Abstract
Resveratrol (RSV), a natural polyphenols, has been suggested to induce cell cycle arrest and activate apoptosis-mediated cell death in several cancer cells, including prostate cancer. However, several molecular mechanisms have been proposed on its chemopreventive action, the precise mechanisms by which RSV exerts its anti-proliferative effect in androgen-independent prostate cancer cells remain questionable. In the present study, we show that RSV activates autophagic cell death in PC3 and DU145 cells, which was dependent on stromal interaction molecule 1 (STIM1) expression. RSV treatment decreases STIM1 expression in a time dependent manner and attenuates STIM1 association with TRPC1 and Orai1. Furthermore, RSV treatment also decreases ER calcium storage and store operated calcium entry (SOCE), which induces endoplasmic reticulum (ER) stress thereby activating AMPK and inhibiting the AKT/mTOR pathway. Similarly, inhibition of SOCE by SKF-96365 decreases the survival and proliferation of PC3 and DU145 cells and inhibits AKT/mTOR pathway and induces autophagic cell death. Importantly, SOCE inhibition and subsequent autophagic cell death caused by RSV was reversed by STIM1 overexpression. STIM1 overexpression restored SOCE, prevent the loss of mTOR phosphorylation and decreased the expression of CHOP and LC3A in PC3 cells. Taken together, for the first time, our results revealed that RSV induces autophagy mediated cell death in PC3 and DU145 cells through regulation of SOCE mechanisms, including down regulating STIM1 expression and trigger ER stress by depleting ER calcium pool.
Keywords: Resveratrol, SOCE, TRPC1, Orai1, STIM1, ER stress, autophagy and prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed cancer in the male population of western decent and is the leading cause of cancer related death in men who are older than 65 [1,2]. Anti-androgen therapy is remarkable in the early stages of prostate tumors development since the growth is completely dependent on androgens. However, in majority of cases, the tumor progress to androgen insensitive stage where androgen is not required for their growth, for which currently no successive therapy is available [3]. Therefore, a better understanding of the mechanism(s) and identification of novel target molecules that control the androgen insensitive prostate cancer cell proliferation and the development of novel therapeutic drugs, are essentially needed in the near future.
Prostate epithelial cells express a large number of Ca2+ permeable channels that are required to regulate different cellular functions including proliferation and differentiation. Loss of Ca2+ homeostasis modulation, could be at least in part, the mechanisms where the prostate cancer epithelial cells become resistant to apoptotic stimuli which ultimately leads to uncontrolled proliferation and invasion [4,5]. Recent studies have suggested that intracellular Ca2+ as well as Ca2+ channels play a major role in PCa progression [6,7]. For example, double knockdown of alpha1D-adrenergic receptor (alpha1D-AR) and transient receptor potential vanilloid type 1 (TRPV1) inhibit noradrenalin induced PC3 cell proliferation by suppressing Ca2+ influx, and reducing the activation of phospholipase C (PLC), protein kinase C (PKC) and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways [8]. Similarly, other studies have shown that calcium influx through TRPC6, TRPV6, TRPM8 and T-type Ca2+ channels participate in the proliferation of prostate cancer cells [9-12]. On the other hand, massive Ca2+ influx via the store operated Ca2+ entry (SOCE) mechanism induces apoptosis mediated cell death in several cancer cells [5,13]. Therefore studying the Ca2+ permeable channels and its downstream signaling pathways may be effective in the identification of novel drug targets that could be used for tumor regression.
SOCE is an important cellular mechanism that ensure the optimal refilling of endoplasmic reticulum (ER) /sarcoplasmic reticulum (SR) Ca2+ stores, activation of several Ca2+ mediated pro and anti-survival mechanisms and gene transcription. In excitable and non-excitable cells, SOCE is mediated by store operated Ca2+ channels (SOCs) which are located in the plasma membrane and depletion of ER Ca2+ stores activates the SOCs mediated Ca2+ influx (known as SOCE). Interestingly, changes in ER Ca2+ levels and SOCs function play a crucial role in the establishment of an androgen independent apoptosis resistance phenotype of prostate cancer [5,14,15]. Members of TRPC family particularly, TRPC1 and TRPC4 have been identified as the promising molecular candidates for SOCs in androgen-dependent LNCaP cells [16,17] and notably, recent studies have identified two other molecular components of SOCs, Orai1 and STIM1 [18,19]. Stromal interaction molecule 1 (STIM1) is a single transmembrane ER/SR resident protein with NH2-terminal Ca2+ binding EF hand directed to the lumen of the ER that functions as a signal transducer from ER to SOCs. Upon store depletion, STIM1 translocates to plasma membrane where it interacts with TRPC1 and/or Orai1 to initiate SOCE [18]. Several studies have shown that SOCE and SOCs are involved in cell cycle progression, proliferation and migration [9,20], but its role in prostate cancer as well as downstream signaling mechanism is still not yet established.
Dietary polyphenols are the most abundant natural antioxidants in the human food and recent reports suggested the efficacy of natural antioxidants in controlling the growth and migration of various cancers [21]. Studies have suggested that dietary intake of natural antioxidants could halt cancer progression. Resveratrol (trans-3,4′ ,5-trihydroxystilbene; RSV), a natural polyphenolic antioxidant found in a wide variety of fruits including grapes, raspberries, and other plants. Several in vitro and in vivo studies have suggested the growth inhibitory efficacy of RSV in several types of cancer cells, notably leukemia, breast, colon, liver, lung, thyroid and other epithelial cancers [21,22]. Importantly, the anti-carcinogenic effect of RSV in prostate cancer has been shown and it’s widely associated with changes in the expression of cell cycle regulating molecules such as, cyclin A, cyclin E, cdc2 and Rb. Several other studies have also noticed that RSV inhibits the proliferation of androgen dependent and independent prostate cancer cell lines by activating the apoptotic pathway [23,24]. Moreover, RSV regulates the expression of PSA (prostate specific antigen) by an androgen receptor (AR) dependent and AR-independent mechanism, and also RSV enhances the effect of anti-AR drug bicalutamide [23-25]. However, the role of SOCE in RSV mediated anti-cancer effect and the precise mechanisms by which RSV exerts its anti-proliferative effect in androgen-independent prostate cancer cell lines remain unanswered. Thus, we have investigated whether SOCE is involved in prostate cancer cell proliferation and does RSV affects cell viability through SOCs dependent Ca2+ pathways. To address this, we have used two androgen-independent prostate epithelial cells PC3 and DU145 along with control RWPE-1 cells. The results from our study suggest that RSV inhibits prostate cancer proliferation by downregulating STIM1 thereby inhibiting SOCE and initiating autophagic cell death.
MATERIALS AND METHODS
Cell culture reagents and transfection
Control prostate cell line RWPE1 (CRL 11609) and prostate cancer cell line DU145 (HTB-81) and PC3 (CRL1435) cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in their respective medium along with various supplements as suggested by ATCC. Cells were maintained at 37°C with 95% humidified air and 5% CO2 and were passaged as needed. Culture medium was changed twice weekly and cells were maintained in complete media, until reaching 90% confluence. For overexpression HA-STIM1 was used and 5 ug of plasmid DNA was transfected using Lipofectamine 2000 in Opti-MEM medium as per supplier’s instructions and assayed after 24 hours.
Cell Proliferation and Cell Viability assays
The ten thousand cells were plated in 96 well plate and synchronized by stimulating with 10% FBS. Then pulsed with BrdU for 2 h before BrdU incorporation was measured as per manufacturer’s instructions (Roche). For viability assays, cells were seeded on 96-well plates at a density of 0.5×105 cells/well. Cell viability was measured by using the Vybrant MTT cell proliferation assay kit (Molecular Probes, Invitrogen). Absorbance was read at 570 nm (630 nm as a reference) on a microplate reader (Molecular Devices). Cell viability was expressed as a percentage of the control culture.
Immunoprecipitation and Western blotting. Immunoprecipitations were carried out as described earlier [26]. RIPA buffer was used to prepare the total cell lysates. Following stimulation, cells were lysed with RIPA buffer and used for immunoprecipitation. Protein concentrations were determined using the Bradford reagent (Bio-Rad) and and 25-50 ug of proteins were resolved in 4%–12% NuPAGE gels, followed by Western blotting with the desired antibodies. Crude membrane was prepared from DU145 and PC3 cells as described previously [27]. Proteins were resolved on 4-12% Bis-Tris gels (Invitrogen), transferred to PVDF membranes and probed with respective antibodies. Peroxidase conjugated respective secondary antibodies were used to label the proteins. The proteins were detected by enhanced chemiluminescence detection kit (SuperSignal West Pico; Pierce). Antibodies that were used in this study are from Cell Signaling Technology (Danvers, MA) and Alomone Labs (TRPC1 and Orai1 antibodies). All other reagents used were of molecular biology grade obtained from Sigma chemicals unless mentioned otherwise.
Confocal images
PC3 and DU145 cells were transiently transfected with GFP-LC3 by using Lipofectamine 2000 in Opti-MEM medium as described by the manufacturer. After 24 h, cells were treated with RSV for 24h and then fixed in 4% formaldehyde for 10 min. Cells were then washed thrice with PBS and images were collected using a MRC1024-krypton/argon laser scanning confocal equipped with a Zeiss LSM 510 Meta photomicroscope.
Calcium Measurements
Cells were incubated with 2 μM fura-2 (Molecular Probes) for 45 min, washed twice with Ca2+ free SES (Standard External Solution, include: 10 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM glucose, pH 7.4) buffer. For fluorescence measurements, the fluorescence intensity of Fura-2-loaded control cells was monitored with a CCD camera-based imaging system (Compix) mounted on an Olympus XL70 inverted microscope equipped with an Olympus 40× (1.3 NA) objective. A monochrometer dual wavelength enabled alternative excitation at 340 and 380 nm, whereas the emission fluorescence was monitored at 510 nm with an Okra Imaging camera (Hamamatsu, Japan). The images of multiple cells collected at each excitation wavelength were processed using the C imaging, PCI software (Compix Inc., Cranbery, PA), to provide ratios of Fura-2 fluorescence from excitation at 340 nm to that from excitation at 380 nm (F340/F380). Fluorescence traces shown represent [Ca2+]i values that are averages from at least 30-40 cells and are a representative of results obtained in at least 3-4 individual experiments.
Electrophysiology
For patch clamp experiments, coverslips with cells were transferred to the recording chamber and perfused with an external Ringer’s solution of the following composition (mM): NaCl, 145; CsCl, 5; MgCl2, 1; CaCl2, 1; Hepes, 10; Glucose, 10; pH 7.3 (NaOH). Whole cell currents were recorded using an Axopatch 200B (Axon Instruments, Inc.). The patch pipette had resistances between 3 −5 M after filling with the standard intracellular solution that contained the following (mM): cesium methane sulfonate, 150; NaCl, 8; Hepes, 10; EGTA, 10; pH 7.2 (CsOH). With a holding potential 0mV, voltage ramps ranging from −100mV to +100mV and 100ms duration were delivered at 2s intervals after whole cell configuration was formed. Currents were recorded at 2 kHz and digitized at 5–8 kHz. pClamp 10.1 software was used for data acquisition and analysis. Basal leak were subtracted from the final currents and average currents are shown. All experiments were carried out under room temperature.
Statistics
Data analysis was performed using Origin 7.0 (OriginLab). Statistical comparisons were made using Student’s t test (2-tailed). Experimental values are expressed as mean ± SEM. Differences in the mean values were considered to be significant at P < 0.05 or P<0.01 or P < 0.001.
RESULTS
Ca2+ induced regulation of cell proliferation and survival in prostate cancer cells
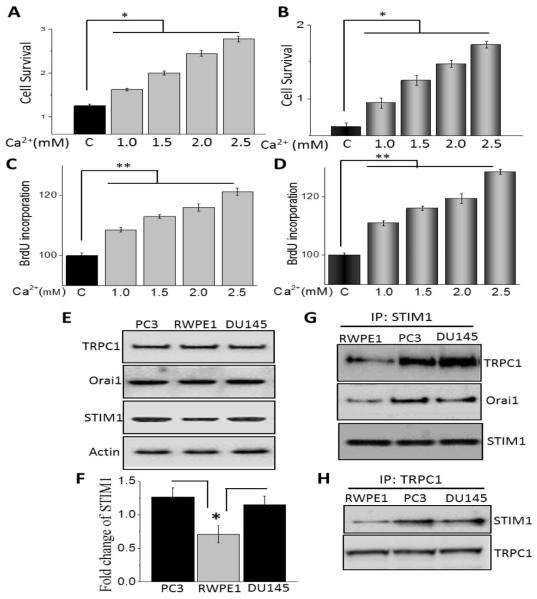
Ca2+ not only function as a second messenger, but have also been shown to be critical for cell proliferation and migration [7]. Importantly, increase in Ca2+ signaling have been shown to activate the immediate early genes that are responsible for inducing resting cells to re-enter the cell cycle and has been suggested to be a target for anticancer therapy [28,29]. We thus evaluated the role of increasing Ca2+ concentration in prostate cancer cell proliferation and/or their survival. Importantly, increasing extracellular Ca2+ concentration showed a dose dependent increase in cell survival in prostate cancerous cells (DU145 and PC3) (Figure 1A and B). Although in the presence of increasing extracellular Ca2+ RWPE1 cells also showed a moderate increase in cell survival, the amount of increase in cell proliferation was relatively less when compared with cancer cells (data not shown). We next evaluated if cell proliferation was also increased under these conditions and again both DU145 and PC3 cells showed a significant increase in BrdU incorporation in the presence of increasing extracellular Ca2+ (Figure 1C and D). Overall, these data suggest that increasing extracellular Ca2+ is important for the increase of cell proliferation of prostate cancer cells.
Figure 1.
Calcium induced regulation of cell proliferation and survival in prostate cancer cells: (A and B) PC3 and DU145 cells were treated with different concentration of Ca2+ for 24h. Cell survival was measured using MTT assay as described in Materials and Methods sections. Values are expressed as mean ± SEM (n=4). *P < 0.05 versus respective control cells. Bar diagram showing the relative absorbance at 450nm of PC3 and DU145 cells after BrDU incorporation under various concentration of calcium are shown in (C and D). Results are expressed as mean ± SEM (n=4) *P< 0.05 and ** P<0.01 verses respective control. (E) Western blotting was showing the expression of TRPC1, Orai1 and STIM1 in PC3, RWPE1 and DU145 cells. (F) Densitometric quantitation for normalized STIM1 relative to β-actin is shown. Values represent mean ± SEM from 3-4 independent experiments (*P<0.05). (G and H) Whole-cell lysates were prepared and immunoprecipitation was performed with anti-STIM1 or anti-TRPC1 antibody as described in Materials and Methods sections. The immunoprecipitates were subjected to immunoblot analysis with anti-STIM1, anti-TRPC1 and anti-Orai1 antibodies.
Agonist-stimulated Ca2+ entry has been suggested to play an essential role in regulating cell survival and increasing cell proliferation. Importantly, in non-excitable cells both Orai1 and TRPC1 has been shown to be important for Ca2+ entry that are activated by agonist-mediated ER store depletion a phenomenon termed as SOCE. Thus, we next evaluated the expression of these Ca2+ entry channels in prostate cells. Although, no change in TRPC1 or Orai1 levels were observed in control and prostate cancer cells, a decrease in STIM1 levels was observed in control RWPE1 cells, when compared with prostate cancer cells (DU145 and PC3) (P<0.05; Figure 1E and F). STIM1 has been recently identified as a key Ca2+ entry regulator that is present in the ER and sense ER Ca2+ levels [19]. Furthermore, upon store depletion STIM1 has been shown to interact with TRPC1 and Orai1 channels to initiate Ca2+ entry via SOCE that could be essential for cell proliferation and survival [18]. Importantly, the functional interaction between STIM1 and TRPC1/Orai1 was also increased in prostate cancer cells when compared with control RWPE1 cells (Figure1G and H). Overall, these data indicate that Ca2+-mediated increase in cell proliferation and survival of prostate cancer cells, is perhaps due to increased STIM1 expression. In addition, increase in STIM1-TRPC1/Orai1 interaction could also facilitate Ca2+ entry via the SOCE mechanism that could promote cell survival and proliferation of prostate cancer cells.
Resveratrol inhibits cell proliferation and survival of prostate cancer cells by down-regulating STIM1
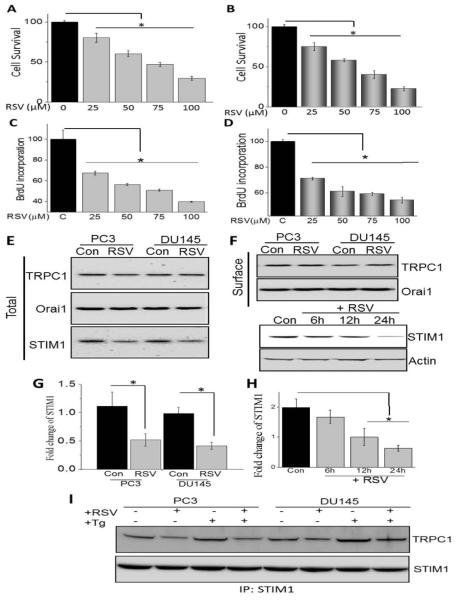
Resveratrol (RSV) an antioxidant agent mainly concentrated in red grapes has been shown to decrease the risk of many cancers [21]; however the mechanism is not well defined. Thus we next evaluated if addition of resveratrol decreases cell proliferation and survival of prostate cancer cells. Cells treated with RSV for 24h showed a dose dependent decrease in the cell survival of prostate cancerous cells (PC3 and DU145) (Figure 2A and B). Similarly, cell proliferation was also decreased and both PC3 and DU145 cells, showed a significant decrease in BrdU incorporation (Figure 2C and D). Data presented in figure 1 suggest that increase in STIM1 expression and its interaction with TRPC1/Orai1 could be critical for increased cell proliferation/survival of prostate cancer cells. Therefore we also evaluated the expression of TRPC1, Orai1 and STIM1 in control and RSV treated prostate cancer cells. Importantly, no change in the overall or surface expression of TRPC1 or Orai1 was observed upon RSV treatment. In contrast a decrease in total STIM1 levels was observed in RSV treated cancer cells (P<0.05; Figure 2E-H). We and others have previously shown that upon store-depletion STIM1 is targeted to the ER-PM junctions where it interacts with TRPC1 channels [30,31]. Thus, co-immunoprecipitation experiments using STIM1 antibodies were performed in control and store-depleted conditions. Addition of thapsigargin (Tg, SERCA pump blocker), showed an increase in STIM1-TRPC1 interaction in both PC3 and DU145 cells, which was also decreased in RSV treatment cells (Figure 2I). These data suggest that RSV inhibit STIM1-TRPC1 functional interaction that could inhibit Ca2+ entry and induce cell death in prostate cancer cells.
Figure 2.
Resveratrol inhibits cell proliferation and survival of prostate cancer cells by down-regulating STIM1: (A and B) PC3 and DU145 cells were treated with different RSV concentrations (dissolved in ethanol) for 24h. Cell survival was measured using MTT assay as described in Materials and Methods sections. Values are expressed as mean ± SEM (n=4). *P < 0.05 versus untreated control cells. Bar diagram showing the relative absorbance at 450nm of PC3 and DU145 cells after BrDU incorporation under various concentration of RSV for 24h are shown in (C and D). Values are expressed as mean ± SEM (n=4). *P < 0.05 versus untreated control cells. (E) PC3 and DU145 cells were treated with vehicle control or RSV for 24h, lysed and proteins were subjected to SDS-PAGE followed by western blotting with respective antibodies as labeled in the Figure. (F) PC3 and DU145 cells were treated with 100 μM RSV for 24h. Crude membrane was prepared and proteins were subjected to SDS-PAGE followed by Western blotting with TRPC1 and Orai1 antibodies. (F) Cells were treated with 100 μM RSV and cells were harvested at 0, 6, 12, or 24 h after RSV treatment. Cell lysates were resolved and analyzed by western blotting. Antibodies used are labeled and actin as used loading control. (G and H) Histogram represents the relative intensity of STIM1 normalized to β-actin. Values represent mean ± SEM from 3-4 independent experiments (*P < 0.05). (I) PC3 and DU145 cells were treated with RSV or Tg in various condition, lysed and proteins were subjected to SDS-PAGE followed by western blotting with respective antibodies.
Store-mediated Ca2+ entry is inhibited upon RSV treatment in prostate cancer cells
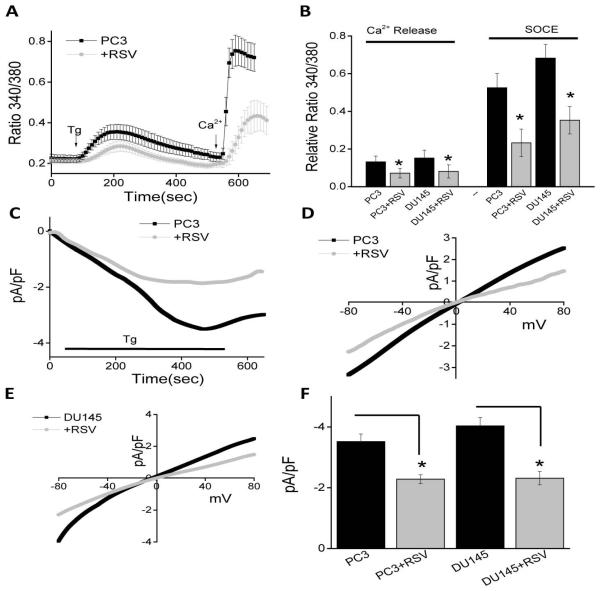
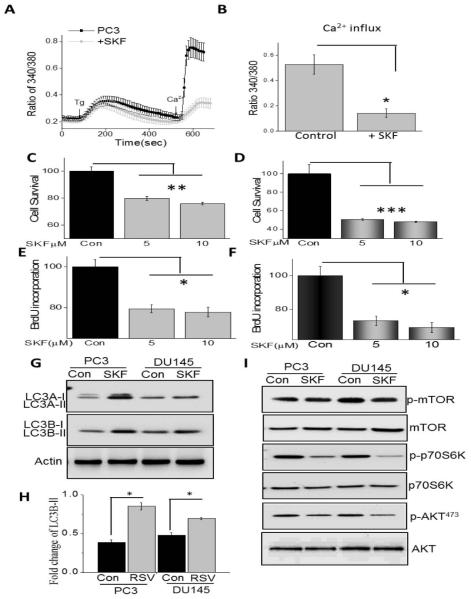
Data presented thus far indicate that Ca2+ is essential for the proliferation/survival of prostate cancer cells and RSV treatment not only inhibit STIM1 expression, but also inhibit Tg-induced association between TRPC1 and STIM1. Furthermore, as TRPC1 is activated via STIM1, and this association is important for SOC-mediated Ca2+ entry, we next evaluated if SOCE is altered upon RSV treatment. To evaluate SOC-mediated Ca2+ entry, ER Ca2+ stores were depleted by the addition of thapsigargin (Tg, 2 μM). Importantly, in the absence of extracellular Ca2+ the increase in [Ca2+]i evoked by Tg (first peak) was significantly decreased in both PC3 and DU145 cells following RSV treatment, when compared with untreated cells (Figure 3A and B). Addition of external Ca2+ (1 mM), which initiates SOC-mediated Ca2+ entry, was also decreased in RSV treated PC3 and DU145 cells (Figure 3A and B), indicating that RSV treatment inhibits SOCE, which could be critical for decreasing cell proliferation/survival.
To establish the identity of the SOC channel, electrophysiological recordings were performed in prostate cancer cells. In PC3 cells, addition of Tg induced an inward current which was non-selective and reversed between 0 and −5mV (P<0.05; Figure 3C, D and F). The currents shown in figure 3C were recorded at a holding potential of −80 mV and I/Vs curves were developed using a ramp protocol where current density was evaluated at various membrane potential and plotted. Importantly, the channel properties were similar to those previously observed with TRPC1 channels [32], suggesting that TRPC1 could contribute to the endogenous SOC-mediated Ca2+ entry channel in PC3 cells. Similar results were also obtained with DU145 cells and the Tg induced inward currents were similar as observed with PC3 cells (data not shown) also the I/V curves were similar between the two cells (Figure 3E). Also, SKF 96365, a nonspecific TRPC channel blocker was used that showed a decrease in these inward currents in both PC3 and DU145 cells (data not shown). Importantly, addition of RSV significantly decreased SOC currents in both PC3 and DU145 cells without altering the current-voltage (I-V) relationship (Figure 3C-F). Collectively, these results suggest that RSV decreases ER Ca2+ by diminishing SOC-mediated Ca2+ entry, which could lead to the activation of the UPR in these cells that together would induce cell death.
RSV treatment induces ER stress followed by the inhibition of the AKT/mTOR pathway
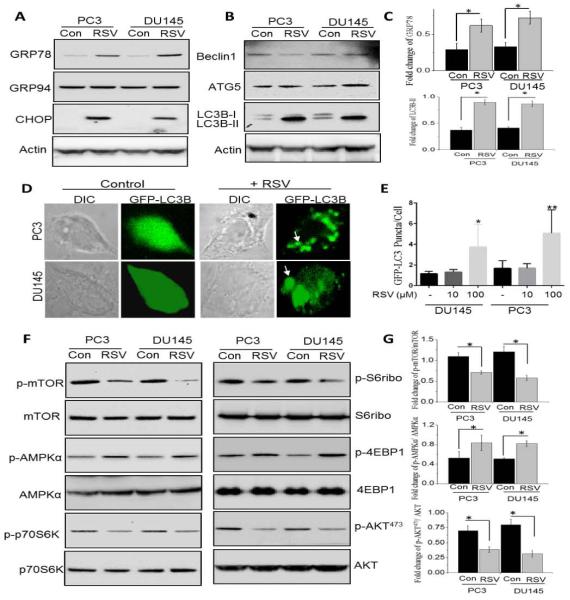
To understand the mechanism(s) of RSV mediated decrease in cell survival, we investigated the unfolded protein response (UPR) as loss of ER-Ca2+ has been shown to induce ER stress [33]. UPR markers (GRP78 and CHOP) were upregulated at the protein levels in RSV-treated cells, whereas no change in GRP94 was observed (Figure 4A). Quantification of GRP78 was shown in Figure 4C (P<0.05). Furthermore, RSV was previously shown to induce autophagy, thus we next evaluated the induction of autophagy in control and RSV treated cancer cells. Importantly, in both PC3 and DU145 cells, RSV treatment showed an increase in LC3B-II, but did not showed an increase in Beclin1 (P<0.05; Figure 4B and C), suggesting that perhaps the autophagy induced by RSV is independent of Beclin1. In addition, 100μM RSV treatment resulted in accumulation of GFP-LC3 puncta in PC3 and DU145 cells, however lower dose of RSV, 10μM failed to show a significant accumulation of GFP-LC3 when compared with control untreated cells (Figure 4D and E). Since the RSV-induced autophagy was independent of Beclin1, we next investigated if it was mediated via the mTOR pathway. Furthermore, Ca2+ entry has been shown to activate the AKT/mTOR pathway, which is essential for inhibition of cell death. Importantly, RSV treatment attenuated the activation of mTOR, a kinase that regulates cell survival, in both PC3 and DU145 cells (Figure 4F). Activation of p70S6K and S6ribo that are downstream of mTOR were also inhibited in the presence of RSV (Figure 4F). Finally, RSV treatment also significantly decreased AKT1 phosphorylation (AKT473) without affecting total AKT1 levels in prostate cancer cells (Figure 4F). Quantification of phospho-mTOR, phospho- AMPKα and phospho-AKT473 were shown in Figure 4G (P<0.05). These results strongly suggest that RSV-mediated loss of Ca2+ influx could be essential for the activation of the protective AKT1/mTOR activation in prostate cancer cells.
Decreasing Ca2+ entry in prostate cancer cells also induce cell death
To better understand the link between Ca2+ influx and cell survival, we inhibited SOCE. Addition of SKF96365 that has been shown to block SOC-mediated Ca2+ entry [34], significantly decreased Tg-induced Ca2+ entry in PC3 cells (Figure 5A and B). Similar results were also obtained with DU145 cells where SKF96365 treatment inhibited SOC-mediated Ca2+ entry (data not shown). Importantly, increasing SKF96365 concentration significantly decreased cell survival in both DU145 and PC3 cells (Figure 5C and D). Similarly, cell proliferation was also decreased under these conditions and again both DU145 and PC3 cells showed a significant decrease in BrdU incorporation in the presence of increasing SKF96365 (Figure 5E and F). Consistent with these data, SKF96365 treatment also showed an increase in LC3A/B in PC3 and DU145 cells (P<0.05; Figure 5G and H); however the effect in PC3 cells was more prominent. Our data presented in figure 4 also showed that the AKT/ mTOR pathway was important and, SKF96365 treatment inhibits the activation of mTOR and AKT1 in both PC3 and DU145 cells (Figure 5I). Together, these data suggest that inhibition of Ca2+ entry leads to decreased cell proliferation/survival of prostate cancer cells via the inhibition of the AKT/ mTOR pathway.
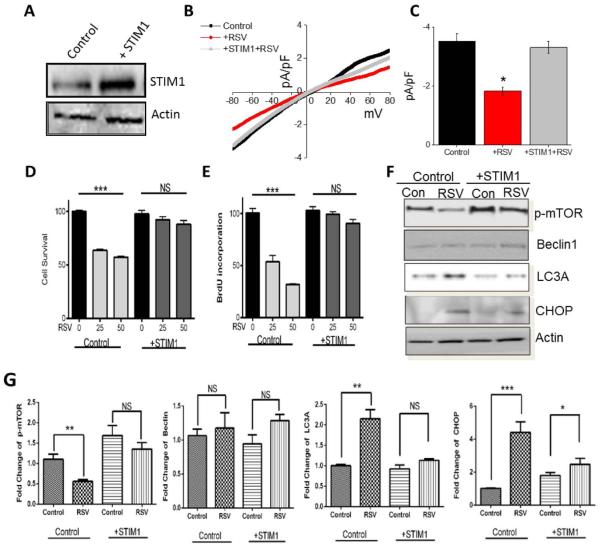
Restoration of STIM1 expression reverts RSV-mediated inhibition of cell proliferation and survival of prostate cancer cells
To further establish that indeed RSV-mediated loss in Ca2+ influx (due to loss of STIM1 expression) is essential for cell death, we overexpressed STIM1 in PC3 cells. Expression of STIM1 plasmid, showed increased STIM1 expression, whereas no increase in control actin levels was observed (Figure 6A). Importantly, RSV-dependent inhibition of SOC-mediated Ca2+ entry was also restored in cells that overexpress STIM1 (Figure 6B and C). Finally, both cell survival and cell proliferation was restored in cells that overexpress STIM1 and were treated with various doses of RSV (Figure 6D and E). Furthermore, phosphorylation of mTOR was also restored in STIM1 overexpressing cells that were treated with RSV (Figure 6F and G). Consistent with these results, both LC3A and CHOP expression that induces cell death, were decreased in STIM1 overexpressing cells that were treated with RSV (Figure 6F and G). Overall, these results suggest that increase in STIM1 expression, restores RSV-dependent loss in Ca2+ entry and inhibits RSV-mediated inhibition of cell proliferation and survival. These results also suggest a mechanism as why some cancer cells are resistant to cell death upon RSV treatment- as a threshold of STIM1 expression is perhaps critical for RSV-mediated cells death.
DISCUSSION
Cytosolic Ca2+ mediates several cellular signaling pathways that are related to proliferation, differentiation, and migration of tumor cells [20]. The Ca2+ influx from the extracellular space and Ca2+ release from the intracellular sources determine the cytosolic Ca2+ concentration. Previously, we have shown that increase in serum Ca2+/Mg2+ ratio promote prostate cancer epithelial cell proliferation by activating TRPM7, which function as an endogenous MagNuM channel in prostate cancer cells. Furthermore, decrease in the Ca2+ to Mg2+ ratio, facilitated Ca2+ entry and led to an increase in cell proliferation of cancer cells [35]. Therefore, changes in the expression or function of Ca2+ channels could affect the cytosolic Ca2+ homeostasis and its dependent cellular process, such as proliferation and survival. Moreover, the role of Ca2+ in autophagic cell death has also been recently elucidated in cancer cells and studies have also shown that the cytosolic Ca2+as a major regulator of both basal and induced autophagy [36].
Interestingly, the anti-carcinogenic activity of dietary polyphenol, RSV has been widely studied both in vitro and in vivo tumor models, including prostate cancer [21,22,25]. It is well known that RSV induces cell cycle arrest, inhibits cell proliferation and activates apoptosis in androgen-independent prostate cancer cells [24,37]. The present study, in conjunction with previous studies, demonstrates that RSV inhibits prostate cancer cell proliferation and induces autophagy in PC3 and DU145 cells. Several mechanisms have been shown to mediate the antiproliferative effects of RSV in prostate cancer cells, however the precise mechanism of RSV-mediated growth inhibition and the involvement of Ca2+ remain unclear. Several phase I and phase II clinical trials are currently in progress at the National Institute of Health (http:/clinicaltrials.gov) for therapeutic effect of RSV against several cancer types including patients with colon cancer and lymphoma [38], however there are no results from prostate cancer patients. Understanding the downstream signaling mechanisms of resveratrol-induced cell death may facilitate the development of additional therapeutic targets for the androgen-independent prostate cancer prevention and treatment. The results of this study demonstrate that STIM1 is an important target of RSV for inhibiting cell proliferation and to activate ER stress mediated autophagic cell death in androgen-independent prostate cancer epithelial cells.
Store operated Ca2+ entry is the predominant Ca2+ influx mechanism in prostate cancer cells. Substantial progress has been made throughout the last two decades in the elucidation of the key players of SOCE and mechanisms responsible for SOCE activation in both excitable and non-excitable cells. Importantly, these studies have identified an involvement of TRPCs and/or Orai1 channels in SOCE. Indeed, Stim1 have also been identified recently as the molecular switch that modulate SOC channels and its significance in SOCE have been elucidated in androgen-dependent prostate cancer cells [15,39]. Nevertheless, it is unclear about the targets and mechanisms by which SOCE exerts its effects on cell function and survival in androgen-independent prostate cancer cells. The data presented here show that SOCE is increased in androgen-independent cells, DU145 and PC3 when compared with normal prostate epithelial cells, RWPE1. MTT assay and BrdU incorporation demonstrated that external Ca2+ mediates the survival and proliferation of DU145 and PC3 cells. These results are consistent with other reports showing that the anti-proliferative effect of external Ca2+ and SOCE in cancer cell [6,20,40]. Moreover, ER Ca2+ depletion and subsequent Ca2+ influx through SOCE have been previously shown to be critical in promoting growth arrest and apoptosis of prostate cancer epithelial cells [4,13].
To address the role of SOCs, we studied the expression of TRPC1, Orai1 and STIM1 level in normal and prostate cancer cell. Our results clearly showed that there were no significant difference in the protein levels of TRPC1 and Orai1; whereas STIM1 levels were increased in cancer cells when compared with control cells. Moreover, the interaction of STIM1 with TRPC1 and Orai1 was also increased in cancer cells. STIM1 is an ER resident protein, monitor the Ca2+ level in the ER by luminal EF Ca2+ binding domain and translocate upon store depletion to plasma membrane to activate SOCE channels [19]. These results suggest that STIM1 interaction with either Orai1 or TRPC1 causes an increase in SOCE in androgen-independent prostate cancer cells. Studies have also shown that molecular components of SOCs, particularly Orai1, TRPC1 and STIM1 seem to play a crucial role in cell proliferation and migration in several human cancers notably, breast, renal, cervical, and lung cancers indicating that inhibition of SOCE suppresses tumorigenicity [41,42]. The data presented here supports the hypothesis that Ca2+ entry through SOCE channels may activate the Ca2+ dependent signaling process such as proliferation and survival that are critical for carcinogenesis, in androgen-independent prostate cancer cells.
Our current study demonstrates that RSV prevents cell proliferation and induces cell death in a dose-dependent manner. Interestingly, 100μM RSV for 24h, abolishes both ER Ca2+ release as well as SOCE in DU-145 and PC-3 cells by decreasing STIM1expression. However, the expression pattern of TRPC1 and Orai1 was not affected upon RSV treatment, but, RSV treatment decreases the STIM1-TRPC1 interaction upon store depletion. Previous study has shown that alteration in the expression of SOC’s, particularly Orai1, contribute to the increased resistance of prostate cancer cell towards induction of apoptosis. Our data substantiate the recent study indicating that STIM1 may play an important role in the development of prostate cancer and inhibition of its expression induces cell death in PC3 cells [43]. A growing number of studies have also shown that blockade or knockdown of STIM1 or TRPC1 significantly inhibits the proliferation and migration of cancer cell [41,43]. However, the underlying mechanisms seem to involve the reduction of SOCE upon STIM1 knockdown which suppress the carcinogenic cytosolic Ca2+ signaling in cancer cells. Our data is quite promising in which we found that RSV induces cell death by decreasing STIM1 expression and subsequent SOCE in androgen independent DU145 and PC3 cells. Several pieces of our data also support the conclusion that RSV inhibits prostate cancer cell proliferation by reducing SOCE. For example, RSV decreases the expression of STIM1 and dissociates the association of TRPC1-STIM1 and Orai1-STIM1 in DU145 and PC3 cells. Inhibition of SOCE by SKF-96365 decreases the survival and proliferation of DU145 and PC3 cells whereas, restoration of SOCE via STIM1 overexpression attenuated the RSV induced cell death. Overall, these results suggested that STIM1 is a primary target of RSV for mediating its anti-carcinogenic activity. However, it should be noted that RSV also regulates several other mechanisms including ER stress and autophagy via STIM1 and SOCE dependent manner.
Resveratrol was shown to induce pro-apoptotic endoplasmic reticulum stress in several cancer cells [44,45]. However, the exact mechanisms that proceed the cancer cells to ER stress under RSV treatment is not yet unveiled. Depletion of ER Ca2+ is known to cause ER stress and the current study have shown that RSV decreases SOCE, a vital mechanisms for ER Ca2+ store refilling, which ultimately could result in the depletion of ER Ca2+ store in DU145 and PC3 cells. As expected, Ca2+ release from the internal stores was significantly decreased upon RSV treatment. The shortage of ER Ca2+ may causes a deterioration in the protein folding in the lumen of the ER and causes ER stress [33,46]. Consistent with this, we found that RSV significantly induced ER stress, such as upregulation of GRP78/Bip, and CHOP/GADD153 protein. Induction of GRP78, also called Bip, may be a pro-survival mechanism of the cells, since it assists in the folding of nascent unfolded proteins, prevents the Ca2+ efflux from the ER to the cytosol, and alleviating ER stress induced apoptotic stimuli [47]. However, if the ER stress persists, cells induce the expression of CHOP, a unique transcription factor that mediates the ER stress induced apoptosis. Induction or overexpression of CHOP sensitizes cells to ER stress mediated apoptosis whereas knockdown or deletion of protects the cell from apoptosis [48]. However, our results have shown that RSV treatment in DU145 and PC3 cells resulted in increased autophagy. Based on our results we may hypothesis that treatment of prostate cancer cells with RSV will lead to ER Ca2+ depletion and subsequent ER stress, which itself might also be a trigger for autophagy.
Autophagy is a catabolic process for the degradation and recycling of macromolecules and organelles, which is activated during stress conditions. Autophagy has a dual role in cancer cells likely this could be a survival or cell death mechanisms. For example, autophagy mediated cell death is the predominant cell death mechanisms in response to certain anti-cancer drugs [49,50]. On the contrary, autophagy activates pro-survival mechanisms that attenuates anti-cancer drug induced apoptosis [51]. Several natural compounds, such as curcumin, paclitaxel, oridonin, quercetin and fisetin, have been shown to induce autophagy mediated cell death in a variety of tumor cells [52,53]. Thus, dietary natural compounds that induce autophagy may be of potential use as anticancer agents. Several studies have also shown that RSV is capable of inducing autophagy in different cancer cell line models [52,53]. Scarlatti and colleagues reported that RSV activates noncanonical Beclin-1–independent autophagy in MCF-7 cells [54]. Similarly, RSV promotes autophagic cell death in chronic myelogenous leukemia cells through both JNK-mediated p62 expression and AMPK activation, and stimulates both apoptosis and autophagy in ovarian carcinoma cells [55,56]. Opipari et al., reported that resveratrol inhibited cell proliferation and induced autophagocytosis in ovarian carcinoma cell lines [57]. Several studies have further shown that resveratrol induces apoptosis mediated cell death in a variety of cancer cells including prostate cancer cell lines [25,52,53]. In this study, we observed that in androgen-independent prostate cancer cells, RSV activates ER stress-mediated autophagy that was independent of Beclin1. LC3 is widely considered the marker for autophagy since during autophagosome formation, LC3 (cytosolic microtubule associated protein light chain 3) is processed and conjugated with phosphatidylethanolamine (PE) and inserted into autophagic vesicle membranes [58,59]. The expression and accumulation of LC3 was robustly increased in RSV treated cells, albeit only at higher doses, which confirms that RSV induced autophagic flux.
Several signaling pathways are known to regulate autophagy including the ERK, PI3K class I and II, and mTOR. The mTOR signaling pathways regulate various cellular process including autophagy through coordinating several signaling molecules such as S6K, 4EBP-1 and AKT. The mTOR/AKT pathway is an important signaling mechanism that regulates different cellular process including cell survival, proliferation, apoptosis and autophagy [60,61]. Indeed it has been shown that the mTOR/AKT pathway is critical for prostate tumor growth and survival, especially when the PTEN, a tumor suppressor gene, is mutated [62]. Moreover, overexpression and uncontrolled activation of AKT have been identified in several human cancers including prostate cancer [63,64]. Similarly, inactivation of mTOR pathway shown to inhibit prostate cancer cell growth, migration and invasion [63]. Interestingly, the role of mTOR in SOCE activation has been elucidated recently in tuberous sclerosis complex-related tumor development. The present study has shown that mTORC1 activates SOCE, and augmented SOCE by hyperactive mTORC1-STIM1 cascade may contribute to the benign nature of tuberous sclerosis complex (TSC)-related tumors. Interestingly, mTORC1 inhibitor, rapamycin suppressed the SOCE by decreasing the expression of STIM1 in tuberous sclerosis complex 2(TSC2) deficient MEF cells [65]. Hence, it is suggested that to develop a drug that can inhibit both mTOR and other signaling molecules PI3K/AKT would be an effective strategy in treating prostate cancer. Consistent with these studies, we observed that RSV treatment activates the AMPK and inhibits the mTOR/AKT phosphorylation. The results suggest that RSV induces autophagy through regulating mTOR/AKT pathways. However, the RSV induced autophagy was abolished in STIM1 overexpressed PC3 cells. The STIM1 overexpression reverts the RSV induced SOCE suppression, ER stress and cell death which indicates that RSV targets SOCE via suppressing STIM1 and deplete ER store causes ER stress induced autophagic cell death in androgen-independent prostate cancer cells. Moreover, the signaling pathways by which RSV regulates autophagy induction and exerts its anti-carcinogenic effects are far from being completely elucidated. Although several studies suggest that increase in intracellular Ca2+ may contribute to anti-carcinogenic effect, this has never been clearly proven. Nevertheless, further investigations are needed to elucidate the molecular mechanisms by which STIM1 regulate prostate cancer cell proliferation and survival.
In conclusion, SOCE is crucially involved in the survival and proliferation of androgen-independent prostate cancer cell lines, DU145 and PC3, and resveratrol treatment attenuates SOCE and induce autophagy mediated cell death. The mechanisms involves altered expression of STIM1 and subsequent SOCE influx which cause an ER stress response in DU145 and PC3 cells. ER stress further potentiate the autophagy mediated cell death through regulating mTOR/AKT pathways. To the best of our knowledge, this is the first report that RSV induces autophagic cell death by regulating the function of STIM1 and subsequent SOCE.
Figure 3.
Store-mediated Ca2+ entry is inhibited upon RSV treatment in prostate cancer cells: (A) Ca2+ imaging was performed in control and in the presence of RSV (100 μM) for 24 hours in PC3 cells. Analog plots of the fluorescence ratio (340/380) from an average of 40-60 cells are shown. (B) Quantification (mean ± SD) of fluorescence ratio (340/380). * indicates significance (p<0.05) versus control. (C) RSV treatment decreased SOCE currents in PC3 cells. Average IV curves under conditions control and RSV treatment are shown in (D). (E) RSV treatment changed IV curves of SOCE currents in DU145 cells. Average (8-10 recordings) current intensity at −80mV under these conditions is shown in (F).
Figure 4.
RSV treatment in prostate cancer cells induces ER stress and autophagy via inhibiting the mTOR/AKT pathway: (A) Western blot images showing the expression of unfolded protein response (UPR) markers (GRP78, GRP94, and CHOP) in the prostate cancer cells after 100μM RSV treatment for 24 hours. (B) Western blot images showing the expression of autophagy protein markers Beclin1, ATG5, LC3B in control and RSV treated cells. (C) Histogram represents the relative intensity of GRP78 and LC3B normalized to β-actin. Values represent mean ± SEM from 3-4 independent experiments (*P < 0.05). (D & E) GFP-LC3 expressing PC3 and DU145 cells were treated with RSV for 24h. Quantitation shown on the right represents mean ± SEM. GFP-positive puncta per cell from three independent experiments (*P < 0.05 and ** P<0.01). (F) Western blot images showing the phosphorylation of mTOR, AMPKα, p70S6K, S6ribo, 4EBP1 and AKT (Cell Signaling Technology, MA.) in both PC3 and DU145 cells after 100μM RSV treatment for 24 hours. (G) Histogram represents the relative intensity of phospho-mTOR, phospho-AMPKα and phospho-AKT normalized to their respective total proteins. Values represent mean ± SEM from 3-4 independent experiments (*P < 0.05).
Figure 5.
Decreasing Ca2+ entry in prostate cancer cells also induce cell death: (A) Ca2+ imaging was performed in control and in the presence of SKF 96365 in PC3 cells. Analog plots of the fluorescence ratio (340/380) from an average of 40-60 cells are shown. (B) Quantification (mean SD) of fluorescence ratio (340/380). * indicates significance (p<0.05) versus control. MTT assays under various concentration of SKF 96365 treated conditions for 24h in PC3 and DU145 cells are shown in (C and D). Bar diagram showing the relative absorbance at 450nm of PC3 and DU145 cells after BrDU incorporation and various SKF concentration are shown in (E and F). Each bar gives the mean ± SEM of 4 separate experiments. *, P< 0.05, **, P<0.01 and ***, P<0.001 respectively. Western blot images showing the expression of autophagy protein markers LC3A and LC3B, mTOR, p70S6K, and AKT in both PC3 and DU145 cells after 10μM SKF 96365 treatment for 24 hours (G-I). Histogram represents the relative intensity of LC3B normalized to β-actin. Values represent mean ± SEM from 3-4 independent experiments (*P < 0.05).
Figure 6.
Restoration of STIM1 expression reverts resveratrol-mediated inhibition of cell proliferation and survival of prostate cancer cells: (A) Western blot image showing the overexpression of STIM1 in PC3 cells. (B) STIM1 overexpression restored IV curve of SOCE currents in PC3 cells. Average (8-10 recordings) current intensity at −80mV under these conditions is shown in (C). MTT assays under various concentration of RSV conditions in control and STIM1 overexpressed PC3 cells are shown in (D). Bar diagram showing the relative absorbance at 450nm of control and STIM1 overexpressed PC3 cells after BrDU incorporation under various concentration of RSV are shown in (E). Each bar gives the mean ± SEM of 4 separate experiments. ***P< 0.001. (F) Western blot images showing the expressions of p-mTOR, Beclin1, CHOP and β-Actin in control and STIM1 overexpressed PC3 cells with or without RSV treatment. Histogram represents the relative intensity of phospho-mTOR, Beclin1, LC3A and CHOP normalized to β-actin. Values represent mean ± SEM from 3-4 independent experiments (*P < 0.05, ** P<0.01, and *** P<0.001).
Acknowledgements
We thank the confocal facility at UND which is partially supported by the NIH COBRE grant (P30GM103329). We duly acknowledge grant support from the NIH (DE017102) awarded to B.B.S.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014 doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Semin Surg Oncol. 2000;18(1):52–74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Prevarskaya N, Skryma R, Shuba Y. Ca2+ homeostasis in apoptotic resistance of prostate cancer cells. Biochem Biophys Res Commun. 2004;322(4):1326–1335. doi: 10.1016/j.bbrc.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Vanoverberghe K, Vanden Abeele F, Mariot P, et al. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 2004;11(3):321–330. doi: 10.1038/sj.cdd.4401375. [DOI] [PubMed] [Google Scholar]
- 6.Shapovalov G, Skryma R, Prevarskaya N. Calcium channels and prostate cancer. Recent Pat Anticancer Drug Discov. 2013;8(1):18–26. doi: 10.2174/15748928130103. [DOI] [PubMed] [Google Scholar]
- 7.Flourakis M, Prevarskaya N. Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta. 2009;1793(6):1105–1109. doi: 10.1016/j.bbamcr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Morelli MB, Amantini C, Nabissi M, et al. Cross-talk between alpha1D-adrenoceptors and transient receptor potential vanilloid type 1 triggers prostate cancer cell proliferation. BMC Cancer. 2014;14:921. doi: 10.1186/1471-2407-14-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thebault S, Flourakis M, Vanoverberghe K, et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66(4):2038–2047. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64(22):8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 11.Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22(49):7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- 12.Weaver EM, Zamora FJ, Puplampu-Dove YA, Kiessu E, Hearne JL, Martin-Caraballo M. Regulation of T-type calcium channel expression by sodium butyrate in prostate cancer cells. Eur J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Skryma R, Mariot P, Bourhis XL, et al. Store depletion and store-operated Ca2+ current in human prostate cancer LNCaP cells: involvement in apoptosis. J Physiol. 2000;527(Pt 1):71–83. doi: 10.1111/j.1469-7793.2000.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Zheng L, Lin P, Danielpour D, Pan Z, Ma J. Overexpression of Bax induces down-regulation of store-operated calcium entry in prostate cancer cells. J Cell Physiol. 2008;216(1):172–179. doi: 10.1002/jcp.21385. [DOI] [PubMed] [Google Scholar]
- 15.Flourakis M, Lehen’kyi V, Beck B, et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanden Abeele F, Lemonnier L, Thebault S, et al. Two types of store-operated Ca2+ channels with different activation modes and molecular origin in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2004;279(29):30326–30337. doi: 10.1074/jbc.M400106200. [DOI] [PubMed] [Google Scholar]
- 17.Vanden Abeele F, Shuba Y, Roudbaraki M, et al. Store-operated Ca2+ channels in prostate cancer epithelial cells: function, regulation, and role in carcinogenesis. Cell Calcium. 2003;33(5-6):357–373. doi: 10.1016/s0143-4160(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 18.Liao Y, Erxleben C, Abramowitz J, et al. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105(8):2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446(7133):284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 20.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11(8):609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 21.Aluyen JK, Ton QN, Tran T, Yang AE, Gottlieb HB, Bellanger RA. Resveratrol: potential as anticancer agent. J Diet Suppl. 2012;9(1):45–56. doi: 10.3109/19390211.2011.650842. [DOI] [PubMed] [Google Scholar]
- 22.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7(4):423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 23.Hudson TS, Hartle DK, Hursting SD, et al. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67(17):8396–8405. doi: 10.1158/0008-5472.CAN-06-4069. [DOI] [PubMed] [Google Scholar]
- 24.Kim YA, Rhee SH, Park KY, Choi YH. Antiproliferative effect of resveratrol in human prostate carcinoma cells. J Med Food. 2003;6(4):273–280. doi: 10.1089/109662003772519813. [DOI] [PubMed] [Google Scholar]
- 25.Jasinski M, Jasinska L, Ogrodowczyk M. Resveratrol in prostate diseases - a short review. Cent European J Urol. 2013;66(2):144–149. doi: 10.5173/ceju.2013.02.art8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pani B, Ong HL, Brazer SC, et al. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009;106(47):20087–20092. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh BB, Lockwich TP, Bandyopadhyay BC, et al. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell. 2004;15(4):635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 29.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 30.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9(6):636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283(25):17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Cheng KT, Bandyopadhyay BC, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A. 2007;104(44):17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13(10):1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 34.Derouiche S, Warnier M, Mariot P, et al. Bisphenol A stimulates human prostate cancer cell migration remodelling of calcium signalling. Springerplus. 2013;2(1):54. doi: 10.1186/2193-1801-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Selvaraj S, Varma A, Derry S, Sahmoun AE, Singh BB. Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels. J Biol Chem. 2013;288(1):255–263. doi: 10.1074/jbc.M112.393918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyer-Hansen M, Bastholm L, Szyniarowski P, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25(2):193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J Androl. 2007;28(2):282–293. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- 38.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2(5):409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 39.Kondratskyi A, Yassine M, Slomianny C, et al. Identification of ML-9 as a lysosomotropic agent targeting autophagy and cell death. Cell Death Dis. 2014;5:e1193. doi: 10.1038/cddis.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borowiec AS, Bidaux G, Pigat N, Goffin V, Bernichtein S, Capiod T. Calcium channels, external calcium concentration and cell proliferation. Eur J Pharmacol. 2013 doi: 10.1016/j.ejphar.2013.10.072. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Luan Y, Yu R, Zhang Z, Zhang J, Wang W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci Trends. 2014;8(1):1–10. doi: 10.5582/bst.8.1. [DOI] [PubMed] [Google Scholar]
- 42.Roberts-Thomson SJ, Peters AA, Grice DM, Monteith GR. ORAI-mediated calcium entry: mechanism and roles, diseases and pharmacology. Pharmacol Ther. 2010;127(2):121–130. doi: 10.1016/j.pharmthera.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Gu P, Zhou YB, Yang DR, Shan YX, Xue BX. [Inhibition of stromal interaction molecule 1 and the expression of apoptosis-related proteins in prostate cancer PC-3 cells] Zhonghua Nan Ke Xue. 2014;20(3):225–228. [PubMed] [Google Scholar]
- 44.Wang FM, Galson DL, Roodman GD, Ouyang H. Resveratrol triggers the pro-apoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp Hematol. 2011;39(10):999–1006. doi: 10.1016/j.exphem.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang TT, Lin HC, Chen CC, et al. Resveratrol induces apoptosis of human nasopharyngeal carcinoma cells via activation of multiple apoptotic pathways. J Cell Physiol. 2011;226(3):720–728. doi: 10.1002/jcp.22391. [DOI] [PubMed] [Google Scholar]
- 46.Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107(7):827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6(1):45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 48.Schonthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol. 2013;85(5):653–666. doi: 10.1016/j.bcp.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10(9):1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12(6):401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo XL, Li D, Hu F, et al. Targeting autophagy potentiates chemotherapy-induced apoptosis and proliferation inhibition in hepatocarcinoma cells. Cancer Lett. 2012;320(2):171–179. doi: 10.1016/j.canlet.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Mukhtar E, Adhami VM, Khan N, Mukhtar H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Curr Drug Targets. 2012;13(14):1831–1841. doi: 10.2174/138945012804545489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B. Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012;45(5):466–476. doi: 10.1111/j.1365-2184.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15(8):1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 55.Kartal M, Saydam G, Sahin F, Baran Y. Resveratrol triggers apoptosis through regulating ceramide metabolizing genes in human K562 chronic myeloid leukemia cells. Nutr Cancer. 2011;63(4):637–644. doi: 10.1080/01635581.2011.538485. [DOI] [PubMed] [Google Scholar]
- 56.Puissant A, Robert G, Fenouille N, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70(3):1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 57.Opipari AW, Jr., Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64(2):696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 58.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 59.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117(Pt 13):2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 60.Sun H, Wang Z, Yakisich JS. Natural products targeting autophagy via the PI3K/Akt/mTOR pathway as anticancer agents. Anticancer Agents Med Chem. 2013;13(7):1048–1056. doi: 10.2174/18715206113139990130. [DOI] [PubMed] [Google Scholar]
- 61.Beauchamp EM, Platanias LC. The evolution of the TOR pathway and its role in cancer. Oncogene. 2013;32(34):3923–3932. doi: 10.1038/onc.2012.567. [DOI] [PubMed] [Google Scholar]
- 62.Persad S, Attwell S, Gray V, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A. 2000;97(7):3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer. 2013;20(3):R83–99. doi: 10.1530/ERC-12-0394. [DOI] [PubMed] [Google Scholar]
- 64.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24(50):7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 65.Peng H, Liu J, Sun Q, et al. mTORC1 enhancement of STIM1-mediated store-operated Ca2+ entry constrains tuberous sclerosis complex-related tumor development. Oncogene. 2013;32(39):4702–4711. doi: 10.1038/onc.2012.481. [DOI] [PMC free article] [PubMed] [Google Scholar]