Abstract
Ceramide is the most abundant lipid in the epidermis and plays a critical role in maintaining epidermal barrier function. Overall ceramide content in keratinocyte increases in parallel with differentiation, which is initiated by supplementation of calcium and/or vitamin C. However, the role of metabolic enzymes responsible for ceramide generation in response to vitamin C is still unclear. Here, we investigated whether vitamin C alters epidermal ceramide content by regulating the expression and/or activity of its metabolic enzymes. When human keratinocytes were grown in 1.2 mM calcium with vitamin C (50 μg/ml) for 11 days, bulk ceramide content significantly increased in conjunction with terminal differentiation of keratinocytes as compared to vehicle controls (1.2 mM calcium alone). Synthesis of the ceramide fractions was enhanced by increased de novo ceramide synthesis pathway via serine palmitoyltransferase and ceramide synthase activations. Moreover, sphingosine-1-phosphate (S1P) hydrolysis pathway by action of S1P phosphatase was also stimulated by vitamin C supplementation, contributing, in part, to enhanced ceramide production. However, activity of sphingomyelinase, a hydrolase enzyme that converts sphingomyelin to ceramide, remained unaltered. Taken together, we demonstrate that vitamin C stimulates ceramide production in keratinocytes by modulating ceramide metabolic-related enzymes, and as a result, could improve overall epidermal barrier function.
Keywords: Ceramide, Ceramide metabolic enzymes, Vitamin C, Calcium, Keratinocyte differentiation, Epidermal barrier
INTRODUCTION
Mammalian epidermis consists of four different layers, stratum basale (SB), stratum spinosum (SP), stratum granulosum (SG), and stratum corneum (SC) (Elias and Menon, 1991; Uchida, 2014). Since the SC is the outermost layer and directly faces the external environment, it functions to serve as a barrier (Elias, 2005). Multiple factors are involved in maintaining epidermal barrier function, of which ceramide is of particular importance (Uchida, 2014). Precursors of ceramide are synthesized during keratinocyte (KC) differentiation and are packaged into lamellar bodies (LB) and eventually secrete their contents at the SG-SC interface (Uchida et al., 2001a; Uchida et al., 2001b). In turn, ceramide is incorporated into the SC, comprising nearly 50% of its lipid mass (Elias and Menon, 1991). In the SC certain species of ceramide, particularly ω-OH-ceramide, covalently attach to the cornified envelope (CE) to form the corneocyte lipid-bound envelope (CLE), a structure that is essential for epidermal barrier function (Wertz and Downing, 1986). As a result, pathologies that affect ceramide content in the SC contribute to a number of barrier abnormalities, including excess water loss (Imokawa et al., 1991; Motta et al., 1994). Considering this, ceramide content is a critical factor for epidermal homeostasis.
While the overall level of ceramide in the epidermis is regulated by several metabolic enzymes, e.g., serine palmitoyltransferase (SPT), ceramide synthase (CerS), sphingomyelinase (SMase), and sphingosine-1-phosphate phosphatase (SPPase), calcium (Ca2+) and vitamin C (Vit C) are known to regulate both KC differentiation and ceramide content (Uchida et al., 2001a; Kim et al., 2011; Uchida, 2014). In particular, prior studies have shown that Vit C treatment significantly increases CerS activity in high Ca2+-supplemented KC, accounting for increased levels of epidermal ceramide (Uchida et al., 2001a).
Although previous studies have provided evidence for the importance of epidermal ceramide in maintaining normal barrier function, quantitative analysis looking into specific levels of metabolic enzymes responsible for ceramide synthesis in KC following Vit C is still unclear. In this study, we show that high Ca2+ (1.2 mM) and Vit C treatment stimulates production of ceramide. This stimulation is mediated by altered activity/ expression of metabolic enzymes involved in ceramide synthesis.
MATERIALS AND METHODS
Cell culture
Human primary KC from Life Technologies (Carlsbad, CA, USA) were maintained in serum-free KC growth medium containing 0.07 mM Ca2+, as described previously (Park et al., 2013b). Early stage of KC differentiation was induced by a switch in extracellular Ca2+concentration from low (0.07 mM) to high (1.2 mM). Moreover, late/terminal differentiated KC were generated by culturing cells in medium containing high Ca2+, along with Vit C (50 μg/ml) treatment for up to 11 days, as described previously (Uchida et al., 2001a; Kim et al., 2011).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using 30 ng cDNA prepared from total RNA fraction of cell lysates, as we described previously (Park et al., 2011). The following primer sets were used: SPPase, 5′-CCATTTCTATGGTCCTCCTCACCT-3′ and 5′-CAATCAGGTCCACAAATG GATAGAA-3′; human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GTCAACG-GATTTGGTCGTATTG-3′ and 5′-GCCATGGGTGGAATCATA-TTG-3′. mRNA expression was normalized to levels of GAPDH.
Western blot analysis
Western blot analysis was performed as previously described (Park et al., 2011). Cell lysates, prepared in radio-immunoprecipitation assay (RIPA) buffer, were resolved by electrophoresis on a 8 to 10% SDS-PAGE gel. The resultant bands were blotted onto nitrocellulose membranes, probed with anti-human β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), keratin 5 (Bioworld, St. Louis Park, MN, USA), keratin 10 (Santa Cruz Biotechnology), loricrin (Abcam, Cambridge, MA, USA), involucrin (Santa Cruz Biotechnology), SPT (Santa Cruz Biotechnology), CerS (Santa Cruz Biotechnology), and SPPase (Abcam), and detected by chemiluminescence reagent (SurModics, Eden Prairie, MN, USA).
Measurement of intracellular levels of ceramide
To assess the levels of cellular ceramide, human KC were incubated with Vit C and washed with phosphate-buffered saline followed by extracting total lipids, as we reported previously (Shin et al., 2012b; Park et al., 2013a). Ceramides were derivatized with o-phthalaldehyde (OPA) reagent and then quantitated using an HPLC system equipped with a fluorometrical detector system (JASCO, Tokyo, Japan), as described previously (Shin et al., 2012b; Park et al., 2013a). Ceramides are expressed as pmol per mg protein.
Enzyme activity assay for serine palmitoyltransferase
SPT activity was determined as previously described with a minor modification (Rutti et al., 2009; Shin et al., 2012a). Briefly, cells were suspended in assay buffer (50 mM HEPES buffer containing 1 mM EDTA, 0.1% sucrose monolaurate, 5 mM L-serine, 50 μM palmitoyl-CoA, and 20 μM pyridoxal 5′-phosphate). The SPT enzymatic reaction was initiated by placing the cell suspension at 37°C for 60 min, followed by the addition of NaBH4 (5 mg/mL) to terminate the reaction. The activity of SPT was analyzed by HPLC system equipped with a fluorometrical detector system (JASCO) as described previously (Shin et al., 2012a).
Enzyme activity assay for ceramide synthase
CerS activity assay was performed as described previously (Kim et al., 2012). Briefly, cell lysates, prepared in assay buffer (20 mM HEPES, pH 7.4, 25 mM KCl, 2 mM MgCl2, 0.5 mM DTT, 0.1% (w/v) fatty acid-free BSA, and 50 μM fatty acid-CoA), were incubated with 10 nmol of C17-sphinganine for 30 min at 37°C. Total lipids were extracted by the addition of CHCl3: MeOH (1:2, v/v), and 100 pmol of C12 ceramide as the internal standard, and applied onto LC-ESI-MS/MS (ABCIEX, Framingham, MA, USA), as described previously (Kim et al., 2012). The activity of CerS is expressed as pmol (dihydroceramide production) per mg protein per min.
Enzyme activity assay for sphingomyelinases
Activities of SMases were assessed as described previously (Loidl et al., 2002). Briefly, cells suspended in appropriate SMases assay buffers (acdic SMase buffer: 250 mM sodium-acetate, 0.2% Triton X-100, pH 4.5 or neutral SMase buffer: 20 mM HEPES, 0.2% Triton X-100, pH 7.4) were incubated with 5 nmol of NBD-sphingomyelin (SM) for 1 h at 37°C. The reaction was stopped by the addition of CHCl3: CH3OH (2:1, v/v) and the organic phases were removed under N2 gas. The residues then were resuspended in MeOH and applied onto HPLC system equipped with a fluorometrical detector system (JASCO). The activities of both SMases are expressed as pmol (NBD-ceramide) per mg protein per min.
Enzyme activity assays for sphingosine-1-phosphate phosphatase
SPPase activity was determined as previously described with a minor modification (Johnson et al., 2003). Briefly, cells were suspended in assay buffer (1 mM EDTA, 0.1% BSA, 10 μM C17 Sphingosine-1-phosphate (S1P) as substrate) and incubated at 37°C for 20 min. The reaction was terminated by the addition of MeOH containing 100 pmoles of dihydrosphingosine as the internal standard and the activity was analyzed by LC-ESI-MS/MS (ABCIEX), as described previously (Johnson et al., 2003). The activity of SPPase is expressed as pmol (C17 sphingosine (SO)) per mg protein per min.
Statistical analysis
Statistical analyses used the unpaired Student t Test. The p values were <0.01 in all cases.
RESULTS
Changes in expression levels of differentiation-associated genes in keratinocyte following Vit C
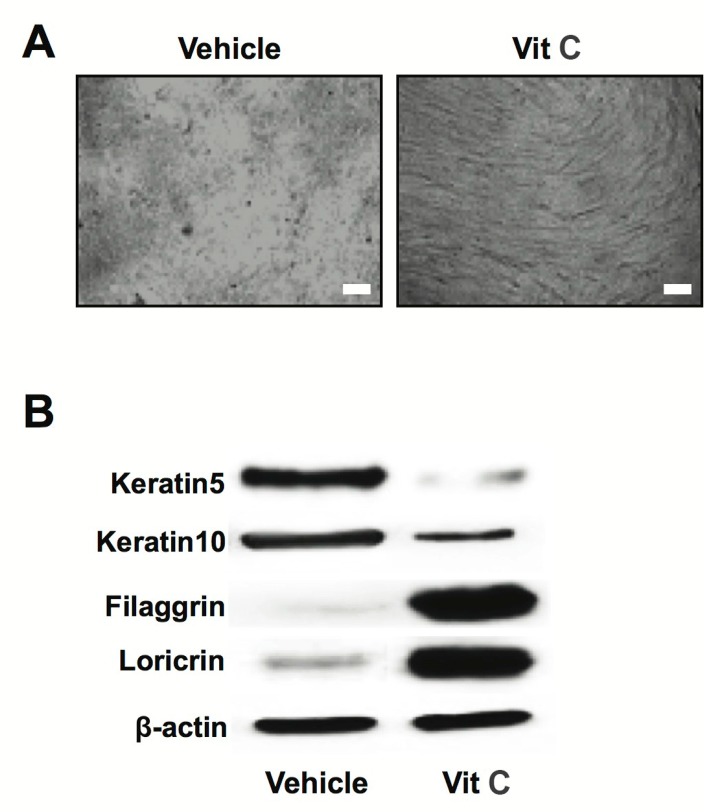
Previous studies have shown high Ca2+ and/or Vit C to alter gene expression associated with KC differentiation (Eichner et al., 1984; Simon and Green, 1985; Mehrel et al., 1990). To evaluate our culture system for KC differentiation induction, we first measured expression of previously identified differentiation marker genes e.g., keratins, involucrin and loricrin, in two distinct KC differentiation stages: 1) incubation with high Ca2+ (early differentiation stage) and 2) incubation with high Ca2+ and Vit C (late/terminal differentiation stage). Our western blot analysis showed that a high Ca2+ environment induced expression of keratin 5 and 10 (Fig. 1B). This increased expression was not seen in KC incubated in high Ca2+ in combination with Vit C (Fig. 1B). Furthermore, our data shows that an environment of high Ca2+ and Vit C, representing a late KC differentiation stage, also induced expression of both involucrin and loricirin (Fig. 1B). Expression of these latter proteins was not seen in KC incubated solely in a high Ca2+ environment (Fig. 1B). Moreover, KC at the early differentiation stage induced by high Ca2+ grew as a monolayer (Fig. 1A). However, cells cultured in high Ca2+ and Vit C grew as multilayered sheets with thicker cell layer, which is morphological features of terminal differentiated KC (Fig. 1A). Together, these results are consistent with previous studies, which showed a reduction in keratin expression and an increase in expression of involucrin and loricrin as KC become terminally differentiated (Eichner et al., 1984; Simon and Green, 1985; Mehrel et al., 1990).
Fig. 1.
Altered levels of differentiation-associated genes during Vit C-induced keratinocyte differentiation. Human primary keratinocytes (KC) were cultured in high Ca2+-supplemented medium with or without Vit C, as described in materials and methods. Phage contrast images of early or terminally differentiated KC (A). Protein expression of differentiation-associated genes was determined by western immunoblot analysis (B). Scale bar=20 μm.
Vit C increases ceramide content by the action of ceramide metabolic enzymes
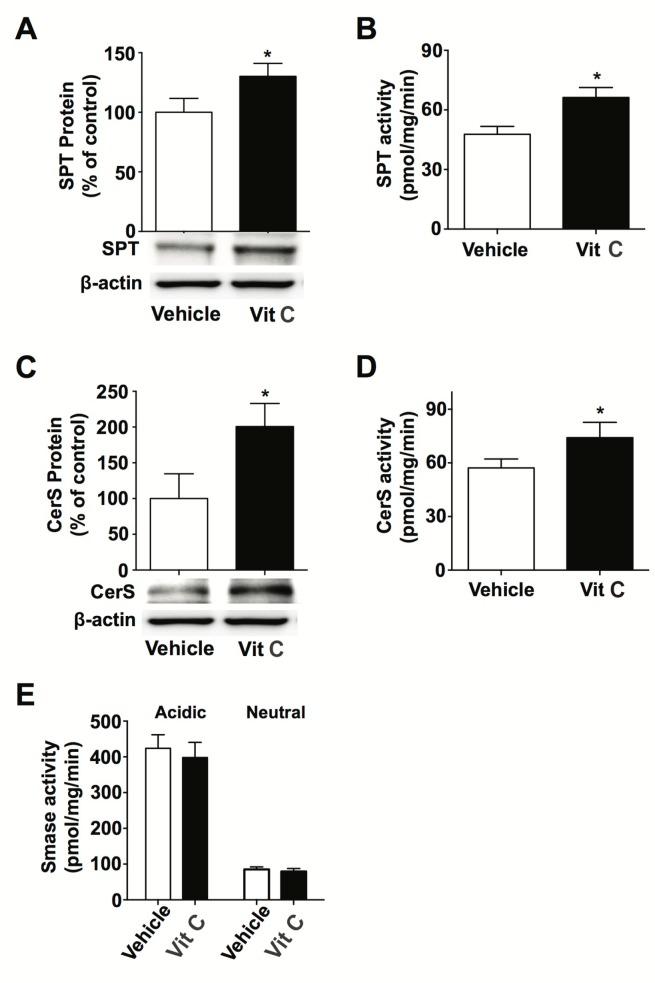
We also assessed ceramide content in KC incubated with Vit C. Our lipid analysis data shows that Vit C treatment significantly increases ceramide content (Table 1). To investigate the metabolic step(s) at which Vit C exhibits its effects on ceramide synthesis, we next measured the expression and activity of key enzymes that are involved in ceramide metabolism, e.g., SPT, CerS, SMase, SPPase, following Vit C treatment. Western blot and enzyme assays showed that both expression and activity of SPT and CerS was significantly increased in cells treated with Vit C (Fig. 2A–2D). Whereas, acidic and neutral SMase activities were not affected by Vit C treatment (Fig. 2E). Furthermore, prior studies have suggested that SPPase expression is a critical factor in regulating ceramide levels. As such, we measured SPPase mRNA and protein expression in KC cultured in Vit C (Fig. 3). Our qPCR and western blot analyses showed that Vit C supplementation significantly increased both SPPase mRNA and protein expression. These results show that altered expression/activity of several ceramide metabolic enzymes account for increased ceramide production that is stimulated by Vit C.
Table 1.
Increased ceramide content in human KC following Vit C
| Treatment | Ceramide (pmol/mg protein ± SD) |
|---|---|
| Vehicle | 1,086.01 ± 92.14 |
| Vit C | 1,431.34 ± 118.44* |
All value are mean ± SD.
Significantly different from Vehicle. p<0.01 (n=3).
Fig. 2.
Vit C treatment alters expression and activity of ceramide metabolic enzymes. Human primary keratinocytes (KC) were cultured in high Ca2+-supplemented medium with or without Vit C, as described in materials and methods. Protein expressions of serine palmitoyltransferase (SPT) (A) and ceramide synthase (CerS) (C) were measured by western immunoblot analysis. Enzyme activities of SPT (B), CerS (D), and sphingomyelinases (SMases) (E) were analyzed by either HPLC or ESI-LC/MS/MS systems. Data are means ± SD (n=3). *p<0.01 versus vehicle control.
Fig. 3.
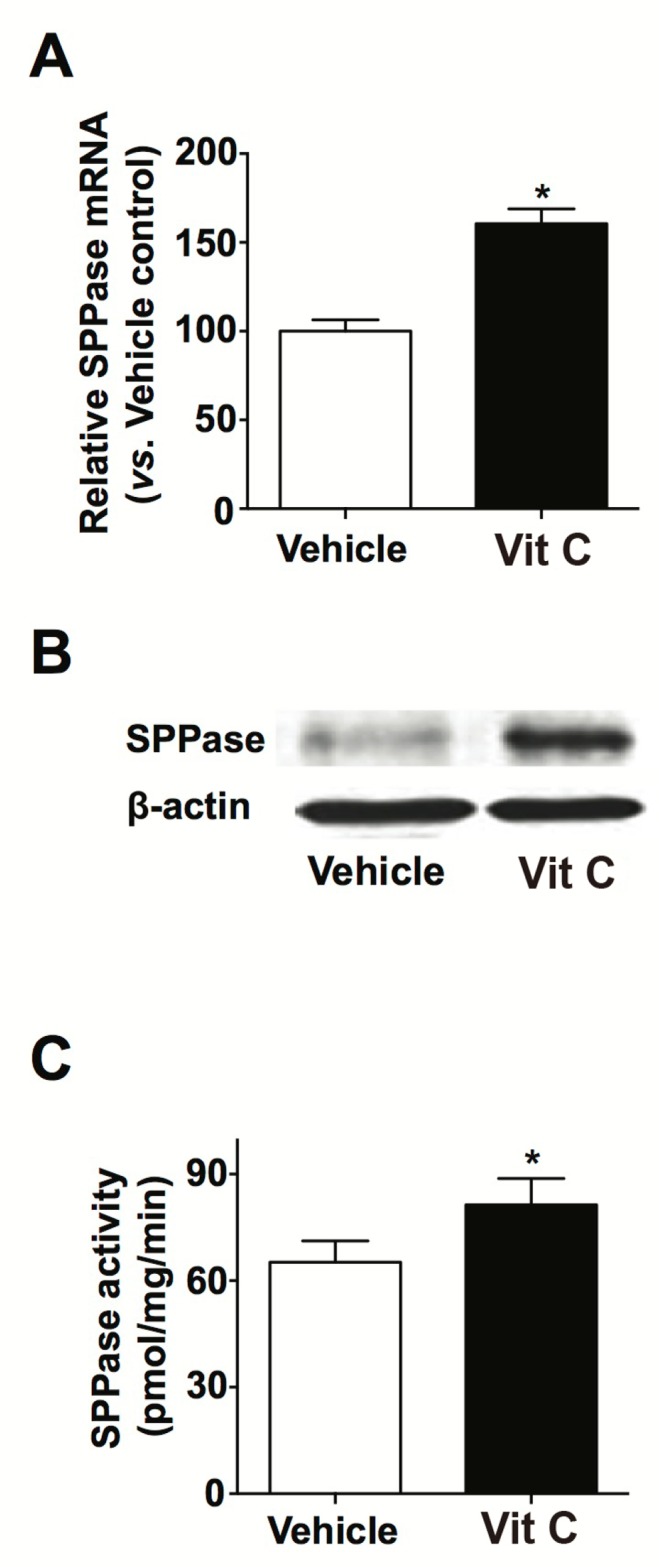
Increased expression and activity of sphingosine-1-phosphate phosphatase in KC following Vit C. Human primary keratinocytes (KC) were cultured in high Ca2+-supplemented medium with or without Vit C, as described in materials and methods. Sphingosine-1-phosphate phosphatase (SPPase) mRNA expression was determined by qRT-PCR (A). Protein expression of SPPase protein was determined by western immunoblot analysis (B). SPPase activity was analyzed by ESI-LC/MS/MS (C). Data are means ± SD (n=3). *p<0.01 versus vehicle control.
DISCUSSION
Of the lipids enriched in the extracellular lamellar membranes, ceramide is the most dominant lipid, which ultimately comprise 50% of total lipid mass in the SC (Elias and Menon, 1991). Prior studies have highlighted the importance of the respective lipid in maintaining barrier function: i) epidermal ceramide synthesis is required for normal barrier recovery after barrier disruption (Holleran et al., 1991), ii) a decrease in content of epidermal ceramide occurs in multiple skin disorders that show barrier abnormalities, e.g., atopic dermatitis and psoriasis (Imokawa et al., 1991; Motta et al., 1994), iii) epidermal ceramide, in particular ω-OH-ceramide, is attached to the CE, forming CLE, a structure that is required for normal barrier function (Wertz and Downing, 1986). We show here that Vit C supplementation increases epidermal ceramide content via specifically increasing the enzymatic activity and expression of SPT, CerS, and SPPase.
It is well established that ceramide content rises in parallel with KC differentiation (Uchida et al., 2001a), the latter being directly modulated by Ca2+. The data in our study is in line with these findings. However, we show that high Ca2+ supplementation (vehicle-treated) has no effect on expression of involucrin and loricrin. Expression of these proteins, which are vital to forming the CE and thus contribute greatly in maintaining the epidermal barrier (Eichner et al., 1984; Simon and Green, 1985; Mehrel et al., 1990), was instead stimulated by Vit C.
Prior studies have demonstrated that overall epidermal ceramide production is stimulated through de novo ceramide synthesis pathway by the action of key enzymes, i.e., SPT and CerS, respectively (Holleran et al., 1990; Uchida et al., 2001a). Consistent with these findings, Vit C-induced elevation in ceramide production appears to be regulated by increased expression/activity of these enzymes. In addition to de novo synthesis pathway, SM hydrolysis is also critical in epidermal production of ceramide (Uchida et al., 2000). SM in human skin is hydrolyzed to ceramide by at least two isoenzymes, a lysosomal type acid-pH optimum SMase (aSMase) and a non-lysosomal, magnesium-dependent neutral-pH optimum SMase (nSMase) (Uchida et al., 2000). However, in this study, Vit C treatment did not alter the activity of either aSMase or nSMase, suggesting that constitutive levels of SMases are likely suffice to stimulate ceramide production in the epidermis.
Moreover, we show here that Vit C-mediated increase in both expression and activity of SPPase, an enzyme which dephosphorylates S1P to SO, contributes, at least in part, to increased epidermal ceramide synthesis. Previous studies using HEK-293 (human embryonic kidney cells) and NIH 3T3 fibroblasts have provided evidence for the important role of SPPase in regulating ceramide levels; i) SPPase regulates ceramide levels in the endoplasmic reticulum (Le Stunff et al., 2004), ii) overexpression of SPPase in cells significantly increased ceramide levels (Le Stunff et al., 2002), iii) exogenous S1P treatment further increased all ceramide species in SPPase-overexpressing cells (Giussani et al., 2006). In agreement with these findings, our results indicate that both expression and activity of SPPase contribute in part to elevating ceramide production in KC following Vit C supplementation.
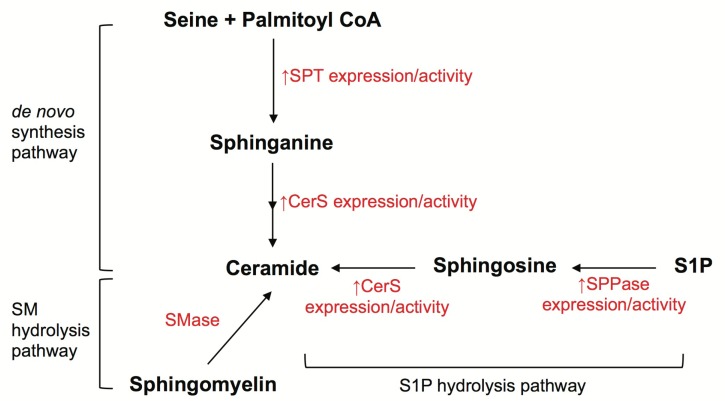
In conclusion, the present studies show that Vit C supplementation increases epidermal ceramide production by three pathways (Fig. 4): 1) increased de novo ceramide synthesis pathway due to activations of SPT and CerS; 2) SM hydrolysis via substrate regulation of constitutively expressed SMases; 3) increased S1P hydrolysis by SPPase to produce SO, followed by ceramide synthesis by CerS. Taken together, our studies suggest that Vit C could be utilized to enhance epidermal barrier function in skin disorders like psoriasis and atopic dermatitis, which are characterized by a significant reduction in epidermal ceramide content.
Fig. 4.
Proposed mechanism of Vit C-mediated increase in ceramide production in human keratinocyte (KC). Vit C supplementation increases epidermal ceramide production by three pathways: i) increased de novo ceramide synthesis pathway due to activations of serine palmitoyltransferase (SPT) and ceramide synthase (CerS); ii) sphingomyelin (SM) hydrolysis via substrate regulation of constitutively expressed sphingomyelinases (SMases); iii) increased sphingosine-1-phosphate (S1P) hydrolysis by sphingosine-1-phosphate phosphatase (SPPase) to produce sphingosine (SO), followed by ceramide synthesis by CerS.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012R1A1A3005669).
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Eichner R, Bonitz P, Sun TT. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J Cell Biol. 1984;98:1388–1396. doi: 10.1083/jcb.98.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- Giussani P, Maceyka M, Le Stunff H, Mikami A, Lepine S, Wang E, Kelly S, Merrill AH, Jr, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-golgi trafficking of ceramide. Mol Cell Biol. 2006;26:5055–5069. doi: 10.1128/MCB.02107-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran WM, Feingold KR, Man MQ, Gao WN, Lee JM, Elias PM. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J Lipid Res. 1991;32:1151–1158. [PubMed] [Google Scholar]
- Holleran WM, Williams ML, Gao WN, Elias PM. Serine-palmitoyl transferase activity in cultured human keratinocytes. J Lipid Res. 1990;31:1655–1661. [PubMed] [Google Scholar]
- Imokawa G, Kuno H, Kawai M. Stratum corneum lipids serve as a bound-water modulator. J Invest Dermatol. 1991;96:845–851. doi: 10.1111/1523-1747.ep12474562. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J Biol Chem. 2003;278:34541–34547. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Qiao Q, Toop HD, Morris JC, Don AS. A fluorescent assay for ceramide synthase activity. J Lipid Res. 2012;53:1701–1707. doi: 10.1194/jlr.D025627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yun H, Cho Y. Analysis of ceramide metabolites in differentiating epidermal keratinocytes treated with calcium or vitamin C. Nutr Res Pract. 2011;5:396–403. doi: 10.4162/nrp.2011.5.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol. 2002;158:1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H, Milstien S, Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem. 2004;92:882–899. doi: 10.1002/jcb.20097. [DOI] [PubMed] [Google Scholar]
- Loidl A, Claus R, Deigner HP, Hermetter A. High-precision fluorescence assay for sphingomyelinase activity of isolated enzymes and cell lysates. J Lipid Res. 2002;43:815–823. [PubMed] [Google Scholar]
- Mehrel T, Hohl D, Rothnagel JA, Longley MA, Bundman D, Cheng C, Lichti U, Bisher ME, Steven AC, Steinert PM, et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-N. [DOI] [PubMed] [Google Scholar]
- Motta S, Monti M, Sesana S, Mellesi L, Ghidoni R, Caputo R. Abnormality of water barrier function in psoriasis. Role of ceramide fractions. Arch Dermatol. 1994;130:452–456. doi: 10.1001/archderm.1994.01690040056007. [DOI] [PubMed] [Google Scholar]
- Park K, Elias PM, Hupe M, Borkowski AW, Gallo RL, Shin KO, Lee YM, Holleran WM, Uchida Y. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol. 2013a;133:1942–1949. doi: 10.1038/jid.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, Uchida Y. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. j Biol Chem. 2011;286:34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, Gallo RL, Saba J, Holleran WM, Uchida Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013b;33:752–762. doi: 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutti MF, Richard S, Penno A, von Eckardstein A, Hornemann T. An improved method to determine serine palmitoyltransferase activity. J Lipid Res. 2009;50:1237–1244. doi: 10.1194/jlr.D900001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KO, Park MY, Seo CH, Lee YI, Kim HS, Yoo HS, Hong JT, Jung JK, Lee YM. Terpene alcohols inhibit de novo sphingolipid biosynthesis. Planta Med. 2012a;78:434–439. doi: 10.1055/s-0031-1298155. [DOI] [PubMed] [Google Scholar]
- Shin KO, Park NY, Seo CH, Hong SP, Oh KW, Hong JT, Han SK, Lee YM. Inhibition of sphingolipid metabolism enhances resveratrol chemotherapy in human gastric cancer cells. Biomol Ther. 2012b;20:470–476. doi: 10.4062/biomolther.2012.20.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Green H. Enzymatic cross-linking of involucrin and other proteins by keratinocyte particulates in vitro. Cell. 1985;40:677–683. doi: 10.1016/0092-8674(85)90216-8. [DOI] [PubMed] [Google Scholar]
- Uchida Y. Ceramide signaling in mammalian epidermis. Biochim. Biophys. Acta. 2014;1841:453–462. doi: 10.1016/j.bbalip.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Behne M, Quiec D, Elias PM, Holleran WM. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol. 2001a;117:1307–1313. doi: 10.1046/j.0022-202x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Hara M, Hamanaka S. [Stratum corneum ceramides: their function and origins] Seikagaku. 2001b;73:268–272. [PubMed] [Google Scholar]
- Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, Suzuki A, Elias PM, Holleran WM, Hamanaka S. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–2082. [PubMed] [Google Scholar]
- Wertz PW, Downing DT. Covalent attachment of omega-hydroxyacid derivatives to epidermal macromolecules: a preliminary characterization. Biochem Biophys Res Commun. 1986;137:992–997. doi: 10.1016/0006-291X(86)90323-2. [DOI] [PubMed] [Google Scholar]