Abstract
Microvascular pericytes take up ascorbic acid on the ascorbate transporter SVCT2. Intracellular ascorbate then protects the cells against apoptosis induced by culture at diabetic glucose concentrations. To investigate whether pericytes might also provide ascorbate to the underlying endothelial cells, we studied ascorbate efflux from human pericytes. When loaded with ascorbate to intracellular concentrations of 0.8–1.0 mM, almost two-thirds of intracellular ascorbate effluxed from the cells over 2 h. This efflux was opposed by ascorbate re-uptake from the medium, since preventing re-uptake by destroying extracellular ascorbate with ascorbate oxidase increased ascorbate loss even further. Ascorbate re-uptake occurred on the SVCT2, since its blockade by replacing medium sodium with choline, by the SVCT2 inhibitor sulfinpyrazone, or by extracellular ascorbate accelerated ascorbate loss from the cells. This was supported by finding that net efflux of radiolabeled ascorbate was increased by unlabeled extracellular ascorbate with a half-maximal effect in the range of the high affinity Km of the SVCT2. Intracellular ascorbate did not inhibit its efflux. To assess the mechanism of ascorbate efflux, known inhibitors of volume-regulated anion channels (VRACs) were tested. These potently inhibited ascorbate transport into cells on the SVCT2, but not its efflux. An exception was the anion transport inhibitor DIDS, which, despite inhibition of ascorbate uptake, also inhibited net efflux at 25–50 µM. These results suggest that ascorbate efflux from vascular pericytes occurs on a DIDS-inhibitable transporter or channel different from VRACs. Further, ascorbate efflux is opposed by re-uptake of ascorbate on the SVCT2, providing a potential regulatory mechanism.
Keywords: SVCT2, ascorbate efflux, voltage-regulated anion channels, ascorbic acid, pericytes
1. Introduction
Pericytes, which surround the endothelial cells of small venules and capillaries, support and interact with underlying endothelial cells, both to regulate blood flow and to tighten endothelial barrier permeability [1–4]. Pericytes are especially important in the brain and retina, where they help to maintain the tightness of the blood-brain barrier. Their loss is one of the hallmarks of early diabetic retinopathy [5–7], which is then followed by dysfunction of endothelial cells, resulting in leakage of serum proteins and lipids into the interstitial space [8–11].
We have shown that human brain pericytes undergo apoptosis following high glucose-induced activation of the Receptor for Advanced Glycation End-products (RAGE). Several days of treatment of pericytes with 100 µM vitamin C (ascorbic acid) increased intracellular ascorbate into the low millimolar range and prevented apoptosis due to RAGE agonists [12]. A subsequent study [13] showed that ascorbate uptake was largely mediated by the Sodium-dependent Vitamin C Transporter 2 (SVCT2) [14,15]. This energy-dependent co-transporter imports ascorbate with a relatively high affinity (apparent Km 20–50 µM) compared to the low millimolar apparent Km of the glucose transporter, which can take up the 2-electron oxidized form of ascorbate, or dehydroascorbate (DHA). The SVCT2 is inhibited by several non-specific anion transport inhibitors, such as sulfinpyrazone [15] and DIDS [16].
At least in the short-term, ascorbate is retained in cells because of its negative charge at physiologic pH. However, significant ascorbate efflux has been demonstrated in hepatocytes [17] and in endothelial cells [17–19]. In astrocytes [16,20] and neurons [21] ascorbate efflux was stimulated by glutamate, an effect attributed to cell-swelling-induced opening of volume-regulated anion channels (VRAC). Hypoosmotic conditions in these and other cells also activates channels with the pharmacologic features of VRAC [22]. However, the relative importance and mechanism by which ascorbate escapes cells under basal conditions has not been determined. Certainly it is important to assess this in epithelial cells of the kidney, intestine, and choroid plexus, which transfer ascorbate across otherwise impermeant barriers, but basal ascorbate efflux and its regulation may also be important in other cells. For example, most non-primate mammals synthesize ascorbate in hepatocytes, and it must somehow leave these cells to circulate and be taken up by other cells. Efflux of ascorbate is also likely to be of importance during deficiency in supporting cells, such as astrocytes for neurons and vascular pericytes for endothelial cells. Such a supportive function of pericytes may be especially relevant for endothelial cells making up the blood-brain barrier. In most brain regions, there is little transit of ascorbate across the blood-brain barrier [23]. This is due to the tightness of the barrier, but may also be due to the finding that brain endothelial cells lack ascorbate transporters, developing the SVCT2 only with time in culture [24]. Under normal conditions, ascorbate enters the brain through the choroid plexus into the cerebrospinal fluid, where it then diffuses to the parenchyma [23]. The ability of a supporting cell such as the microvascular pericyte to both take up and efflux ascorbate thus could provide a dynamic system that helps to ensure a reserve supply of the vitamin to endothelial cells and neurons alike.
In this work we investigated ascorbate efflux from human microvascular pericytes, assessing the role of ascorbate re-uptake and key features of the efflux system.
2. Experimental Procedures
2.1. Materials
Sigma/Aldrich Chemical Co. (St. Louis, MO) supplied the reagent chemicals, including ascorbate, ascorbate oxidase, clomiphene, (4-(2-butyl-6,7-dichloro-2-cyclopentylindan-1-on-5-yl)oxybutyric acid (DCPIB), DHA, 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS), N-2-hydroxyethylpiperazine N’-2-ethanesulfonic acid (Hepes), R(+)-[(6,7-dichloro-2-cyclopentyl-2,3-dihydro-2-methyl-1-oxo-1H-inden-5-yl)-oxy]acetic acid (IAA-94), niflumic acid, 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), and sulfinpyrazone. Perkin-Elmer Life and Analytical Sciences, Inc. (Boston, MA) supplied the [1-14C]ascorbic acid (4.0 µCi/mmol).
2.2. Cell Culture
Human brain vascular pericytes were obtained from Sciencell Research Laboratories (Carlsbad, CA, catalog #1200) and cultured in Pericyte Medium with included supplements from the same company (catalog #1201). Cells were cultured on plates coated with poly-L-lysine at 37°C in humidified air containing 5% CO2. Cells were used within 3–10 passages. It should be noted that the commercial culture medium for these cells contains ascorbate. In these experiments cells were cultured in medium that was stored at 3°C for 2 weeks, which depletes intracellular ascorbate to less than about 2 µM [13].
2.3. Assay of unlabeled ascorbate
To measure unlabeled intracellular ascorbate, pericytes in 6-well plates were rinsed twice with Krebs-Ringer Hepes buffer (KRH; 20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4) and lysed with 25 % metaphosphoric acid (w/v). The lysate was then neutralized with 3 volumes of 100 mM Na2HPO4 and 0.05 mM EDTA (pH 8.0), and cells were scraped from the plate with a rubber spatula. The lysate was centrifuged at 3°C for 1 min at 13,000 × g and the supernatant was taken for assay of ascorbate. Assay of ascorbate was performed in duplicate by ion pair high performance liquid chromatography with electrochemical detection as previously described [25]. Intracellular ascorbate concentrations were calculated based on the intracellular distribution space of 3-O-methylglucose in pericytes, measured as previously described for EA.hy926 endothelial cells [26]. This pericyte distribution space was 6.1 ± 1.6 µl/mg protein (N = 6 determinations).
2.4. Assay of unlabeled ascorbate uptake
Pericytes cultured to confluence in 6-well plates were rinsed twice with 2 ml of 37°C KRH containing 5 mM D-glucose and incubated with the agents indicated in 2 ml of glucose-KRH for 10 min at 37 °C. Unlabeled ascorbate was added to a concentration of 100 µM and the incubation was continued for another 30 min at 37°C. The cells were rinsed twice in 2 ml of ice-cold glucose-KRH and taken for assay of intracellular ascorbate as described above.
2.5. Assay of ascorbate efflux
Pericytes cultured to confluence in 6-well plates in cold-stored (ascorbate-depleted) culture medium were treated with unlabeled ascorbate at the indicated concentrations. After 1 h at 37°C, the medium was aspirated and the cells were gently rinsed twice in 2 ml of KRH that contained 5 mM D-glucose. After the last rinse, the 1 ml of glucose-KRH was added and the cells were incubated at 37°C. At the indicated times, the medium was aspirated and the cells were rinsed twice in 2 ml of ice-cold KRH to stop efflux and taken for assay of intracellular ascorbate as described above.
For assay of radiolabeled ascorbate efflux, confluent cells in 12-well plates cultured in 1 ml of ascorbate-depleted medium were loaded with 0.05 µCi of L-[1-14C]ascorbic acid (10 µM) for 1 h at 37°C. The cells were rinsed twice in 1 ml of glucose-KRH, followed by addition of 1 ml of glucose-KRH at 37 °C. At the indicated times, the medium was removed and the cells were rinsed twice in 2 ml of ice-cold KRH. After the last rinse and removal of KRH, cells were treated with 1 ml of 0.05 N NaOH, scraped from the plate, and the extract was added to 5 ml Ecolume liquid scintillation fluid (ICN, Costa Mesa, CA) and mixed. The radioactivity of duplicate samples was measured in a Packard CA-2200 liquid scintillation counter, after allowing at least 1 h before counting for decay of chemiluminescence. For assay of extracellular radiolabeled ascorbate, at the times indicated, 100 µL aliquots of the medium were removed and treated as above with 1 ml of 0.05 N NaOH. This was added to 5 ml of scintillation fluid, and radioactivity counted as described above.
2.6. Data Analysis
Results are shown as mean + standard error. Statistical comparisons were made using GraphPad Prism version 6.05 for Windows (GraphPad Software, San Diego, CA). Differences between treatments were assessed by the Student’s t-test or one-way ANOVA with post-hoc testing using Tukey’s or Dunnett’s test, as appropriate.
3. Results
3.1. Ascorbate efflux and re-uptake in human brain microvascular pericytes
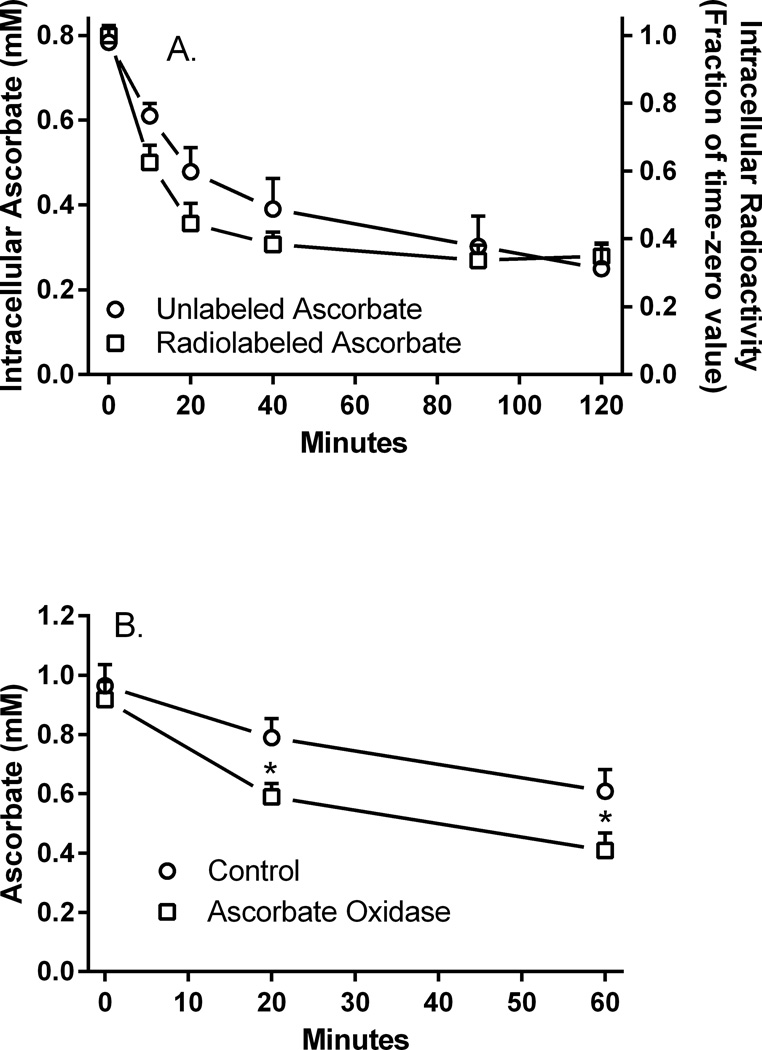
We showed previously that human brain pericytes take up 100 µM ascorbate to an intracellular concentration of about 0.8 mM over 30 min of incubation at 37°C [13]. Similar initial intracellular ascorbate concentrations were found after 60 min of incubation of these cells with ascorbate (Fig. 1A, circles, left-hand Y-axis). After cells were rinsed twice in glucose-containing KRH and then incubated over 120 min with this KRH, intracellular ascorbate progressively decreased to about 300 µM. Similar results were observed when the cells were loaded with 10 µM radiolabeled ascorbate, with intracellular ascorbate amounts plateauing at about 30% of the initial loading value (Fig. 1A, squares, right-hand axis). Efflux of ascorbate was slightly more rapid for the lower amounts of radiolabeled ascorbate than for unlabeled ascorbate (Fig. 1A, compare circles to squares). Thus, despite loading to intracellular ascorbate concentrations several-fold more than initially present in the medium, the cells could only retain about one third of the initial intracellular levels attained.
Figure 1. Intracellular ascorbate concentrations.
Panel A. Efflux ascorbate from cells loaded with 100 µM ascorbate (circles) or 10 µM [1-14C]ascorbate (squares) was measured as described under Materials and methods. Results for radiolabeled ascorbate efflux are expressed as a fraction of the time zero value, which was 49 ± 5 pmol/well. N = 6 plates for each form of ascorbate. Panel B. Efflux of unlabeled ascorbate in the absence (circles) or presence (squares) of 1.6 U/ml ascorbate oxidase was measured as described in Materials and methods. Results are shown from 8 plates, with p < 0.05 compared to the corresponding control sample from the same plate lacking ascorbate oxidase.
To determine whether extracellular ascorbate might re-enter cells during efflux, cells loaded with 100 µM ascorbate as described in Fig. 1 were also treated with 1.6 U/ml ascorbate oxidase during efflux. Ascorbate oxidase will not enter the cells over this time period and will destroy extracellular ascorbate, thus preventing its re-uptake on the ascorbate transporter. As shown in Fig. 1B, the presence of ascorbate oxidase outside the cells enhanced apparent ascorbate efflux over 60 min compared to cells not treated with the enzyme. This result supports the notion that the apparent increase in efflux with ascorbate oxidase was due to loss of ascorbate re-uptake, possibly on the SVCT2 ascorbate transporter. If so, then known inhibitors of the SVCT2 uptake should also accelerate apparent ascorbate efflux.
3.2. Mechanism of ascorbate re-uptake
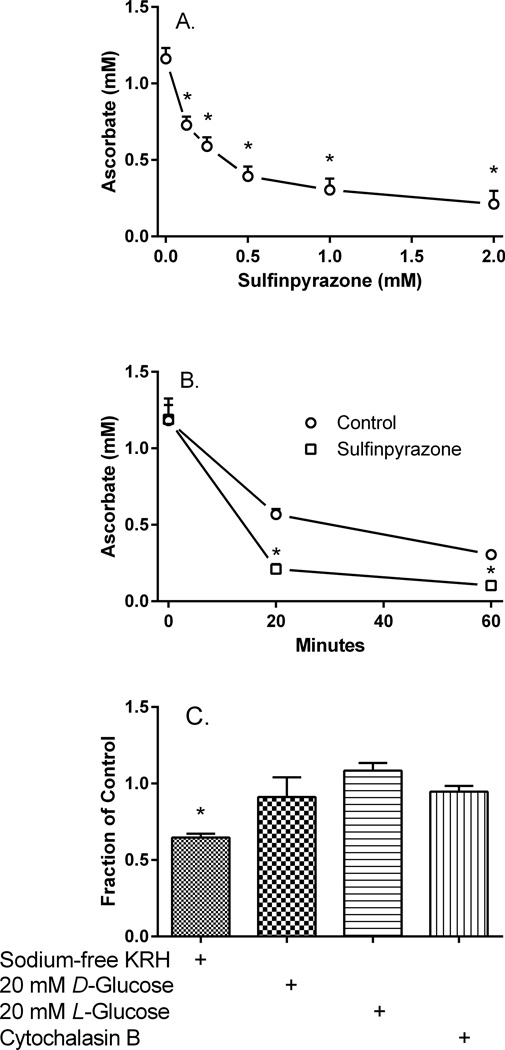
Sulfinpyrazone, a non-specific anion channel inhibitor that is known to inhibit ascorbate uptake in other cells [27,28], inhibited uptake of unlabeled ascorbate to about 16% of control at 1 mM (Fig. 2A). When a concentration of 1 mM sulfinpyrazone was present during efflux of unlabeled ascorbate (Fig. 2B, squares), the apparent efflux of ascorbate was more rapid over 1 h than in its absence (Fig. 2B, circles). Since inhibition of ascorbate efflux would have increased retention of ascorbate in the cells, the finding of a decrease supports the notion that a substantial amount of ascorbate re-enters cells during efflux. It also suggests that it could be on the SVCT2, notwithstanding the relatively low specificity of sulfinpyrazone for the SVCT2.
Figure 2. Effects of SVCT2 and glucose transport inhibition on intracellular ascorbate concentrations.
Panel A. Pericytes were incubated at 37 °C with the indicated concentrations of sulfinpyrazone, followed in 10 min by 100 µM ascorbate. After 30 min the cells were rinsed and taken for assay of intracellular ascorbate as described in Materials and methods. Results are shown from 4 experiments with an “*” indicating p < 0.05 compared to the sample not treated with the inhibitor. Panel B. Cells loaded for 60 min with 100 µM ascorbate were treated without (circles) or with 1 mM sulfinpyrazone at 37 °C during efflux and intracellular ascorbate was measured as described in Materials and methods. Results are shown from 6 experiments, with an “*” indicating p < 0.05 compared to the corresponding time point sample without the inhibitor. Panel C. Cells loaded with 100 µM ascorbate for 60 min were rinsed twice in 37 °C KRH containing 5 mM D-glucose and either 128 mM sodium or 128 mM choline chloride. Cells were incubated at 37°C during ascorbate efflux in sodium-free KRH (first bar) or in sodium-containing KRH with 20 mM D-glucose (second bar), 20 mM L-glucose plus 5 mM D-glucose (third bar), or 5 mM D-glucose plus 10 µM cytochalasin B and 1.6 U/ml ascorbate oxidase (fourth bar). After 30 min, the medium was aspirated, the cells were rinsed twice in the respective KRH and were then taken for assay of intracellular ascorbate. Results are shown from 4–5 experiments, expressed as a fraction of the 5 mM D-glucose control that was carried out in each experiment. An “*” indicates p < 0.01 compared to that control.
Further suggesting that the SVCT2 mediated ascorbate re-uptake was the finding that replacement of sodium with choline in the medium KRH during rinses and efflux also decreased intracellular ascorbate by 35% compared to that seen in sodium-containing KRH at 30 min (Fig. 2C, first bar). Inhibition of ascorbate transport on the SVCT2 due to lack of sodium in the medium would prevent its re-uptake and thus increase apparent net efflux, reflected by decreased cellular ascorbate.
It is likely that extracellular ascorbate will also be oxidized to DHA outside cells during efflux. This is especially relevant to the experiment in Fig. 1B using ascorbate oxidase, which will convert extracellular ascorbate to DHA. This DHA could also re-enter the cells on the ubiquitous glucose transporter. The efflux experiments were carried out in 5 mM D-glucose to provide ongoing cellular energy. This glucose concentration would likely inhibit DHA uptake on the GLUT1 transporter, which is the major glucose transporter in bovine retinal pericytes, with an EC50 of 0.5–2 mM [29]. To examine whether a higher concentration of D-glucose might further inhibit ascorbate uptake, cells that had been loaded with 100 µM ascorbate were rinsed twice and incubated in KRH that contained 5 mM d-glucose, 20 mM D-glucose, or both 5 mM d-glucose and 20 mM l-glucose. After 30 min of efflux, there was no difference in intracellular ascorbate (Fig. 2C, second and third bars). Similarly, the known glucose transport inhibitor cytochalasin B at 10 µM had no effect on net ascorbate efflux (Fig. 2C, fourth bar). Together, these results suggest that the apparent ascorbate re-uptake was not due to uptake of DHA on the glucose transporter.
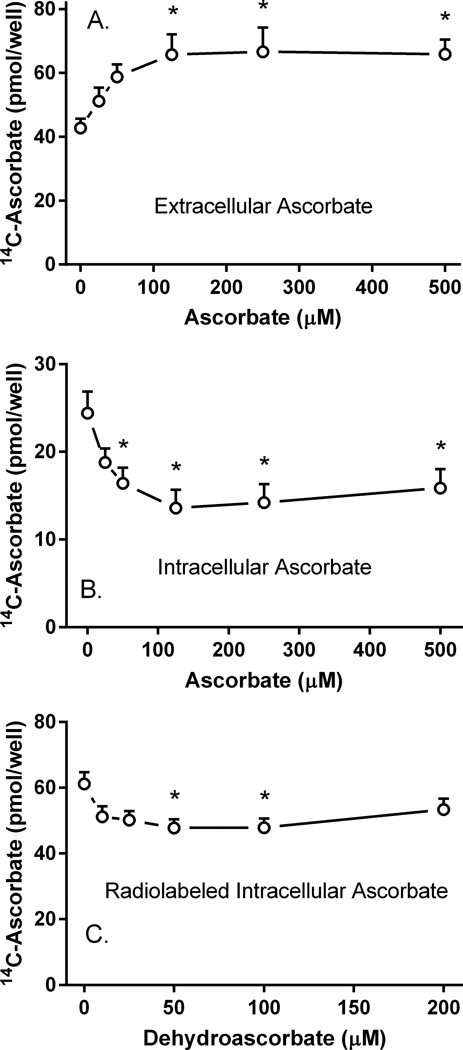
To determine whether this re-uptake is in fact on the SVCT2, cells were first loaded with 10 µM radioactive ascorbate for 60 min, rinsed to remove extracellular radioactivity, and incubated for 60 min at 37°C in the presence of increasing concentrations of unlabeled ascorbate. The presence of unlabeled extracellular ascorbate during efflux of radiolabeled ascorbate significantly increased extracellular radiolabeled ascorbate (Fig. 3A) and decreased intracellular radiolabeled ascorbate (Fig. 3B). These results can be explained by positing that increasing concentrations of unlabeled extracellular ascorbate competitively prevent re-uptake of radiolabeled ascorbate, both increasing extracellular radiolabeled ascorbate and decreasing intracellular radiolabeled ascorbate. The effect on each phase was saturable, with a half-maximal response of about 25 µM. The maximal effect was about 50% of the control value in the absence of added ascorbate during efflux. These results support the notion of substantial ascorbate re-uptake on the SVCT2 during efflux.
Figure 3. Effects of intra- and extracellular unlabeled ascorbate on radiolabeled ascorbate efflux.
Pericytes in 12-well plates were incubated for 60 min with 10 µM radiolabeled ascorbate, the medium was aspirated, and the cells were rinsed twice in KRH containing 5 mM D-glucose. The cells were incubated with 1 ml of glucose-KRH containing the indicated concentration of unlabeled ascorbate. After 60 min, radiolabeled ascorbate was measured in the medium (Panel A) or in the cells (Panel B) as described in Materials and methods. Results are shown from 6 experiments, with an “*” indicating p < 0.05 compared to the sample not treated with unlabeled ascorbate. Panel C. Pericytes were loaded with 10 µM radiolabeled ascorbate for 20 min in complete medium, followed by addition of the indicated concentration of DHA. After another 30 min at 37°C, the medium was removed and the cells were rinsed twice in 37°C glucose-KRH. Following addition of 1 ml of glucose-KRH at 37°C, efflux was measured at 30 min as described in Materials and methods. Results are shown from 6 experiments, with an “*” indicating p < 0.05 compared to the sample not treated with DHA.
3.3. Mechanism of ascorbate efflux by pericytes
To determine whether intracellular ascorbate affects its efflux, cells were treated for 20 min with 10 µM radiolabeled ascorbate to load the cells, followed by increasing concentrations of DHA. After 30 min, the cells were rinsed twice with KRH and incubated for another 30 min at 37°C to allow efflux. Conversion of unlabeled DHA to unlabeled ascorbate within cells should increase the intracellular concentration of unlabeled ascorbate, which would then be present during the efflux of radiolabeled ascorbate. As shown in Fig. 3C, there was a modest increase of apparent efflux. This was likely due to inhibition of radiolabeled ascorbate re-uptake by effluxed ascorbate. Had there been inhibition of radiolabeled efflux, one would have expected intracellular radiolabeled ascorbate to increase, not decrease. Thus, intracellular ascorbate has little effect on its efflux.
As noted in the Introduction, previous studies have suggested that ascorbate might leave cells on an ion channel under hypo-osmotic conditions, with VRAC being the most likely candidate. We therefore tested several known inhibitors of VRACs and other anion channels, including niflumic acid, DCPIB, clomiphene, NPPB, and IAA-94 at concentrations known to inhibit anion efflux in VRACs. However, as shown in Fig. 4A, all of these inhibitors also partially blocked uptake of unlabeled ascorbate into pericytes. Each inhibitor was then tested at the same concentrations on unlabeled ascorbate efflux (Fig. 4B–F). Rather than inhibit ascorbate efflux (which would have retained intracellular ascorbate after 30 to 60 min), these agents either had no effect (niflumic acid, Fig. 4B) or increased apparent efflux (DCPIB, Fig. 4C; clomiphene, Fig. 4D; NPPB, Fig. 4E; and IAA-94, Fig. 4F). This suggests that as with sulfinpyrazone, the effect of most of the agents was to block ascorbate re-uptake, making it unlikely that VRAC or related channels mediate the efflux of basal ascorbate in these cells.
Figure 4. VRAC inhibitor effects on ascorbate uptake and efflux.
Panel A. After 2 rinses in KRH containing 5 mM D-glucose at 37°C, pericytes were treated with the inhibitors as noted for 10 min, followed by addition of 100 µM ascorbate. After 30 min at 37°C, the cells were rinsed and taken for assay of intracellular ascorbate as described in Methods. The inhibitor concentrations were: niflumic acid, 100 µM; DCPIB, 20 µM; clomiphene, 100 µM; NPPB, 100 µM, and IAA-94, 100 µM. Results are shown from 4 experiments, with an “*” indicating p < 0.05 compared to the sample not treated with an inhibitor. Panels B – F. Pericytes were loaded with 100 µM ascorbate for 60 min in medium at 37°C. After medium aspiration and 2 rinses in glucose-KRH, the cells were treated with glucose-KRH containing the inhibitor noted in each panel at the same concentration used in the uptake studies. Efflux of unlabeled ascorbate was measured at each time point indicated. Results are shown from 3–4 experiments, with an “*” indicating p < 0.05 compared to the corresponding control at 20 or 60 min of efflux.
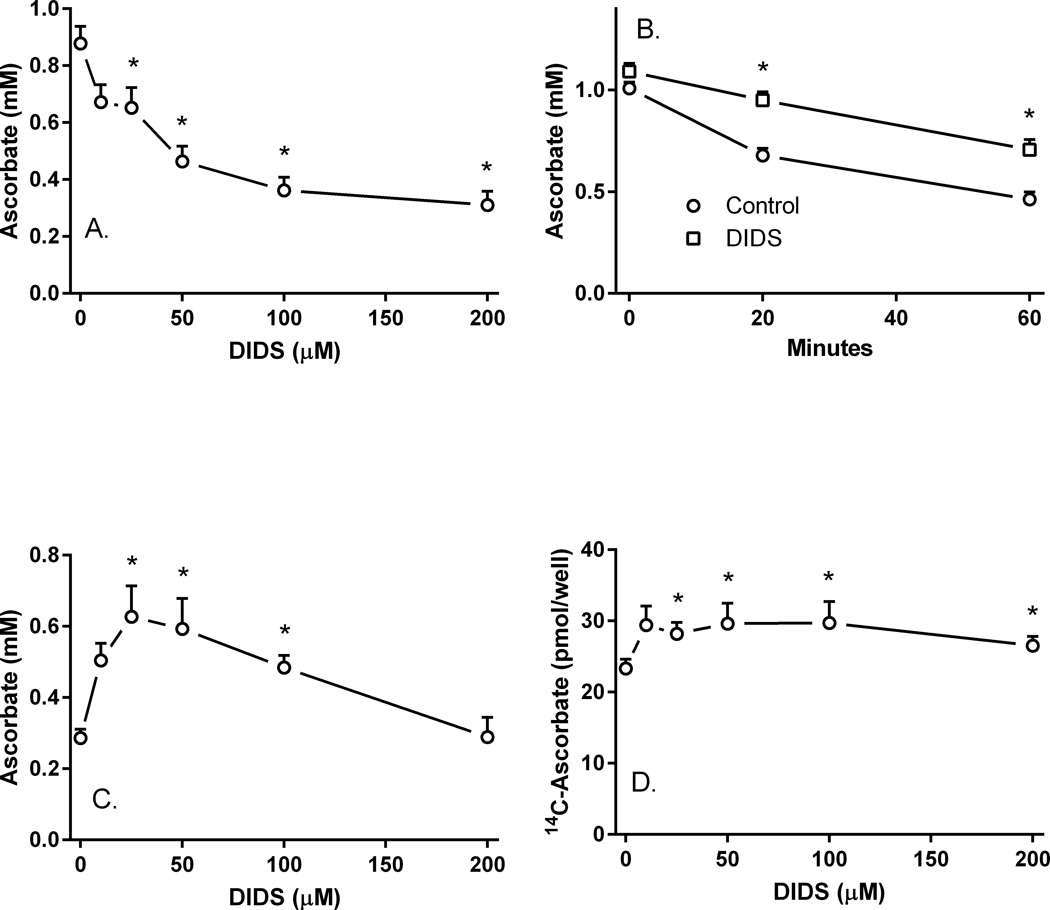
However, different results were obtained with the stilbene disulfonate anion transport inhibitor DIDS. As expected, DIDS inhibited ascorbate uptake into pericytes with a near-maximal effect at 100 µM (Fig. 5A). When cells were loaded with 100 µM unlabeled ascorbate followed by efflux over 60 min, the presence of 100 µM DIDS in the efflux medium significantly retarded apparent efflux at both 20 and 60 min (Fig. 5B). The effect of DIDS to maintain intracellular ascorbate was biphasic, with a maximal effect at 25–50 µM, decreasing back to baseline at higher DIDS concentrations (Fig. 5C). When cells were loaded with 10 µM radioactive ascorbate instead of higher concentrations of unlabeled ascorbate, a similar retention of intracellular ascorbate was induced by the presence of increasing concentrations of DIDS (Fig. 5D).
Figure 5. DIDS inhibition of ascorbate uptake and efflux.
Panel A. Pericytes were rinsed and treated in KRH containing 5 mM D-glucose with the indicated concentrations of DIDS at 37°C for 30 min before rinsing and assay of intracellular ascorbate. Results are from 6 experiments, with an “*” indicating p < 0.05 compared to a sample not treated with DIDS. Panel B. Pericytes loaded with 100 µM unlabeled ascorbate for 60 min in culture medium at 37°C were rinsed twice in 37°C glucose-KRH and treated without (circles) or with (squares) 100 µM DIDS in 1 ml of glucose-KRH. At the indicated times, the medium was aspirated and the cells were rinsed twice in 2 ml of ice-cold KRH before assay of intracellular ascorbate. Results are from 5 experiments, with an “*” indicating p < 0.05 compared to the sample not treated with DIDS at the same time point. Panel C. Pericytes were loaded with 100 µM unlabeled ascorbate for 60 min, rinsed twice in glucose-KRH, and treated in 37 °C glucose-KRH with the indicated concentration of DIDS. After 30 min, the cells were rinsed again in ice-cold KRH and taken for assay of intracellular ascorbate. Results are shown from 6 experiments with an “*” indicating p < 0.05 compared to the sample not treated with DIDS. Panel D. Pericytes in culture medium were loaded with 10 µM radiolabeled ascorbate for 60 min, rinsed twice in glucose-KRH, and treated with the indicated concentration of DIDS in glucose-KRH. After 30 min, the cells were rinsed and taken for assay of intracellular radiolabeled ascorbate. Results are shown from 7 experiments, with an “*” indicating p < 0.05 compared to the sample not treated with DIDS.
4. Discussion
The present studies show that ascorbate effluxes from human brain microvascular pericytes and that this effect is counteracted by substantial re-uptake of the vitamin from the surrounding medium. This effect is evident both at low millimolar intracellular ascorbate concentrations and much lower intracellular concentrations when the cells are loaded with tracer amounts of radioactive ascorbate. Multiple features of the re-uptake suggest that it occurs on the SVCT2 transporter. First, loss of intracellular ascorbate is increased when extracellular ascorbate is destroyed with ascorbate oxidase. Second, inhibition of the SVCT2 by substitution of medium sodium with choline or by the known inhibitor sulfinpyrazone enhances apparent efflux. Third, ascorbate uptake is not affected by high concentrations of d- or l-glucose or by cytochalasin B, indicating that the observed re-uptake was not due to uptake of DHA on glucose transporters. Fourth, increasing concentrations of extracellular ascorbate during efflux caused the cells to retain less radiolabeled ascorbate during efflux. Because of the concentration-dependence of the effect, this likely reflects inhibition of radiolabeled ascorbate re-uptake. A specific role for the SVCT2 in ascorbate re-uptake was further supported by the finding that the effects of increasing extracellular ascorbate on the retention of radiolabeled ascorbate were half-maximal at extracellular concentrations of about 25 µM, which are in the range of the Km for the high affinity SVCT2 [15]. Finally, we have shown that these human pericytes express the SVCT2 [13].
If extracellular DHA inhibits the SVCT2, this could affect re-uptake of ascorbate, especially in the presence of added ascorbate oxidase, which will generate DHA from ascorbate. Others have shown in cell lines [30] and bovine retinal pericytes [31]. However, we did not observe this for 10 µM radiolabeled ascorbate uptake in the same primary culture pericytes used in this study over a range of added DHA concentrations (10–200 µM) [13]. Thus, we do not think that inhibition of the SVCT2 by DHA is a factor in the current experiments.
As noted in the Introduction, other cells, including hepatocytes and endothelial cells are known to efflux ascorbate. When directly compared, hepatocytes have higher relative rates of efflux than do endothelial cells [17]. This might be expected, given that at least in non-primates, hepatocytes synthesize ascorbate and release it for use by the rest of the body. In the absence of ascorbate re-uptake, pericytes in the present studies had released from 50 to 85% of their intracellular ascorbate in 60 min. These results suggest that ascorbate re-uptake serves as a compensatory mechanism for the otherwise relatively rapid loss of ascorbate from cells.
Whereas extracellular ascorbate enhanced ascorbate release from pericytes, intracellular ascorbate did not. In hepatocytes efflux was inhibited about 50% by 4 mM intracellular ascorbate [17]. Since we did not achieve intracellular ascorbate concentrations much above 1.5 mM in pericytes in this study, we may have missed this effect. However, we failed to observe an effect on ascorbate efflux even at intracellular ascorbate concentrations as high as 3 mM in EA.hy926 cells [19].
When cultured cells are placed in conditions of low osmolality, VRAC are activated and release increased amounts of ascorbate and other organic anions [20,32]. Similarly, it has been shown that glutamate treatment of astrocytes causes cell swelling and activation of VRACs to release more ascorbate [33]. How ascorbate effluxes from cells in the absence of such stimuli, as studied in this work, remains an enigma. We tested several known VRAC inhibitors and found that they all inhibited ascorbate uptake at concentrations usually used to inhibit VRACs. For the most part, they also accelerated apparent ascorbate efflux. This could be due to activation of efflux or to inhibition of re-uptake. Since these agents are known as inhibitors of anion channels, we favor the latter explanation and conclude that VRACs are not involved in the mechanism of basal ascorbate efflux.
In contrast to the VRAC inhibitors, low concentrations (25–50 µM) of the generic anion transport inhibitor DIDS decreased both unlabeled and radiolabeled ascorbate efflux from pericytes. This, along with previous results that ascorbate efflux is regulated by intracellular calcium in endothelial cells [18,19], suggests that the efflux occurs on a channel or transport protein and is not due to simple diffusion. Because of its two sulfonic acid groups, DIDS should not penetrate the cells to an appreciable extent during the time course of efflux, suggesting that it interacts with the exofacial portion of the efflux channel/transporter. At higher concentrations, the inhibitory effect of DIDS on efflux of unlabeled ascorbate declines, although there is only a trend for radiolabeled ascorbate. This suggests that another process increasing efflux is induced by concentrations of DIDS 100 µM and higher. Certainly inhibition of any re-uptake on the SVCT2 by DIDS could contribute to this effect, as could opening of another efflux channel by higher DIDS concentrations.
The results of this study show that brain microvascular pericytes release ascorbate and that this release is counteracted in part by re-uptake on the SVCT2. Ascorbate efflux likely occurs on a channel/transporter that does not resemble a VRAC in terms of its response to inhibitors. Nonetheless, ascorbate efflux is substantial and could acutely supply the vitamin to neighboring endothelial cells when extracellular concentrations of the vitamin are sharply decreased in response to oxidative stress.
Acknowledgements
This work was supported by National Institutes of Health grant DK050435 and by the Cell Culture Core of the Vanderbilt Diabetes Research and Training Center (DK020593).
Abbreviations
- DCPIB
(4-(2-butyl-6,7-dichloro-2-cyclopentylindan-1-on-5-yl)oxybutyric acid
- DIDS
4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid
- DHA
dehydroascorbate
- Hepes
N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid
- KRH
Krebs-Ringer Hepes
- IAA-94
R(+)-[(6,7-dichloro-2-cyclopentyl-2,3-dihydro-2-methyl-1-oxo-1H-inden-5-yl)-oxy]acetic acid
- NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid
- VRAC
voltage-regulated anion channels.
References
- 1.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol. Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci. Methods. 2011;199:223–229. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Wisniewska-Kruk J, Hoeben KA, Vogels IM, Gaillard PJ, Van Noorden CJ, Schlingemann RO, Klaassen I. A novel co-culture model of the blood-retinal barrier based on primary retinal endothelial cells, pericytes and astrocytes. Exp. Eye Res. 2012;96:181–190. doi: 10.1016/j.exer.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira F. Pericytes in diabetic retinopathy. Br. J Ophthalmol. 1966;50:134–143. doi: 10.1136/bjo.50.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Yanoff M, Liu X, Ye X. Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin Med. J (Engl.) 1997;110:659–663. [PubMed] [Google Scholar]
- 7.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mimura K, Umeda F, Yamashita T, Kobayashi K, Hashimoto T, Nawata H. Effects of glucose and an aldose reductase inhibitor on albumin permeation through a layer of cultured bovine vascular endothelial cells. Horm. Metab Res. 1995;27:442–446. doi: 10.1055/s-2007-979998. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita T, Mimura K, Umeda F, Kobayashi K, Hashimoto T, Nawata H. Increased transendothelial permeation of albumin by high glucose concentration. Metabolism. 1995;44:739–744. doi: 10.1016/0026-0495(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 10.Stauber WT, Ong SH, McCuskey RS. Selective extravascular escape of albumin into the cerebral cortex of the diabetic rat. Diabetes. 1981;30:500–503. doi: 10.2337/diab.30.6.500. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzi M, Healy DP, Hawkins R, Printz JM, Printz MP. Studies on the permeability of the blood-brain barrier in experimental diabetes. Diabetologia. 1986;29:58–62. doi: 10.1007/BF02427282. [DOI] [PubMed] [Google Scholar]
- 12.May JM, Jayagopal A, Qu ZC, Parker WH. Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes. Biochem. Biophys. Res. Commun. 2014;452:112–117. doi: 10.1016/j.bbrc.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker WH, Qu ZC, May JM. Ascorbic acid transport in brain microvascular pericytes. Biochem. Biophys. Res. Commun. 2015;458:262–267. doi: 10.1016/j.bbrc.2015.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol. Membr. Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 15.May JM. The SLC23 family of ascorbate transporters: ensuring that you get and keep your daily dose of vitamin C. Br. J Pharmacol. 2011;164:1793–1801. doi: 10.1111/j.1476-5381.2011.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson JX, Dixon SJ. Ascorbic acid transport in mouse and rat astrocytes is reversibly inhibited by furosemide, SITS, and DIDS. Neurochem. Res. 1989;14:1169–1175. doi: 10.1007/BF00965504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upston JM, Karjalainen A, Bygrave FL, Stocker R. Efflux of hepatic ascorbate: a potential contributor to the maintenance of plasma vitamin C. Biochem. J. 1999;342:49–56. [PMC free article] [PubMed] [Google Scholar]
- 18.Davis KA, Samson SE, Best K, Mallhi KK, Szewczyk M, Wilson JX, Kwan CY, Grover AK. Ca(2+)-mediated ascorbate release from coronary artery endothelial cells. Br. J. Pharmacol. 2006;147:131–139. doi: 10.1038/sj.bjp.0706492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May JM, Qu ZC. Ascorbic acid efflux and re-uptake in endothelial cells: maintenance of intracellular ascorbate. Mol. Cell Biochem. 2009;325:79–88. doi: 10.1007/s11010-008-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siushansian R, Dixon SJ, Wilson JX. Osmotic swelling stimulates ascorbate efflux from cerebral astrocytes. J. Neurochem. 1996;66:1227–1233. doi: 10.1046/j.1471-4159.1996.66031227.x. [DOI] [PubMed] [Google Scholar]
- 21.May JM, Li L, Hayslett K, Qu ZC. Ascorbate transport and recycling by SH-SY5Y neuroblastoma cells: Response to glutamate toxicity. Neurochem. Res. 2006;31:785–794. doi: 10.1007/s11064-006-9077-z. [DOI] [PubMed] [Google Scholar]
- 22.Al-Nakkash L, Iserovich P, Coca-Prados M, Yang H, Reinach PS. Functional and molecular characterization of a volume-activated chloride channel in rabbit corneal epithelial cells. J Membr. Biol. 2004;201:41–49. doi: 10.1007/s00232-004-0706-5. [DOI] [PubMed] [Google Scholar]
- 23.Harrison FE, May JM. Vitamin C function in the brain: Vital role of the ascorbate transporter (SVCT2) Free Radic. Biol Med. 2009;45:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao H, May JM. Development of ascorbate transport in brain capillary endothelial cells in culture. Brain Res. 2008;1208:79–86. doi: 10.1016/j.brainres.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May JM, Qu Z-C, Mendiratta S. Protection and recycling of a-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch. Biochem. Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 26.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of a-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 27.Siushansian R, Wilson JX. Ascorbate transport and intracellular concentration in cerebral astrocytes. J. Neurochem. 1995;65:41–49. doi: 10.1046/j.1471-4159.1995.65010041.x. [DOI] [PubMed] [Google Scholar]
- 28.Holmes ME, Samson SE, Wilson JX, Dixon SJ, Grover AK. Ascorbate transport in pig coronary artery smooth muscle: Na+ removal and oxidative stress increase loss of accumulated cellular ascorbate. J. Vasc. Res. 2000;37:390–398. doi: 10.1159/000025755. [DOI] [PubMed] [Google Scholar]
- 29.Mandarino LJ, Finlayson J, Hassell JR. High glucose downregulates glucose transport activity in retinal capillary pericytes but not endothelial cells. Invest. Ophthalmol. Vis. Sci. 1994;35:964–972. [PubMed] [Google Scholar]
- 30.Fiorani M, Azzolini C, Guidarelli A, Cerioni L, Cantoni O. A novel biological role of dehydroascorbic acid: Inhibition of Na(+)-dependent transport of ascorbic acid. Pharmacol. Res. 2014;84:12–17. doi: 10.1016/j.phrs.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Khatami M, Li WY, Rockey JH. Kinetics of ascorbate transport by cultured retinal capillary pericytes. Inhibition by glucose. Invest Ophthalmol. Vis. Sci. 1986;27:1665–1671. [PubMed] [Google Scholar]
- 32.Lane DJ, Lawen A. The glutamate aspartate transporter (GLAST) Mediates L-glutamate-stimulated ascorbate-release via swelling-activated anion channels in cultured neonatal rodent astrocytes. Cell Biochem. Biophys. 2013;65:107–119. doi: 10.1007/s12013-012-9404-8. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JX, Peters CE, Sitar SM, Daoust P, Gelb AW. Glutamate stimulates ascorbate transport by astrocytes. Brain Res. 2000;858:61–66. doi: 10.1016/s0006-8993(99)02433-6. [DOI] [PubMed] [Google Scholar]