Abstract
Background
To determine whether matted nodes (MNs) uniquely identify HPV+ oropharyngeal cancer (OPC) patients at disproportionately high distant failure (DF) risk who may benefit from intensified systemic therapy.
Methods
178 stage III/IV HPV+ OPC patients who completed definitive chemoradiotherapy were stratified by risk-group (low-risk=T1-3/N0-2c/<10 pack-years; intermediate-risk=T1-3/N0-2c/≥10 pack-years; high-risk=T4 or N3). Prognostic impact of MNs was assessed.
Results
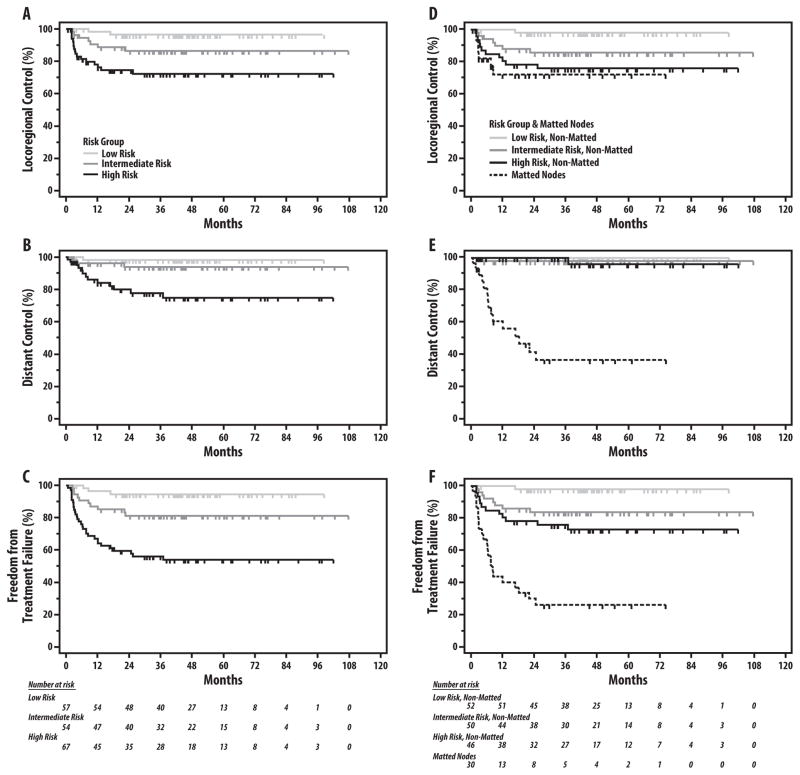
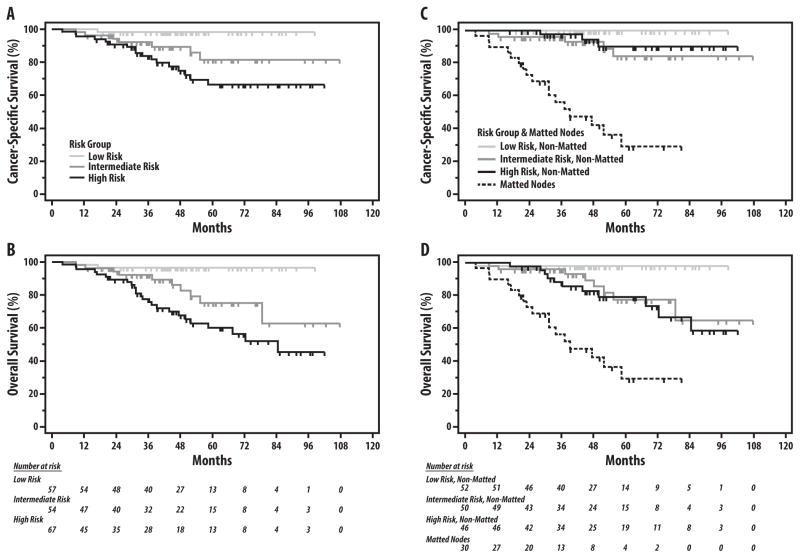
At 52-months median follow-up, event rates with and without MNs were: locoregional failure (LRF): 23.3% vs. 12.8%(p=0.16), DF: 50.0% vs. 1.4%(p<0.01), any failure: 73.3% vs. 14.2%(p<0.01); cause-specific-mortality: 56.7% vs. 5.4%(p<0.01), and death: 56.7% vs. 13.5%(p<0.01). In multivariate analyses including risk-group and individual risk-factors, MNs were the strongest predictor for all endpoints except LRF. Among patients without MNs, risk-group discriminated LRF (at 3-years: low-risk=2.0%, intermediate-risk=14.4%, high-risk=24.2%; p<0.01), but not DF (low-risk=0.0%, intermediate-risk=2.1%, high-risk=3.8%; p=0.53).
Conclusions
MNs portended dramatically increased DF and death risks in HPV+ OPC, identifying a candidate population for consideration of chemo-intensification.
Keywords: Matted nodes, human papillomavirus, oropharyngeal cancer, distant metastases
Background
The rising incidence of oropharyngeal cancer (OPC) in the United States, in contrast to the decreasing incidence of other head and neck squamous cell carcinomas (HNSCC), has attracted increased attention to current management paradigms for OPC (1). The recognition of the favorable prognosis of OPC related to human papillomavirus (HPV), which represents a growing majority of OPC diagnoses, combined with the morbidity of current curative multimodality approaches for locally advanced disease has prompted clinical trials of de-intensification trials for patients with HPV-related (+) OPC (2–4). While consideration of treatment de-intensification based on the expectation of high rates of cure may be appropriate for certain subgroups of patients with locally advanced HPV-related OPC, populations with a substantial risk of treatment failure after standard-intensity chemoradiotherapy (CRT) are inappropriate candidates for reduced intensity therapy. Specifically, patients with T4 primary tumors, N3 nodal classification, and significant smoking histories have been identified as being at significantly increased risk of both locoregional failure (LRF) (5) and distant failure (DF) (6). Although such higher risk patients have been included in ongoing prospective studies of treatment de-escalation for HPV+ OPC, intensification of therapy may in fact be the more appropriate modification of standard therapy for those at highest risk of failure. Accurate identification of patients with a sufficiently poor prognosis after standard intensity therapy is necessary, however, in order to consider treatment intensification within the HPV+ population.
Radiographically matted lymph nodes, defined as three nodes abutting one another with radiographic extracapsular spread (rECS) replacing the intervening fat planes, have been identified as a poor prognostic factor in HPV+ oropharyngeal cancer, independent of T-classification, N-classification, and smoking status (7, 8). In particular, the presence of matted nodes appears to identify patients with an especially high risk of DF(8). Whether matted nodes can improve risk stratification for treatment failure, pattern of failure, and death, compared to previously proposed HPV-related OPC risk-groups (5), however, has not been assessed. We therefore sought to determine whether matted nodes could identify patients at highest risk of DF, independent of potential surrogates for high risk of DF such as HPV+ risk-group and rECS (9), and thereby serve as a robust selection criterion for evaluation of intensification of systemic therapy in future randomized studies.
Methods
Patients
Under an Institutional Review Board-approved protocol, the records of 233 consecutive patients with previously untreated, histologically confirmed, AJCC/UICC stage III or IV oropharyngeal SCC who completed definitive CRT at our institution between 7/2003 and 12/2010 were retrospectively reviewed. HPV detection for all patients was performed on prospectively collected primary tumor tissue using either multiplex polymerase chain reaction (PCR) MassArray following DNA extraction from a core tissue sample or in-situ hybridization (ISH) for high-risk HPV genotypes 16, 18, 33, 35, 39, 45, 51, 52, 56, and 66 on paraffin-embedded tissue as previously described (10). Patients eligible for the present analysis included those with histologically confirmed HPV-related oropharyngeal cancers who had pretreatment computed tomography imaging available for review. After exclusion of patients with HPV-negative OPC or indeterminate HPV status and those who underwent surgical neck treatment prior to initiation of CRT, 178 meeting eligibility criteria were included in the present analysis.
Treatment
After staging consisting of clinical examination, direct laryngoscopy, dedicated head and neck protocol CT or fused PET/CT with contrast enhancement, and chest imaging, patients underwent CT simulation in a 5 point mask followed by intensity-modulated radiation therapy (IMRT) with concurrent chemotherapy, as previously described (10–12). IMRT prescription doses were 70 Gy to the primary and nodal gross tumor volumes (GTVs) and 56–64 Gy to the at-risk clinical target volumes (CTVs). GTVs and CTVs were uniformly expanded 3–5 mm to create planning target volumes. IMRT was delivered in 35 daily fractions to 178 patients (99%) and in twice-daily fractionation to 2 patients (1%). Concurrent chemotherapy consisted of weekly carboplatin (AUC 1) and paclitaxel (30 mg/m2) in 175 patients (98%) and cisplatin-based regimens in 3 patients (2%).
Post-Treatment Surveillance and Surgical Management
All patients were routinely seen in follow-up in the Departments of Radiation Oncology, Otolaryngology, and Hematology/Oncology, with clinical examination performed every 6–12 weeks and post-treatment CT or PET/CT imaging at 3 months. In the earlier years of the study period, patients with advanced nodal disease often underwent planned neck dissection, while in later years patients were clinically and radiographically observed, with neck dissection reserved for clinical or PET-based suspicion of residual disease after CRT. Forty patients (22%) underwent adjuvant neck dissection as part of their initial course of therapy (i.e. within 6 months within completion of CRT) as either planned therapy (n=23) or due to clinical, radiographic, or scintigraphic suspicion for residual disease (n=17).
Radiographic Assessment of Matted Nodes and Extracapsular Spread
Pretreatment CT or CT/PET scans within four weeks of initiation of CRT for all patients were reviewed by either a neuroradiologist or in conjunction by a head and neck surgeon and radiation oncologist; reviewers were blinded to the clinical outcome of each patient. Matted nodes were defined as three nodes abutting one another with rECS replacing the intervening fat planes, as previously detailed (Figure 1) (7, 8). rECS was defined by CT evidence of loss of the sharp plane between the lymph node capsule and the surrounding fat.
Figure 1.
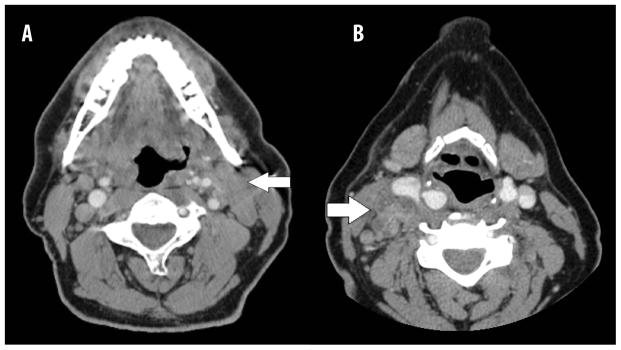
Matted nodes in HPV+ oropharyngeal cancer. (A) 57 year-old male never smoker with HPV+ cT2N2cM0 squamous cell carcinoma of the left tonsil with matted nodes in left level II. (B) 60 year-old female never smoker with HPV+ cT2N2bM0 of the right tonsil with matted nodes in right level II & III.
Statistical Analysis
The outcomes of interest in the present analysis were LRF, DF, any failure, cause-specific mortality (CSM) and OS. Due to the evolving neck dissection practices over the time period of the study, gross or microscopic residual disease present in the adjuvant neck dissection specimen was counted as a LRF event in order to provide the most conservative estimate of treatment failure, as detailed previously (5). For the endpoints LRF, DF, and any failure, patients were censored at date of last follow-up, treatment failure at another site, second primary tumor, or death, while for CSM only non-OPC related deaths were censored as a competing event. Patients with synchronous LRF and DF were counted as experiencing each endpoint.
Patients were stratified into previously proposed HPV+ risk-groups (5). Low risk was classified as those with AJCC stage T1-3 N0-2c disease and a smoking history of < 10 pack-years, intermediate risk as T1-3 N0-2c stages and ≥ 10 pack-years, and high risk as T4 or N3 classifications, irrespective of smoking history. The rate of each endpoint was estimated using Kaplan-Meier methods with log-rank test used for comparison of survival trends across groups (13, 14), before and after adjustment for the presence of matted nodes. Univariable associations between risk-group and each endpoint were tested using the log-rank test, with hazard ratios (HRs) for pairwise comparisons between risk-groups calculated using the methods of Klein & Moeschberger (15). Cox proportional-hazards regression models were used to assess the impact of HPV-positive risk group, matted nodes, and rECS on each endpoint. Proportional hazard assumptions for co-variates in Cox models were tested using the non-zero slope method in a generalized linear regression of the scaled Schoenfeld residuals on functions of time (16), and were met for all Cox models (p>0.05). Baseline characteristics of patients with and without matted nodes were compared using either independent samples t-test or Chi-square test. A two-sided p-value ≤0.05 was used to denote statistical significance for all analyses. Statistical analyses were performed using predominantly MedCalc (v12.2.1.0, MedCalc Software, Mariakerke, Belgium), with RStudio (v 0.98.507, RStudio, Boston, MA) used for proportional hazard assumption testing.
Results
Baseline characteristics for the study population, including when stratified by the presence of matted lymph nodes, are shown in Table 1. Matted nodes were present in 30 patients (16%) (Figure 1). Consistent with the definition of matted nodes (at least three lymph nodes with confluent rECS), advanced nodal classification (i.e. N2b-N3) (p<0.001) and rECS (100% vs. 57%, p<0.001) were more commonly present in patients with matted nodes, as was T4 classification (p=0.047). However, only a minority of patients with advanced nodal classification (7/74 N2b patients, 12/42 N2c patients, and 11/25 N3 patients) or rECS (30/115) had matted nodes present. Patients with matted nodes present were more likely than those without matted nodes to be classified as high-risk by HPV+ risk-classification (70% vs. 31.1%, respectively, p<0.001), though two-thirds of high-risk patients did not have matted nodes present. Other characteristics, including age, smoking history, and use of adjuvant neck dissection did not differ significantly between patients with and without matted nodes
Table 1.
Baseline characteristics.
| CHARACTERISTIC | ALL HPV+ PATIENTS (N=178) | NO MATTED NODES (N=148) | MATTED NODES (N=30) | ||||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | p-value* | |
| Age (years) | 55.2 | 34.1–78.1 | 56.3 | 34.1 – 78.1 | 53.9 | 42.0 – 75.1 | 0.24 |
| Smoking Pack-years | 6 | 0–140 | 10 | 0 – 140 | 1.5 | 0 – 100 | 0.20 |
| No. of pts | % | No. of pts | % | No. of pts | % | ||
| Male | 160 | 89.9% | 131 | 88.5% | 29 | 96.7% | 0.26 |
| Smoking History | |||||||
| <10 pk yrs | 92 | 51.7% | 75 | 50.7% | 19 | 63.3% | 0.31 |
| ≥ 10 pk yrs | 86 | 48.3% | 73 | 49.3% | 11 | 36.7% | |
| Never | 66 | 37.1% | 52 | 35.1% | 14 | 46.7% | 0.27# |
| Former | 61 | 34.3% | 52 | 35.1% | 9 | 30.0% | |
| Current | 51 | 28.7% | 44 | 29.7% | 7 | 23.3% | |
| T-classification | |||||||
| T1 | 27 | 15.2% | 21 | 14.2% | 6 | 20.0% | 0.37# 0.047** |
| T2 | 69 | 38.8% | 60 | 40.5% | 9 | 30.0% | |
| T3 | 34 | 19.1% | 32 | 21.6% | 2 | 6.7% | |
| T4 | 48 | 27.0% | 35 | 23.6% | 13 | 43.3% | |
| N-classification | |||||||
| N0 | 10 | 5.6% | 10 | 6.8% | 0 | 0.0% | <0.001# |
| N1 | 13 | 7.3% | 13 | 8.8% | 0 | 0.0% | |
| N2a | 14 | 7.9% | 14 | 9.5% | 0 | 0.0% | |
| N2b | 74 | 41.6% | 67 | 45.3% | 7 | 23.3% | |
| N2c | 42 | 23.6% | 30 | 20.3% | 12 | 40.0% | |
| N3 | 25 | 14.0% | 14 | 9.5% | 11 | 36.7% | |
| Radiographic Extracapsular Spread | 115 | 64.6% | 85 | 57.4% | 30 | 100.0% | <0.001 |
| Consolidative Neck Dissection | 40 | 22.5% | 30 | 20.3% | 10 | 33.3% | 0.19 |
| HPV+ Risk-Group | |||||||
| Low Risk (T1-3, N0-2c, <10 pk-yrs) | 57 | 32.0% | 52 | 35.1% | 5 | 16.7% | <0.001# |
| Intermediate Risk (T1-3, N0-2c, ≥10 pk-yrs) | 54 | 30.3% | 50 | 33.8% | 4 | 13.3% | |
| High Risk (T4 or N3) | 67 | 37.6% | 46 | 31.1% | 21 | 70.0% | |
Independent samples T-test or Chi-square test for comparison between patients with and without matted lymph nodes
Chi-square for trend
Comparison of T4 vs. T1-3 classifications
Median follow-up from the start of RT for the study population is 51.7 months (interquartile range 35.4 – 67.6 months). Crude incidence for each endpoint for patients with and without matted nodes present was as follows: LRF 23.3% vs. 12.8% (Chi-square p=0.16); DF 50.0% vs. 1.4% (p<0.001), any failure 73.3% vs. 14.2% (p<0.001); CSM 56.7% vs. 5.4% (p<0.001), and death from any cause 56.7% vs. 13.5% (p<0.001), respectively. Among 74 patients with N2b nodal classification, DF occurred in 1/67 without matted nodes versus 3/7 with matted nodes (p=0.002). Among 42 patients with N2c disease, DF occurred in 0/30 without matted nodes versus 6/12 with matted nodes (p<0.001), whereas among 25 N3 patients, DF occurred in 0/14 patients without versus 6/11 patients with matted nodes (p=0.003). The presence of matted nodes was significantly associated with all endpoints on univariable Cox regression, including LRF (HR 2.7, p=0.024), DF (HR 66.3, p<0.001), any failure (HR 8.3, p<0.001), CSM (HR 15.1, p<0.001) and OS (HR 6.8, p<0.001). The presence of rECS on univariable analysis was significantly associated with DF (HR 10.7, p=0.021), any failure (HR 2.9, p=0.007), and OS (HR 2.2, p=0.045), with a borderline trend for CSM (HR 2.4, p=0.075) and a weak trend for LRF (HR 1.74, p=0.21). The magnitude of the univariable association between ECS and each endpoint, however, was notably much weaker than the comparable endpoint’s HR for matted nodes. Among 148 patients without matted nodes, the presence of rECS was not associated with any endpoint (3-year Kaplan-Meier estimates: locoregional control 85.7% vs. 88.7% [log-rank p=0.50]; distant control 98.7% vs. 100% [p=0.90]; freedom from treatment failure 84.6% vs. 88.8% [p=0.54]; cause-specific survival 98.8% vs. 96.7% [p=0.25]; OS 90.5% vs. 96.7% [p=0.83] for patients with and without rECS present, respectively). Stratification of rECS by the presence of matted nodes in a Cox regression model further demonstrates the lack of prognostic impact of rECS without matted nodes on LRF, DF, any failure, CSM, and OS (p>0.25), in contrast to the statistically significant association between matted nodes and each of these endpoints (Table 2).
Table 2.
Cox Regression Model of Radiographic Extracapsular Spread (rECS) When Stratified by Presence of Matted Nodes
| Locoregional Failure | Distant Failure | Any Failure | Cause-Specific Mortality | All-Cause Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVARIATE | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| rECS (−) | Ref | - | - | Ref | - | - | Ref | - | - | Ref | - | - | Ref | - | - |
| rECS (+); Matted Nodes (−) | 1.4 | 0.5 – 3.5 | 0.51 | 0.83 | 0.1– 13.1 | 0.90 | 1.3 | 0.5– 3.2 | 0.54 | 0.5 | 0.1– 1.9 | 0.28 | 1.1 | 0.5– 2.7 | 0.81 |
| rECS (+); Matted Nodes (+) | 3.3 | 1.2– 9.5 | 0.026 | 60.2 | 8.0– 455.3 | <0.001 | 9.8 | 4.4– 22.1 | <0.001 | 10.4 | 3.9– 28.2 | <0.001 | 7.2 | 3.1– 16.9 | <0.001 |
CI = confidence interval; HR = hazard ratio; Ref = reference
As shown in Figures 2A–C and 3A–B, stratification of the study population by the previously defined HPV+ OPC risk-groups effectively discriminated risk of LRF, DF, any failure, CSM and OS (log-rank p≤0.002 for all comparisons; Table 3). After adjustment for risk-group using Cox regression, the presence of matted nodes remained a significant predictor of DF (HR 52.2, p<0.001), any failure (HR 6.2, p<0.001), CSM (HR 12.5, p<0.001), and OS (HR 5.1, p<0.001), and trended towards significance for LRF (HR 2.0, p=0.15) (Table 4). Adjustment by Cox regression for previously identified individual risk factors(5, 6) in HPV-associated OPC (T4-classification, N3-classification, and ≥10 smoking pack-years) yielded similar results, with matted nodes remaining the strongest prognostic factor for all endpoints (HR 152.4 for DF, HR 7.2 for any failure, HR 14.1 for CSM, and HR 6.4 for OS; all p<0.001) except for LRF (HR 1.9, p=0.2) (Table 5). Varying the stratification of nodal classification by other cutpoints (i.e. N0-N2a vs. N2b-N3, N0-N2b vs. N2c-N3) did not change the prognostic value of matted results for each endpoint (data not shown). Categorizing patients with matted nodes into a separate fourth risk group (Table 3, Figures 2D–E and 3C–D) highlights the dramatic increase in risk of treatment failure and death among patients with matted nodes compared to patients without matted nodes, even among those with T4 or N3 disease classifications. Compared to high-risk patients without matted nodes, patients with matted nodes (irrespective of T-classification, N-classification, and smoking status) experienced a substantially increased risk of both DF (63.9% vs. 3.8% at 5 years, HR 34.5, 95% CI 6.2 – 190.6) and any failure (74.1% vs. 24.2% at 5 years, HR 4.1, 95% CI 1.4 – 11.7) with correspondingly higher rates of CSM (5-year CSM: 71.0% vs. 9.8%, HR 12.6, 95% CI 3.4 – 47.4) and OS (5-year OS: 71.0% vs. 21.0%, HR 3.9, 95% CI 1.3 – 11.9), while the increase in LRF risk was marginal and non-significant (5-year LRF: 28.2% vs. 24.2%, HR 1.4, 95% CI 0.4 – 5.3). Among patients without matted nodes (n=148), HPV+ risk-group remained prognostic for risk of LRF (5-year LRF: 2.0% vs. 14.4% vs. 24.2%, respectively; p=0.005), but did not discriminate for risk of DF, which was minimal in all risk-groups (5-year DF: 0.0% vs. 2.1% vs. 3.8% for low-, intermediate-, and high-risk patients without matted nodes, respectively; p=0.53).
Figure 2.
Risk of locoregional failure (A, D), distant failure (B, E), and any failure (C, F) by HPV+ risk-group, without and with adjustment for matted nodes, respectively. Kaplan-Meier estimates and p-values for all comparisons shown in Table 2.
Figure 3.
Cause-specific survival (A, C) and overall survival (B, D) by HPV+ risk-group, without and with adjustment for matted nodes, respectively. Kaplan-Meier estimates and p-values for all comparisons shown in Table 2.
Table 3.
Kaplan-Meier Estimates of Locoregional Failure, Distant Failure, Any Failure, Cause-Specific Mortality Outcome by HPV+ Risk-Group, Before and After Accounting for Presence of Matted Nodes
| HPV+ RISK STRATIFICATION | HPV+ RISK STRATIFICATION, ADJUSTED FOR PRESENCE OF MATTED NODES | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Endpoint | Low Risk (n=57) (T1-3, N0-2c, <10 pk-yrs) | Intermediate Risk (n=54) (T1-3, N0-2c, ≥10 pk-yrs) | High Risk (n=67) (T4 or N3) | Log-rank p-value | Low Risk, Non-matted nodes (n=52) | Intermediate Risk, Non-matted nodes (n=50) | High Risk, Non-matted nodes (n=46) | Matted Nodes (n=30) | Log-rank p-value | |
| 3 YEAR ESTIMATE (± 95% CI ) | Locoregional Failure | 3.6% (0.0 – 8.1%) | 13.6% (4.2 – 23.0%) | 27.6% (16.2 – 39.0%) | <0.001 | 2.0% (0.0 – 5.7%) | 14.4% (4.4 – 24.4%) | 24.2% (11.7 – 36.7%) | 28.2% (10.0 – 46.4%) | 0.001 |
| Distant Failure | 1.8% (0.0 – 5.3%) | 6.1% (0.0 – 12.8%) | 22.3% (10.9 – 33.7%) | <0.001 | 0.0% (0.0 – 0.0%) | 2.1% (0.0 – 6.2%) | 0.0% (0.0 – 0.0%) | 63.9% (43.7 – 84.1%) | <0.001 | |
| Any Failure | 5.4% (0.0 – 11.3%) | 18.8% (8.2 – 29.4%) | 43.8% (31.9 – 55.8%) | <0.001 | 2.0% (0.0 – 5.7%) | 16.2% (5.8 – 26.6%) | 24.2% (11.7 – 36.7%) | 74.1% (58.2 – 90.0%) | <0.001 | |
| Cause-Specific Mortality | 1.8% (0.0 – 5.3%) | 7.8% (0.4 – 15.2%) | 16.3% (6.9 – 25.7%) | 0.002 | 0.0% (0.0 – 0.0%) | 4.0% (0.0 – 9.5%) | 2.4% (0.0 – 7.1%) | 43.9% (24.9 – 62.9%) | <0.001 | |
| All-Cause Mortality | 3.5% (0.0 – 8.2%) | 7.8% (0.4 – 15.2%) | 22.5% (12.1 – 32.9%) | <0.001 | 1.9% (0.0 – 5.6%) | 4.0% (0.0 – 9.5%) | 11.9% (2.3 – 21.5%) | 43.9% (24.9 – 62.9%) | <0.001 | |
| 5 YEAR ESTIMATE (± 95% CI ) | Locoregional Failure | 3.6% (0.0 – 8.1%) | 13.6% (4.2 – 23.0%) | 27.6% (16.2 – 39.0%) | <0.001 | 2.0% (0.0 – 5.7%) | 14.4% (4.4 – 24.4%) | 24.2% (11.7 – 36.7%) | 28.2% (10.0 – 46.4%) | 0.001 |
| Distant Failure | 1.8% (0.0 – 5.3%) | 6.1% (0.0 – 12.8%) | 25.2% (13.0 – 37.4%) | <0.001 | 0.0% (0.0 – 0.0%) | 2.1% (0.0 – 6.2%) | 3.8% (0.0 – 11.2%) | 63.9% (43.7 – 84.1%) | <0.001 | |
| Any Failure | 4.8% (0.0 – 10.1%) | 18.8% (8.2 – 29.4%) | 45.9% (33.6 – 58.2%) | <0.001 | 2.0% (0.0 – 5.3%) | 16.2% (5.8 – 26.6%) | 27.1% (13.8 – 40.4%) | 74.1% (58.2 – 90.0%) | <0.001 | |
| Cause-Specific Mortality | 1.8% (0.0 – 5.3%) | 18.6% (5.3 – 31.9%) | 33.6% (19.9 – 47.3%) | 0.002 | 0.0% (0.0 – 0.0%) | 15.7% (2.2 – 29.2%) | 9.8% (0.0 – 20.6%) | 71.0% (50.4 – 91.6%) | <0.001 | |
| All-Cause Mortality | 3.5% (0.0 – 8.2%) | 24.7% (9.8 – 39.6%) | 40.0% (26.5 – 53.5%) | <0.001 | 1.9% (0.0 – 5.6%) | 22.5% (7.2 – 37.8%) | 21.0% (7.9 – 34.1%) | 71.0% (50.4 – 91.6%) | <0.001 | |
CI = confidence interval; HR = hazard ratio; Ref = reference
Table 4.
Cox Regression Model for Recurrence and Survival Endpoints with Adjustment for Matted Nodes and Risk Group in HPV-Related Oropharyngeal Cancer
| Locoregional Failure | Distant Failure | Any Failure | Cause-Specific Mortality | All-Cause Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVARIATE | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| Low-Risk Group (T1-3, N0-2c, <10 pk-yrs) | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – | Ref | – | – |
| Intermediate Risk Group (T1-3, N0-2c, ≥10 pk-yrs) | 5.0 | 0.8–18.9 | 0.087 | 3.7 | 0.4–35.5 | 0.25 | 3.9 | 1.1–14.1 | 0.039 | 7.5 | 0.9–60.6 | 0.059 | 5.5 | 1.2–24.8 | 0.028 |
| High Risk Group (T4 or N3) | 8.2 | 1.9–35.6 | 0.005 | 7.5 | 1.0 –57.9 | 0.054 | 7.70 | 2.3–25.4 | <0.001 | 7.1 | 0.9–53.9 | 0.060 | 7.1 | 1.7–30.4 | 0.009 |
| Matted Nodes (yes vs. no) | 2.0 | 0.8–4.8 | 0.15 | 52.2 | 11.6–234.8 | <0.001 | 6.15 | 3.3–11.6 | <0.001 | 12.5 | 5.1–30.4 | <0.001 | 5.1 | 2.5–10.2 | <0.001 |
CI = confidence interval; HR = Hazard Ratio; Ref = Reference
Table 5.
Cox Regression Model for Recurrence and Survival Endpoints with Adjustment for Matted Nodes and Individual Risk Factors in HPV-Related Oropharyngeal Cancer
| Locoregional Failure | Distant Failure | Any Failure | Cause-Specific Mortality | All-Cause Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVARIATE | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| T-classification (T4 vs. T1-3) | 2.0 | 0.9–4.4 | 0.091 | 14.3 | 3.6–56.5 | <0.001 | 3.0 | 1.6–5.7 | <0.001 | 2.6 | 1.1–6.1 | 0.025 | 2.9 | 1.5–5.6 | 0.002 |
| N-classification (N3 vs. N0-2c) | 3.2 | 1.3–7.8 | 0.013 | 1.5 | 0.5–4.2 | 0.49 | 2.2 | 1.1–4.3 | 0.033 | 1.3 | 0.4–3.3 | 0.65 | 1.4 | 0.6–3.1 | 0.44 |
| Smoking Pack-years (≥10 vs. <10) | 1.2 | 0.6–2.6 | 0.66 | 5.6 | 1.7–18.8 | 0.006 | 1.7 | 0.9–3.2 | 0.090 | 1.5 | 0.7–3.4 | 0.32 | 1.6 | 0.8–3.2 | 0.15 |
| Matted Nodes (yes vs. no) | 1.9 | 0.7– 4.8 | 0.20 | 152.4 | 26.9–864.3 | <0.001 | 7.2 | 3.7 –14.3 | <0.001 | 14.1 | 5.8–34.2 | <0.001 | 6.4 | 3.1 – 13.0 | <0.001 |
CI = confidence interval; HR = Hazard Ratio
Discussion
The primary finding of the present study is that the presence of matted lymph nodes in patients with HPV-related OPC identified those patients with a greater than 60% risk of DF, while the absence of matted nodes was associated with a minimal DF risk. The prognostic impact of matted nodes on DF remained statistically and clinically significantly even after adjustment for HPV+ risk group and for previously identified risk factors in HPV+ OPC(2, 5, 6), among which matted nodes were the strongest predictor for DF, any failure, CSM, and OS. In contrast, the association between matted nodes and LRF was only marginal and non-significant after separate adjustment for both risk group and individual risk factors. Finally, among patients without matted nodes, risk group was of minimal utility for stratifying risk of DF (<4% irrespective of risk group), though remained highly prognostic for LRF.
These present findings are highly pertinent to ongoing efforts to refine patient selection for treatment modification in HPV-related OPC. Although the prognosis for this overall patient population is generally favorable, 15–25% of patients with HPV-related OPC nonetheless experience disease recurrence despite treatment with full-intensity CRT (2, 5, 17, 18). DF accounts for approximately 50% of treatment failures in HPV+ OPC, compared to a substantially lower proportion in patients with traditional smoking-related HNSCC (2, 6). These shifting patterns of failure have placed heightened importance on distant control in this population. While advanced T- and N-disease classifications are established risk factors for DF, the incidence of DF in patients with T4 or N3 disease nonetheless remains less than 30% at 3 years in both the present study and prior reports (6). Therefore, the characterization of a population of patients with matted nodes whose risk of DF exceeds 60% despite treatment with full-intensity CRT, represents an advance in our ability to identify patients with exceptionally poor distant control rates for whom escalation of treatment directed at reducing DF may be warranted.
Efforts to intensify systemic therapy in locally advanced head and neck cancer (LAHNC) to-date have been largely hampered by lack of a survival benefit and increased toxicity (19, 20) compared to the standard of concurrent CRT. A meta-analysis of randomized trials of chemotherapy in HSNCC demonstrated that while the addition of induction chemotherapy to locoregional therapy (without concomitant chemotherapy) reduced DF, no improvement in survival could be detected (21). The DeCIDE study, which randomized 280 patients with N2/3 nodal classifications to definitive chemoradiation with or without induction docetaxel, cisplatin, and 5-fluorouracil, similarly demonstrated an absolute 9% reduction in DF at 3 years with induction chemotherapy, though without any overall survival benefit, possibly due to competing events and low total event rates(22). In contrast, the PARADIGM study, which enrolled 145 LAHNC patients with all extents of nodal involvement, failed to show a significant improvement in any endpoint, including distant control, though the low DF rate in both the induction chemotherapy (7%) and control arms (11%) may have limited the power of study to detect a difference (23). Importantly, neither these studies nor the meta-analysis suggested any improvement in LRF, confirming that the sole potential benefit of induction chemotherapy in LAHNC is in improvement of distant control. Therefore, if any role for induction chemotherapy exists for patients with LAHNC, it is most likely in those patients at highest risk of DF with comparatively low rates of competing LRF events, for whom improved distant control will be most likely to translate into improved survival. Our data suggest that patients with HPV-related OPC with matted nodes represent precisely such a population, and provide the rationale for future studies that evaluate the role of systemic therapy intensification in this especially high risk group.
An additional important finding of the present study is that the exceedingly low rate of DF among patients without matted nodes underscores the lack of any role for induction chemotherapy or other intensification of systemic therapy in such patients. Patients with low-, intermediate-, and high-risk HPV-related OPC without matted nodes had 5-year DF rates of 0%, 2%, and 4%, respectively. These distant control rates leaves little, if any, room for improvement, and therefore provide little rationale for the systemic therapy intensification in the absence of matted nodes. In contrast, 3-year LRF (including residual cells at adjuvant neck dissection) in these HPV+ risk groups among patients without matted nodes was 2.0%, 14.4%, and 24.2%, respectively. Therefore, patients in the intermediate and high risk groups, even in the absence of matted nodes, remain at significant risk of LRF despite concurrent chemoradiation, and argue against the inclusion of such patients in studies of treatment de-intensification for HPV-related OPC.
The present study has several strengths, specifically the inclusion of an unselected and uniformly treated population of HPV+ OPC patients, long-term follow-up period, and detailed information on pattern of failure, and is among the largest studies to-date reporting outcomes of patients with HPV+ OPC treated with uniform chemoradiation. Furthermore, this study builds on our previously published work identifying the prognostic import of matted nodes in OPC (7, 8, 24) within an expanded uniform cohort of HPV+ OPC patients with longer term follow-up, stratified by previously proposed HPV+ risk-group(5), and analyzed for pattern of first failure endpoints. The present study differs from this prior work by focusing only those patients with HPV+ OPC after stratification by risk group based upon T/N classification and smoking history, an analysis not performed by Spector et al. The previously unreported survival and pattern of failure data included herein should contribute to the design of future clinical trials focusing on modification of both locoregional and systemic therapy for HPV+ OPC patients, as well as inform counseling of such patients, both issues of increasing importance given the ongoing growth of this patient population(25). Future studies to expand on the current work should include independent validation of the prognostic value of both matted nodes and the presently proposed risk stratification in patients with HPV+ OPC. Validation of inter-observer agreement in identifying radiographically matted nodes, presently ongoing in a multi-institutional collaboration, will additionally be required in order for the presence of matted nodes to guide future clinical management decisions. Analysis of patterns of failure among patients with matted nodes who are treated with induction chemotherapy may additionally shed further light on the potential of intensification of systemic therapy to improve these patients’ outcomes. Finally, limitations applicable to all retrospective studies pertain to the present study, including insufficient power to detect differences between risk-groups with limited number of events.
In summary, the present study demonstrates that patients with HPV-related OPC with matted nodes experience exceedingly high rates of DF after concurrent chemoradiation, and therefore represent a novel candidate population for assessing the efficacy of systemic therapy intensification. By comparison, the virtual absence of distant metastases in patients without matted nodes highlights the lack of rationale for induction chemotherapy or other intensified systemic therapy is such patients, irrespective of T-or N-classification. Finally, matted nodes did not significantly impact risk of LRF, suggesting that decisions regarding the intensity of locoregional therapy can be made irrespective of the presence of matted nodes.
Acknowledgments
Supported in part by The University of Michigan Head and Neck Specialized Program of Research Excellence (SPORE): P50CA097248. The Molecular Basis of Head and Neck Cancer Biology, and by the Newman Family Research Fund
The authors thank Cui Guo for supplementary statistical support.
Footnotes
Presented in part at the 2014 Multidisciplinary Head and Neck Cancer Symposium, Scottsdale, AZ and the 2014 American Society for Radiation Oncology (ASTRO) Annual Meeting
Conflicts of interest: none
Contributor Information
Jeffrey M. Vainshtein, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
Matthew E. Spector, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Mohannad Ibrahim, Department of Radiology, University of Michigan, Ann Arbor, MI.
Carol R. Bradford, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Gregory T. Wolf, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Matthew H. Stenmark, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
Francis P. Worden, Division of Hematology Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan.
Jonathan B. McHugh, Department of Pathology, University of Michigan, Ann Arbor, MI.
Mark E. Prince, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Thomas Carey, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Douglas B. Chepeha, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Avraham Eisbruch, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quon H, Forastiere AA. Controversies in treatment deintensification of human papillomavirus-associated oropharyngeal carcinomas: should we, how should we, and for whom? J Clin Oncol. 2013;31(5):520–2. doi: 10.1200/JCO.2012.46.7746. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Ang KK. The epidemic of HPV-associated oropharyngeal cancer is here: is it time to change our treatment paradigms? J Natl Compr Canc Netw. 2011;9(6):665–73. doi: 10.6004/jnccn.2011.0055. [DOI] [PubMed] [Google Scholar]
- 5.Vainshtein JM, Spector ME, McHugh JB, et al. Refining risk stratification for locoregional failure after chemoradiotherapy in human papillomavirus-associated oropharyngeal cancer. Oral oncology. 2014;50(5):513–9. doi: 10.1016/j.oraloncology.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 7.Spector ME, Gallagher KK, Light E, et al. Matted nodes: poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck. 2012;34(12):1727–33. doi: 10.1002/hed.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spector ME, Chinn SB, Bellile E, et al. Matted Nodes Predict Distant Metastasis in Advanced Stage III/IV Oropharyngeal Squamous Cell Carcinoma. Head Neck. 2014 doi: 10.1002/hed.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kann BH, Buckstein M, Carpenter TJ, et al. Radiographic extracapsular extension and treatment outcomes in locally advanced oropharyngeal carcinoma. Head Neck. 2013 doi: 10.1002/hed.23512. [DOI] [PubMed] [Google Scholar]
- 10.Vainshtein JM, Spector ME, Stenmark MH, et al. Reliability of post-chemoradiotherapy F-18-FDG PET/CT for prediction of locoregional failure in human papillomavirus-associated oropharyngeal cancer. Oral oncology. 2014;50(3):234–9. doi: 10.1016/j.oraloncology.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28(16):2732–8. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68(5):1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Altman DG. Practical statistics for medical research. London ; New York: Chapman and Hall; 1991. p. xii.p. 611. [Google Scholar]
- 14.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29–41. [Google Scholar]
- 15.Klein JP, Moeschberger ML. Survival analysis : techniques for censored and truncated data. 2. New York: Springer; 2003. p. xv.p. 536. [Google Scholar]
- 16.Grambsch PM, Therneau TM. Proportional hazard tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 17.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226–35. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22(5):1071–7. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko EC, Genden EM, Misiukiewicz K, et al. Toxicity profile and clinical outcomes in locally advanced head and neck cancer patients treated with induction chemotherapy prior to concurrent chemoradiation. Oncology reports. 2012;27(2):467–74. doi: 10.3892/or.2011.1512. [DOI] [PubMed] [Google Scholar]
- 20.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 21.Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Cohen EE, Karrison TG, Kocherginsky M, et al. Phase III Randomized Trial of Induction Chemotherapy in Patients With N2 or N3 Locally Advanced Head and Neck Cancer. J Clin Oncol. 2014;32(25):2735–43. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddad RIR, Guilherme, Tishler RB, Adkins D, et al. The PARADIGM trial: A phase III study comparing sequential therapy (ST) to concurrent chemoradiotherapy (CRT) in locally advanced head and neck cancer (LANHC) J Clin Oncol. 2012;30(15_suppl):5501. [Google Scholar]
- 24.Spector ME, Gallagher KK, Bellile E, et al. Patterns of nodal metastasis and prognosis in human papillomavirus positive oropharyngeal squamous cell carcinoma. Head Neck. 2013 doi: 10.1002/hed.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fakhry C, D’Souza G. Discussing the diagnosis of HPV-OSCC: common questions and answers. Oral oncology. 2013;49(9):863–71. doi: 10.1016/j.oraloncology.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]