Abstract
Background
It is not clear if cross-reactivity or co-sensitization to glutathione S-transferases (GST) occurs in tropical and subtropical environments. In the United States, Bla g 5 is the most important GST allergen, and lack of co-exposure to GST from certain species allows a better assessment of cross-reactivity.
Objectives
To examine the molecular structure of GST allergens from cockroach (Bla g 5), dust mites (Der p 8, Blo t 8) and helminth (Asc s 13) for potential cross-reactive sites, and to assess the IgE cross-reactivity of sensitized patients from a temperate climate for these allergens for molecular diagnostic purposes.
Methods
Four crystal structures were determined. Sera from cockroach and mite allergic patients were tested for IgE reactivity to these GST. A panel of six murine anti-Bla g 5 mAb was assessed for cross-reactivity with the other three GST using antibody binding assays.
Results
Comparisons of the allergen structures, formed by two-domain monomers that dimerize, revealed few contiguous regions of similar exposed residues, rendering cross-reactivity unlikely. Accordingly, anti-Bla g 5 or anti-Der p 8 IgE from North American patients did not recognize Der p 8 or Bla g 5, respectively, and neither showed binding to Blo t 8 or Asc s 13. A weaker binding of anti-Bla g 5 IgE to Der p 8 versus Bla g 5 (~100-fold) was observed by inhibition assays, similar to a weak recognition of Der p 8 by anti-Bla g 5 mAb. Patients from tropical Colombia had IgE to all four GST.
Conclusions
The lack of significant IgE cross-reactivity among the four GST is in agreement with the low shared amino acid identity at the molecular surface. Each GST is needed for accurate molecular diagnosis in different geographic areas.
Keywords: Cockroach, house dust mite, helminth, glutathione S-transferases (GST), cross-reactivity, diagnosis
Introduction
Cockroach allergy is an important risk factor for emergency room admissions with asthma in the U.S., especially in inner city-areas, where cockroach infestation is common.1-5 Each patient has a unique profile of sensitization to allergens identified from cockroach.6 Although none of the allergens are dominant in the U.S. population, Bla g 5 is one of the most prevalent among cockroach-allergic asthmatic patients, with an IgE antibody reactivity against the natural allergen of 68%, measured by radioimmunoassay.7 Bla g 5 belongs to a group of enzymes, the glutathione S-transferases (GST), which primarily catalyze the addition of a glutathione molecule (GSH) to another (commonly toxic) compound destined for removal from the cell, thus having a detoxifying function.7,8 Glutathione S-transferase homologs to Bla g 5 have been described among mite allergens and nematode parasite proteins. Mite allergens Der p 8 and Blo t 8 are GST from Dermatophagoides pteronyssinus and Blomia tropicalis, respectively, and are also clinically relevant. Interestingly, Der p 8 is a major allergen in sub-tropical and tropical areas, although lower prevalence has been reported in temperate areas.9-13 Infection with the nematode Ascaris lumbricoides (ascariasis) induces IgE synthesis against parasite antigens that may cross-react with homologous allergens. Cross-reactivity of allergens from nematodes and other sources, especially described for tropomyosins, is thought to aggravate the allergic response.14-17
Diagnosis often becomes complex when patients are sensitized to clinically cross-reactive allergens. The identification of the primary source of sensitization is difficult when skin tests are positive to extracts from different sources containing cross-reactive allergens. The use of purified species-specific allergens for molecular diagnosis facilitates the process of differentiation. IgE cross-reactivity has been reported in tropical and subtropical areas for GST from the American cockroach and the mite homolog Der p 8.9 However, different classes of GST exist, and it is unknown whether IgE cross-reactivity occurs between Bla g 5 and Der p 8, the most relevant and common GST from cockroach and mite in the U.S.. A recent study showed simultaneous IgE reactivity to Bla g 5, Der p 8, Blo t 8 and Asc s 13 in some Ascaris-infected patients from Colombia.18 Asc s 13 represents the Ascaris suum GST1, which shares 100% identity in 203 residues to the native Ascaris lumbricoides GST allergen Asc l 13, from which five N-terminal residues are unknown.19 It was not possible to distinguish whether the IgE reactivity to these GST resulted from co-sensitization or from cross-reactivity, because in tropical areas cosensitization to D. pteronyssinus and B. tropicalis is common, and may also coincide with sensitization to cockroaches and/or helminth infections.
Here, we investigated the IgE reactivity of cockroach and mite allergic patients from the U.S. to four GST: Bla g 5, Der p 8, Blo t 8 and Asc s 13. First, the X-ray crystal structures of the four GST were determined in order to compare the solvent accessible areas in common that could be responsible for cross-reactivity. Second, the IgE antibody recognition of the four GST was assessed by using sera from U.S. patients who are naturally exposed to Bla g 5 and Der p 8, but not to Blo t 8 and Asc s 13 present in tropical areas. Reactions to the tropical GST by patients from a temperate area would most likely indicate that cross-reactivity rather than co-sensitization occurred. This study is an analysis at the molecular level of the IgE cross-reactivity among three GST inhalant allergens and a GST allergen from a helminth parasite among patients from a temperate area, for the design of more accurate molecular diagnosis techniques.
Methods
Sera from cockroach and mite allergic patients
Sera from cockroach allergic patients (n = 31) were kindly provided by Dr. Robert Wood, from The Johns Hopkins University, Baltimore, MD, as part of a collaborative study with the Inner City Asthma Consortium (ICAC).20 Twenty-two sera were selected for their sensitivity to either Bla g 5 (n = 15) and/or Dermatophagoides pteronyssinus extracts (n = 18). Eight more Bla g 5 positive sera (3 of which were also Der p 8 positive) were identified from 12 sera from cockroach allergic patients additionally provided by ICAC. Fourty seven plasma from mite allergic patients were obtained from PlasmaLab International (Everett, WA), making a total of 68 samples from dust mite allergic patients from temperate areas of the U.S. Additional 32 sera from a tropical area (Colombia) were used to assess IgE immunoreactivity of the four GST. Details are provided in the Online Repository.
Expression, purification and determination of the X-ray crystal structures of Bla g 5, Der p 8, Blo t 8 and Asc s 13
The four GST were expressed in E. coli and purified by affinity chromatography. Their three-dimensional structures were determined by X-ray crystallography. Details are provided in the Online Repository.
Production of anti-Bla g 5 monoclonal antibodies
Balb/c mice were immunized with recombinant Bla g 5 (E. coli expressed). Splenocytes from mice with the highest Bla g 5 titers were fused with myeloma cells. Resulting hybridomas were screened against Bla g 5 by ELISA and cloned by limiting dilution analysis. The recognition of the four GST by Bla g 5-specific monoclonal antibodies (mAb) was assessed by enzyme-linked immunosorbent assay (ELISA), either by direct binding or by inhibition assays as described in the Online Repository.
Analysis of IgE antibody binding to GST
IgE antibody binding was analyzed by ELISA using direct or inhibition binding assays as described in the Online Repository.
Results
X-ray crystal structures of Bla g 5, Der p 8, Blo t 8 and Asc s 13
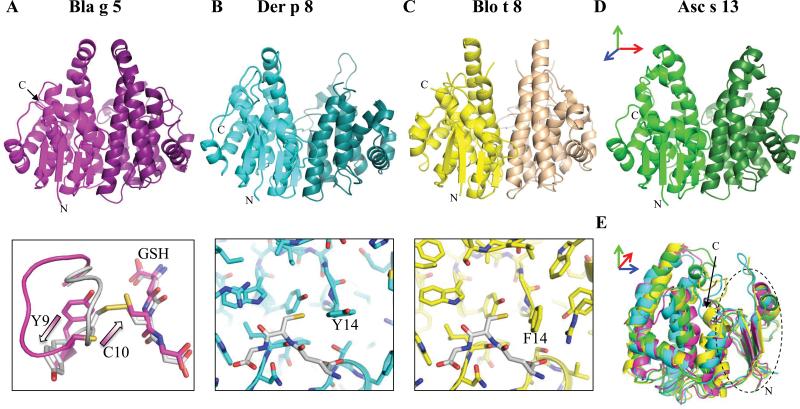
The X-ray crystal structures of Bla g 5, Der p 8, Blo t 8 and Asc s 13 were determined. The structures were submitted to the PDB, under the ID codes shown in Table E4 (Online Repository). The four GST are dimeric and have an overall fold typical of this group of enzymes (Fig. 1A-E). Each monomer (one from each GST shown superimposed in Fig. 1E) is formed by two domains: 1) a small thioredoxin-like N-terminal domain (~ 80 residues) made of a combination of 3 α-helices and a 4 stranded β-sheet; and 2) a larger helical C-terminal domain made entirely of α-helices (up to ~120 residues) (Fig. 1A-D).
Figure 1. GST Allergen Structures.
A-C (top) and D) Ribbon diagrams of the dimer. E) Superposition of monomers, and N-terminal thioredoxin-like sub-domain (dashed oval). Two active site conformations of Bla g 5, and the active site loop of Der p 8 and Blo t 8 (GSH molecule in white) are shown in bottom of A, B and C, respectively. Arrows in three colors indicate how the structures in A-D are rotated with respect to E. Black arrows in A and E show the C-termini.
The allergens belong to different classes of GST enzymes based on the structure and sequence analysis (Fig. E1, Tables E2 and E3, Online Repository). 21 The catalytic site between both domains had specific features depending on the class of GST. Bla g 5 and Asc s 13 are structurally related to the sigma class of GST enzymes (also known as Class II in studies of insect GST) (Fig. 1A, 1D). In a structure search, the best matches are to other GST from nematodes. Structural comparisons show that Bla g 5 (Fig. 1A) closely resembles the drosophila GST: dmGST-2. Initial studies confirm that Bla g 5 efficiently catalyzes the GSH-hydroxynonenal (NHE) conjugation similar to dmGST-2 (data not shown).8 In addition, Bla g 5 is unique among GST enzymes with a Cys at position 10 in the active site loop. In one molecule out of six in the crystal lattice, Cys10 was covalently linked to a GSH molecule, which remained in the canonical GSH position. However, to accommodate this linkage, the active site loop flipped to an alternate conformation that is expected to occlude an additional HNE substrate. Figure 1A (bottom) shows the typical GST conformation (magenta) and an alternate conformation (white) where Tyr9, which is usually the activating hydroxyl, is flipped away from the GSH and Cys10 forms a disulfide bond with the GSH.
Der p 8 and Blo t 8 are similar to the mu class of mammalian GST (Fig. 1B, C). Mu class enzymes typically attach GSH to highly electrophilic compounds. A distinctive difference of the mite GST appears to be the rare occurrence of a large aromatic moiety at position 14 in the active site loop instead of the usual leucine. This indicates that these enzymes may have different substrate specificities from other mu-class GST (Fig. 1B, 1C; bottom).
Lack of significant cross-reactivity with IgE from allergic patients and anti-Bla g 5 mAb
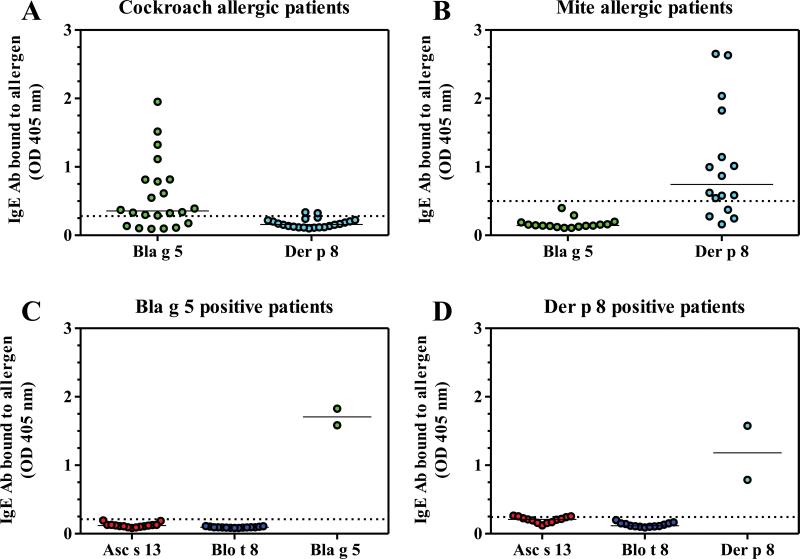
Originally, 22 sera from cockroach allergic patients and 16 plasma from mite allergic patients from North America were tested for direct binding to Bla g 5 and Der p 8. Fifteen of 22 sera from cockroach allergic patients had Bla g 5-specific IgE, in agreement with the streptavidin ImmunoCAP analysis20. None of the 15 sera showed significant IgE reactivity to Der p 8, despite the fact that most sera (18/22) had IgE against D. pteronyssinus extracts (15.0 ± 25.8 kU/L; range 0.46 to >100 kU/L) (Fig. 2A). Similarly, 12 out of 16 plasma/sera from dust mite allergic patients, with IgE specific for D. pteronyssinus allergens, reacted with Der p 8, and none significantly reacted with Bla g 5 (despite IgE reactivity to Bla g 1 and/or Bla g 2 in n = 6) (Fig. 2B). No significant IgE cross-reactivity was observed among the four GST tested, since none of the Bla g 5 or Der p 8-positive IgE antibodies reacted to the three remaining GST (Fig. 2C and D). Additional sera/plasma were tested to identify samples suitable for IgE antibody binding inhition assays (Fig. E2 Online Repository). Considering all samples tested, the prevalences of IgE sensitization to Bla g 5 and Der p 8 were 68% (23/34) and 49% (33/68), respectively. The immunoreactivity of each of the four GST to IgE from patients allergic to the respective source was proven for Bla g 5 and Der p 8 (Fig. 2, E2), and for Blo t 8 and Asc s 13 (using sera from South American patients from tropical Colombia; Fig. E3 Online Repository).18
Figure 2. IgE reactivity to GST.
A) IgE reactivity to Bla g 5 and none to Der p 8 in 15/22 cockroach allergic patients (from which 18 had also IgE reactivity to D. pteronyssinus by ImmunoCAP). B) IgE reactivity to Der p 8 and none to Bla g 5, in 12/16 mite allergic patients. C, D) IgE from 15 Bla g 5-positive sera (C) and 12 Der p 8-positive sera (D) did not bind Asc s 13 and Blo t 8. Two positive controls are on the right. Horizontal bars represent the median of the values. Dotted lines represent a cutoff value for a positive ELISA reading as defined in the methods.
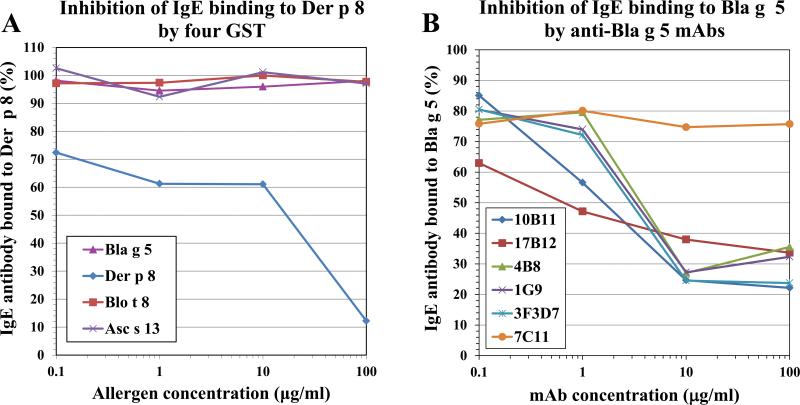
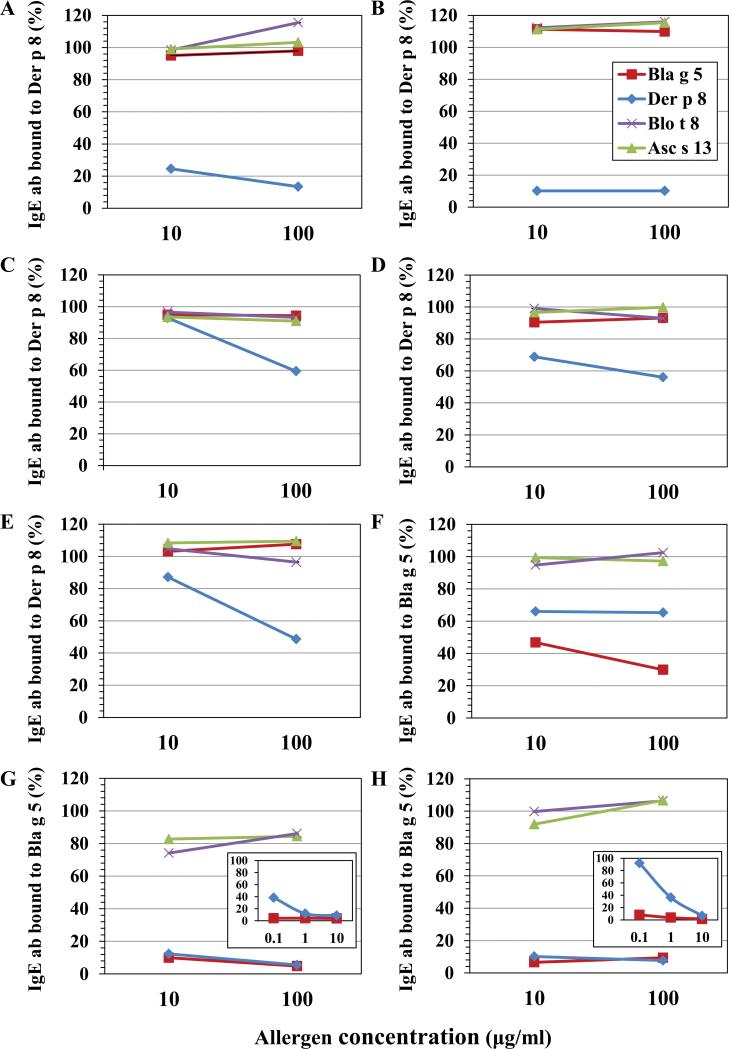
IgE binding to Der p 8 was only inhibited by Der p 8, but not by Bla g 5, Blo t 8 or Asc s 13 using either a sera pool (Fig. 3A) or plasma from individual mite allergic patients from North American patients (Fig. 4A-E). Among cockroach allergic patients, Bla g 5 inhibited IgE antibody binding to Bla g 5, as expected, but so did Der p 8, partially or totally (Fig. 4F-H). However, this binding by IgE from cockroach allergic patients to Der p 8 was at least ~100-fold weaker than to Bla g 5 (Fig 4G-H insets). This low level of cross-reactivity only for cockroach allergic patients is similar to what was observed for anti-Bla g 5 murine mAb reacting with Der p 8 at very high mAb concentrations (Fig. 5, see below).
Figure 3. Inhibition of IgE reactivity to GST.
A) Inhibition of IgE binding to Der p 8 by Der p 8, but not the other three GST using a pool of three sera from mite allergic patients from North America. B) Inhibition of IgE binding by anti-Bla g 5 mAb, but not by the anti-Bla g 1 mAb 7C11 negative control. Serum was from a patient highly allergic to cockroach (>100kU/L) sensitized to Bla g 1 (29.6kU/L) and Bla g 5 (94.5kU/L).
Figure 4. Inhibition of IgE reactivity to GST by Bla g 5 and Der p 8.
A-E) IgE reactivity to Der p 8 was only inhibited by Der p 8 among mite allergic patients from North America. F-H) IgE reactivity to Bla g 5 was inhibited by Bla g 5, and at ~100-fold less affinity by Der p 8 among cockroach allergic patients. (insets show larger range of inhibitor concentrations).
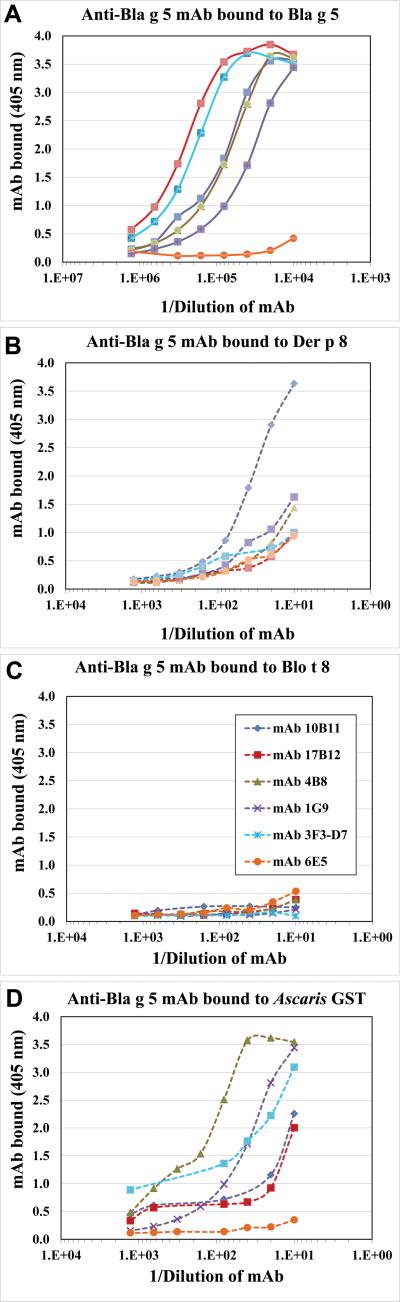
Figure 5. Recognition of GST by anti-Bla g 5 mAb.
Comparison of dose-response curves of six anti-Bla g 5 mAb binding to A) Bla g 5 (curves in solid lines) and to each of the other three GST: B) Der p 8, C) Blo t 8 and D) Asc s 13 (curves in dashed lines). Note the differences in the X-axis: there was a 1,000-fold lower concentration of mAb required for binding to Bla g 5, compared to the other GST.
Six mAb raised against Bla g 5 were tested for their reactivity to Der p 8, Blo t 8 and Asc s 13, to search for common antigenic determinants. The anti-Bla g 5 mAb showed minimal cross-reactivity to Der p 8 and Asc s 13 at very high concentrations of antibody (at least 1,000-fold more than for binding Bla g 5) (Fig. 5). Four of the mAb were ~10-fold more reactive to Asc s 13 than to Der p 8. No cross-reactivity was observed between Bla g 5 and Blo t 8 (Fig. 5). Inhibition assays showed that the six mAb bound to at least 4 different overlapping areas of Bla g 5. The mAb 4B8, 1G9 and 3F3D7 were in the same cross-reactive group (data not shown). Assuming that each epitope measures about 900 Å2, four non-overlapping epitopes would cover an important part of the immunogenic Bla g 5 surface (approximately 3,600 Å2 from 8,500 Å2, or 42% of the Bla g 5 monomer surface area). All tested mAb were able to individually inhibit IgE antibody binding to Bla g 5, showing their relevance for IgE antibody binding (Fig. 3B). The mAb 6E5 was not tested due to the low expression by the hybridoma.
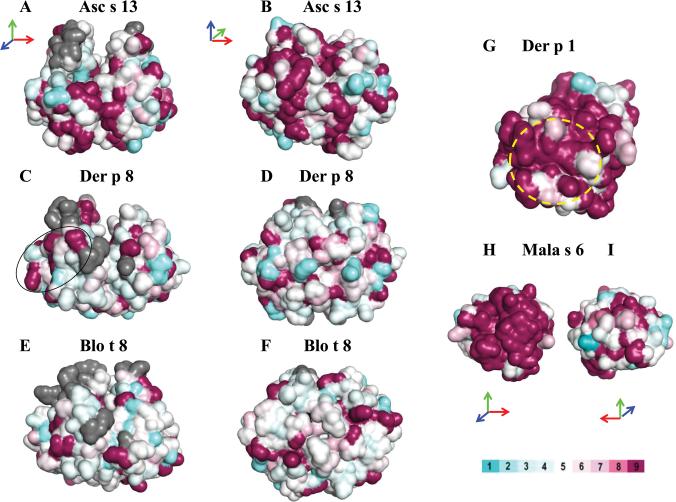
Potential cross-reactive surface residues
In order to assess the similarity of the residues on the surface of the four GST, their structures were aligned and then colored for residue similarity (Fig. 6). In panels A-F, Asc s 13, Der p 8, and Blo t 8 were compared to Bla g 5 and shaded accordingly. None of the structures display any substantial contiguous surface with amino acid identity to Bla g 5. The oval in Fig. 6C identifies 4 contiguous and two adjacent residues in common between Bla g 5 and Der p 8, which appears to be the most similar area between the two allergens that may account for the observed weak cross-reactivity (Fig. 6B, E4). This similarity is not present in the other two GST. The lack of similar surface residues is in agreement with the absence of substantial cross-reactivity or antibody binding of the six anti-Bla g 5 mAb tested for binding to Asc s 13, Der p 8 and Blo t 8.
Figure 6. Surface residue comparisons.
Residue similarity of Asc s 13 (A, B), Der p 8 (C, D), and Blo t 8 (E, F) to Bla g 5. G) Der p 1 similarity to Der f 1, and epitope for cross-reactive mAb 4C1 (circle).37 H-I) Mala s 6 similarity to Cat r 1.23 Color bar represents residue similarity from low (light blue) to high (maroon) (Online Repository). Grey represents gaps or insertions in sequence alignment. Arrows indicate how the structures are rotated with respect to each other.
From the structures, a Surface Area Similarity (SAS) index based on the surface exposed residues was calculated (Tables E1, E2, Online Repository). The weak mAb binding to Asc s 13 was in line with a larger number of identical residues on the Bla g 5 surface when one compares Asc s 13 versus Der p 8 and Blo t 8 (SAS: 58% versus 47% and 46% respectively (Fig. 6, Table E2).
For a comparison where there is known cross-reactivity, Der p 1 is colored in Figure 6G with the similarity to Der f 1 (SAS: 86%), showing many identical residues. The structure of the epitope for the cross-reactive mAb 4C1, known to interfere with IgE antibody binding, is indicated in Figure 6G.22 Similarly, it was recently demonstrated that patient IgE antibodies cross-react with the cyclophilin allergens Cat r 1 and Mala s 6.23 Both allergens share surface residue similarity (Fig. 6H-I). Near the cyclophilin active site, there is a substantial contiguous surface of residue identity that is likely to be the source of the cross reactivity (SAS: 75%) (Fig. 6H).
Discussion
This study is a comparative analysis of four glutathione-S-transferase allergens at the atomic level, coupled with assessment of IgE cross-reactivity to these GST in U.S. allergic patients for diagnostic purposes. Most studies of GST allergens have been performed in tropical or sub-tropical areas, where cross-reactivity among certain GST has been suggested.9,18 The species producing these allergens often co-exist, and the distinction between co-sensitization and cross-reactivity is controversial.24 For example, dual sensitization to Blomia tropicalis and Dermatophagoides pteronyssinus is common. IgE cross-reactivity between both species is generally low and unique allergenic specificities of B. tropicalis allergens have been found.25 B. tropicalis allergens share little cross-reactivity with the three major Dermatophagoides pteronyssinus allergens Der p 1, Der p 2 and Der p 5.24-26 In temperate areas like the U.K., for patients naturally exposed only to D. pteronyssinus, a low cross-reactivity was found. Cross-reactivity in this population was not due to group 5 allergens, which were species-specific.27
Four GST molecular structures were determined here, including the first two published structures of GST enzymes from the class Arachnida (Der p 8 and Blo t 8). The structures revealed a similar overall fold, typical of this group of enzymes, but low overall amino acid identities (23-37%). It has been estimated that cross-reactivity is rare below 50% identity at the level of the entire protein, and requires more than 70% identity in most cases.28 This is illustrated by the observation that Tyr p 8 cross-reacts with Der p 8 (which shares 83% identity). In contrast, two different classes of GST from Blattella germanica (mu Bla g 5 and a delta GST, sharing only 14% identity), showed IgE reactivity that was attributed to co-sensitization.29,30 Despite this general rule, an unexpected cross-reactivity between Bla g 5 and a GST from Wuchereria bancrofti (WbGST), a major lymphatic filarial pathogen of humans, was reported, with only a 27.9% identity between both proteins (alignment in Fig. E1, Online Repository).31 Although the authors identified shared linear epitopes possibly responsible of a low level of cross-reactivity, the corresponding peptides were unable to fully inhibit the IgE antibody binding to the protein.
The fundamental determinant of cross-reactivity is the presence of clusters of identical amino acids on the molecular surface that are accessible to antibodies; these clusters are not always readily apparent in a sequence comparison. A low level of homology, without clusters of high identity, was found on the molecular surface of the four GST structures analyzed, in contrast with the positive control comparison of Der p 1 and Der f 1.22 In agreement with these findings, the immunological analysis showed no significant cross-reactivity among the four GST using either IgE antibodies from U.S. patients sensitized to Bla g 5 and/or Der p 8, or murine anti-Bla g 5 mAbs that bind to epitopes overlapping with IgE binding sites, by direct and/or indirect binding assays.
Regarding direct binding assays, it has been reported that passive adsorption on polystyrene results in partial loss of protein function. Therefore, allergens coating the plates may not always be present in their “native state”.32 The plates were coated with a large amount of allergen (10 μg/ml). Even a large loss of 90% of protein, would leave 100 ng/well in the native conformation. In addition, NMR experiments showed that there was no effect of pH changes (mimicking the ones that occur due to allergen adsorption to plates in direct binding assays) on the allergen conformation (data not shown). The levels of IgE antibody binding measured strongly suggest the presence of correctly folded IgE conformational epitopes in the recombinant GST used for the direct antibody binding assays.
IgE were specific for Der p 8 among mite allergic patients from temperate areas. The epitopes for these IgE antibodies may either be located in parts of the allergen surface non-structurally equivalent, or in structurally equivalent parts of the surface that have different amino acids. On the other hand, IgE against Bla g 5 showed weak binding to Der p 8 by antibody inhibition assays. This low level of IgE cross-reactivity between Bla g 5 and Der p 8, only among cockroach allergic patients, was similar to the one observed for anti-Bla g 5 murine mAb reacting with Der p 8. There are residues in common between Bla g 5 and Der p 8 (i.e. 18-25 and 65-80 by Bla g 5 sequence numbering (Fig. E1 Online Repository), but they are mostly not accessible for antibody binding in Der p 8. A structural alignment revealed a possible discontinuous epitope on Der p 8 that could be responsible for this low level cross-reactivity (Asp 28, Asp 31, Arg 33, Gln 35, Asn 49 and Lys 201 of Der p 8, Fig. E4). Since a lack of cross-reactivity was observed among mite allergic patients, these results suggest a low level of asymmetric cross-reactivity between Bla g 5 and Der p 833, and confirm a lack of significant IgE cross-reactivity among the four GST. Although Aalberse et al. suggested that complete absence of cross-reactivity cannot be proven, the results clearly show a lack of significant in vitro IgE cross-reactivity, that should translate into a lack of clinical cross-reactivity (whereas the reverse does not always occur).34 This observation was facilitated by using sera from patients living in temperate areas, who are not co-sensitized to allergens or parasite antigens present only in tropical environments (i.e. Blo t 8, Asc s 13).
The strongest IgE responses in patients from temperate areas were to Bla g 5 and Der p 8. The lack of significant cross-reactivity between both allergens was proven by direct binding and further confirmed by inhibition of IgE antibody binding. Although a moderate cross-reactivity has been reported between Der p 8 and GST from American cockroach (Periplaneta americana) whole body extract,9 this result cannot be extrapolated to Bla g 5 for several reasons. First, the affinity purification performed in the study by Huang et al., using glutathione agarose beads, would have resulted in the isolation of many GST (such as the ones described from delta and theta classes, that share only 10-22% identity with Bla g 5) (details in Online Repository). Second, Bla g 5 belongs to a different class (sigma) than other GST from German cockroach (delta and theta) and Der p 8 (mu). The identity between Bla g 5 and these GST is low (~20% with German cockroach GST and 28% with Der p 8), below the 35% estimated threshold under which high-affinity cross-reactivity is unlikely.33
The GST positive patients from the U.S. did not recognize the tropical allergens Blo t 8 and Asc s 13, in agreement with the low homologies between Bla g 5 or Der p 8 and either Blo t 8 or Asc s 13 (range 23-37%) (Table E2). Cross-reactivity among GST needs to be further evaluated in tropical environments, where evidence of cross-inhibition of a GST band using extracts from Ascaris and B. tropicalis has been observed.15 In tropical areas, the simultaneous exposure to cockroach, mites and Ascaris could generate a stronger and more diverse response with an increased repertoire of recognized epitopes.35 A lack of cross-reactivity between GST inhalant allergens and the Asc s 13 has implications for the interpretation of interactions between disease mechanisms of helminth IgE-binding components and allergens. These interactions are likely not to be relevant for GST compared to other highly cross-reactive proteins such as tropomyosins.14-17
Diagnosis of cockroach allergy relies on the use of cockroach extracts by either skin test or in vitro ImmunoCAP. Molecular allergy diagnosis can also be performed, using a panel of four cockroach allergen components (Bla g 1, Bla g 2, Bla g 5 and Bla g 7) available on microarray (ImmunoCAP-ISAC). To design a panel of cockroach allergens for molecular diagnosis, the aspartic protease Bla g 2 and the GST Bla g 5 are the main allergens of choice due to their highest IgE prevalence, and their specificity for German cockroach.3,6 Allergens from groups 1 and 7 are clinically cross-reactive and will not allow distinction of IgE sensitization between common species such as German and American cockroaches. The importance of Bla g 5 in cockroach allergy is additionally supported by: 1) the strength of the specific IgE response, with the highest IgE antibody titers compared to allergens from groups 1, 2, 4 and 7; and 2) the highest correlation between skin test reactivity to cockroach extract and specific IgE to Bla g 5.6,36 The homologous mite allergen Der p 8 is a major allergen in tropical and sub-tropical areas.9 The IgE reactivity to native Der p 8 was 96% among mite-sensitized subjects from sub-tropical Taiwan, and 75% and 65% in the tropical countries of Malaysia and Singapore, respectively.9 Lower IgE prevalences have been measured for Der p 8 in Australia (25% and 40%) and in European countries with temperate climates (9-16%), and for Blo t 8 (25%).9-12 To diagnose mite allergy, a panel of three allergens from D. pteronyssinus is available by ImmunoCAP ISAC: Der p 1 and Der p 2 (which are immunodominant) and the minor allergen Der p 10. Although the addition of mite GST may not be required in temperate areas due to its low prevalence, further studies will be required to assess if their addition will improve molecular diagnosis in tropical and sub-tropical areas.
In conclusion, consistent evidence for a lack of cross-reactivity among GST observed in the present study is especially relevant for the development of more accurate molecular diagnostic techniques, by which assessment of specific IgE responses to isolated natural or recombinant allergens is carried out in single tests or microarrays and multiplex assays. The use of species-specific GST should be included in diagnostic panels for evaluating allergies, especially to cockroach, for the identification of sources of IgE cross-reactivity to be avoided and for the development of immunotherapy. The non-cross-reactive antibodies identified in this study will also be useful for assessment of exposure and sensitization to GST allergens.
Supplementary Material
Clinical Implications.
The lack of significant in vitro IgE cross-reactivity to Bla g 5, Der p 8, Blo t 8 and Asc s 13 in allergic patients from temperate climates highlights that species-specific GST are required for molecular diagnostics.
Capsule Summary.
A low identity at the molecular surface of four GST from cockroach, mite and Ascaris is reflected in a lack of significant cross-reactivity of anti-Bla g 5 murine mAb and IgE from patients living in a temperate environment.
Acknowledgements
We thank Dr. Robert Wood for kindly providing sera from cockroach allergic patients as part of a collaboration with the Inner City Asthma Consortium. The authors appreciate the assistance of Jason Williams, Ph.D. with mass spectrometry, and thank Aaron Manning for technical assistance in protein purification.
Research in this publication was supported in part by Research Project Number Z01- ES102885-01 to REL, and ZIA- ES102645 to LCP in the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health, and in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI077653 (AP and MDC). Use of the Advance Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences contract W-31-109-Eng-38. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research was also supported by the Administrative Department of Science Technology and Innovation (Colciencias) Contract 406-2011 and 201-2015 (Colombia).
Abbreviations
- Ab
Antibody
- CR
Cockroach
- Der p
Dermatophagoides pteronyssinus
- ELISA
Enzyme-linked immunosorbent assay
- GST
Glutathione S-transferase/s
- GSH
Glutathione mAb Monoclonal antibody/ies
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- PDB
Protein Data Bank
- RMSD
Root-mean-square deviation
- WbGST
GST from Wuchereria bancrofti
References
- 1.Pollart SM, Chapman MD, Fiocco GP, Rose G, Platts-Mills TA. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J Allergy Clin Immunol. 1989;83:875–82. doi: 10.1016/0091-6749(89)90100-0. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 3.Arruda LK, Barbosa MC, Santos AB, Moreno AS, Chapman MD, Pomés A. Recombinant allergens for diagnosis of cockroach allergy. Curr Allergy Asthma Rep. 2014;14:428. doi: 10.1007/s11882-014-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huss K, Adkinson NF, Jr., Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 5.Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Satinover SM, Reefer AJ, Pomés A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. 2005;115:803–9. doi: 10.1016/j.jaci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Arruda LK, Vailes LD, Platts-Mills TA, Hayden ML, Chapman MD. Induction of IgE antibody responses by glutathione S-transferase from the German cockroach (Blattella germanica). J Biol Chem. 1997;272:20907–12. doi: 10.1074/jbc.272.33.20907. [DOI] [PubMed] [Google Scholar]
- 8.Agianian B, Tucker PA, Schouten A, Leonard K, Bullard B, Gros P. Structure of a Drosophila sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidation products. J Mol Biol. 2003;326:151–65. doi: 10.1016/s0022-2836(02)01327-x. [DOI] [PubMed] [Google Scholar]
- 9.Huang CH, Liew LM, Mah KW, Kuo IC, Lee BW, Chua KY. Characterization of glutathione S-transferase from dust mite, Der p 8 and its immunoglobulin E cross-reactivity with cockroach glutathione S-transferase. Clin Exp Allergy. 2006;36:369–76. doi: 10.1111/j.1365-2222.2006.02447.x. [DOI] [PubMed] [Google Scholar]
- 10.Hales BJ, Martin AC, Pearce LJ, Laing IA, Hayden CM, Goldblatt J, et al. IgE and IgG anti-house dust mite specificities in allergic disease. J Allergy Clin Immunol. 2006;118:361–7. doi: 10.1016/j.jaci.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill GM, Donovan GR, Baldo BA. Cloning and characterization of a major allergen of the house dust mite, Dermatophagoides pteronyssinus, homologous with glutathione S-transferase. Biochim Biophys Acta. 1994;1219:521–8. doi: 10.1016/0167-4781(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 12.Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, et al. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004;34:597–603. doi: 10.1111/j.1365-2222.2004.1930.x. [DOI] [PubMed] [Google Scholar]
- 13.Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, et al. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008;38:959–65. doi: 10.1111/j.1365-2362.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 14.Santos AB, Rocha GM, Oliver C, Ferriani VP, Lima RC, Palma MS, et al. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. 2008;121:1040–6. doi: 10.1016/j.jaci.2007.12.1147. [DOI] [PubMed] [Google Scholar]
- 15.Acevedo N, Sanchez J, Erler A, Mercado D, Briza P, Kennedy M, et al. IgE cross-reactivity between Ascaris and domestic mite allergens: the role of tropomyosin and the nematode polyprotein ABA-1. Allergy. 2009;64:1635–43. doi: 10.1111/j.1398-9995.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9:29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–86. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acevedo N, Mohr J, Zakzuk J, Samonig M, Briza P, Erler A, et al. Proteomic and immunochemical characterization of glutathione transferase as a new allergen of the nematode Ascaris lumbricoides. PLoS ONE. 2013;8:e78353. doi: 10.1371/journal.pone.0078353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebau E, Eckelt VH, Wildenburg G, Teesdale-Spittle P, Brophy PM, Walter RD, et al. Structural and functional analysis of a glutathione S-transferase from Ascaris suum. Biochem J. 1997;324(Pt 2):659–66. doi: 10.1042/bj3240659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oseroff C, Sidney J, Tripple V, Grey H, Wood R, Broide DH, et al. Analysis of T cell responses to the major allergens from German cockroach: Epitope specificity and relationship to IgE production. J Immunol. 2012;189:679–88. doi: 10.4049/jimmunol.1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa H, Holm L. Advances and pitfalls of protein structural alignment. Curr Opin Struct Biol. 2009;19:341–8. doi: 10.1016/j.sbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Chruszcz M, Pomés A, Glesner J, Vailes LD, Osinski T, Porebski PJ, et al. Molecular determinants for antibody binding on group 1 house dust mite allergens. J Biol Chem. 2012;287:7388–98. doi: 10.1074/jbc.M111.311159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh D, Mueller GA, Schramm G, Edwards LL, Petersen A, London RE, et al. Primary identification, biochemical characterization, and immunologic properties of the allergenic pollen cyclophilin cat R 1. J Biol Chem. 2014;289:21374–85. doi: 10.1074/jbc.M114.559971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo IC, Cheong N, Trakultivakorn M, Lee BW, Chua KY. An extensive study of human IgE cross-reactivity of Blo t 5 and Der p 5. J Allergy Clin Immunol. 2003;111:603–9. doi: 10.1067/mai.2003.167. [DOI] [PubMed] [Google Scholar]
- 25.Arruda LK, Vailes LD, Platts-Mills TA, Fernandez-Caldas E, Montealegre F, Lin KL, et al. Sensitization to Blomia tropicalis in patients with asthma and identification of allergen Blo t 5. Am J Respir Crit Care Med. 1997;155:343–50. doi: 10.1164/ajrccm.155.1.9001334. [DOI] [PubMed] [Google Scholar]
- 26.Chew FT, Yi FC, Chua KY, Fernandez-Caldas E, Arruda LK, Chapman MD, et al. Allergenic differences between the domestic mites Blomia tropicalis and Dermatophagoides pteronyssinus. Clin Exp Allergy. 1999;29:982–8. doi: 10.1046/j.1365-2222.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 27.Simpson A, Green R, Custovic A, Woodcock A, Arruda LK, Chapman MD. Skin test reactivity to natural and recombinant Blomia and Dermatophagoides spp. allergens among mite allergic patients in the UK. Allergy. 2003;58:53–6. doi: 10.1034/j.1398-9995.2003.23354.x. [DOI] [PubMed] [Google Scholar]
- 28.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–38. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 29.Liao EC, Lin YH, Chiu CL, Lin TC, Tsai JJ. Identification of allergenic component Tyr p 8 from Tyrophagus putrescentiae and cross-reactivity with Der p 8. Clin Vaccine Immunol. 2013;20:506–12. doi: 10.1128/CVI.00633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong KY, Jeong KJ, Yi MH, Lee H, Hong CS, Yong TS. Allergenicity of sigma and delta class glutathione S-transferases from the German cockroach. Int Arch Allergy Immunol. 2009;148:59–64. doi: 10.1159/000151506. [DOI] [PubMed] [Google Scholar]
- 31.Santiago HC, Leevan E, Bennuru S, Ribeiro-Gomes F, Mueller E, Wilson M, et al. Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J Allergy Clin Immunol. 2012;130:248–56. doi: 10.1016/j.jaci.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler JE, Ni L, Nessler R, Joshi KS, Suter M, Rosenberg B, Chang J, Brown WR, Cantarero LA. The physical and functional behavior of capture antibodies adsorbed on polystyrene. J Immunol Methods. 1992;150:77–90. doi: 10.1016/0022-1759(92)90066-3. [DOI] [PubMed] [Google Scholar]
- 33.Aalberse RC. Assessment of allergen cross-reactivity. Clin Mol Allergy. 2007;5:2. doi: 10.1186/1476-7961-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aalberse RC, Akkerdaas J, van Ree R. Cross-reactivity of IgE antibodies to allergens. Allergy. 2001;56:478–90. doi: 10.1034/j.1398-9995.2001.056006478.x. [DOI] [PubMed] [Google Scholar]
- 35.Acevedo N, Caraballo L. IgE cross-reactivity between Ascaris lumbricoides and mite allergens: possible influences on allergic sensitization and asthma. Parasite Immunol. 2011;33:309–21. doi: 10.1111/j.1365-3024.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 36.Slater JE, James R, Pongracic JA, Liu AH, Sarpong S, Sampson HA, et al. Biological potency of German cockroach allergen extracts determined in an inner city population. Clin Exp Allergy. 2007;37:1033–9. doi: 10.1111/j.1365-2222.2007.02751.x. [DOI] [PubMed] [Google Scholar]
- 37.Chruszcz M, Chapman MD, Vailes LD, Stura EA, Saint-Remy JM, Minor W, et al. Crystal structures of mite allergens Der f 1 and Der p 1 reveal differences in surface-exposed residues that may influence antibody binding. J Mol Biol. 2009;386:520–30. doi: 10.1016/j.jmb.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.