Abstract
Background
Whether there is a kidney function threshold to statin effectiveness in patients with acute myocardial infarction is poorly understood. Our study sought to help fill this gap in clinical knowledge.
Methods
We undertook a new-user cohort study of the effectiveness of statin therapy by level of estimated glomerular filtration rate (eGFR) in adults who were hospitalized for myocardial infarction (MI) between 2000 and 2008. Data came from the Cardiovascular Research Network.
The primary clinical outcomes were one-year all-cause mortality and cardiovascular hospitalizations, with adverse outcomes of myopathy and development of diabetes mellitus.
We calculated incidence rates, the number needed to treat (NNT) and used Cox proportional hazards regression with propensity score matching and adjustment to control for confounding, with testing for variation of effect by level of kidney function.
Results
Compared with statin non-initiators (n=5,583), statin initiators (n=5,597) had a lower propensity score-adjusted risk for death (HR, 0.79; 95% Confidence Interval [CI], 0.71, 0.88) and cardiovascular hospitalizations (HR, 0.90; 95% CI, 0.82, 1.00). We found little evidence of variation in effect by level of eGFR (p=0.86 for death; p=0.77 for cardiovascular hospitalization). Adverse outcomes were similar for statin initiators and statin non-initiators. The NNT to prevent one additional death over 1 year of follow-up ranged from 15 (95% CI 11, 28) for eGFR <30 ml/min/1.73 m2 requiring statin treatment over 2 years to prevent one additional death, to 67 (95% CI 49, 118) for patients with eGFR >90 ml/min/1.73m2.
Conclusions
Our findings suggest that there is potential for important public health gains by increasing the routine use of statin therapy for patients with lower levels of kidney function.
Keywords: Statins, eGFR, chronic kidney disease, Comparative Effectiveness
Introduction
Patients with chronic kidney disease are at high risk for developing cardiovascular disease.(1) Randomized trials have demonstrated the efficacy of statins in primary and secondary prevention for reducing cardiovascular disease risk in patients without chronic kidney disease.(2) Trials however, have shown disappointing results in secondary prevention with statins in patients on renal dialysis.(3;4) Two recent meta-analyses(5;6) examined subgroups from randomized trials and found evidence of efficacy among patients with non-dialysis requiring chronic kidney disease.(5) Importantly, however, these analyses used data that were not sufficiently granular to assess statin effects by level of kidney function— particularly relevant as more than 30% of patients presenting with an acute coronary syndrome have stage 3 chronic kidney disease or worse.(7)
One potential explanation for the lack of statin effectiveness in chronic dialysis is physiological differences in the mechanism of development of cardiovascular diseaseCVD with different levels of kidney function. Calcification of coronary arteries in the general population is often characterized by vascular intimal injury within the vessel followed by development of a lipomatous plaque that can rupture, leading to platelet aggregation, vessel occlusion, and acute myocardial infarction. While statins may be most effective in reducing vascular disease with a strong lipomatous component, vascular disease among patients with chronic kidney disease appears to arise, at least in part, from changes in regulation of mineral metabolism in the setting of damaged kidneys, which ultimately causes medial calcification.(8;9) The vascular injury resulting from calcification among chronic kidney disease patients may not be as amenable to treatment with statin therapy. These issues clearly indicate research is needed to guide evidence-based clinical decision-making across the spectrum of chronic kidney disease severity.
To fill this need, we examined the use and impact of statins for secondary prevention by level of kidney function across a broad spectrum of patients who were recently hospitalized for acute myocardial infarction. We hypothesized that the effectiveness of statins would be lower with worsening kidney function.
Methods
Setting
Patients were drawn from geographically and demographically diverse integrated health care delivery systems participating in the Cardiovascular Research Network, a consortium of investigators and health plans funded by the National Heart, Lung, and Blood Intitute. Data for this analysis were from CVRN sites with the necessary data and included 5 Kaiser Permanente regions, Northwest, Northern California, Southern California, Colorado, Hawaii, and the Group Health Cooperative (Seattle, WA). The institutional review boards at each site approved the study and a waiver of informed consent.
Participants
Patients were identified and followed using electronic data from health care encounters contained in each site’s virtual data warehouse.(10) We defined the index event as hospitalization between January 2000 and December 2008 with a primary discharge diagnosis of myocardial infarction (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 410.xx, excluding 410.x2 [follow-up care]). Previous work has shown these codes have a positive predictive value of 95% (95% CI 91-98).(11) We excluded patients if, during the 365 days before hospital discharge from their index event, there was any inpatient or outpatient visit for myocardial infarction (ICD-9-CM 410.xx), dispensing of a lipid-lowering agent (statins, bile acid sequestrants, fibrates, cholesterol absorption inhibitors, nicotinic acids), or evidence of renal dialysis. We excluded patients younger than 18 years, those with no outpatient serum creatinine measurement during the year before their index event, those who died during the 90 days following their index event, and patients without at least 12 months of continuous health system membership and pharmacy benefit before their index event.
We first analyzed the entire eligible cohort and then took advantage of the availability of important potential confounder data on baseline body mass index (BMI) and systolic blood pressure (SBP) in a subgroup analysis. Other clinical data were not available. This subset of 2091 eligible subjects’ index events occurred during the time period when their health plan used an electronic medical record system that provided access to BMI and SBP, so they were more likely to have recent events (>80% of the subcohort had an index date between 2006 and 2008).
Study design: statin exposure ascertainment and follow-up
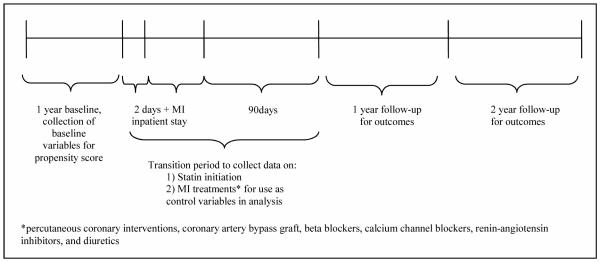
Figure 1 illustrates our new-user study design. Statin exposure was ascertained through pharmacy records. We divided patients into statin initiators and non-initiators based on whether they had a new statin dispensing during the two days before leaving the hospital (to capture discharge statin dispensing), or up to 90 days after their index hospitalization. Time zero for follow-up began at 90 days post-discharge and we followed patients for both one-year and two-year timeframes using an intent-to-treat design. We chose this window for identification of statin dispensing and start of follow-up so that all patients would be followed from a common point in their natural history, and because we wanted to compare our results to clinical trials of secondary prevention with statins. These trials typically enroll patients who are at least 3 months post-MI.(12;13)
Figure 1.
Study design diagram
Clinical Outcomes
Outcomes included cardiovascular disease -related hospitalization (primary discharge ICD-9-CM codes 390-459) and all-cause death. Deaths were identified from the each site’s vital statistics files. Our primary outcome analysis was one year because of concern over statin initiation among the non-initiator group with time.
Adverse Outcomes
We included known statin-related adverse outcomes of diabetes mellitus(14) and myopathy.(15) Diabetes was identified using an ICD-9-CM code-based approach modified from Nichols and colleagues(16) (1 inpatient stay or at least 2 outpatient visits coded with any of 250.x, 357.2, 366.41, 362.01–362.07). Myopathy was identified using ICD-9-CM codes that have a positive predictive value of 74%(17) (inpatient stay coded with 791.3, or inpatient stay with primary discharge code 728.89, or secondary discharge code of 728.89 along with a creatine kinase test performed within 7 days of the hospitalization, or code 584.xx along with secondary discharge code of 728.89).
Covariates
We measured all baseline characteristics during the year before the index date. We classified patients into groups based on their level of kidney function using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation(18) to estimate glomerular filtration rate (eGFR) in mL/min per 1.73 m2. We used the serum creatinine measurement from the ambulatory setting that was closest to, and before, a patient’s index date. Consonant with staging recommendations, (19) we present within-group comparisons for patients with eGFR (in units of mL/min per 1.73 m2) in categories of >=90, 60-89, 45-59, 30-44 and <30.
Propensity Scores and adjustment for confounding
To balance the groups of statin initiators and non-initiators on observed variables, thus reducing the impact of selection, we used logistic regression to estimate study site-specific propensity scores (PS)(20;21) for the initiation of statin therapy (0.05 caliper).. These PS were also used as adjustment variables in our models. The PS we constructed for the primary analysis included the following potential confounders, selected on clinical grounds (i.e.potentially associated with exposure and outcome) : age, sex, race, income and education (from 2000 US Census data), selected laboratory tests and findings (low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), total cholesterol, triglyceride, blood glucose, hemoglobin, glycated hemoglobin (HbA1c), and urinary albumin to creatinine ratio (ACR)), specific medications (beta blockers, calcium channel blockers, angiotensin-converting-enzyme inhibitor or angiotensin receptor blockers (ACE/ARB), and diuretics), diagnoses (hypertension, diabetes, dyslipidemia, heart failure, ischemic stroke/transient ischemic attack, atrial fibrillation/flutter, mitral and/or aortic valvular disease, peripheral arterial disease, dementia, chronic lung disease and depression) and baseline prevalence of healthcare utilization (defined as counts of lipid-related laboratory tests; unique medications dispensed; unique cardiovascular diagnoses; total unique diagnoses; cardiovascular hospitalizations, all-cause hospitalizations, cardiology visits; emergency department visits; and ambulatory office visits). The PS constructed for the subcohort included the most recent pre-index date ambulatory BMI and SBP, as well as the variables included in the whole-cohort propensity score. As shown in Figure 1, PS variables were all evaluated during a one-year baseline. We matched statin initiators to statin non-initiators using a 1:1 ‘greedy’ matching algorithm to match statin initiators and non-initiators on the propensity score(21); we also undertook a 10:1 match with nearly identical results. Balance was assessed using absolute standardized percent differences, and as suggested by other investigators,(22;23) we considered standardized differences of < 0.1 to support the assumption of balance between the groups.
We also constructed a site-specific “high-dimensional” propensity score (hd-PS) to help balance the groups by matching and to adjust for confounding.(20) The hd-PS included all the variables listed above, plus more than 400 variables constructed from cardiovascular medication dispensings, diagnosis codes and procedure codes. The hd-PS algorithm first screens candidate confounders by estimating unconditional associations of individual potential confounders with the exposure and then separately with the outcome. Covariates were then ranked by their confounding potential; we included the top 500. (24) We had to omit lipid values measured after index hospital discharge because these values may have reflected intermediate, treated valuesthat could bias estimates of comparative effectiveness.(25) During the ‘transition period’ (see Figure 1), we collected data on important co-interventions including use of other medications (i.e., beta blockers, calcium channel blockers, ACE/ARB, and diuretics) and coronary revascularization (percutaneous coronary intervention and coronary artery bypass grafting). Because these variables are likely not intermediates , we controlled for them in the analysis using stratification (by estimating a separate baseline hazard).
Statistical analysis
We calculated incidence rates and 95% confidence intervals (CIs) for all outcomes by statin exposure status and level of eGFR. We also constructed Kaplan-Meier survival curves by level of kidney function, and then estimated the relative hazard for each outcome using Cox proportional hazards regression models. These models included a term for statin initiation, propensity to receive statin, co-intervention adjustments, level of kidney function, the interaction of kidney function with statin initiation, and were stratified by receipt of important co-interventions during the transition period. We used a ‘missing’ category for variables with missing data.
We calculated the proportion of cross overs—non-initiators who initiated statin therapy after the start of follow-up —and conducted a separate analysis for which cross-overs were censored when they crossed over. An additional analysis included an interaction term for statin initiation with proteinuria defined by a urine dipstick being positive for protein of 1+ or greater (yes/no). We also estimated the number needed to treat (NNT) with a statin to prevent one outcome, by level of kidney function.(26)
Results
Baseline characteristics
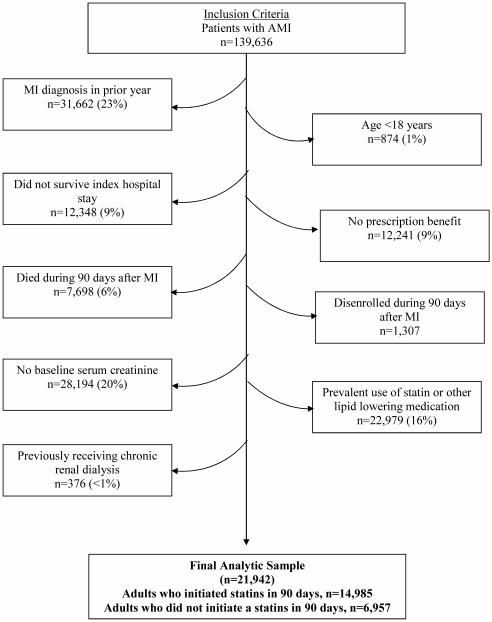
We initially identified a total of 139,636 patients hospitalized for myocardial infarction between January 2000 and December 2008, and 21,942 (14,985 statin initiators; 6,957 non-initiators) were included in our analysis sample (Figure 2). The most common reasons for exclusion were previous myocardial infarction (23%), no serum creatinine measurement before myocardial infarction (20%), and pre-existing use of lipid-lowering medication (16%).
Figure 2.
Cohort Assembly
Before matching, statin initiators tended to be younger than non-initiators, have better baseline kidney function, less diabetes and less prior inpatient health care utilization, but a higher mean LDL-C level (Table 1). Standardized differnces indicated imbalance (i.e. >0.1) before matching, but the propensity score matched sample of 5,597 statin initiators and 5,583 non-initiators was well-balanced.
Table 1.
Baseline characteristics of Propensity Score-Matched Statin Initiators and Statin Non-initiators
| Whole Cohort |
1:1 Propensity Score Matched Cohort |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Statin Initiators | Statin Non-Initiators | Standardized Difference |
Statin Initiators | Statin Non-Initiators | Standardized Difference |
|||||
| n=14,985 | n=6,957 | n=5,597 | n=5,583 | |||||||
| Age | ||||||||||
| Mean (SD) age, years |
67.62 | (13.2) | 73.16 | (13.8) | −0.41 | 71.96 | (12.5) | 71.64 | (14.1) | 0.02 |
| Age <45 (n, %) |
582 | (3.9) | 241 | (3.5) | 0.02 | 101 | (1.8) | 228 | (4.1) | −0.14 |
| Age 45− 54 |
2088 | (13.9) | 536 | (7.7) | 0.20 | 475 | (8.5) | 511 | (9.2) | −0.02 |
| Age 55− 64 |
3558 | (23.7) | 996 | (14.3) | 0.24 | 974 | (17.4) | 911 | (16.3) | 0.03 |
| Age 65− 74 |
3683 | (24.6) | 1450 | (20.8) | 0.09 | 1409 | (25.2) | 1219 | (21.8) | 0.08 |
| Age 75− 84 |
3519 | (23.5) | 2226 | (32.0) | −0.19 | 1733 | (31.0) | 1657 | (29.7) | 0.03 |
| Age 85+ | 1555 | (10.4) | 1508 | (21.7) | −0.31 | 905 | (16.2) | 1057 | (18.9) | −0.07 |
| Female, n (%) |
5813 | (38.8) | 3544 | (50.9) | −0.25 | 2748 | (49.1) | 2699 | (48.3) | 0.02 |
| Race, n (%) | ||||||||||
| White | 10543 | (70.4) | 4941 | (71.0) | −0.01 | 4085 | (73.0) | 3927 | (70.3) | 0.06 |
| Non-white | 2826 | (18.9) | 1196 | (17.2) | 0.04 | 936 | (16.7) | 1016 | (18.2) | −0.04 |
| Missing | 1616 | (10.8) | 820 | (11.8) | −0.03 | 576 | (10.3) | 640 | (11.2) | −0.03 |
| Education level, % with <college degree (mean, SD)a |
72.5 | (17.1) | 72.9 | (17.2) | −0.02 | 73.1 | (17.1) | 73.2 | (17.2) | 0.00 |
| Median family income, $ (mean, SD)a |
$61,310 | $24,920 | $59,792 | $24,462 | 0.06 | $59,753 | $25,030 | $59,894 | $25,077 | −0.01 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) |
||||||||||
| Mean (SD) |
70.7 | (21.2) | 63.1 | (23.2) | 0.34 | 65.5 | (21.9) | 65.1 | (23.2) | 0.02 |
| >90, n (%) |
2942 | (19.6) | 926 | (13.3) | 0.17 | 789 | (14.1) | 854.0 | (15.3) | −0.03 |
| 60-89 | 7425 | (49.6) | 2814 | (40.5) | 0.18 | 2525 | (45.1) | 2360.0 | (42.3) | 0.06 |
| 45-59 | 2722 | (18.2) | 1599 | (23.0) | −0.12 | 1208 | (21.6) | 1214.0 | (21.7) | 0.00 |
| 30-44 | 1402 | (9.4) | 1081 | (15.5) | −0.19 | 759 | (13.6) | 786.0 | (14.1) | −0.02 |
| <30 | 494 | (3.3) | 537 | (7.7) | −0.19 | 316 | (5.7) | 369.0 | (6.6) | −0.04 |
| Baseline Dipstick Proteinuriab n (%) |
||||||||||
| Negative or trace |
3202 | (21.4) | 1501 | (21.6) | −0.01 | 1240 | (22.2) | 1123 | (20.1) | 0.05 |
| 1+ and greater |
105 | (0.7) | 72 | (1.0) | −0.04 | 40 | (0.7) | 44 | (0.8) | −0.01 |
| Not tested | 11678 | (77.9) | 5384 | (77.4) | 0.01 | 4317 | (77.1) | 4416 | (79.1) | −0.05 |
| Baseline hemoglobin (g/dL) |
||||||||||
| Mean (SD) |
12.81 | (3.1) | 12.04 | (2.9) | 0.25 | 12.2 | (3.0) | 12.2 | (3.0) | 0.03 |
| ≥15.0 n, (%) |
3342 | (22.3) | 840 | (12.1) | 0.27 | 822 | (14.7) | 755 | (13.5) | 0.03 |
| 14.0-14.9 | 2386 | (15.9) | 892 | (12.8) | 0.09 | 794 | (14.2) | 760 | (13.6) | 0.02 |
| 13.0 - | 2152 | (14.4) | 1095 | (15.7) | −0.04 | 949 | (17.0) | 875 | (15.7) | 0.03 |
| 13.9 11.0- 12.9 |
2138 | (14.3) | 1652 | (23.8) | −0.24 | 1148 | (20.5) | 1232 | (22.1) | −0.04 |
| <11 | 2758 | (18.6) | 1761 | (25.3) | −0.16 | 1325 | (23.7) | 1317 | (23.6) | 0.00 |
| Not tested | 2182 | (14.6) | 717 | (10.3) | 0.13 | 559 | (10.0) | 644 | (11.5) | −0.05 |
| HDL cholesterol (g/dL) |
||||||||||
| Mean (SD) |
43.0245 | 3(18.7) | 44.04 | (21.3) | −0.05 | 43.2 | (20.2) | 43.57 | (21.3) | −0.02 |
| ≥60 n, (%) |
1482 | (9.9) | 691.00 | (9.9) | 0.00 | 535 | (9.6) | 554 | (9.9) | −0.01 |
| <60 | 8038 | (53.6) | 2743.00 | (39.4) | 0.29 | 2396 | (42.8) | 2400 | (43.0) | 0.00 |
| Not tested | 5465 | (36.5) | 3523.00 | (50.6) | −0.29 | 2666 | (47.6) | 2629 | (47.1) | 0.01 |
| LDL cholesterol (g/dL) |
||||||||||
| Mean (SD) |
132.07 | (35.0) | 114.28 | (36.2) | 0.50 | 118.9 | (35.7) | 117.87 | (35.8) | 0.03 |
| ≤200 n, (%) |
293 | (2.0) | 60 | (0.9) | 0.09 | 68 | (1.2) | 57.00 | (1.0) | 0.02 |
| 160-199.9 | 1516 | (10.1) | 262 | (3.8) | 0.25 | 256 | (4.6) | 252.00 | (4.5) | 0.00 |
| 130-159.9 | 2819 | (18.8) | 620 | (8.9) | 0.29 | 591 | (10.6) | 597.00 | (10.7) | 0.00 |
| 100-129.9 | 2753 | (18.4) | 1062 | (15.3) | 0.08 | 942 | (16.8) | 960.00 | (17.2) | −0.01 |
| 70-99.9 | 1303 | (8.7) | 873 | (12.6) | −0.13 | 672 | (12.0) | 678.00 | (12.1) | 0.00 |
| <70 | 250 | (1.7) | 275 | (4.0) | −0.14 | 174 | (3.1) | 178.00 | (3.2) | 0.00 |
| Not tested | 6051 | (40.4) | 3805 | (54.7) | −0.29 | 2894 | (51.7) | 2861.00 | (51.2) | 0.01 |
| Total cholesterol (g/dL) |
||||||||||
| Mean (SD) |
214.63 | (42.4) | 196.13 | (44.1) | 0.43 | 201.9 | (43.5) | 199.81 | (43.5) | 0.05 |
| ≥240 n, (%) |
2409 | (16.1) | 496 | (7.1) | 0.28 | 489 | (8.7) | 469 | (8.4) | 0.01 |
| 200-239 | 3664 | (24.5) | 1030 | (14.8) | 0.24 | 930 | (16.6) | 941 | (16.9) | −0.01 |
| <200 | 3519 | (23.5) | 2012 | (28.9) | −0.12 | 1567 | (28.0) | 1591 | (28.5) | −0.01 |
| Not tested | 5393 | (36.0) | 3419 | (49.1) | −0.27 | 2611 | (46.7) | 2582 | (46.3) | 0.01 |
| Triglycerides, n (%) |
||||||||||
| Mean (SD) |
179.67 | (132.0) | 169.26 | (132.8) | 0.08 | 173.3 | (133.0) | 171.30 | (135.8) | 0.01 |
| >=150 (%) |
4049 | (27.0) | 1237 | (17.8) | 0.22 | 1154 | (20.6) | 1093 | (19.6) | 0.03 |
| <150 | 4108 | (27.4) | 1614 | (23.2) | 0.10 | 1305 | (23.5) | 1369 | (24.5) | −0.02 |
| Not tested | 6828 | (45.6) | 4106 | (59.0) | −0.27 | 3138 | (56.1) | 3121 | (55.9) | 0.00 |
| Glucose n, (% tested) |
10199 | (68.1) | 4530 | (65.1) | 0.06 | 3504 | (62.6) | 3526 | (63.2) | −0.01 |
| Glycated hemoglobin n, (% tested) |
4105 | (27.4) | 2208 | (31.7) | −0.10 | 1760 | (31.5) | 1742 | (31.2) | 0.01 |
| Albumin to creatinine ratio (ACR) |
||||||||||
| n, (% tested) | 1033 | (6.9) | 479 | (6.9) | 0.00 | 383 | (6.8) | 380 | (6.8) | 0.00 |
| Medication Use Renin- angiotensin system inhibitor use, n (%) |
5217 | (34.8) | 2886 | (41.5) | −0.14 | 2222 | (39.7) | 2201 | (39.4) | 0.01 |
| Beta- blocker use, n (%) |
4612 | (30.8) | 2438 | (35.0) | −0.09 | 1942 | (34.7) | 1902 | (34.1) | 0.01 |
| Calcium channel blocker use, n (%) |
2951 | (19.7) | 1705 | (24.5) | −0.12 | 1350 | (24.1) | 1304 | (23.4) | 0.02 |
| Diuretic use, n (%) |
5730 | (38.2) | 3564 | (51.2) | −0.26 | 2615 | (46.7) | 2613 | (46.8) | 0.00 |
| Medical History, n (%) |
||||||||||
| Heart Failure |
1072 | (7.2) | 1568 | (22.5) | −0.44 | 875 | (15.6) | 880 | (15.8) | 0.00 |
| Coronary artery bypass surgery |
21 | (0.1) | 27 | (0.4) | −0.05 | 14 | (0.3) | 15 | (0.3) | 0.00 |
| Percutane ous coronary intervention |
35 | (0.2) | 37 | (0.5) | −0.05 | 23 | (0.4) | 28 | (0.5) | −0.01 |
| Ischemic stroke or transient ischemic attack |
145 | (1.0) | 163 | (2.3) | −0.11 | 89 | (1.6) | 100 | (1.8) | −0.02 |
| Cerebrov ascular disease |
595 | (4.0) | 667 | (9.6) | −0.22 | 391 | (7.0) | 416 | (7.5) | −0.02 |
| Atrial fibrillation or flutter |
559 | (3.7) | 703 | (10.1) | −0.25 | 399 | (7.1) | 397 | (7.1) | 0.00 |
| Mitral and/or aortic valvular disease |
262 | (1.7) | 309 | (4.4) | −0.16 | 186 | (3.3) | 184 | (3.6) | −0.02 |
| Peripheral arterial disease |
268 | (1.8) | 260 | (3.7) | −0.12 | 165 | (3.0) | 173 | (3.1) | −0.01 |
| Diagnosed dyslipide mia |
2431 | (16.2) | 885 | (12.7) | 0.10 | 747 | (13.4) | 744 | (13.3) | 0.00 |
| Hypertension | 8026 | (53.6) | 4067 | (58.5) | −0.10 | 3218 | (57.5) | 3170 | (56.8) | 0.01 |
| Diabetes mellitus |
2238 | (14.9) | 1471 | (21.1) | −0.16 | 1093 | (19.5) | 1073 | (19.2) | 0.01 |
| Diagnosed dementia |
561 | (3.7) | 608 | (8.7) | −0.21 | 370 | (6.6) | 371 | (6.7) | 0.00 |
| Diagnosed depression |
627 | (4.2) | 411 | (5.9) | −0.08 | 296 | (5.3) | 304 | (5.5) | −0.01 |
| Chronic lung disease |
2189 | (14.6) | 1443 | (20.7) | −0.16 | 1080 | (19.3) | 1038 | (18.6) | 0.02 |
| Chronic liver disease |
187 | (1.2) | 178 | (2.6) | −0.10 | 59 | (1.1) | 145 | (2.6) | −0.12 |
| Mechanical fall |
66 | (0.4) | 92 | (1.3) | −0.09 | 46 | (0.8) | 58 | (1.0) | −0.02 |
| Medical Care Utilization (mean, SD) |
||||||||||
| Count of lipid tests |
2.09 | (2.2) | 1.81 | (2.5) | 0.12 | 1.89 | (2.4) | 1.91 | (2.4) | −0.01 |
| Count of unique ICD9 diagnosis |
161.52 | (119.5) | 196.00 | (157.1) | −0.25 | 185.29 | (133.9) | 185.03 | (138.1) | 0.00 |
| Count of cardiovascular hospitalization days |
0.75 | (3.1) | 2.20 | (6.0) | −0.30 | 1.42 | (4.4) | 1.71 | (5.3) | −0.06 |
| Countof inpatient stays |
0.32 | (0.9) | 0.77 | (1.5) | −0.36 | 0.57 | (1.2) | 0.63 | (1.4) | −0.05 |
| Count of emergency department visits |
0.63 | (1.4) | 1.25 | (2.4) | −0.31 | 1.00 | (1.8) | 1.04 | (2.1) | −0.02 |
| Count of cardiology outpatient visits |
0.43 | (1.2) | 0.79 | (2.0) | −0.22 | 0.63 | (1.5) | 0.68 | (1.9) | −0.03 |
From 2000 census block data
Completely missing for one site
Clinical Outcomes
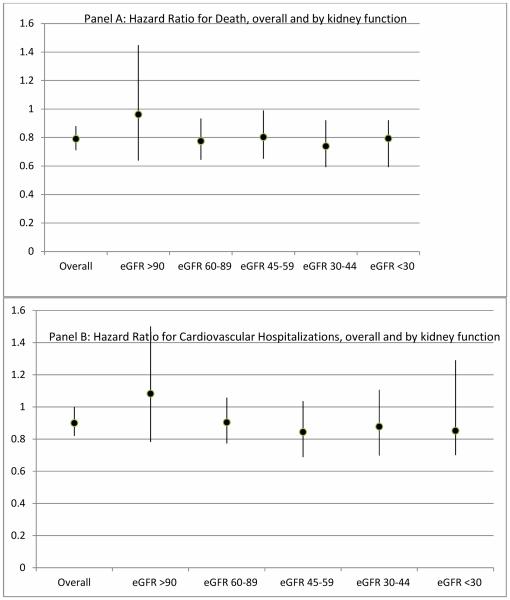
We found that the rate of clinical outcomes increased with lower kidney function at both one and two years; within any given level of kidney function, the rate was always lower for statin initiators. For example, among patients with eGFR >90ml/min/1.73m2, the one-year death rate for statin initiators was 5.1 per 100 person-years compared to 7.7per 100 person-years for non-initiators. For patients with eGFR <30ml/min/1.73m2, the death rate was 367.2 per 100 person-years for statin initiators, and 49.5 per 100 person-years for non-initiators. The differential with cardiovascular hospitalization was less stark; among patients with an eGFR <30ml/min/1.73m2, the rate over one year for statin initiators was 33.9 per 100 person years, compared with 35.6 per 100 person years for statin non-initiators. The HR for death at one year for statin initiation compared to non-initiation was 0.79 (95% CI, 0.71-0.88), and 0.9 (95% CI 0.82, 1.00) for cardiovascular hospitalizationThe p-value for the interaction term between eGFR and statin initiation was not significant for death or for cardiovascular hospitalization. Because our main research question involves whether statin effects vary by level of renal function we also report the estimated effect HR for statin initiation versus non-initiation by eGFR category. Those HR show no clinically important variation across level of eGFR for death or cardiovascular hospitalization (Figure 3).
Figure 3.
Hazard ratio for Death and Cardiovascular hospitalization, by level of renal function
Adverse outcomes
We found few diagnosed myopathy events in the propensity score matched cohort (54 among statin initiators and 66 among statin non-initiators). Diabetes incidence was similar between initiators and non-initiators, within level of kidney function (Table 3).
Table 3.
Adverse Outcomes Over One Year: Statin initiators compared to statin non-initiators, overall and by level of kidney function. Hazard ratios and 95% confidence intervals were estimated from Cox proportional hazards regression
| Statin Initators (n=5,597; 4,504 without diabetes at baseline) |
Statin Non-initiators (n=5,583; 4,510 without diabetes at baseline) |
PS Matched and Adjusted Hazard Ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Events | Events per 100 person years |
Events | Events per 100 person years |
||
| Diabetes* | 447 | 11.52 | 400 | 10.71 | 0.97 (0.84, 1.12) |
| P-value for interaction of statin initiation by level of kidney function |
0.58 | ||||
| Myopathy | 54 | 1.34 | 66 | 1.04 | 0.82 (0.57, 1.20) |
| P-value for interaction of statin initiation by level of kidney function |
0.98 | ||||
among patients without diabetes at baseline
The HR for myopathy at one year (0.82, 95% CI 0.57-1.20) and diabetes development (0.97, 95% CI 0.84-1.12) showed the risk for both outcomes was similar between statin initiators and non-initiators; the risk did not vary by level of kidney function.
Our results were robust with respect to variations in kidney function; patients without dipstick proteinuria (negative or trace) had HR for death at one year of 0.85 (95% CI 0.68-1.06) for statin initiators compared to non-initiators, and patients with positive dipstick proteinuria (1+ or greater) had a HR of 0.55 (95% CI 0.20-1.56).
The use of the hd-PS adjustment yielded nearly identical findings (HR for death= 0.81, 95% CI 0.72-0.91: HR for cardiovascular hospitalization 0.88, 95% CI 0.78, 0.98)), as did the analysis in the subcohort that additionally adjusted for BMI and BP. Censoring at statin cross-over yielded results similar to the primary analysis, and our two year results also closely mirrored the one year findings for all analyses and outcomes.
Our NNT estimates (Table 4) suggest that 31 patients in our sample would need statin therapy to prevent one death and that 65 patients in our sample would prevent one cardiovascular hospitalization. However, because the rate of death and cardiovascular hospitalization was greater with worsening kidney function, the NNT was much more favorable at lower levels of eGFR.
Table 4.
Survival Probability, and Numbers Needed to Treat (NNT) at One Year to Prevent One Outcome
| Survival probability at 1 year (non-initiators) |
Number needed to treat to benefit (95% CI*) |
|
|---|---|---|
| Death | ||
| Overall | 0.83 | 31 (22, 55) |
| eGFR >90 | 0.93 | 67 (49, 118) |
| eGFR 60-89 | 0.87 | 40 (29, 70) |
| eGFR 45-59 | 0.82 | 28 (20, 50) |
| eGFR 30-44 | 0.74 | 21 (15, 37) |
| eGFR <30 | 0.63 | 15 (11, 28) |
| CV Hospitalization | ||
| Overall | 0.83 | 65 |
| eGFR >90 | 0.91 | 114 |
| eGFR 60*89 | 0.85 | 71 |
| eGFR 45*59 | 0.82 | 60 |
| eGFR 30*44 | 0.78 | 50 |
| eGFR <30 | 0.72 | 41 |
The risk difference for CV hospitalization was not statistically significant therefore we cannot calculate a 95% CI on for CV hospitalization
Discussion
Our findings suggest that patients with mild to moderate chronic kidney disease enjoy the same relative cardioprotective and mortality reduction benefits of statin use as do patients with preserved eGFR. These findings are consonant with recent reports from systematic reviews of clinical trial subgroups.(5;6) However, our study also confirms that the absolute rate of death and cardiovascular hospitalization varies by level of kidney function, so the constant relative risk reduction from statin therapy translates into a greater absolute risk reduction with worsening kidney function.
The variable benefit – on the absolute scale - of statin therapy by level kidney function may be especially important because patients with kidney dysfunction are known to have a lower probability of being treated with cardioprotective therapy, including statins.(27) Evidence suggests this treatment disparity has attenuated,(28) so we examined data from the last year of our study (2008) and found that 71% of our patients with eGFR <60ml/min/1.73m2 initiated statin therapy within the 90days following an MI, compared to 90% of patients with eGFR >90 ml/min/1.73m2. Thus, while relative statin treatment effects may not differ by level of renal function, poor outcome rates and treatment rates do differ by level of renal function, suggesting that attention could be usefully focused on increasing treatment rates among patients with pre-dialysis chronic kidney disease. Substantial public health gains might be made by eliminating this ‘treatment-risk paradox’.(29)
Our findings were similar in magnitude and direction to recent meta-analyses by Palmer and colleagues of subgroups from clinical trials in patients with chronic kidney disease. For example, statins were estimated to reduce all-cause mortality (relative risk [RR] 0.81 [95% CI, 0.74, 0.88]) and cardiovascular events (RR, 0.76 [95% CI, 0.73, 0.80]).(5) While informative, those studies had to combine all pre-dialysis chronic kidney disease patients together, regardless of disease stage, leaving the residual question of whether there is a renal function threshold below which statins are not effective. This is an important limitation of those meta-analyses because the physiologic disruption of mineral metabolism thought to cause the medial calcification in chronic kidney disease may increase with worsening stage of kidney disease. Our study lends reassurance to treatment decisions because of findings of consistent relative effects across the spectrum of pre-dialysis chronic kidney disease.
The Study of Heart and Renal Protection (SHARP) was conducted among patients with stage 3 chronic kidney disease or worse, and the investigators showed that compared to placebo, simvastatin plus ezetimibe was effective at reducing LDL cholesterol(30) and have reported benefits of preventing major atherosclerotic events (the combination of MI, coronary death, ischemic stroke, or revascularization), but the findings were not significant for mortality. While important, SHARP did not include patients with known atherosclerotic disease, and tested a combination of ezetimibe and simvastatin. Our study expands on SHARP by examining the use and impact of statins for secondary cardiovascular event prevention across a broad spectrum of chronic kidney disease patients, and stratifying more finely by level of eGFR. Several observational studies have reported on the effectiveness of secondary prevention with statin treatment in patients with chronic kidney disease, but these reports have important limitations including small samples resulting in aggregation across stage of chronic kidney disease,(31) or lack of information about baseline medication use,(32) resulting in prevalent user bias.(33)
We did not observe a significantly higher risk of potential statin-related adverse effects of myopathy and development of diabetes, although we had limited precision in our estimates at lower levels of eGFR and follow-up was limited to two years. However, our findings are similar to recent meta-analyses regarding the safety of statins in clinical trial participants.
An inherent observational study limitation is potential for residual confounding and selection bias. Of particular note is the ‘healthy user bias’ that has been observed in statin studies.(34) We attempted to mitigate this through use of propensity score methods, including using a ‘high-dimensional’ propensity score. However, residual confounding may still exist for example, because statin initiators had a lower comorbidity load than non-initiators. Our study may be particularly susceptible to this bias because we lacked an active control.(35) Our study focused on secondary prevention patients and only included statin initiators who started after their MI. These design decisions strengthened our study, but lead to information most generalizable to the subset of patients with chronic kidney disease who were not taking statin therapy prior to being hospitalized for MI.
We found that initiation of statin therapy after myocardial infarction was associated with similar relative reductions in death and cardiovascular disease hospitalizations in patients across the range of eGFR. However, given the higher absolute rates of these events with worse kidney function, and their lower propensity to be prescribed a statin, our study suggests potential for public health gains by decreasing the gap in statin treatment among patients with chronic kidney disease.
Clinical Significance.
Our findings illustrate that patients with chronic kidney disease (CKD) can enjoy similar benefits from statin treatment as patients without CKD. Because cardiovascular event rates increase with decreasing kidney function the number needed to treat (NNT) is smaller (more favorable) with decreasing level of kidney function. Thus there is potential for public health gains from decreasing the existing disparity in statin treatment by level of CKD.
Table 2.
Clinical Outcomes Over One Year: Analysis of secondary prevention following myocardial infarction for statin initiators compared to statin non-initiators. Hazard ratios and 95% confidence intervals were estimated from Cox proportional hazards regression
| Statin Initators (n=5,597) | Statin Non-initiators (n=5,583) |
PS Matched and Adjusted Hazard Ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Events | Events per 100 person years |
Events | Events per 100 person years |
||
| Death | 614 | 11.93 | 904 | 18.47 | 0.79 (0.71, 0.88) |
| P-value for interaction of statin initiation by level of kidney function |
0.86 | ||||
| CV Hospitalization | 805 | 16.84 | 850 | 18.74 | 0.90 (0.82, 1.00) |
| P-value for interaction of statin initiation by level of kidney function |
0.77 | ||||
Acknowledgments
The National Institutes of Health (National Heart, Lung and Blood Institute) funded the study. The Center for Health Research, Kaiser Permanente Northwest worked through a subcontract to Kaiser Foundation Research Institute. ARRA Award No. 1RC2HL101666. The funding source had no involvement in the study design; in the collection, analysis and interpretation of data; in writing the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors had a role in writing this manuscript and had access to the data
Conflict of Interest: None
Reference List
- (1).Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- (2).Smith M, Lee N, Haney E, Carson S. Drug class review: HMG-CoA reductase inhibitors (statins) Update 5. World Wide Web 2010;Available from: URL: http://www.ohsu.edu/drugeffectiveness/reports/final.cfm. [PubMed]
- (3).Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci. D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, et al. Rosuvastatin and Cardiovascular Events in Patients Undergoing Hemodialysis. N Engl J Med. 2009 Apr 2;360(14):1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- (4).Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005 Jul 21;353(3):238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- (5).Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012 Aug 21;157(4):263–75. doi: 10.7326/0003-4819-157-4-201208210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Upadhyay A, Earley A, Lamont JL, Haynes S, Wanner C, Balk EM. Lipid-lowering therapy in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012 Aug 21;157(4):251–62. doi: 10.7326/0003-4819-157-4-201208210-00005. [DOI] [PubMed] [Google Scholar]
- (7).Freeman RV, Mehta RH, Al BW, Cooper JV, Kline-Rogers E, Eagle KA. Influence of concurrent renal dysfunction on outcomes of patients with acute coronary syndromes and implications of the use of glycoprotein IIb/IIIa inhibitors. J Am Coll Cardiol. 2003 Mar 5;41(5):718–24. doi: 10.1016/s0735-1097(02)02956-x. [DOI] [PubMed] [Google Scholar]
- (8).Al-Aly Z. Vascular calcification in uremia: what is new and where are we going? Adv Chronic Kidney Dis. 2008 Oct 15;(4):413–9. doi: 10.1053/j.ackd.2008.07.011. [DOI] [PubMed] [Google Scholar]
- (9).Lu KC, Wu CC, Yen JF, Liu WC. Vascular Calcification and Renal Bone Disorders. ScientificWorldJournal. 2014 Jul 17;2014:637065. doi: 10.1155/2014/637065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hornbrook MC, Hart G, Ellis JL, Bachman DJ, Ansell G, Greene SM, Wagner EH, Pardee R, Schmidt MM, Geiger A, Butani AL, Field T, Fouayzi H, Miroshnik I, Liu L, Diseker R, Wells K, Krajenta R, Lamerato L, Neslund DC. Building a virtual cancer research organization. J Natl Cancer Inst Monogr. 2005;(35):12–25. doi: 10.1093/jncimonographs/lgi033. [DOI] [PubMed] [Google Scholar]
- (11).Varas-Lorenzo C, Castellsague J, Stang MR, Tomas L, Aguado J, Perez-Gutthann S. Positive predictive value of ICD-9 codes 410 and 411 in the identification of cases of acute coronary syndromes in the Saskatchewan Hospital automated database. Pharmacoepidemiol Drug Saf. 2008 Aug 17;(8):842–52. doi: 10.1002/pds.1619. [DOI] [PubMed] [Google Scholar]
- (12).Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996 Oct 3;335(14):1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- (13).Liem AH, van Boven AJ, Veeger NJ, Withagen AJ, Robles de Medina RM, Tijssen JG, van Veldhuisen DJ. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J. 2002 Dec 23;(24):1931–7. doi: 10.1053/euhj.2002.3291. [DOI] [PubMed] [Google Scholar]
- (14).Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010 Feb 27;375(9716):735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- (15).Graham DJ. INcidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA: The Journal of the American Medical Association. 2004 Dec 1;292(21):2585–90. doi: 10.1001/jama.292.21.2585. SJS. [DOI] [PubMed] [Google Scholar]
- (16).Nichols GA, Desai J, Elston LJ, Lawrence JM, O'Connor PJ, Pathak RD, Raebel MA, Reid RJ, Selby JV, Silverman BG, Steiner JF, Stewart WF, Vupputuri S, Waitzfelder B. Construction of a Multisite DataLink Using Electronic Health Records for the Identification, Surveillance, Prevention, and Management of Diabetes Mellitus: The SUPREME-DM Project. Prev Chronic Dis. 2012 Jun;9:E110. doi: 10.5888/pcd9.110311. Epub@2012 Jun 7.:E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Andrade SE, Graham DJ, Staffa JA, Schech SD, Shatin D, La GL, Goodman MJ, Platt R, Gurwitz JH, Chan KA. Health plan administrative databases can efficiently identify serious myopathy and rhabdomyolysis. J Clin Epidemiol. 2005 Feb;58(2):171–4. doi: 10.1016/j.jclinepi.2004.10.004. [DOI] [PubMed] [Google Scholar]
- (18).Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).K/DOQI K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(Supplement 1):S1–266. [PubMed] [Google Scholar]
- (20).Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009 Jul;20(4):512–22. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Boston, MA: 2013. Pharmacoepidemiology Toolbox [computer program]. Version 2.4.11. [Google Scholar]
- (22).Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001 Apr;54(4):387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- (23).Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, Rochon PA, Anderson GM. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005 Apr 23;330(7497):960–2. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010 Jun;19(6):537–54. doi: 10.1002/pds.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 4th Springer; New York: 2010. Issues in data analysis; pp. 345–98. [Google Scholar]
- (26).Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999 Dec 4;319(7223):1492–5. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Keough-Ryan TM, Kiberd BA, Dipchand CS, Cox JL, Rose CL, Thompson KJ, Clase CM. Outcomes of acute coronary syndrome in a large Canadian cohort: impact of chronic renal insufficiency, cardiac interventions, and anemia. Am J Kidney Dis. 2005 Nov;46(5):845–55. doi: 10.1053/j.ajkd.2005.07.043. [DOI] [PubMed] [Google Scholar]
- (28).Cardinal H, Bogaty P, Madore F, Boyer L, Joseph L, Brophy JM. Therapeutic management in patients with renal failure who experience an acute coronary syndrome. Clin J Am Soc Nephrol. 2010 Jan;5(1):87–94. doi: 10.2215/CJN.04290609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ko D, Mamdani M, Alter D. Lipid-lowering therapy with statins in high-risk elderly patients: The treatment-risk paradox. JAMA. 2004 Apr 21;291(15):1864–70. doi: 10.1001/jama.291.15.1864. [DOI] [PubMed] [Google Scholar]
- (30).Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011 Jun 25;377(9784):2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kaneko H, Yajima J, Oikawa Y, Tanaka S, Fukamachi D, Suzuki S, Sagara K, Otsuka T, Matsuno S, Funada R, Kano H, Uejima T, Koike A, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Yamashita T. Effects of statin treatment in patients with coronary artery disease and chronic kidney disease. Heart Vessels. 2013 Feb 21; doi: 10.1007/s00380-013-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Natsuaki M, Furukawa Y, Morimoto T, Sakata R, Kimura T. Renal function and effect of statin therapy on cardiovascular outcomes in patients undergoing coronary revascularization (from the CREDO-Kyoto PCI/CABG Registry Cohort-2) Am J Cardiol. 2012 Dec 1;110(11):1568–77. doi: 10.1016/j.amjcard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- (33).Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003 Nov 1;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- (34).Dormuth CR, Patrick AR, Shrank WH, Wright JM, Glynn RJ, Sutherland J, Brookhart MA. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009 Apr 21;119(15):2051–7. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Setoguchi S, Gerhard T. Comparator Selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith S, Torchia M, editors. Developing a Protocol for Observational Comparative Effectiveness Research. Agency for Healthcare Research and Quality; Rockville, MD: 2013. pp. 59–70. AHRQ Publication No. 12(13)-EHC099. [PubMed] [Google Scholar]