits, or Glaucoma Hemifield Test outside normal limits. Glaucoma suspects were diagnosed if they presented history of elevated IOP (> 21 mmHg) and/or suspicious or glaucomatous appearance of the optic nerve, but normal and reliable visual field results.
For the purposes of the analysis, subjects with both eyes eligible from DIGS were included and those with only one eye eligible were excluded. To be included in the analysis, each patient was required to have at least 5 SAP tests and 5 SDOCT tests over a duration of at least one year of follow-up. The images of RNFL with Spectralis and visual field tests with SAP 24-2 were obtained at 6-month intervals during follow-up. The study included a total of 2646 Spectralis visits, with an average of 752 visits per year.
Stereophotographs
All patients had stereoscopic optic disc photographs repeated at least every 12 months during follow-up. The images were reviewed with a stereoscopic viewer (Screen-VU stereoscope, PS Manufacturing, Portland, OR, USA) by 2 or more experienced graders masked to the subjects’ identity and any other test results. Details of the methodology used to grade the optic disc photographs at UCSD Optic Disc Reading Center have been described elsewhere 16, 17. Discrepancies between the 2 graders were resolved by consensus or adjudication by a third experienced grader. Only photographs with adequate quality were utilized.
Progression assessment was based on focal and/or diffuse thinning of neuroretinal rim, increased excavation, appearance or enlargement of RNFL defects. Change in rim color, presence of disc hemorrhage, or progressive parapapillary atrophy was not sufficient for characterization of progression.
Optical Coherence Tomography
Spectralis SDOCT was used to measure global RNFL in this study. Details of operation have been described previously 18, 19. All images were reviewed by experienced technicians at the Imaging Data Evaluation and Assessment (IDEA) Center. To be included, images had to be centered, with accurate segmentation, and have a signal strength >15 dB. For this study we used the global RNFL thickness, which corresponds to the average RNFL thickness in the 3.4 mm diameter peripapillary circle around the optic nerve head. This parameter has been shown to perform well in the assessment of rates of change in previous studies 17, 20.
Standard Automated Perimetry
All visual field results were reviewed by UCSD Visual Field Assessment Center (VisFACT).16 Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors, were excluded. Glaucomatous visual field progression was evaluated by Humphrey Field Analyzer Guided Progression Analysis (GPA) software. For each individual point on visual field, GPA compares the sensitivity of a follow-up test with the one from averaging 2 baseline visits at the same location. It flags points whose changes are greater than expected variability (at 95% significance level). If significant change is detected in more than 3 points and repeated at the same location in 2 consecutive follow-up tests, GPA will flag the last test as “Possible Progression”. If more than 3 points have significant changes detected and repeated in 3 consecutive follow-ups at the same location, GPA will flag the last one as “Likely Progression”. For the purpose of the study, only the classification of “Likely Progression” was considered as visual field progression.
In the current study, the baseline was chosen as the test closest to SDOCT date and the last visual field test was also the one closest to the last available SDOCT date.
Definitions of groups
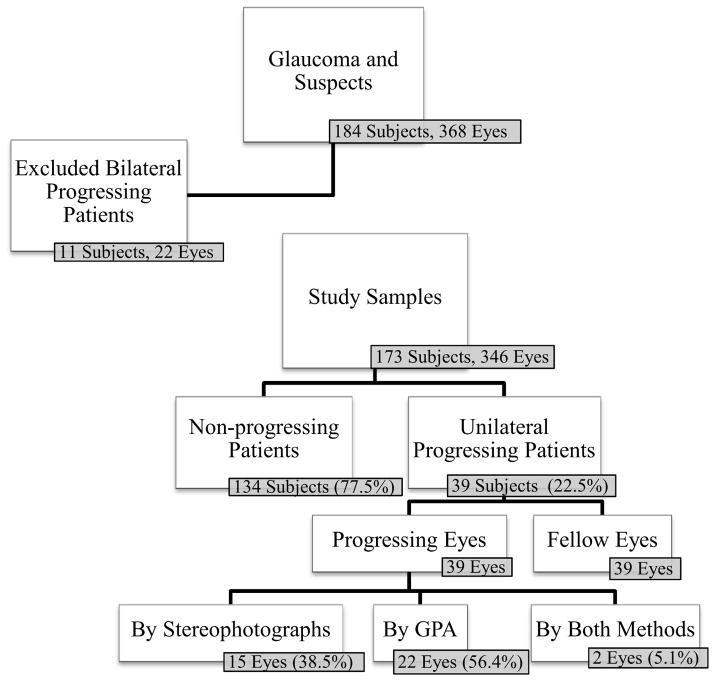
Figure 1 shows a flowchart depicting the selection of eyes and subjects for the study. As the purpose of this study was to evaluate the global RNFL thickness changes in the contralateral eye of subjects with unilateral progression, those subjects with both eyes progressing by conventional methods were excluded from further analyses. Therefore, we denominated progressing patients as those patients who had only one eye progressing by SAP and/or stereophotographs, whereas their contralateral eye did not show progression by these methods. In contrast, we denominated non-progressing patients as those who did not have progression by conventional methods in either eye. The eyes of these non-progressing patients were labeled as control eyes.
Figure 1.
Flow chart showing the number of patients enrolled and analyzed.
Statistical Analysis
Linear mixed effects models were used to evaluate the relationship between change in global RNFL thickness over time and progression by conventional parameters. Details of usage of these models for evaluation of rate of change in glaucoma have been reported previously 8, 21.
We considered measurements of global RNFL thickness as the dependent variable. The variable Time (time from baseline in years) was included as a continuous predictor. Significance of the coefficient associated with the variable Time indicated whether there was a significant trend in RNFL thickness measurements over time, that is, whether RNFL thickness measurements tend to decrease or increase significantly over time. Progression as defined by stereophotographs and/or SAP GPA was depicted as the fixed-effect covariate (variable Progression) with the value of “1” if the eye progressed by stereophotographs and/or SAP GPA, and the value of “0” if the eye did not show progression by either of the methods. The 2-way interaction between time and progression (variable Progression × Time) was also included in the model to evaluate whether there is significant difference in the longitudinal RNFL thickness measurements over time between progressing eyes and control eyes. The following random components for both the intercept and slope were added to the model: random patient-specific effects and random eye-specific effects (i.e., progressing in one eye but non-progressing in the fellow eye) for each eye nested within each patient. The inclusion of random intercepts allows for the variation in baseline global RNFL measurements, whereas the random slopes allow for the variation in the rate of progressive RNFL loss among eyes and patients.
Subsequent models were built taking into account other possible predictors such as ancestry, gender, baseline age, baseline SAP 24-2 mean deviation (MD), central corneal thickness (CCT), mean IOP during follow-up, as well as their interactions with variable Time. Initially, univariable models were constructed, containing only one putative predictor along with its Time-interaction term. Subsequently, more complex models comprising multiple predictors and interaction terms were created. Significance of the predictors was assessed using Wald tests and deviance statistics to reach the most parsimonious final model. When the final model was built, estimates of rate of RNFL loss for individual eyes were obtained by best linear unbiased prediction (BLUP) 3, 22.
All statistical analyses were performed with commercially available software (STATA, version 13.1; StataCorp LP, College Station, TX, USA). The alpha level (type I error) was set at 0.05.
Results
Study Sample
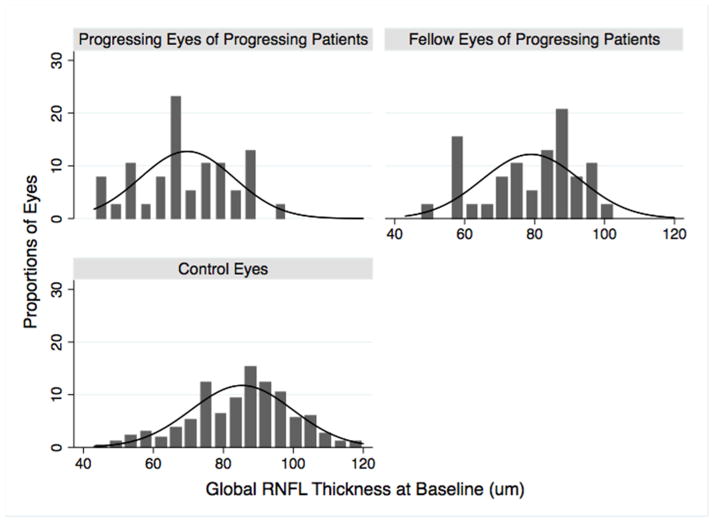
Overall there was an average of 8.3 OCT examinations (range 5–16) per eye during an average follow-up of 3.5 ± 0.7 years. Table 1 shows the demographic and clinical characteristics of all subjects at baseline. Progressing eyes of progressing patients had worse MD and thinner global RNFL thickness at baseline compared to control eyes (P < 0.001 for both comparisons). Global RNFL thickness was also thinner in the progressing eyes of unilateral progressing patients compared to their fellow eyes (P = 0.004). Figure 2 shows the distribution of baseline global RNFL thickness in progressing eyes, fellow eyes of progressing patients and control eyes at baseline.
Table 1.
Demographic and clinical characteristics at baseline for all subjects.
| Non- progressing Patients (n=134) | Unilateral Progressing Patients (n=39) | P Value | ||
|---|---|---|---|---|
| Control Eyes (n=268) | Progressing Eyes (n=39) | Fellow Eyes (n=39) | ||
| Baseline Age (yrs) | 65.7 ± 10.4 | 70.6 ± 9.7 | 0.003a,d | |
| Gender | ||||
| Female | 84 (63%) | 14 (36%) | <0.001b,d | |
| Ancestry | ||||
| European | 88 (66%) | 26 (67%) | 0.978b,d | |
| African | 46 (34%) | 13 (33%) | ||
| Baseline MD (dB) | −1.80 (−2.30 to 0.27) | −5.13 (−7.99 to −1.40) | −3.26 (−3.47 to −0.54) | <0.001a,c,d 0.169a,c,e |
| Baseline gRNFL thickness (μm) | 85 (75 to 95) | 70 (63 to 79) | 79 (70 to 90) | <0.001a,c,d 0.004a,c,e |
| Mean IOP during follow- up (mmHg) | 18 (14 to 21) | 16 (12 to 20) | 17 (14 to 19) | 0.296 a,c,d 0.623 a,c,e |
| CCT (μm) | 555 (526 to 581) | 548 (518 to 579) | 543 (513 to 579) | 0.099a,c,d 0.723a,c,e |
| Follow-up (yrs) | 3.5 ± 0.7 | 3.5 ± 0.7 | 0.825d,f | |
yrs = years; dB = decibels; MD = mean deviation; gRNFL = global retinal nerve fiber layer; IOP = intraocular pressure; CCT = central cornea thickness
Wilcoxon rank-sum test
Fisher exact test
Median (interquatile range)
Progressing eyes vs. Control eyes
Progressing eyes vs. Fellow eyes
t-test
Figure 2.
Histogram showing the distribution of global retinal nerve fiber layer thickness in progressing eyes, fellow eyes of unilateral progressing patients, and control eyes at baseline.
Rates of Global RNFL Loss
Eyes progressing according to GPA or changes on optic disc stereophotographs had statistically significant rates of global RNFL thickness loss during follow-up with an average loss of −0.89 ± 0.22 μm/year (± standard error of the coefficient) (P < 0.001) (Table 2). The fellow eyes of progressing patients also had significant decline of global RNFL thickness over time, with an average loss of −1.00 ± 0.20 μm/year (P < 0.001). In 18 (46%) of these eyes, the slopes were statistically significant and the mean rate of change in these eyes with statistically significant slopes was −1.81 ± 0.43 μm/year. There was also a decrease in global RNFL thickness in eyes of patients without progression by conventional methods in either eye, with an average decrease in global RNFL thickness of −0.71 ± 0.09 μm/year (P < 0.001). The average rate of RNFL progression of the progressing eyes of progressing patients was significantly faster than that of control eyes [−0.89 μm/year (95% CI: −1.33 to −0.45 μm/year) vs. −0.71 μm/year (95% CI: −0.86 to −0.56 μm/year); P < 0.001]. In addition, the average rate of RNFL progression in the fellow eye of progressing patients was also faster than that of control eyes [−1.00 μm/year (95% CI: −1.39 to −0.61 μm/year) vs. −0.71 μm/year (95% CI: −0.86 to −0.56 μm/year); P < 0.001]. A significant correlation in rates of change was observed between the two eyes of patients with unilateral progression (r = 0.41; P = 0.009, Pearson’s correlation).
Table 2.
Results of the mixed effects model investigating longitudinal changes in global retinal nerve fiber layer thickness in progressing and fellow eyes of patients with progression by guided progression analysis or disc stereophotographs (unilateral progressors) compared to rates in non-progressing patients.
| Parameters | Control Eyes (n=268) | Unilateral Progressing Patients (n=39)
|
P Value | ||||
|---|---|---|---|---|---|---|---|
| Progressing Eyes (n=39) | Fellow Eyes (n=39) | ||||||
|
| |||||||
| Mean | SE | Mean | SE | Mean | SE | ||
| Intercept | 84.71 | 1.16 | 69.37 | 2.11 | 78.62 | 2.23 | <0.001a,d <0.001b,d 0.003c,e |
| Change of gRNFL thickness (μm/year) | −0.71 | 0.09 | −0.89 | 0.22 | −1.00 | 0.20 | <0.001a,d <0.001b,d 1.000c,e |
g RNFL = global retinal nerve fiber layer
Progressing eyes of unilateral progressing patients vs. Control eyes
Fellow eyes of unilateral progressing patients vs. Control eyes
Progressing eyes vs. Fellow eyes
Linear mixed model
Sign test
It was also important to consider the effect of possible confounding variables on differences in rate of global RNFL loss between fellow eyes of progressing patients versus control eyes. This was evaluated using a multivariable random coefficient model including progression, baseline age, baseline MD, gender, race, CCT, mean IOP during follow-up and their interactions with Time (Table 3). The model showed that there was a significant difference in rates of RNFL loss over time between fellow eyes of unilateral progressing patients and control eyes, even after accounting for the effects of potentially confounding variables. The adjusted rates of progression for these two groups were −1.21 μm/year (−1.82 to −0.58 μm/year) vs. −0.73 μm/year (−0.97 to −0.48 μm/year), respectively (P = 0.013). Both better baseline MD and higher mean IOP were also significantly associated with faster RNFL thickness loss over time.
Table 3.
Results of the random coefficient model investigating rates of change in global retinal nerve fiber layer thickness in control eyes compared to the fellow eyes of patients progressing in one eye. The model adjusted for potential confounding factors and their interactions with time.
| Parameters | Coefficient | 95% CI | P value |
|---|---|---|---|
| Time (years) | −0.73 | −0.97 to −0.48 | <0.001 |
| Progression (yes) | −4.78 | −9.24 to −0.32 | 0.036 |
| Progression × Time | −0.48 | −0.85 to −0.10 | 0.013 |
| Age at Baseline (years) | −0.09 | −0.10 to 0.02 | 0.344 |
| Age × Time | 0.01 | 0.00 to 0.04 | 0.258 |
| MD at Baseline (dB) | 1.56 | 1.25 to 1.87 | <0.001 |
| MD × Time | −0.09 | −0.12 to −0.05 | <0.001 |
| IOP (mmHg) | −0.13 | −0.41 to 0.15 | 0.358 |
| IOP × Time | −0.03 | −0.06 to 0.00 | 0.034 |
| CCT (per 100 μm thicker) | 0.04 | 0.00 to 0.07 | 0.038 |
| CCT × Time | 0.00 | 0.00 to 0.00 | 0.423 |
| Race (African Descent) | 3.88 | 0.03 to 7.72 | 0.048 |
| Race × Time | −0.20 | −0.53 to 0.12 | 0.219 |
| Gender (Male) | −5.62 | −9.28 to −1.97 | 0.003 |
| Gender × Time | −0.10 | −0.41 to 0.20 | 0.509 |
| Intercept | 93.80 | 81.72 to 105.88 | <0.001 |
MD = mean deviation; IOP = intraocular pressure; CCT = central corneal thickness; CI = Confidence Interval. The variable Progression was assigned the value of 1 if the patient had shown progression using GPA or optic disc stereophotographs in either eye or the value of 0 if neither eye had shown progression using these conventional parameters.
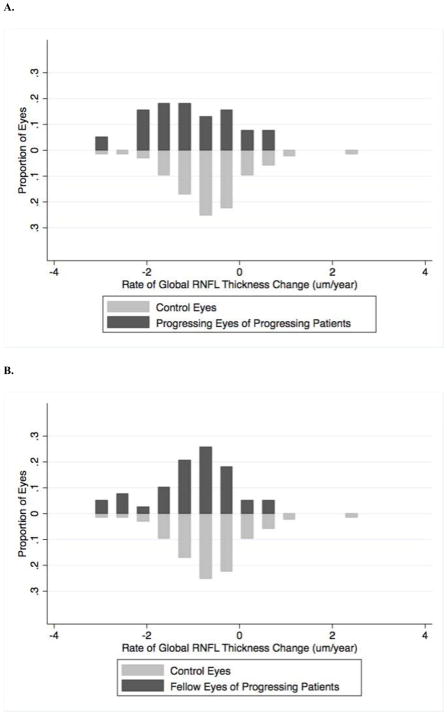
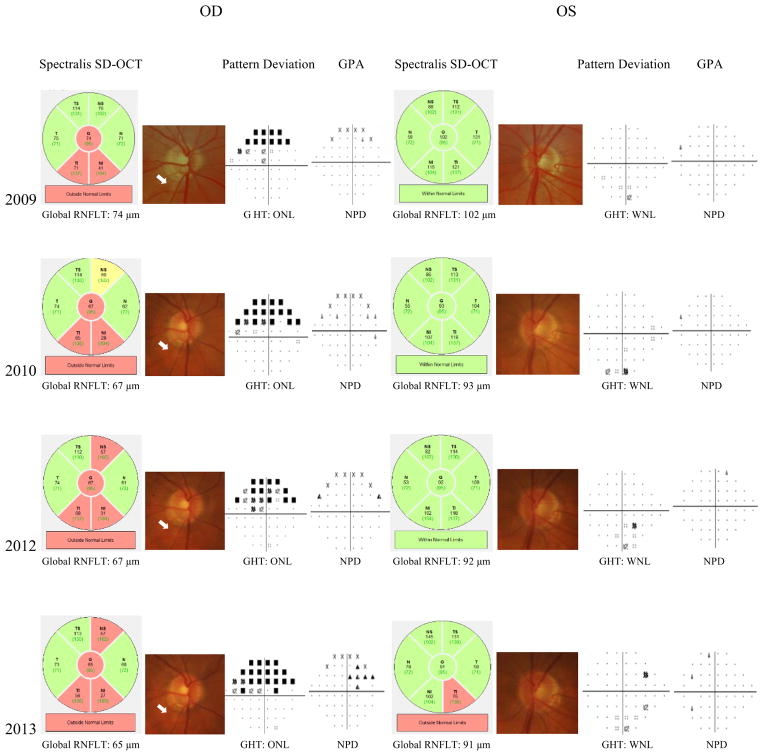
Figure 3 shows the distribution of rates of RNFL loss of progressing eyes and fellow eyes of progressing patients as well as of the control eyes during follow-up. Figure 4 shows an example of a patient included in the study whose right eye showed progression on GPA and had a rate of RNFL thickness change of −2.25 μm/year. The left eye did not show progression on GPA or optic disc stereophotographs, however this eye had a rate of RNFL loss of −2.75 μm/year, which was even faster than the right eye.
Figure 3.
Histogram showing the distribution of rates of change in global retinal nerve fiber layer thickness during follow-up in control eyes compared to progressing eyes of progressing patient (A) and in control eyes compared to fellow eyes of progressing patients (B).
Figure 4.
Example of a patient included in the study with progression by Guided Progression Analysis in the right eye (OD).
The fellow eye (left eye, OS) showed no progression by conventional parameters, however, spectral domain optical coherence tomography (SDOCT) showed a significant slope of change in global retinal nerve fiber layer thickness during follow-up in the fellow eye (OS).
Abbreviations: GHT: Glaucoma Hemifield Test; GPA: Guided Progression Analysis; ONL: Outside Normal Limits; WNL: Within Normal Limits; NPD: No Progression Detected.
Discussion
In this study we found that a substantial proportion of the fellow eyes of patients showing unilateral progression by conventional methods had significant decline of RNFL thickness over time. When adjusted for confounding factors, the mean rate of global RNFL loss in the fellow eyes of patients showing unilateral progression was −1.21 μm/year compared to −0.73 μm/year for the eyes of patients that did not show progression by conventional methods in either eye. Almost half of the fellow eyes of patients with unilateral progression by conventional methods showed statistically significant progression by SDOCT. This finding may have significant implications for clinical practice as it suggests that the contralateral eye of some patients with apparent unilateral progression may also have progressive glaucomatous damage, even in the absence of progression using conventional measures.
Although, after adjustment for confounding variables, we found contralateral eyes of patients with unilateral progression by conventional tests had an average rate of RNFL loss of −1.21 μm/year (Table 3), there was a wide range in rates of RNFL loss in these eyes, varying from −3.20 μm/year to 0.52 μm/year. Therefore some fellow eyes were actually progressing very quickly, despite absence of progression on conventional tests. Conversely, some contralateral eyes of patients with unilateral progression had slow rates of change in RNFL thickness with rates similar to control eyes (Figure 3B). It is important to differentiate eyes experiencing fast and slow rates of RNFL loss as previous studies have shown that faster rates of RNFL thinning are associated with increased risk of future visual field progression 11, 12. Evaluation of rate of RNFL loss may therefore be a useful parameter for discriminating eyes at higher risk of progression to functional impairment or blindness, which may benefit from more aggressive and earlier treatment escalation.
When measuring rates of change in either structural or functional indices it is important to consider the stage of disease. In more advanced glaucoma a floor may be reached in structural or functional measurements and as this floor is approached the rate of change is likely to decrease. In the present study, fellow eyes had thicker RNFL at baseline than eyes progressing on GPA or stereophotographs (79 μm vs. 70 μm, P = 0.004). Moreover, the coefficient for MD × Time in Table 3 also denoted that for each dB better MD, the rate of RNFL thickness change was 0.09 μm/year faster. Therefore, we would infer that the “room” for fellow eyes to decrease in RNFL thickness would be larger than eyes already showing progression on GPA or stereophotographs. This may explain why the average rate of change in RNFL thickness in the fellow eyes was actually somewhat faster than in eyes progressing by conventional methods.
Previous studies have also examined rates of change in RNFL thickness in glaucoma 10, 11, 21 and the slope for the progressing eyes in the current study (−0.89 μm/year) was similar. Studies using time-domain OCT (TDOCT) 10 in a mixed population of glaucoma patients and glaucoma suspects have reported RNFL losses of −0.72 μm/year. Using SDOCT, Miki and colleagues 12 found a faster rate of RNFL loss in patients showing progression on SAP, with losses of −2.02 μm/year, which was a faster rate than that of progressing patients in the current study. This is not surprising, because their study only included glaucoma suspects, which may imply more room for RNFL thickness to decrease.
RNFL thickness is known to decrease with age, with a recent longitudinal study in healthy subjects, reporting a −0.52 μm/year decrease in RNFL thickness using Cirrus SDOCT 23. The control eyes in our cohort had faster rates of RNFL loss than would be expected with normal aging, losing on average 0.71 μm/year, which may support the hypothesis that some of these control eyes had actually glaucomatous progression that had not yet resulted in detectable changes by conventional methods. Future longitudinal follow-up in a cohort of these eyes is needed to clarify the hypothesis. This rate was similar to that reported by Miki et al (−0.82 μm/year) 12 and Sung et al (−0.90 μm/year) 24 for control eyes in glaucoma suspects and advanced glaucoma, respectively.
It is important to consider the influence of possible confounding factors on rates of RNFL loss. Better baseline SAP MD was significantly associated with faster rates of RNFL thinning (P < 0.001). This observation may have been due to eyes with more advanced disease receiving more aggressive treatment, or having RNFL thicknesses closer to the floor of OCT measurements, as previously discussed. Higher mean IOP during follow-up was also significantly associated with faster rates of RNFL loss (P = 0.034). In contrast, age, race, gender and CCT were not associated with the rate of RNFL losses.
The current results should be interpreted in the context of some limitations. First as SDOCT is a relatively new technology, the study had a relatively short follow-up period. With longer follow-up, additional patients may have shown progression on conventional parameters, which may have influenced average rates of change between groups. However, a potential advantage of SDOCT is that it may allow earlier detection of change and hence shorten the duration of follow-up needed to determine if an eye is progressing. Despite this it would be interesting to see if the fellow eyes with fast rates of change in global RNFL thickness did indeed develop visual field endpoints with further follow-up. Another limitation of this study was the relatively small sample size, which limited the power to evaluate the effect of some possible confounding variables on the rate of global RNFL thinning. Furthermore the relationship between measurements from structural and functional tests in glaucoma is complex and not fully understood. It is possible that some eyes show progression on visual field indices such as GPA, without evidence of progressive RNFL loss and for this reason a combination of structural and functional assessments is likely to be needed to provide optimal detection of progression 24–27.
In conclusion, this study has shown that RNFL thickness loss occurs in a substantial number of the contralateral eyes of patients with glaucoma showing unilateral progression by conventional methods. These findings indicate that assessment of RNFL thickness by SDOCT may detect progressive glaucomatous damage that is not detected by visual fields or optic disc stereophotography. As damage from glaucoma is irreversible, prevention of visual impairment depends on identification of progression at an early stage. When one eye is noted to have progressive optic disc or visual field changes, our results suggest that the other eye may also be progressing.
Highlights.
Loss of retinal nerve fiber layer was observed in substantial number of contralateral eyes of glaucoma patients showing unilateral progression by conventional means. Optical coherence tomography may detect progressive damage prior to conventional methods.
Acknowledgments
Financial Support
National Institutes of Health/National Eye Institute grant EY021818, EY11008, U10EY14267, EY019869 and core grant P30EY022589; participant retention incentive grants in the form of glaucoma medication at no cost from Alcon Laboratories Inc, Allergan, Pfizer Inc, and Santen Inc; unrestricted grant from Research to Prevent Blindness, New York; fellowships from Brazilian National Research Council-CAPES 12309-13-3 (CPBG)
Footnotes
Conflict of Interest
T.L. – none; A.J.T. – research support from Heidelberg Engineering; C.P.B.G. – none; L.M.Z. - research support from National Eye Institute, Carl Zeiss Meditec, Heidelberg Engineering, Optovue, Topcon; R.N.W. – research support from Carl Zeiss Meditec, Genentech, Heidelberg Engineering, Nidek, Novartis, Optovue, Topcon; Consultant for Alcon, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Topcon; F.A.M. – research support from Alcon Laboratories, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Heidelberg Engineering, Merck, Reichert, Sensimed, Topcon, National Eye Institute; Consultant for Alcon, Allergan, Reichert.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medeiros FA, Weinreb RN, Moore G, et al. Integrating event-and trend-based analyses to improve detection of glaucomatous visual field progression. Ophthalmology. 2012;119:458–67. doi: 10.1016/j.ophtha.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. J Glaucoma. 2012;21:147–54. doi: 10.1097/IJG.0b013e31820bd1fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artes PH, O’leary N, Nicolela MT, et al. Visual field progression in glaucoma: what is the specificity of the guided progression analysis? Ophthalmology. 2014;121:2023–7. doi: 10.1016/j.ophtha.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 6.Leung CK, Choi N, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: pattern of RNFL defects in glaucoma. Ophthalmology. 2010;117:2337–44. doi: 10.1016/j.ophtha.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros FA, Alencar LM, Zangwill LM, et al. The relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116:1125–33. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medeiros FA, Lisboa R, Zangwill LM, et al. Evaluation of progressive neuroretinal rim loss as a surrogate end point for development of visual field loss in glaucoma. Ophthalmology. 2014;121:100–9. doi: 10.1016/j.ophtha.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medeiros FA, Zangwill LM, Alencar LM, et al. Detection of glaucoma progression with stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009;50:5741–8. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meira-Freitas D, Lisboa R, Tatham A, et al. Predicting progression in glaucoma suspects with longitudinal estimates of retinal ganglion cell counts. Invest Ophthalmol Vis Sci. 2013;54:4174–83. doi: 10.1167/iovs.12-11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121:1350–8. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatham AJ, Boer ER, Rosen PN, et al. Glaucomatous Retinal Nerve Fiber Layer Thickness Loss Is Associated With Slower Reaction Times Under a Divided Attention Task. Am J Ophthalmol. 2014;158:1008–17. doi: 10.1016/j.ajo.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gracitelli CPB, Abe RY, Tatham AJ, et al. Association between progressive retinal nerve fiber layer loss and longitudinal change in quality of life in glaucoma. JAMA Ophthalmol. 2015;133:384–90. doi: 10.1001/jamaophthalmol.2014.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mckean-Cowdin R, Wang Y, Wu J, et al. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:941–8. doi: 10.1016/j.ophtha.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sample PA, Girkin CA, Zangwill LM, et al. The african descent and glaucoma evaluation study (ADAGES): Design and baseline data. Arch Ophthalmol. 2009;127:1136–45. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medeiros FA, Vizzeri G, Zangwill LM, et al. Comparison of retinal nerve fiber layer and optic disc imaging for diagnosing glaucoma in patients suspected of having the disease. Ophthalmology. 2008;115:1340–6. doi: 10.1016/j.ophtha.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leite MT, Rao HL, Zangwill LM, et al. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011;118:1334–9. doi: 10.1016/j.ophtha.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatham AJ, Weinreb RN, Zangwill LM, et al. Estimated retinal ganglion cell counts in glaucomatous eyes with localized retinal nerve fiber layer defects. Am J Ophthalmol. 2013;156:578–87. doi: 10.1016/j.ajo.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoesl LM, Tornow RP, Schrems WA, et al. Glaucoma diagnostic performance of GDxVCC and spectralis OCT on eyes with atypical retardation pattern. J Glaucoma. 2013;22:317–24. doi: 10.1097/IJG.0b013e318237c8c5. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50:1675–81. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros FA, Alencar LM, Sample PA, et al. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology. 2010;117:2061–6. doi: 10.1016/j.ophtha.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257–63. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Sung KR, Sun JH, Na JH, et al. Progression detection capability of macular thickness in advanced glaucomatous eyes. Ophthalmology. 2012;119:308–13. doi: 10.1016/j.ophtha.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros FA, Leite MT, Zangwill LM, et al. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci. 2011;52:5794–803. doi: 10.1167/iovs.10-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medeiros FA, Lisboa R, Weinreb RN, et al. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology. 2013;120:736–44. doi: 10.1016/j.ophtha.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medeiros FA, Zangwill LM, Anderson DR, et al. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. 2012;154:814–24. doi: 10.1016/j.ajo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]