Abstract
Human mesenchymal stem cells (MSCs) have been used in cell-based therapy to promote revascularization after peripheral or myocardial ischemia. High levels of reactive oxygen species (ROS) are involved in the senescence and apoptosis of MSCs, causing defective neovascularization. Here, we examined the effect of the natural antioxidant lycopene on oxidative stress-induced apoptosis in MSCs. Although H2O2 (200 μM) increased intracellular ROS levels in human MSCs, lycopene (10 μM) pretreatment suppressed H2O2-induced ROS generation and increased survival. H2O2-induced ROS increased the levels of phosphorylated p38 mitogen activated protein kinase (MAPK), Jun-N-terminal kinase (JNK), ataxia telangiectasia mutated (ATM), and p53, which were inhibited by lycopene pretreatment. Furthermore, lycopene pretreatment decreased the expression of cleaved poly (ADP ribose) polymerase-1 (PARP-1) and caspase-3 and increased the expression of B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein (Bax), which were induced by H2O2 treatment. Moreover, lycopene significantly increased manganese superoxide dismutase (MnSOD) expression and decreased cellular ROS levels via the PI3K-Akt pathway. Our findings show that lycopene pretreatment prevents ischemic injury by suppressing apoptosis-associated signal pathway and enhancing anti-oxidant protein, suggesting that lycopene could be developed as a beneficial broad-spectrum agent for the successful MSC transplantation in ischemic diseases.
Keywords: Lycopene, MSC, Oxidative stress, Apoptosis, Anti-oxidant reagent
INTRODUCTION
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can differentiate into multiple cell types (Castro-Manrreza and Montesinos, 2015) such as neurons, hepatocytes, cardiomyocytes, and epithelial cells. Transplantation of MSCs has been used in the treatment of certain tissue injuries such as ischemic heart failure and hind-limb ischemia (Monsel et al., 2014). However, survival of the incorporated MSCs is reduced by the hostile microenvironment of ischemic tissue (characterized by hypoxia and free radical damage), thus inhibiting vasculogenesis and tissue repair. This, therefore, presents a significant MSC-based therapeutic challenge.
Researchers are attempting to enhance stem cell survival and function to overcome this problem; however, solutions re main limited. Recent evidence has suggested that ROS play a major role in the pathogenesis of hypertension and atherosclerosis in animals and humans (Engelhard et al., 2006; Fearon and Faux, 2009; Rodrigo et al., 2011). A high level of ROS causes endothelial dysfunction and impairs vasodilation, thus contributing to the development of cardiovascular disease. In patients with heart failure who were treated by MSC transplantation, high levels of reactive oxygen species (ROS) are associated with significantly lower MSC counts than those observed in patients treated with an antioxidant. MSCs exposed to prolonged oxidative stress may be functionally impaired (Jin et al., 2010). Survival of MSCs after intramyocardial transplantation can be a strong indicator of a favorable cardiovascular prognosis in cell-based therapy (Bhang et al., 2011). These studies suggest that the ischemic microenvironment, including the adverse oxidative stress response, has a deleterious effect on MSC survival and function. Therefore, protection of MSCs from ischemia-induced apoptosis may prove beneficial for cell therapy.
Lycopene, a naturally occurring carotenoid found in tomatoes and tomato-plant extracts, exhibits potent free radical-scavenging activity (Kelkel et al., 2011). Lycopene modulates redox-sensitive molecular pathways by inhibiting the production of ROS (Palozza et al., 2011; Chao et al., 2014). Cell culture studies have shown that lycopene protects endothelial cells (ECs) against oxidative injury (Palozza et al., 2010). Although many studies have shown the beneficial effects of lycopene, the protective effect of lycopene on oxidative stress and the mechanism underlying anti-oxidant property of lycopene in a variety of stem/progenitor cells have not been well studied. In this study, we assessed the protective effect of lycopene on ischemic conditions in MSCs and elucidated the anti-oxidant mechanism of lycopene against oxidative stress.
MATERIALS AND METHODS
Materials
Human MSCs (hMSCs) were obtained from American Type Culture Collection (Manassas, VA, USA). Fetal bovine serum (FBS) was purchased from Biowhittaker (Walkersville, MD, USA). Hydrogen peroxide solution was obtained from the Sigma Chemical Company (St. Louis, MO, USA). Phospho-p38 mitogen-activated protein kinase (MAPK), p38 MAPK, phospho-c-Jun N-terminal kinase (JNK), JNK, phospho-ataxia telangiectasia mutated (ATM), ATM, phospho-p53, p53, phospho-PI3K, PI3K, phospho-Akt, and Akt antibodies were obtained from New England BioLabs (Hertfordshire, UK). Manganese superoxide dismutase (MnSOD), Bcl-2, BAX, cleaved caspase-3 (c-caspase-3), and poly (ADP ribose) polymerase-1 (PARP-1) were purchased from Santa Cruz Biotechnology (Delaware, CA, USA). Goat anti-rabbit or mouse IgG antibody was purchased from Jackson ImmunoResearch (West Grove, PA, USA). Lycopene and Akt inhibitor were purchased from Sigma (St. Louis, MO, USA).
Human MSC cultures
Human adipose tissue-derived MSCs were obtained from the American Type Culture Collection (Manassas, VA, USA). MSCs were cultured in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 100 U / ml of penicillin/streptomycin. The cells were placed in a 5% CO2 incubator with saturated humidity at 37°C.
Chemicals treatment of MSCs
MSCs were washed twice with PBS, and the medium was exchanged with fresh minimum essential medium (MEM)-alpha supplemented with 10% FBS. To investigate the apoptosis signaling pathway, MSCs were pretreated with lycopene (10 μg/ml) at 37°C for 30 min, and then treated with H2O2 (200 μM) for the indicated time periods (0, 1, 2, 3, and 4 h). To assess various cell signaling pathways, MSCs were treated with lycopene along for time periods (0, 15, 30, 60, and 120min or 0, 24, and 48 h). Treatment with an Akt inhibitor (10−6 M) was carried out before lycopene treatment, at 37°C for 30 min.
Intracellular reactive oxygen species (ROS) assay
CM-H2DCFDA (DCF-DA), which acts as an H2O2-sensitive fluorophore, was used to detect the H2O2-induced production of ROS. DCF-DA (10 μM) was added to the cells and incubated for 30 min at room temperature in the dark. The cells were then viewed with a laser confocal microscope (600×; Fluoview 1000, Olympus, Tokyo, Japan) with excitation and emission wavelengths of 488 nm and 515–540 nm, respectively.
Cell viability assay
At subconfluency, exponentially growing MSCs were incubated in a 96-well plate with lycopene for various durations. Cell viability were determined using a modified 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which is based on the conversion of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2-tetrazolium to formazan by mitochondrial NAD(P)H-dependent oxidoreductase enzymes. Formazan levels were quantified by measuring the absorbance at 575 nm using a microplate reader (Tecan, Männedorf, Switzerland).
TUNEL assay
TdT-mediated dUTP Nick End Labeling (TUNEL) assay was performed using TdT Fluorescein In Situ Apoptosis Detection Kit (Trevigen Inc, Gaithersburg, MD, USA). The MSCs were pre-treated lycopene (30 min) and after treated H2O2 (4 h). Then, MSCs were labelled in according to manufacturer’s instructions. Stained MSCs were visualized with fluorescent microscope (ZEISS, Oberkochen, Germany).
Western blot analysis
Cell homogenates (20 μg protein) were separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were transferred to a nitrocellulose membrane for antibody probing. After the blots had been washed with TBST (10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20), the membranes were blocked with 5% skim milk for 1 h and incubated with the appropriate primary antibodies at the dilutions recommended by the supplier. The membranes were then washed, and the primary antibodies were detected using goat anti-rabbit IgG or goat anti-mouse IgG conjugated to horseradish peroxidase. The bands were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech, England, UK).
Statistical analysis
The results are presented as means ± standard error. All experiments were analyzed by analysis of variance (ANOVA), followed by a comparison of the treatment and control groups using the Bonferroni-Dunn test. A p-value of <0.05 was considered statistically significant.
RESULTS
The protective effect of lycopene on H2O2-induced apoptosis
To investigate the protective effect of lycopene against oxidative stress-induced cell death, MSCs were treated with H2O2 (200 μM) for the indicated periods (0, 1, 2, 3, and 4 h). The maximal effect of H2O2-induced cell death in MSC was observed after 4 h of treatment (Fig. 1A), and pre-treatment with lycopene increased the viability of MSC at concentration ≥10 μg/mL (Fig. 1B).
Fig. 1.
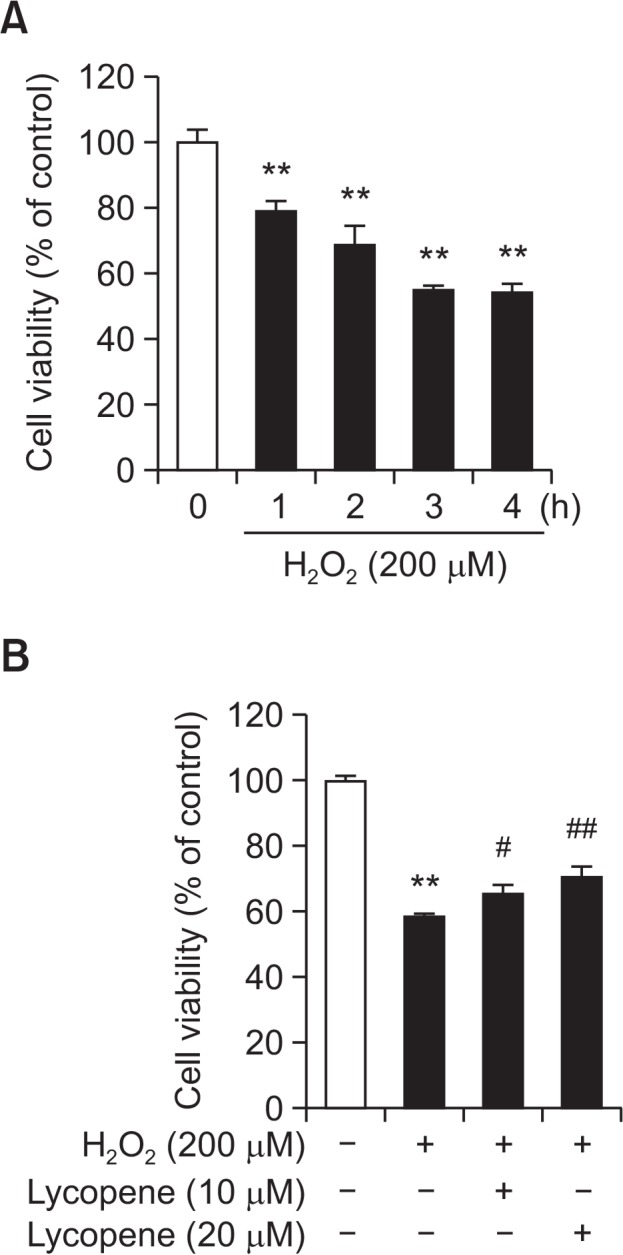
Effect of lycopene on H2O2-induced apoptotic cell death in MSCs. (A) MSCs were treated with H2O2 (200 μM) for 0–4 h, and cell viability was assessed. Values are expressed as the mean ± SD. **p<0.01 vs. control. (B) After lycopene pretreatment (0–20 μM) for 30 min, cell viability was assessed followed by 4 h of exposure to H2O2. Values are expressed as the mean ± SD. **p<0.01 vs. control, #p<0.05 and ##p<0.01 vs. H2O2 alone.
Lycopene-mediated inhibition of H2O2-induced ROS generation and phosphorylation of MAPK and ATM
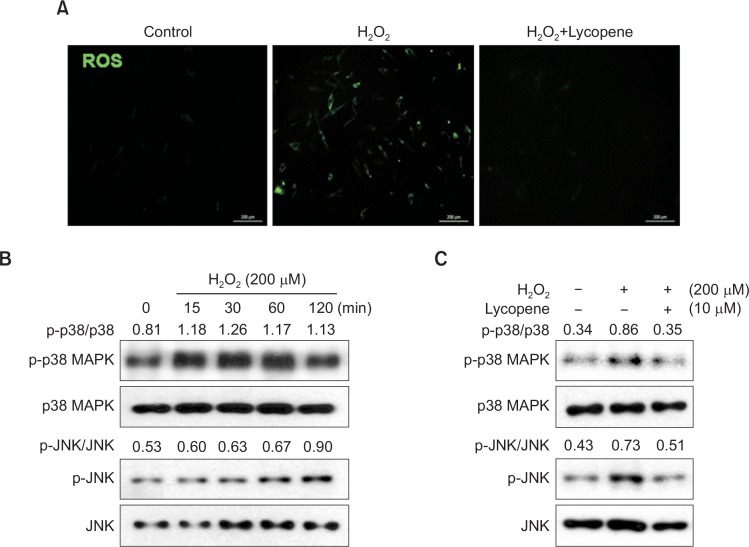
To determine the degree of involvement of ROS in H2O2-induced apoptosis, DCF-DA was used as an indicator of ROS production, and changes in the intracellular peroxide levels were measured. As shown in Fig. 2A, when MSCs were exposed to 200 μM H2O2 for 1 h, intracellular ROS levels were significantly increased compared to those in untreated cells, indicating that H2O2 enhances ROS production. However, treatment with lycopene (10 μM) significantly attenuated the observed increase in ROS levels. To assess the involvement of MAPKs in H2O2-induced cell injury, cells were treated with H2O2 for different periods (0–120 min). H2O2 increased JNK and p38 MAPK phosphorylation (Fig. 2B), and activation of these MAPKs was inhibited by lycopene treatment (Fig. 2C). Next, the involvement of ATM and p53 activation in H2O2-induced cell injury was evaluated. H2O2 increased ATM and p53 phosphorylation in a time-dependent manner (Fig. 3A, 3B). However, ATM and p53 activation was also inhibited by lycopene treatment (Fig. 3C, 3D). These results indicated that lycopene decreased oxidative stress-induced ROS levels and inhibited the phosphorylation of apoptosis-induced cell signaling pathways.
Fig. 2.
Lycopene inhibits intracellular ROS generation and activation of p38 MAPK and JNK. (A) MSCs were treated with lycopene for 30 min prior to H2O2 (200 μM) treatment, and cellular ROS levels were measured using the ROS-sensitive fluorophore CM-H2DCFDA (DCF-DA) by confocal microscopy. The image is representative of four independent experiments. (B) MSCs were treated with H2O2 (200 μM) for 0–120 min, and the levels of phosphorylated p38 MAPK and JNK were detected by western blot analysis. (C) MSCs were treated with lycopene (10 μM), followed by H2O2, and the levels of phosphorylated p38 MAPK and JNK were detected by western blot analysis.
Fig. 3.
Lycopene inhibits the ATM and p53 pathways. (A–B) MSCs were treated with H2O2 (200 μM) for 0–120 min, and the levels of phosphorylated ATM and p53 were detected by western blot analysis. (C–D) MSCs were treated with lycopene (10 μM), followed by H2O2, and the levels of phosphorylated ATM and p53 were detected by western blot analysis.
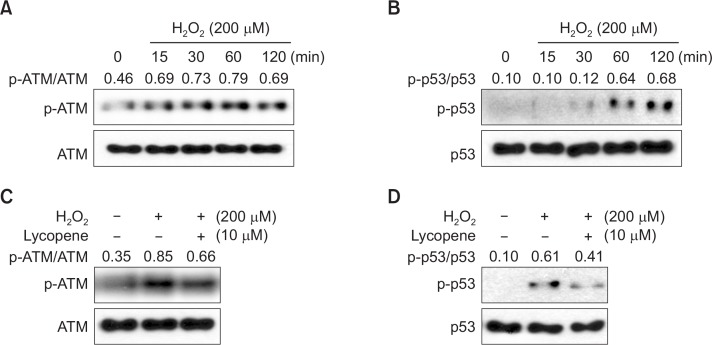
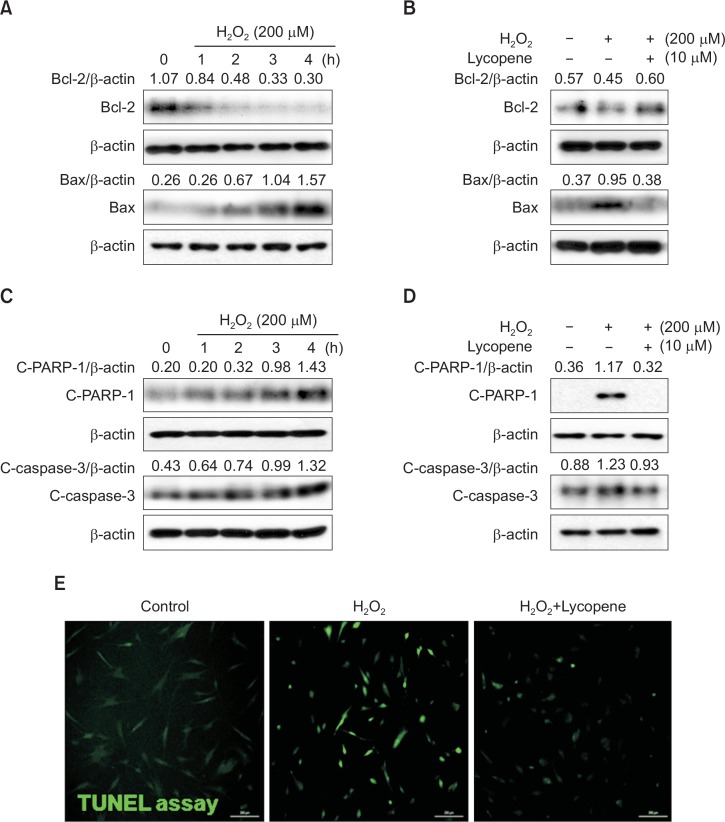
Lycopene pretreatment affects H2O2-induced expression of apoptotic proteins in MSCs
To investigate the effect of lycopene on regulation of apoptosis-associated protein in ischemic condition, MSCs were treated with H2O2 for 0–4 h, and the expression of Bcl-2 (anti-apoptotic protein) and Bax (pro-apoptotic protein was evaluated by western blot analysis. H2O2 increased Bax protein levels and decreased Bcl-2 expression in MSCs in a time dependent manner, compared with that observed in the control (Fig. 4A). However, pretreatment with lycopene significantly increased Bcl-2 expression and decreased Bax expression (Fig. 4B). To further examine the signaling pathways involved in the protective effect of lycopene against H2O2-induced apoptosis in MSCs, the expression of the pro-apoptotic regulators, cleaved caspase-3 (C-Caspase-3) and PARP-1 (C-PARP-1), was assessed by western blot analysis. H2O2 treatment resulted in the up-regulation of C-Caspase-3 and C-PARP-1 (Fig. 4C), but pretreatment with lycopene significantly decreased their expressions (Fig. 4D). In addition, TUNEL assay indicated that pretreatment with lycopene significantly attenuated H2O2-induced apoptosis in MSCs, compared with other group (Fig. 4E). These findings suggested that the anti-apoptotic effects of lycopene were involved in the regulation of apoptosis-associated protein expressions.
Fig. 4.
Effect of lycopene on H2O2-induced regulation of Bcl-2, Bax, and cleaved PARP-1 and caspase-3. (A) MSCs were treated with H2O2 for 0–4 h, and then the expression levels of Bcl-2 and Bax were assessed by western blot analysis. (B) MSCs were treated with lycopene (10 μM), followed by H2O2, and the levels of Bcl-2 and Bax were detected by western blot analysis. (C) MSCs were treated with H2O2 for 0–4 h, and the amounts of the cleaved caspase-3 and PARP-1 were assessed by western blot analysis. (D) MSCs were treated with lycopene (10 μM), followed by H2O2, and the amounts of cleaved caspase-3 and PARP-1 were detected by western blot. (E) MSCs were treated with lycopene for 30 min prior to H2O2 (200 μM) for 4 h treatment, and DNA damage was analyzed using TUNEL assay. Representative images of TUNEL positive cells (green) show H2O2-induced apoptotic MSCs.
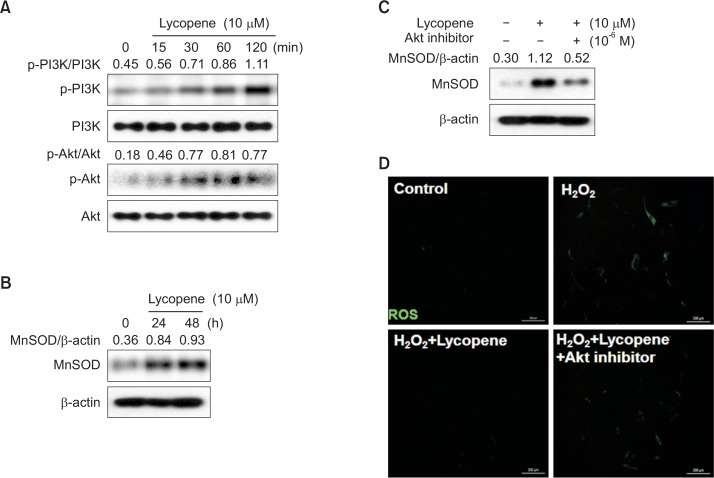
Akt regulates MnSOD expression in lycopene-stimulated MSCs under oxidative stress conditions
To elucidate the mechanism involved in the protective effects of lycopene, we tested the hypothesis that lycopene induced the attenuation of ROS generation by activating Akt signaling pathways. Using western blot analysis, we assessed the phosphorylation of PI3K and Akt in MSCs treated with lycopene for various periods (0–120 min). The results showed that phosphorylation of PI3K and Akt was increased in lycopene-treated MSCs within 15 min post treatment, and this activation was sustained for 120 min (Fig. 5A). MnSOD is an antioxidant protein that eliminates superoxides. To investigate whether the antioxidant effects of lycopene are associated with the Akt and MnSOD signaling pathways in MSCs, the cellular expression level of MnSOD in response to lycopene was assessed by western blot analysis. Lycopene increased MnSOD expression at 24 h post treatment (Fig. 5B), and this increase in MnSOD expression was suppressed by Akt inhibition (Fig. 5C). Moreover, lycopene-induced reduction in ROS levels was also abrogated by Akt inhibition (Fig. 5D), indicating that Akt activation plays a key role in the anti-oxidant effect of lycopene in MSCs via the expression of MnSOD.
Fig. 5.
Effect of lycopene-mediated induction of the Akt pathway on MnSOD expression. (A) MSCs were treated with lycopene (10 μM) for 0–120 min. The levels of phosphorylated PI3K and Akt were detected by western blot analysis. (B) MSCs were treated with lycopene for 0–48 h. Total cell lysates were subjected to SDS-PAGE and probed with an anti-MnSOD antibody. (C) MSCs were treated with an Akt inhibitor prior to lycopene treatment (48 h), after which the expression of MnSOD was detected. (D) MSCs were treated with lycopene and/or an Akt inhibitor prior to H2O2 treatment, and then DCF-DA was assessed by confocal microscopy.
DISCUSSION
Previous studies demonstrated that transplantation of stem/ progenitor cells such as MSCs, bone marrow stem cells, and cardiac stem cells reduces ischemia-induced myocardial tissue injury and improves left ventricular function (Hare et al., 2012; Karantalis et al., 2014; Suncion et al., 2014). However, stem cells transplanted into the ischemic myocardium are susceptible to an adverse tissue microenvironment that has reduced oxygen supply and free radical damage. Exposure to these adverse conditions compromises the full therapeutic benefits of transplanted MSCs. Thus, protection of MSCs from cell death in the ischemic microenvironment is crucial for the successful advancement of such stem cell-based therapies in the clinical setting. Lycopene is a carotenoid that belongs to a series of pigments (Holzapfel et al., 2013). Carotenoids are naturally occurring products that are widespread in vegetables and fruits (Shi and Le Maguer, 2000; Engelmann et al., 2011). Lycopene is reported to be one of the most potent antioxidants among the carotenoids (Krinsky, 1998). Furthermore, lycopene can restore free radical-induced reactions such as the production of ROS or other peroxy radicals (Di Mascio et al., 1989; Krinsky, 1998). However, these beneficial effects of lycopene in stem/progenitor cells have not been well reported. Therefore, in the present study, we evaluated the ability of lycopene to protect MSCs from ischemia-induced apoptosis. We found that lycopene restored the viability of MSCs, which was adversely affected by H2O2, in a dose-dependent manner. Moreover, lycopene blocked the generation of ROS in H2O2-treated MSCs, suggesting that lycopene might exert anti-apoptotic activities via inhibition of ROS generation.
Addition of extracellular H2O2 increased the intracellular levels of ROS, which subsequently activated p38MAPK and JNK. These signaling kinases transduce extracellular stress stimuli into cellular responses via phosphorelay cascades. These MAPK components function in a pleiotropic manner to regulate a broad variety of physiological functions such as cell growth, differentiation, and apoptosis (Huwiler and Pfeilschifter, 1994; Frey and Mulder, 1997). ATM is one of the key regulatory molecules in oxidative stress-induced apoptosis (Chung et al., 2012). The H2O2-mediated increase in ROS production results in ATM phosphorylation, which, in turn, activates p53. In ischemic condition, we observed that lycopene treatment inhibited H2O2-induced activation of these signaling molecules, suggesting that lycopene protected MSCs against ROS damages through the regulation of extracellular stress-associated signal pathway.
Apoptosis plays a significant role in the pathophysiology of cardiovascular injury (Nakka et al., 2008; Galluzzi et al., 2009) and occurs via a cascade of cellular events involving several apoptosis-regulatory genes. The Bcl-2 family of proteins represents a critical checkpoint in major apoptotic signal transduction cascades by acting upstream of irreversible damage to cellular constituents (Weyhenmeyer et al., 2012) (Yang et al., 2009). The Bcl-2/Bax ratio is a determining factor in the regulation of apoptotic cell death (Bar-Am et al., 2005). Caspase-3, one of the key executors of apoptosis (Cohen, 1997), is essential for DNA fragmentation and the morphological changes associated with apoptosis (Janicke et al., 1998). Caspase-3 is activated during apoptosis and is responsible for the processing of cellular proteins, including the cleavage of PARP to generate 85- and 31-kDa fragments (Chen et al., 1998). PARP cleavage is therefore a hallmark of apoptosis (Los et al., 2002). In the present study, H2O2 treatment resulted in decreased expression of the Bcl-2/Bax ratio at the protein level, activation of caspase-3, and cleavage of PARP-1 in MSCs. Pretreatment with lycopene restored the Bcl-2/BAX ratio and inhibited caspase-3 activation and PARP cleavage. Taken together, our results suggest that inhibition of apoptosis may be a key mechanism underlying the cyto-protective effect of lycopene.
Although several studies have reported the beneficial effects of lycopene in various biological processes, lycopene’s mechanism of action has not been fully elucidated. Lycopene treatment enhanced heme oxygenase-1 (HO-1) gene expression via activation of the PI3K/Akt pathway (Sung et al., 2015). The present study demonstrated a new mechanism of action for lycopene against oxidative stress. Our findings show that its pro-survival effect in MSCs is mediated by activation of Akt signaling. In addition, lycopene treatment enhanced the survival of MSCs in ischemic condition via the increase in Akt-associated MnSOD expression. These results are in agreement with those of a previous report, which demonstrated that Akt pathway engagement prevents increases in intracellular ROS levels, activation of the pro-apoptotic molecule JNK, and consequently apoptosis (Li et al., 2014). These findings suggest that lycopene reduced the cellular ROS levels of MSCs in ischemic condition via the Akt-MnSOD secretion circuit.
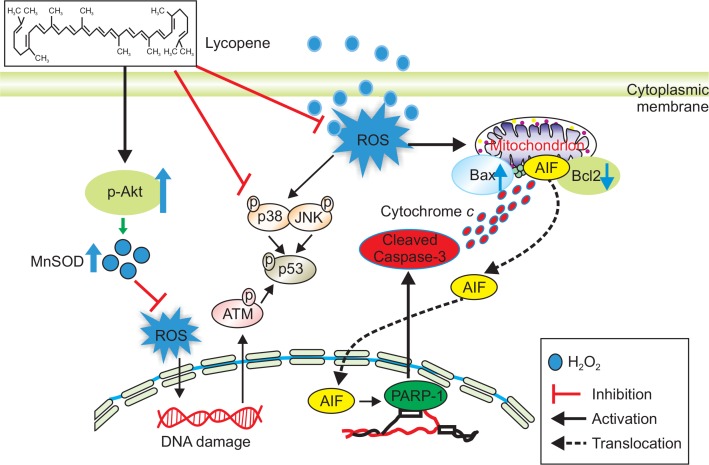
Fig. 6 summarizes the multiple effects of lycopene on oxidative stress in MSCs. In oxidative stress condition, lycopene inhibits extracellular stress signal pathway, such as p38 MAPK, JNK, ATM, and p53, and regulates the expression of anti- and pro-apoptosis associated proteins. Moreover, to eliminate oxidative stress-induced cellular ROS, lycopene increases in the level of MnSOD via PI3K-Akt phosphorylation cascade, resulting in augmentation of MSCs survival against ischemic injury.
Fig. 6.
Schematic representation of proposed mechanisms by which lycopene prevents oxidative stress in MSCs. Oxidative stress triggers the induction of reactive oxygen species (ROS) in MSCs. ROS lead to the phosphorylation of ATM and apoptosis-associated MAPK (p38 and JNK) signaling pathway, resulting in augmentation of the phosphorylation of p53. Moreover, ROS break the balance between Bax and Bcl2, resulting in the induction of apoptosis via the cleavage of PARP-1 and Caspase-3. Lycopene suppresses oxidative stress-induced apoptosis through Akt-MnSOD axis. In addition, lycopene decreases the phosphorylation of apoptosis-associated MAPK (p38 and JNK). These effects of lycopene on oxidative stress induce the decrease in the phosphorylation of ATM-p53 signaling pathway and the protection of cleavage PARP-1 and caspase-3, resulting in prevention of apoptosis and augmentation of survival in MSCs. ATM; ataxia telangiectasia mutated serine/threonine protein kinase, MAPK; mitogen-activated protein kinases, PARP-1; poly [ADP-ribose] polymerase 1, PI3K; phosphatidylinositol-4,5-bisphosphate 3-kinase.
In conclusion, the present study demonstrates that lycopene can protect MSCs from ischemia-induced apoptosis by inhibiting ROS generation, possibly via the Akt/MnSOD pathway, indicating that lycopene may reduce the spread of ischemic injury and rescue dying MSCs in the ischemic region. Lycopene can, therefore, be developed as a cyto-protective agent in ischemic diseases.
Acknowledgments
This study was supported by a National Research Foundation (NRF) grant funded by the Korean government (MEST) (2011-0009610) and a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C2253). The funders had no role in study design, data collection or analysis, the decision to publish, or preparation of the manuscript.
REFERENCES
- Bar-Am O, Weinreb O, Amit T, Youdim MB. Regulation of Bcl-2 family proteins, neurotrophic factors, and APP processing in the neurorescue activity of propargylamine. FASEB J. 2005;19:1899–1901. doi: 10.1096/fj.05-3794fje. [DOI] [PubMed] [Google Scholar]
- Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HH, Sung LC, Chen CH, Liu JC, Chen JJ, Cheng TH. Lycopene Inhibits Urotensin-II-Induced Cardiomyocyte Hypertrophy in Neonatal Rat Cardiomyocytes. Evid Based Complement Alternat Med. 2014;2014:724670. doi: 10.1155/2014/724670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chung YM, Park SH, Tsai WB, Wang SY, Ikeda MA, Berek JS, Chen DJ, Hu MC. FOXO3 signalling links ATM to the p53 apoptotic pathway following DNA damage. Nat Commun. 2012;3:1000. doi: 10.1038/ncomms2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Engelhard YN, Gazer B, Paran E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: a double-blind, placebo-controlled pilot study. Am Heart J. 2006;151:100. doi: 10.1016/j.ahj.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Engelmann NJ, Clinton SK, Erdman JW., Jr Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr. 2011;2:51–61. doi: 10.3945/an.110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: novel tools give (free) radical insight. J Mol Cell Cardiol. 2009;47:372–381. doi: 10.1016/j.yjmcc.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Frey RS, Mulder KM. Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor beta in the negative growth control of breast cancer cells. Cancer Res. 1997;57:628–633. [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochem. Biophys. Acta. 2009;1787:402–413. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci. 2013;14:14620–14646. doi: 10.3390/ijms140714620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J. Transforming growth factor beta 2 stimulates acute and chronic activation of the mitogen-activated protein kinase cascade in rat renal mesangial cells. FEBS Lett. 1994;354:255–258. doi: 10.1016/0014-5793(94)01132-X. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Jin Y, Kato T, Furu M, Nasu A, Kajita Y, Mitsui H, Ueda M, Aoyama T, Nakayama T, Nakamura T, Toguchida J. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun. 2010;391:1471–1476. doi: 10.1016/j.bbrc.2009.12.096. [DOI] [PubMed] [Google Scholar]
- Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkel M, Schumacher M, Dicato M, Diederich M. Antioxidant and anti-proliferative properties of lycopene. Free Radic Res. 2011;45:925–940. doi: 10.3109/10715762.2011.564168. [DOI] [PubMed] [Google Scholar]
- Krinsky NI. The antioxidant and biological properties of the carotenoids. Ann NY Acad Sci. 1998;854:443–447. doi: 10.1111/j.1749-6632.1998.tb09923.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Xue F, Xu SZ, Wang XW, Tong X, Lin XJ. Lycopene protects bone marrow mesenchymal stem cells against ischemia-induced apoptosis in vitro. Eur Rev Med Pharmacol Sci. 2014;18:1625–1631. [PubMed] [Google Scholar]
- Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, Herceg Z, Wang ZQ, Schulze-Osthoff K. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell. 2002;13:978–988. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsel A, Zhu YG, Gennai S, Hao Q, Liu J, Lee JW. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology. 2014;121:1099–1121. doi: 10.1097/ALN.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- Palozza P, Parrone N, Simone RE, Catalano A. Lycopene in atherosclerosis prevention: an integrated scheme of the potential mechanisms of action from cell culture studies. Arch Biochem Biophys. 2010;504:26–33. doi: 10.1016/j.abb.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Palozza P, Simone R, Catalano A, Monego G, Barini A, Mele MC, Parrone N, Trombino S, Picci N, Ranelletti FO. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-kappaB nuclear binding and increase in PPARgamma expression. J Nutr Biochem. 2011;22:259–268. doi: 10.1016/j.jnutbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- Shi J, Le Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr. 2000;40:1–42. doi: 10.1080/10408690091189275. [DOI] [PubMed] [Google Scholar]
- Suncion VY, Ghersin E, Fishman JE, Zambrano JP, Karantalis V, Mandel N, Nelson KH, Gerstenblith G, DiFede Velazquez DL, Breton E, Sitammagari K, Schulman IH, Taldone SN, Williams AR, Sanina C, Johnston PV, Brinker J, Altman P, Mushtaq M, Trachtenberg B, Mendizabal AM, Tracy M, Da Silva J, McNiece IK, Lardo AC, George RT, Hare JM, Heldman AW. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114:1292–1301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung LC, Chao HH, Chen CH, Tsai JC, Liu JC, Hong HJ, Cheng TH, Chen JJ. Lycopene inhibits cyclic strain-induced endothelin-1 expression through the suppression of reactive oxygen species generation and induction of heme oxygenase-1 in human umbilical vein endothelial cells. Clin Exp Pharmacol Physiol. 2015;42:632–639. doi: 10.1111/1440-1681.12412. [DOI] [PubMed] [Google Scholar]
- Weyhenmeyer B, Murphy AC, Prehn JH, Murphy BM. Targeting the anti-apoptotic Bcl-2 family members for the treatment of cancer. Exp Oncol. 2012;34:192–199. [PubMed] [Google Scholar]
- Yang TM, Barbone D, Fennell DA, Broaddus VC. Bcl-2 family proteins contribute to apoptotic resistance in lung cancer multicellular spheroids. Am J Respir Cell Mol Biol. 2009;41:14–23. doi: 10.1165/rcmb.2008-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]