Abstract
Background
Bony non-unions arising in the aftermath of collateral radiation injury are commonly managed with vascularized free tissue transfers. Unfortunately, these procedures are invasive and fraught with attendant morbidities. This study investigates a novel, alternative treatment paradigm utilizing adipose derived stem cells (ASCs) combined with angiogenic deferoxamine (DFO) in the rat mandible.
Methods
Rats were exposed to a bioequivalent dose of radiation and mandibular osteotomy. Those exhibiting non-unions were subsequently treated with surgical debridement alone or debridement plus combination therapy. Radiographic and biomechanical outcomes were assessed after healing.
Results
Significant increases in biomechanical strength and radiographic metrics were observed in response to combination therapy (p<0.05). Importantly, combined therapy enabled a 65% reduction in persisting non-unions when compared to debridement alone.
Conclusions
We support the continued investigation of this promising combination therapy in its potential translation for the management of radiation-induced bony pathology.
Keywords: radiotherapy, osteoradionecrosis, pathologic fracture, adipose-derived stromal cell, deferoxamine
INTRODUCTION
The current arsenal for managing non-unions in previously irradiated bone involves the removal of necrotic tissue and vascularized free tissue transfers. These surgical procedures are complex, arduous and introduce significant donor site morbidity.1-6 Further, there are no accepted reconstructive options to turn to once autologous tissue transplantation fails. Efficacious treatment alternatives for managing these events are therefore highly sought after.
Radiation exerts its damaging effects on bone through mechanisms of vascular diminution, depletion of cellular volume and attenuation of existing osteocyte function.7-11 Previously, we have utilized a mandibular rat model of radiation-induced pathologic fracture healing to investigate these ravaging effects. We demonstrated diminished metrics of vascularity, osteocyte viability, callus mineralization and biomechanical strength.12-14 Further, we evidenced a reproducible non-union rate of 75-80%.12-14
Our current investigation seeks to address the mechanisms of vascular diminution and depletion of viable cells at the site of non-union by therapeutically re-establishing vascularity and replacing necrotic tissue with viable progenitor cells. To do this, we utilize adipose-derived stem cells (ASCs) in combination with angiogenic deferoxamine (DFO) in our established animal model.
ASCs represent a multipotent, abundant stromal cell type with demonstrated capacity to differentiate along an osteogenic lineage through stimulation of the BMP pathway, making them suitable for filling bony defects.15-25
DFO is an iron-chelating compound currently on formulary for treating patients with transfusion related iron-overload.26 It has also been shown to invoke angiogenic responses when utilized in minute, intermittent doses in a localized environment.27-29 Our laboratory has since extended the use of this powerful strategy to improve pathologic bone healing after radiation injury in the rat mandible.12-14,26
We hypothesize that the combined utility of ASCs and DFO will rescue non-unions arising in the aftermath of radiotherapy, evidencing enhanced metrics of radiographic mineralization and biomechanical strength.
MATERIALS AND METHODS
Experimental Design
Animal experimentation was conducted in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4. Protocols were approved by the University of Michigan's Committee for the Utilization and Care of Animals prior to implementation. Forty-eight adult male Lewis rats (300-350g) were obtained through the University of Michigan's Unit for Laboratory Animal Medicine. All animals were caged in a restricted, pathogen-free facility and maintained on a 12-hour light/dark schedule. Prior to handling and radiation, a 7-day acclimation period was allowed. Thirty-six rats were subjected to a human equivalent dose of radiation (HEDR) and osteotomy surgery, followed by external fixation and a subsequent 40 day fracture healing period. After radiographical assessment, those exhibiting non-unions (n=30) were further subdivided randomly into two groups for a second surgery: surgical debridement (n=15) and surgical debridement with combination therapy (n=15). Defects of animals in the combination therapy group were implanted with ASC-loaded scaffolds, and these animals received postoperative DFO injections. All animals were then allowed a second 40 day healing period after which the animals were sacrificed and their mandibles harvested for further analysis.
Animal Radiation Protocol
All radiation procedures were conducted in the Irradiation Core at the University of Michigan. After transient induction of anesthesia with an oxygen/isoflurane mixture, left hemi-mandibles were irradiated using a Philips RT250 orthovoltage unit (250 kV X-rays, 15 mA; Kimtron Medical, Woodbury, CT), which delivers ionizing radiation through a filtered system to a specified region of interest (ROI). Our selected ROI spans a 2 mm distance posterior to the third molar and correlates to the anatomic site of the osteotomy. Lead shielding was provided to ensure localized delivery and protection of surrounding tissues. A previously described HEDR developed with the guidance of the Department of Radiation Oncology at the University of Michigan was utilized. Briefly, a fractionated dose of 7 Gy per day was administered over 5 days for a total of 35 Gy. This is comparable to 70 Gy in human mandibular high-dose radiotherapy. This dose was designed to predictably replicate pathologies analogous to those observed in the setting of clinically advanced mandibular osteoradionecrosis, while taking the diminutive size of the mandible and surrounding tissues into consideration.30-32
Peri-Operative Care
Gentamicin (30 mg/kg SQ) was administered once prior to surgery and twice post-operatively. To ensure adequate analgesia, hydration and anesthesia, rats were delivered buprenorphine (0.15 mg/kg SQ) and subcutaneous Lactated Ringer's solution (25 cc/kg SQ), and then anesthetized using an inhalational oxygen/isoflurane mixture throughout the surgical procedure. Post-operatively, animals were placed on warming blankets and monitored for heart and respiratory rates. Post-operative analgesia with buprenorphine was continued twice daily through post-operative day 4, and as needed thereafter. Weight gain, porphyrin staining and nutritional intake were assessed to determine the need for continued analgesia.
Osteotomy and Fixator Placement
After standard preparation and draping, a 2 cm midline incision was performed ventrally from the anterior submentum to the neck crease. A custom titanium fixator device was placed as previously described.33 After stabilization of the fixator, a vertical osteotomy was created using a reciprocating blade (Stryker, Kalamazoo, MI, USA) directly behind the third molar on the left hemi-mandible. After reduction of the osteotomy edges, wounds were irrigated, hemostasis verified and incisions were closed in layers. Four hours after osteotomy, the fixator device was set to a 2 mm fixed distance and a 40 day healing period was allowed prior to radiographic evaluation.
Adipose-Derived Stromal Cell Culturing
ASCs were isolated from the inguinal fat pads of 12 adult male, isogenic Lewis rats, as previously described.34 Rats were euthanized with inhalational isoflurane, and then fat pads were cautiously dissected from donor rats, washed sequentially in serial dilutions of betadine and then mechanically digested in Hank's Balanced Salt solution (HBSS, no calcium, no magnesium; Life Technologies, Grand Island, NY, USA). The minced pads were exposed to 0.075% collagenase A (C5894; Sigma-Aldrich, St. Louis, MO, USA) while shaken briskly in a 37°C water bath for 30 minutes. Dulbecco's Modified Eagle Medium (DMEM; Sigma-Aldrich) with 10% fetal bovine serum (FBS; Life Technologies) was then used to neutralize the collagenase A, and the cell suspension was passed through a 100 μm cell strainer, ensuring removal of the larger undigested tissue. Cells were centrifuged at 1,000 rpm for 5 minutes, and the cell pellet was re-suspended in a fresh growth medium (DMEM with 10% FBS, 100 IU/mL penicillin and 100 IU/mL streptomycin). Cells less than passage 3 were utilized in stem cell implantation during a second surgery.
Adipose-Derived Stromal Cell Scaffold Preparation
Each gelatin sponge (Surgifoam; Ethicon, Somerville, NJ, USA) was designed to be a 2×9×2-mm rectangle to match the maximum critical size defect of our fracture gap. The sponges were pre-wetted in the complete medium (DMEM with 10% FBS, 100 IU/mL penicillin and 100 IU/mL streptomycin) and air bubbles were removed by applying gentle pressure on the sponge between two pieces of sterile filter paper. Two million ASCs were collected and suspended in 50μL of collagen (2.5mg/mL, rat tail collagen, type I;BD Biosciences, Franklin Lakes, NJ, USA), and loaded onto each sponge using capillary action. Previous experiments have shown via direct cell counts (Coulter counter, model ZBI; Coulter Electronics, Hialeah, FL, USA) of the residual suspension after removal of the gelatin sponge, that when utilizing this procedure, greater than 95% of the cells entered the sponges .35 After loading the sponges with the cells, all vehicles were incubated at 37°C for 30 min before implantation.
Debridement, ASC Implantation and Deferoxamine Administration
Following the initial 40 day healing period, mandibles were assessed radiographically (MX20; Faxitron X-Ray, Lincolnshire, IL, USA) (Figure 1). Animals exhibiting unions (n=6) were excluded from the remainder of the study. Rats displaying non-unions were randomly divided into two groups. Both groups (n=15/group) underwent surgical debridement, consisting of sharp dissection and removal of necrotic soft tissue by scalpel. The combination therapy group received implantation of ASC-loaded gelatin sponges into the fracture gap prior to masseter closure (See Figure 2). The combination therapy group was then subjected to localized injections of DFO (200μM, 300μL) directly into the fracture gap by palpating the defect through the overlying skin. These injections were administered every other day from POD 4-12, for a total of 5 doses as previously described, in order to coincide with the peak of angiogenesis in a murine fracture model.12-14,26-29,36,37 After a second 40 day healing period, animals were sacrificed by isoflurane overdose followed by thoracotomy, and left hemi-mandibles were dissected en bloc for outcomes testing.
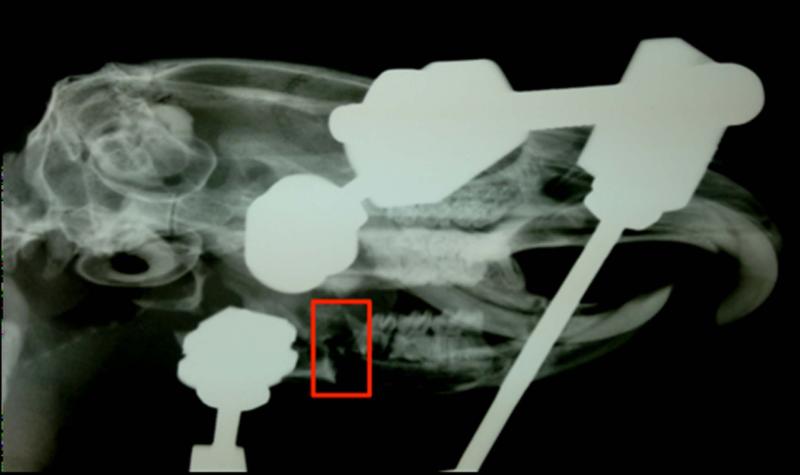
Figure 1.
Faxitron confirmation of established non-union (outlined in red) in the left hemimandible following an initial 40 day healing period.
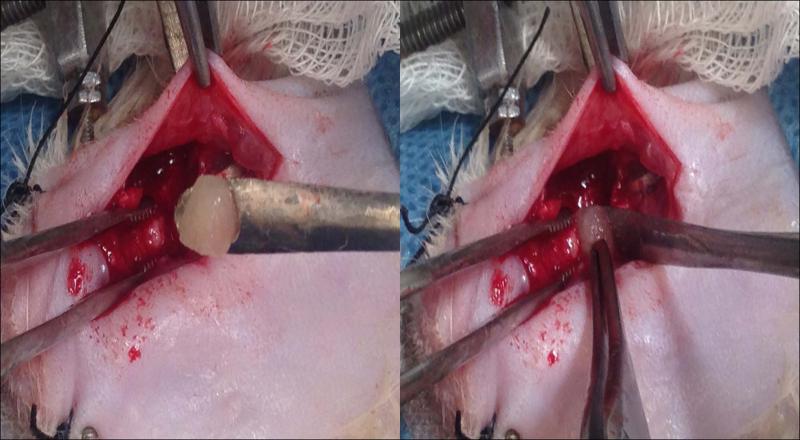
Figure 2.
Implantation of the ASC scaffold into the fracture site. The rat is lying on his dorsum, with the inferior border of the left mandible exposed.
Microcomputed Tomography Analysis
Dissected mandibles were assessed for incidence of bony union, which was clinically defined as bone bridging and absence of movement across the fracture site upon manipulation. Micro-CT images were obtained using 80kVp, 80mA and 1100 ms exposures. Three hundred ninety two projections were taken at a 45-micron voxel size for bone analysis. GE's Microview 2.2 software was used to generate the ROI and derive metrics of total volume (TV), bone volume (BV), bone volume fraction (BVF), tissue mineral density (TMD) and bone mineral density (BMD).
Biomechanical Testing
After imaging, both the anterior and posterior ends of the mandibles were potted in bismuth and loaded to failure in uniaxial monotonic tension at 0.5 mm/s using a servohydraulic testing machine (858 Minibiox II; MTS Systems Corporation, Eden Prairie, MN, USA). Crosshead displacement was recorded by using an external variable differential transducer (LVDT; Lucas Schavitts, Hampton, VA, USA), and load data were collected with a 100-lb load cell (Sensotec, Columbus, OH, USA). Data were sampled at 200 Hz on a TestStar system (TestStar IIs System version 2.4; MTS Systems Corporation, Minneapolis, MN, USA). Load-displacement curves were analyzed for whole bone yield load (Y), failure energy (E), ultimate load (UL), failure load (FL) and elastic energy (EE) using custom computational code (MATLAB 7.11; Mathworks Inc., Natick, MA, USA).
Statistical Analysis
An independent samples t-test was used for analyzing both the mineralization and biomechanical data (SPSS version 16.0; SPSS, Inc., Chicago, IL, USA). Results were accepted as statistically significant at a value of p<0.05.
RESULTS
Microcomputed Tomography Results
Radiomorphometric analysis of the ROI indicated significant differences in mineralization parameters between the combination therapy group and surgical debridement group (See Table 1). Specifically, the combination therapy group yielded significantly higher values for BVF (p=0.034), BMD (p=0.038) and TMC (p=0.013) as compared to the surgical debridement group. These enhanced metrics suggest that the combination therapy facilitates more robust mineralization in the healing mandible when compared to surgical debridement alone (See Figure 3).
Table 1.
Micro-Computed Tomography (μCT ) Radiomorphometrics for Bone Volume Fraction (BVF), Bone Mineral Density (BMD) and Tissue Mineral Content (TMC).
| μCT Radiomorphometrics | |||
|---|---|---|---|
| Debridement | ASCs + DFO | P-Value | |
| BVF | 0.78±0.069 | 0.85±0.073 | *0.034 |
| BMD (mg/cc) | 665.64±56.23 | 722.08±65.20 | *0.038 |
| TMC (mg) | 16.18±4.14 | 20.42±3.29 | *0.013 |
Means ± standard deviations for μCT callus mineralization metrics for each group. P- values also given for independent t-test between the groups.
Denotes significance at p < 0.05.
Note: Adipose Derived Stromal Cells (ASCs) and deferoxamine (DFO).
Figure 3.
Representative μCT scans of Debridement vs. ASC + DFO mandibles demonstrating superior mineralization at the fracture site in treated mandibles.
Biomechanical Testing Results
Metrics for biomechanical strength paralleled the radiomorphometric data, exhibiting significantly higher values for the combination therapy group than the surgical debridement group (See Table 2). Mandibles exposed to combination therapy evidenced significantly greater values for Y (p=0.015) and E (p=0.026). Additional differences trending toward statistical significance further suggested enhanced biomechanical integrity in the treatment group. These trending differences included UL (p=0.066), FL (p=0.095) and EE (p=0.057). These biomechanical data imply that the introduction of this angiogenic and stem cell based therapy enhances the tensile strength of healing mandibles above those receiving only surgical debridement.
Table 2.
Means ± standard deviations for Yield (Y) in Newtons, Energy (E) in Joules, Ultimate Load (UL) in Newtons, Failure Load (FL) in Newtons and Elastic Energy (EE) in Joules for each group.
| Biomechanical Metrics | |||
|---|---|---|---|
| Debridement | ASCs + DFO | P-Value | |
| Y (N) | 35.06±14.91 | 65.39±28.55 | *0.015 |
| E (J) | 6.67±4.45 | 16.96±11.78 | *0.026 |
| UL (N) | 50.17±22.93 | 75.82±32.03 | 0.066 |
| FL (N) | 50.17±22.93 | 73.99±33.82 | 0.095 |
| EE (J) | 2.96±3.38 | 10.70±10.92 | 0.057 |
P-values also given for independent t-test between the groups.
Denotes significance at p < 0.05.
Note: Adipose Derived Stromal Cells (ASCs) and deferoxamine (DFO).
Bony Union Results
Perhaps most clinically significant, the combination therapy group demonstrated a 65% improvement in the remediation of non-unions compared to the surgical debridement group. Specifically, the combination therapy group displayed a 7% incidence of non-union, while the surgical debridement group exhibited a 20% incidence of non-union.
DISCUSSION
Despite advancements in the delivery of radiotherapy for Head and Neck Cancer (HNC), advanced osteoradionecrosis and associated non-unions still occur.7,38,39 Furthermore, there remains a grave disparity between the complex, severe and corrosive collateral damage imposed by radiotherapy and the limitations in our current diagnostic and reconstructive armaments for managing these pernicious side effects. The goal of this experiment was to investigate a therapeutic strategy designed to restore both the cellularity and vascularity at the site of mandibular non-union in rat mandibles exposed to radiation injury. We posited that the cellular replenishment afforded by ASCs, in combination with the angiogenic stimulus of DFO would rescue non-unions arising in the aftermath of radiotherapy, evidencing enhanced metrics of radiographic mineralization and biomechanical strength.
We previously established diminished metrics of vascularity, osteocyte viability, callus mineralization and biomechanical strength in a rat model of radiation-induced mandibular pathologic fracture healing.12-14 We further evidenced a reproducible non-union rate of 75-80% due to the administration of a HEDR.12-14 The aim of our previous studies concerned the prevention of radiation-induced bone related pathologies such as non-union with the use of DFO therapy. DFO exerts its angiogenic function by stimulating the hypoxia inducible factor (HIF 1-alpha) pathway. Under normoxic conditions, HIF 1-alpha undergoes prolyl-hydroxylation in a reaction requiring iron as a co-factor, ultimately leading to its degradation. Acting as an iron chelator, DFO prevents this degradation by removing iron from the equation. The sustained presence of HIF 1-alpha allows for the activation of a transcriptional cascade, upregulating VEGF and other angiogenic factors. Utilizing this approach to augment angiogenesis and subsequent vascularity, investigators have demonstrated the ability to accelerate normal fracture healing and distraction osteogenesis in long bone animal models.27-29 We have utilized this powerful technique to prevent the development of non-unions in the irradiated rat mandible. We have demonstrated improved metrics of vascularity, osteocyte viability, callus mineralization and biomechanical strength as follows:
220% increase in vessel number12
122% increase in osteocyte count14
53% increase in bone mineral density13
147% increase in ultimate load13
Collectively, we observed a prevention of non-union formation by 45% over non-treated controls (non-unions control = 22% vs. DFO = 67%) in a model where non-unions are the expected outcome.12-14
The present study represents an extension of our previous line of inquiry that seeks to treat non-unions once already established. In order to do this we modified our experimental study design by selecting out only animals exhibiting non-unions and subjecting them to further experimentation as previously described.
While our selection of DFO is based on our prior promising results concerning the prevention of non-unions, the addition of ASCs to our current treatment paradigm was warranted due to the extent of tissue necrosis. Tissue necrosis after radiation potentiates the formation of volumetric voids that may presumably be filled by introducing viable progenitors to the site of injury. Recent studies have shown promise in the treatment of radiation tissue injury with lipoaspirate transplantation. ASCs have been hypothesized to target damaged areas, release angiogenic factors, form new vessels, and increase tissue oxygenation.40-42 Recent improvements in isolation techniques and processing mechanisms have made ASCs an attractive reproducible means for tissue engineering strategies. ASCs are easily accessible, available in large numbers, attach and proliferate rapidly in culture, and have demonstrated the capacity to successfully fill critical sized bone defects in calvarial rat models.22 Levi et al. demonstrated that ASCs undergo rapid osteogenic differentiation to successfully ossify critical sized mouse calvarial defects.23 Based on collective observations, other investigators have posited that even a relatively large sized defect might be successfully repaired via ASC implantation. Small clinical studies have already investigated the potential for ASCs to heal defects in the human skeleton. In fact, successful or expedited healing of defects in the human mandible, maxilla and cranium were reported with the implementation of ASC-based treatment paradigms.43-45
Our results demonstrate that the combination of the cellular regeneration from ASCs and the angiogenic impact of DFO efficaciously bolsters the treatment of non-unions developing secondary to a high dose of radiation. While 80% of non-unions were healed with debridement alone, the addition of ASCs and DFO allowed for a remarkable 93% union rate in comparison. Further, our outcomes of μCT and biomechanical testing reveal that the unions in the treatment group demonstrated enhanced mineralization and mechanical strength in comparison to their non-treated counterparts. These data, in addition to gross examination of more robust callus formation, indicate that the functionality of these mandibles may be meaningfully improved with ASC and DFO combination therapy, which may have important clinical ramifications. Specifically, radiomorphometric analysis indicated a 9% increase in both BVF and BMD, as well as a 26% increase in TMC in the treatment group. Biomechanical tension testing further substantiated the efficacy of combined therapy as demonstrated by significant improvements in Y by 87% and E by 154% beyond debridement alone. Biomechanical enhancements of 51% for UL, 47% for FL and 261% for EE were trending, but not significant due to large standard deviations. These data suggest that because of their inferior biomechanical strength, unions that had only received debridement may be more susceptible to re-injury.
We wish to address certain limitations to this study that should be considered when interpreting our findings within the context of clinical applications. While we focused our investigation on the healing of bone, clinically, patients presenting with ORN and pathologic fractures often undergo extensive debridement of necrotic tissue including large sections of soft tissues. While our results with regards to bony healing are promising, they do not address the need for soft tissue reconstruction. We therefore advocate future investigation of these applications for the enhancement of soft tissue healing after radiation injury. Secondly, while our objective was to investigate the use of a combined therapeutic approach for the treatment of radiation-induced non-unions, subsequent studies are necessary to elucidate the specific etiology of the reconstructive ability of the stem cells. Currently, it is unclear whether the cells differentiate into osteoblasts and directly grow bone, or differentiate into endothelial cells and contribute to the vasculature, or produce pro-regenerative cytokines, or most likely, a combination of these events. Future studies aimed at determining the specific mechanism of how ASCs incorporate into the irradiated endogenous tissue will utilize ubiquitously GFP-tagged cells to attempt to track the fate of the implanted stem cells.
Although these results are promising, it is important to address the potential clinical use of stem cell therapies and angiogenic factors in the reconstructive management of HNC patients. We envision the use of this therapeutic strategy for reconstructive purposes to be used judiciously in a select group of patients who have developed sustained bone pathology beyond conventional care that remain cancer free at the time of reconstruction. It is also important to note that the incidence of pathologic fractures due to advanced osteoradionecrosis is typically a late finding, manifesting even years after reconstruction.7,39 This disease free period is advantageous to our treatment paradigm since it temporally limits any interaction stem cells or angiogenic factors may have with malignant cells. Lastly, although anti-angiogenic therapies have been successful in halting the progression of various tumors, no known studies have reported an associated incidence linking the development of tumors to the use of angiogenic therapies or ASCs.
Nevertheless, the results of this experiment demonstrate promising evidence in an animal model of a combined alternative therapeutic approach for the treatment of radiotherapy-induced non-unions. Specifically, quantifiable measures of bone density and bone strength are evidenced in the rat mandible. Based on our findings, we support the continued optimization of this promising strategy for translation to the clinical arena, and assert that the ability to change a radiated environment to allow for the healing of non-unions would make a substantial contribution to the reconstructive surgeon's current management arsenal.
Acknowledgements
The authors thank Mary Davis and Dave Karnak for assistance with the delivery of radiotherapy and Charles Roehm for fixator device fabrication. The authors also thank Cheyenne Vasseli for his laboratory contributions. This work was supported by grants from the National Institutes of Health (CA12587-01 and CA12587-06) to S.R.B., T32-GM008616 to Cynthia L. Marcelo for A.D. and The American Academy of Otolaryngology-Head and Neck Surgery Foundation: Percy Memorial Award for grant titled “A Combined Approach for Managing Mandibular Non-Unions after Radionecrosis.”
Financial Disclosure and Products: Funding supported by the following grants from the National Institutes of Health: “Translational Optimization of Bone Regeneration in the Irradiated Mandible” (CA12587-06) to S.R. Buchman and “Training Grant in Trauma, Burn, and Wound Healing Research” (T32-GM008616) for A. Donneys and The American Academy of Otolaryngology-Head and Neck Surgery Foundation: Percy Memorial Award to A. Donneys for grant titled “A Combined Approach for Managing Mandibular Non-Unions after Radionecrosis.”
REFERENCES
- 1.Urken ML, Buchbinder D, Costantino PD, et al. Oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Arch Otolaryngol Head Neck Surg. 1998;124:46–55. doi: 10.1001/archotol.124.1.46. [DOI] [PubMed] [Google Scholar]
- 2.Peled M, El-naaj IA, Lipin Y, Ardekian L. The use of free fibular flap for functional mandibular reconstruction. J Oral Maxillofac Surg. 2005;63:220–224. doi: 10.1016/j.joms.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 3.McCrory AL, Magnuson JS. Free tissue transfer versus pedicled flap in head and neck reconstruction. Laryngoscope. 2002;112:2161–2165. doi: 10.1097/00005537-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Nakamizo M, Yokoshima K, Yagi T. Use of free flaps for reconstruction in head and neck surgery: a retrospective study of 182 cases. Auris Nasus Larynx. 2004;31:269–273. doi: 10.1016/j.anl.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Ross DA, Hundal JS, Son YH, et al. Microsurgical free flap reconstruction outcomes in head and neck cancer patients after surgical extirpation and intraoperative brachytherapy. Laryngoscope. 2004;114:1170–1176. doi: 10.1097/00005537-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Shaari CM, Buchbinder D, Costantino PD, Lawson W, Biller HF, Urken ML. Complications of microvascular head and neck surgery in the elderly. Arch Otolaryngol Head Neck Surg. 1998;124:407–411. doi: 10.1001/archotol.124.4.407. [DOI] [PubMed] [Google Scholar]
- 7.Sciubba J, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006;7:175–183. doi: 10.1016/S1470-2045(06)70580-0. [DOI] [PubMed] [Google Scholar]
- 8.Xie XT, Qiu WL, Yuan WH, Wang ZH. Experimental study of radiation effect on the mandibular microvasculature of the guinea pig. Chin J Dent Res. 1998;1:46–51. [PubMed] [Google Scholar]
- 9.Johnsson AA, Jacobsson M, Granström G, Johansson CB, Strid KG, Turesson I. A microradiographic investigation of cancellous bone healing after irradiation and hyperbaric oxygenation: a rabbit study. Int J Radiat Oncol Biol Phys. 2000;48:555–563. doi: 10.1016/s0360-3016(00)00638-6. [DOI] [PubMed] [Google Scholar]
- 10.Maeda M, Earle JD. Effects of irradiation on cortical bone and their time-related changes. A biomechanical and histomorphological study. J Bone Joint Surg Am. 1988;70:392–399. [PubMed] [Google Scholar]
- 11.Takahashi S, Sugimoto M, Kotoura Y, Sasai K, Oka M, Yamamuro T. Long-term changes in the haversian systems following high-dose irradiation. An ultrastructural and quantitative histomorphological study. J Bone Joint Surg. 1994;76:722–738. doi: 10.2106/00004623-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Donneys A, Weiss DM, Deshpande SS, et al. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone. 2013;52:318–325. doi: 10.1016/j.bone.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donneys A, Ahsan S, Perosky JE, et al. Deferoxamine restores callus size, mineralization, and mechanical strength in fracture healing after radiotherapy. Plast Reconstr Surg. 2013;131:711e–719e. doi: 10.1097/PRS.0b013e3182865c57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donneys A, Nelson NS, Page EE, et al. Targeting angiogenesis as a therapeutic means to reinforce osteocyte survival and prevent non-unions in the aftermath of radiotherapy. Head Neck. 2014 doi: 10.1002/hed.23744. Advance online publication: doi:10.1002/hed.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi B, Longaker MT. Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells. 2011;29:576–582. doi: 10.1002/stem.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi B, Longaker MT. Osteogenic differentiation of adipose-derived stromal cells in mouse and human: in vitro and in vivo methods. J Craniofac Surg. 2011;22:388–391. doi: 10.1097/SCS.0b013e318207b72b. [DOI] [PubMed] [Google Scholar]
- 17.Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 18.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 19.Niemeyer P, Fechner K, Milz S, et al. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31:3572–3579. doi: 10.1016/j.biomaterials.2010.01.085. [DOI] [PubMed] [Google Scholar]
- 20.Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 21.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 22.Yoon E, Dhar S, Chun DE, et al. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Engineering. 2007;13:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 23.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS ONE. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 25.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 26.Farberg AS, Jing XL, Monson LA, et al. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone. 2012;50:1184–1187. doi: 10.1016/j.bone.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen X, Wan C, Ramaswamy G, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–1305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan C, Gilbert SR, Wang Y, et al. Activation of the hypoxia- inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchanque–Fossuo CN, Monson LA, Farberg AS, et al. Dose-response effect of human equivalent radiation in the murine mandible: part I. A histomorphometric assessment. Plast Reconstr Surg. 2011;128:114–121. doi: 10.1097/PRS.0b013e31821741d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchanque–Fossuo CN, Monson LA, Farberg AS, et al. Dose-response effect of human equivalent radiation in the murine mandible: part II. A biomechanical assessment. Plast Reconstr Surg. 2011;128:480e–487e. doi: 10.1097/PRS.0b013e31822b67ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monson LA, Jing XL, Donneys A, Farberg AS, Buchman SR. Dose-response effect of human equivalent radiation in the mandible. J Craniofac Surg. 2013;24:1593–1598. doi: 10.1097/SCS.0b013e31826cfeea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchman SR, Ignelzi MA, Jr, Radu C, et al. A unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511–519. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Levi B, Nelson ER, Brown K, et al. Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plastic and reconstructive surgery. 2011;128(2):373–386. doi: 10.1097/PRS.0b013e31821e6e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 36.Donneys A, Farberg AS, Tchanque-Fossuo CN, Deshpande SS, Buchman SR. Deferoxamine enhances the vascular response of bone regeneration in mandibular distraction osteogenesis. Plast Reconstr Surg. 2012;129(4):850–856. doi: 10.1097/PRS.0b013e31824422f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glowacki J. Angiogenesis in fracture repair. Clinical orthopaedics and related research. 1998;355:S82–S89. doi: 10.1097/00003086-199810001-00010. [DOI] [PubMed] [Google Scholar]
- 38.Dimery W, Hong WK. Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst. 1993;85(2):95–111. doi: 10.1093/jnci/85.2.95. [DOI] [PubMed] [Google Scholar]
- 39.Notani K, Yamazaki Y, Kitada H, et al. Management of osteoradionecrosis corresponding to the severity of osteoradionecrosis and the method of radiotherapy. Head Neck. 2003;25:181–186. doi: 10.1002/hed.10171. [DOI] [PubMed] [Google Scholar]
- 40.Broughton G, II, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117:1e–S-32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 41.Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 42.Levi B, Nelson ER, Hyun JS, et al. Enhancement of human adipose-derived stromal cell angiogenesis through knockdown of a BMP-2 inhibitor. Plastic and reconstructive surgery. 2012;129(1):53. doi: 10.1097/PRS.0b013e3182361ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulakov AA, Goldshtein DV, Grigoryan AS, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med. 2008;146:522–525. doi: 10.1007/s10517-009-0322-8. [DOI] [PubMed] [Google Scholar]
- 44.Lendeckel S, Jödicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Mao JJ, Giannobile WV, Helms JA, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]