Abstract
Preceding infection or inflammation such as bacterial meningitis has been associated with poor outcomes after stroke. Previously, we reported that intracorpus callosum microinjection of lipopolysaccharides (LPS) strongly accelerated the ischemia/reperfusion-evoked brain tissue damage via recruiting inflammatory cells into the ischemic lesion. Simvastatin, 3-hydroxy-3-methylgultaryl (HMG)-CoA reductase inhibitor, has been shown to reduce inflammatory responses in vascular diseases. Thus, we investigated whether simvastatin could reduce the LPS-accelerated ischemic injury. Simvastatin (20 mg/kg) was orally administered to rats prior to cerebral ischemic insults (4 times at 72, 48, 25, and 1-h pre-ischemia). LPS was microinjected into rat corpus callosum 1 day before the ischemic injury. Treatment of simvastatin reduced the LPS-accelerated infarct size by 73%, and decreased the ischemia/reperfusion-induced expressions of pro-inflammatory mediators such as iNOS, COX-2 and IL-1β in LPS-injected rat brains. However, simvastatin did not reduce the infiltration of microglial/macrophageal cells into the LPS-pretreated brain lesion. In vitro migration assay also showed that simvastatin did not inhibit the monocyte chemoattractant protein-1-evoked migration of microglial/macrophageal cells. Instead, simvastatin inhibited the nuclear translocation of NF-κB, a key signaling event in expressions of various proinflammatory mediators, by decreasing the degradation of IκB. The present results indicate that simvastatin may be beneficial particularly to the accelerated cerebral ischemic injury under inflammatory or infectious conditions.
Keywords: Simvastatin, Cerebral Stroke, Cytokine, Inflammation, Macrophages, Microglia
INTRODUCTION
Preceding infection or inflammation such as bacterial meningitis has been associated with poor outcome after cerebral ischemic insult (Emsley and Hopkins, 2008; Katchanov et al., 2010; Heikinheimo et al., 2013; Fugate et al., 2014). Brain damage following cerebral ischemia/reperfusion is mediated by a cascade of diverse injury process and preceding infection or inflammation prior to cerebral ischemic insult may worsen the brain damage by affecting this cascade (Emsley and Tyrrell, 2002; Emsley and Hopkins, 2008).
Lipopolysaccharide (LPS), a bacterial endotoxin, has been widely used to activate inflammatory cells in experimental models (Ji et al., 2007; Kang et al., 2012). In our previous study, we successfully activated microglia/macrophages throughout the whole ipsilateral hemisphere by microinjecting LPS into rat corpus callosum (Lee et al., 2005). In this experimental model, activated microglia/macrophages highly accelerated the brain tissue damage after middle cerebral artery occlusion (MCAO) followed by reperfusion (Lee et al., 2005). Thus, LPS microinjection into corpus callosum may serve as a good experimental model for the study of the effect of preceding inflammation on the brain ischemic insult.
Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase) inhibitors, have been reported to present anti-inflammatory and immunomodulatory effect. Thus, Simvastatin treatment was reported to alter the release of cytokines and trophic factors including interleukin-1β, tumor necrosis factor-α, and brain derived neurotrophic factor and the phagocytotic activity of microglia (Churchward and Todd, 2014). Statins have a variety of direct effects via blockade of GTPase isoprenylation on the gene expression and function of cells of both the innate and adaptive immune systems, including endothelial cells, macrophages, dendritic cells and T cells (Bu et al., 2011). In addition, statins have protective effect in cerebral vessels and brain ischemia (Giannopoulos et al., 2012; Chróinín et al., 2013). Patients who discontinued the use of statins had a significantly increased mortality during the first year after the acute cerebrovascular event (Colivicchi et al., 2007). However, despite the anti-inflammatory activities of statins, little study has been done to investigate the usefulness of statins on cerebral ischemic injury with preceding infection or inflammatory conditions.
In the present study, therefore, we evaluated the protective effect of simvastatin on the evolution of LPS-accelerated cerebral ischemic brain damage via anti-inflammatory activities.
MATERIALS AND METHODS
Animals and drug treatment
Sprague-Dawley male rats, weighing between 260 and 270 g, were purchased from Charles River Laboratories (Seoul, Korea) and kept on a 12 h light/dark cycle with ad libitum access to food and water. Rats were acclimated to their environment for 5 d before use for experiments. All experimental procedures using animals were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Committee of Korea University College of Medicine. For simvastatin treatment, rats were treated with vehicle alone or simvastatin 20 mg/kg (Zocor, Merck and Co, Whitehouse Station, NJ, USA). Simvastatin was administrated orally 4 times at 72, 48, 25 and 1 h before the induction of the cerebral ischemic insult.
Intracorpus callosum microinjection of LPS and transient focal ischemia in rat
Rats were anesthetized with chloral hydrate (300 mg/kg, i.p.) and positioned in a small-animal stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). Microinjection of LPS (5 μg/5 μl; Escherichia coli serotype 055:B5; Sigma, St. Louis, MO) into the corpus callosum was performed, as described before (Lee et al., 2005). One day later, focal cerebral ischemia was achieved by right-sided endovascular MCAO, as described before (Lee et al., 2005). After 2 h of ischemia, the suture was pulled back and the animal was allowed to recover. A rectal temperature probe was introduced, and body temperature was maintained at 37°C with a heating pad during whole surgery period.
Physiological parameters
A left femoral artery was catheterized for continuous blood pressure monitoring and periodic blood sampling. Physiological values were measured 30 min before MCAO and 30 min after reperfusion. Mean arterial blood pressure (MABP) was monitored for 5 min using a DigiMed blood pressure analyzer (MicroMed, Louisville, KY, USA). Blood samples were taken to measure pH, PaO2, PaCO2 and blood glucose using an automatic pH/blood gas analyzer (Ciba Corning Diagnostics Corp., Medfield, MA, USA).
Measurement of infarct volume
Rats were anesthetized with chloral hydrate and decapitated at 3 h after MCAO. Rat brains were cut into coronal slices of 2 mm in thickness using a rat brain matrix (Ted Pella, Redding, CA, USA). Cerebral infarct volume was determined using 2% triphenyltetrazolium chloride (TTC, Sigma), as described before (Lee et al., 2005). The total volume of infarction was determined by integrating six chosen sections and expressed as percentage of the total brain volume. Because post ischemic brain edema increases brain volume in the infarct area, the corrected infarct volumes were calculated to compensate for brain edema, as previously described (Li et al., 1997). Thereafter, tissues were frozen, cut into 10 or 30 μm coronal sections on a cryostat (Leica 3050, Leica, Nussloch, Germany) and stored at −20°C. Some tissues were quickly stored at −70°C for RT-PCR and Western blot analyses.
Identification of microglia/macrophages
Microglia/macrophages were identified by staining with Griffonia simplicifolia isolectin B4 (isolectin B4). After blocking endogenous peroxidase with 0.3% hydrogen peroxide (H2O2) in 0.1 M phosphate buffer for 10 min, sections were incubated in 10% normal horse serum-supplemented 0.1 M phosphate buffered saline (PBS) for 30 min. Sections were then incubated overnight at 4°C with biotinylated isolectin B4 (diluted 1:100, Vector Laboratories, Burlingame, CA, USA) in PBS containing 0.3% triton X-100 and 2% normal horse serum. After washing three times with PBS for 10 min each, sections were incubated with peroxidase-conjugated streptavidin (diluted 1:200, Vector Laboratories) for 1–2 h at room temperature. Subsequently, sections were washed in PBS and then rinsed in 0.05 M Tris-HCl buffer (pH 7.6). Isolectin B4-positive cells were visualized by 5 min of incubation in 0.05 M Tris-HCl buffer containing 0.02% diaminobenzidine and 0.0045% hydrogen peroxide at 37°C. Finally, sections were dehydrated, mounted by Canada balsam, and then analyzed under a bright-field microscope (Olympus BX 51, Olympus Co., Japan).
Immunohistochemistry
For immunohistochemistry, sections were treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min, and then incubated in 10% normal horse serum-supplemented PBS for 30 min. Sections were next incubated overnight at room temperature with polyclonal antibodies directed against iNOS (diluted 1:1,000, Chemicon, Temecula, CA, USA), COX-2 (diluted 1:500; Pharmingen, San Diego, CA, USA), IL-1β (diluted 1:100; Biosource, Camarillo, CA, USA), or NF-κB p65 (diluted 1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in PBS containing 0.3% triton X-100 and 1% normal horse serum. After washing three times with PBS for 10 min each, sections were incubated sequentially in biotinylated goat anti-rabbit IgG (Vector Laboratories) and peroxidase-conjugated streptavidin (Vector Laboratories), diluted 1:200 in the same solution as the primary antiserum. Between the incubations, the tissues were washed three times with PBS for 10 min each. Sections were visualized with 3,3-diaminobenzidine (0.5 μg/ml) in 0.1 M Tris buffer and mounted by Canada balsam (Junsei Chemial Co., Japan). Stained sections were subsequently examined under a conventional light microscope (Olympus BX 51). For cell type identification of iNOS and NF-κB p65, sections were double-stained with polyclonal antibodies directed against iNOS or NF-κB p65 and a microglia-specific marker biotinylated isolectin B4 (diluted 1:50, Vector Laboratories). Immunoreactivity was visualized by using rhodamine-conjugated goat anti-rabbit IgG (diluted 1:50, Jackson ImmunoResearch Inc., West Grove, PA, USA), FITC-conjugated donkey anti-mouse IgG (diluted 1:50, Jackson ImmunoResearch Inc., for NeuN) and FITC-conjugated streptavidin (diluted 1:50, Jackson ImmunoResearch Inc., for isolectin B4). In order to verify the specificity of primary antibody, control sections were treated in the same manner with omission of the primary antibody. Stained sections were subsequently examined with a conventional epifluorescence microscope (Olympus BX 51).
Western blot analysis
Brain samples were homogenized in 50 mM Tris buffer containing 50 mM HEPES (pH 7.4), 0.1 mM EGTA (pH 8.0), 0.2% NP-40, 10 mM EDTA (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthvanadate, 1 mM PMSF, and 1 mM DTT. After centrifugation at 12,000×g, protein concentration in the supernatant was determined using the Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce, Rockford, IL, USA). Aliquot containing 50 μg of total protein was boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. Then, each aliquot was loaded onto a 10% or 12% polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose membranes (Schleicher and Schuell Bioscience, Inc., Riviera Beach, FL, USA). To reduce background staining, the filters were incubated with 5% non-fat dry milk in Tween 20 (0.1%)-containing TBS for 45 min, followed by incubation with primary antibodies directed against IκB (diluted 1:1,000; Santa Cruz Biotechnology Inc.), COX-2 (diluted 1:1,500; Pharmingen, San Diego, CA, USA) or IL-1β (diluted 1:1,000; Biosource, Camarillo, CA, USA), and then with ECL kit (Amersham, Piscataway, NJ, USA).
RT-PCR
Rats were sacrificed with chloral hydrate, and brains were removed and sliced into six 2 mm-thick coronal sections using a brain matrix. Total RNA was extracted from the third section of coronal sections using Trizol reagent according to the manufacturer’s instructions (Sigma). The following primers were used for iNOS: forward; 5′-ACACAGCCTCAGAGTCCTTC-3′ and reverse; 3′-GAACTGAGGGTACATGCTGG-5′ which yielded products of 593 bp. PCR products were separated by electrophoresis through 1.2% agarose containing 0.5 μg/ml ethidium bromide and imaged using a BioDoc-IT imaging system (Bio-Rad, Hercules, CA, USA). Band intensities were determined using GS-710 calibrated imaging densitometer (Bio-Rad). All signals were standardized against GAPDH mRNA signal, and results are expressed as each mRNA/GAPDH mRNA ratio.
Microglial cell culture and chemotaxis assay
Pure microglial cells were prepared by mild shaking (37°C, for 2 min at 200 r.p.m.) from primary mixed glial cell cultures. In brief, cerebral cortices of 1–2 days-old neonatal rats were triturated to single cells. They were then plated into poly-D-lysine (1 μg/ml) coated 75 cm2 T-flask and maintained in MEM containing 10% fetal bovine serum (FBS). Microglia were detached from the flasks by mild shaking (37°C, for 2 min at 200 r.p.m.) and plated. After 6 h, microglia were replaced with serum-free MEM and then used for experiment after overnight.
A 48-well microchemotaxis chamber (Neuroprobe, Gaithersburg, MD, USA) was used to measure the migration of microglia or monocytes towards assay medium or the chemoattractant monocyte chemoattractant protein-1 (MCP-1, 20 ng/ml). In brief, the upper and lower compartments of the chamber were separated by 8 μm polyvinylpyrrolidone-free filter. Microglia (1×105 cells/ml; 50 μl/wells) were added to the upper chamber and after 2 h of incubation period, non-migrating cells were gently scraped from the upper surface of the filter. Cells on the lower surface were fixed in methanol and stained with Diff Quik (Baxter, McGaw Park, IL, USA). The number of cells migrating to the underside of the filter was microscopically counted by an examiner blinded to the experimental condition.
Statistical analysis
Results are expressed as mean ± standard deviation (S.D.) or standard error of the mean (S.E.M.) and analyzed for statistical significance by using one-way ANOVA, followed by Scheffe’s test for multiple comparisons. A p value<0.05 was considered significant.
RESULTS
Blockade of the LPS-accelerated ischemic injury by simvastatin
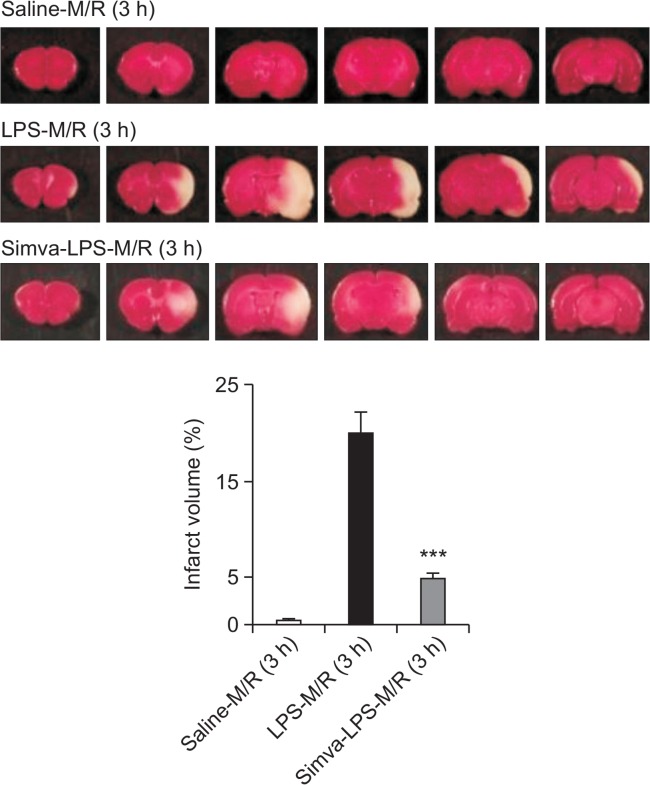
To induce infiltration/migration of inflammatory cells such as microglia and monocytes, LPS (5 μg/5 μl) was microinjected into rat corpus callosum 1 d before the ischemic insult. Pre-injection of LPS into corpus callosum did not result in the change of physiological parameters such as mean arterial pressure, pH, arterial partial CO2 and O2 pressures and blood glucose concentration (Table 1). Also, physiological measures were not significantly different between saline- and simvastatin-treated groups. In line with our previous study (Lee et al., 2005), injection of LPS 1 d prior to MCAO/reperfusion accelerated the ischemic injury (Fig. 1). Thus, marked brain tissue damage was observed at 3 h after reperfusion. In comparison, little damage was obtained 3 h after MCAO/reperfusion in saline-injected rat brains. Treatment of rats with simvastatin markedly reduced the LPS-accelerated ischemic injury. Photo images of cresyl violet-stained brain slice showed that the cytoprotective effect of simvastatin on neuronal cells was markedly observed in both cortical and subcortical lesions (data not shown).
Table 1.
Physiological values
| MABP | pH | PaCO2 | PaO2 | Glucose | |
|---|---|---|---|---|---|
| 30 min before occlusion | |||||
| Saline | 109.9 ± 15.6 | 7.47 ± 0.02 | 47.5 ± 3.2 | 181.0 ± 17.5 | 145.0 ± 9.7 |
| Simva | 112.4 ± 13.2 | 7.46 ± 0.02 | 46.1 ± 3.2 | 179.4 ± 13.1 | 132.0 ± 12.7 |
| LPS (1d) | 118.7 ± 14.6 | 7.47 ± 0.02 | 45.8 ± 3.7 | 179.0 ± 14.9 | 137.0 ± 14.7 |
| Simva-LPS (1d) | 114.4 ± 13.0 | 7.46 ± 0.03 | 47.1 ± 4.5 | 178.0 ± 11.3 | 139.0 ± 17.1 |
| 30 min after reperfusion | |||||
| Saline | 116.1 ± 17.3 | 7.47 ± 0.03 | 43.3 ± 2.9 | 177.9 ± 17.3 | 141.4 ± 11.1 |
| Simva | 114.1 ± 16.1 | 7.46 ± 0.03 | 46.8 ± 3.3 | 175.2 ± 18.5 | 139.1 ± 14.7 |
| LPS (1d) | 107.5 ± 14.6 | 7.47 ± 0.04 | 44.7 ± 2.7 | 179.6 ± 21.6 | 143.0 ± 15.5 |
| Simva-LPS (1d) | 111.3 ± 10.4 | 7.47 ± 0.04 | 43.3 ± 3.1 | 179.7 ± 16.4 | 140.0 ± 13.7 |
MABP indicates mean arterial blood pressure; PaCO2, partial arterial pressure of CO2; PaO2, partial arterial pressure of oxygen. Data are mean ± S.D. n=7
Fig. 1.
Blockade of the LPS-increased infarct volume by simvastatin. Representative TTC-stained coronal brain sections with six slices (2 mm-thick) each between 4 and 16 mm from the frontal pole. Rats were treated with simvastatin (Simva), as described in the Materials and Methods. One day after saline or LPS microinjection into the corpus callosum, rat brain was exposed to MCAO (M) for 2 h and reperfusion (R) for 3 h. The graphs show percentage changes of infarct volume. Each bar represents mean ± S.D. of 12 rats. ***p<0.001, compared with the infarct volume obtained in LPS-M/R (3 h) group.
Inhibition of iNOS by simvastatin
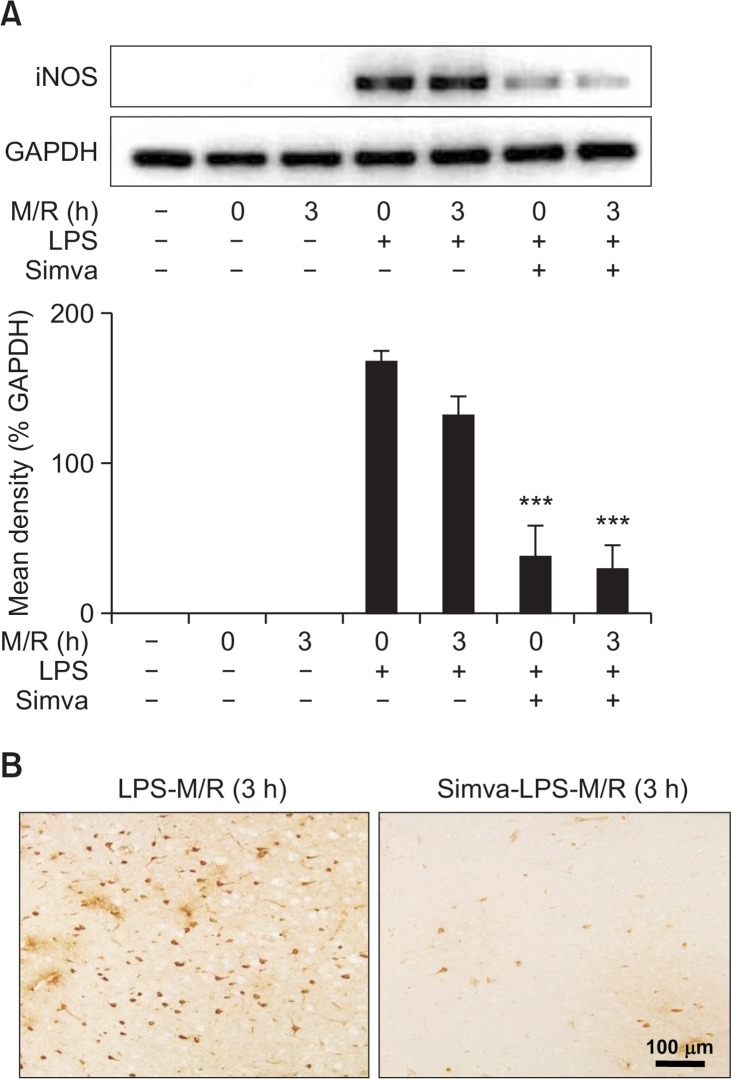
We previously reported that increased iNOS expression played a crucial role in the LPS-accelerated ischemic injury (Lee et al., 2005). Similarly, in the present study, RT-PCR and immunohistochemical studies showed that iNOS expression was intensely enhanced by MCAO/reperfusion in LPS pre-injected rats (Fig. 2). iNOS immunoreactivity appeared in round-shaped cells as well as shrunken or triangular neurons surrounding the vessels throughout the infarct zone (Fig. 2B). In saline-injected rat brains, however, iNOS immunoreactivity was much lower even after 2-h MCAO/3-h reperfusion (data not shown). Simvastatin strongly attenuated the iNOS immunoreactivity enhanced by MCAO/reperfusion in LPS pre-injected rats (Fig. 2).
Fig. 2.
Inhibition of iNOS by simvastatin. (A and B) Rats were treated with simvastatin, saline or LPS, as described in Fig. 1. Rat brain was exposed to MCAO for 2 h and reperfusion for 0 or 3 h. Brain slices were used for RT-PCR analysis (A) or immunohistochemical staining for iNOS (B). (A) Each bar represents mean ± S.D. of 8 rats. ***p<0.001, compared between indicated groups. (B) Microphotographs are representatives from 8 independent experiments.
Inhibition of COX-2 and IL-1β expression by simvastatin
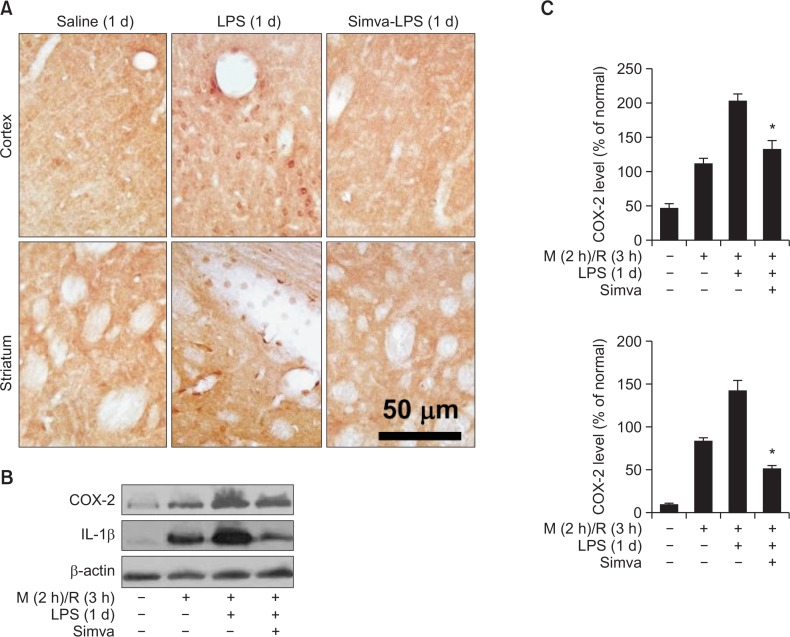
We further investigated whether simvastatin decreased the expression of other proinflammatory mediators COX-2 and IL-1β. The expression of COX-2 (Fig. 3A) and IL-1β (data not shown) was largely induced, but blocked by simvastatin, in round-shaped cells in LPS-pretreated rat brains. Western blot analysis showed that the levels of COX-2 and IL-1β proteins were enhanced in LPS-microinjected rat brains and further more intensely increased by ischemia/reperfusion (Fig. 3B and C). Simvastatin blocked the expression of COX-2 and IL-1β increased by MCAO/reperfusion in LPS-injected rats (Fig. 3B and C).
Fig. 3.
Inhibition of COX-2 and IL-1β expression by simvastatin. Rats were treated with simvastatin (Simva), saline or LPS, as described in Fig. 1. (A) Photomicrographs of COX-2 immunoreactive cells were taken in ipsilateral brain (cortex and striatum, respectively) at 1 d after LPS injection. Microphotographs are representatives from 6 independent experiments. (B and C) Rats were treated with simvastatin (Simva), saline or LPS, as described in Fig. 1. (B) Rat brain was exposed to MCAO (M) for 2 h and reperfusion (R) for 0 or 3 h. Brain slices were used for western blot analysis for COX-2 and IL-1β. (C) Quantitative analysis of the gel represented in A. Each bar represents mean ± SEM of 8 rats. *p<0.05, compared between indicated groups.
No inhibition of infiltration or migration of isolectin B4-positive cells by simvastatin
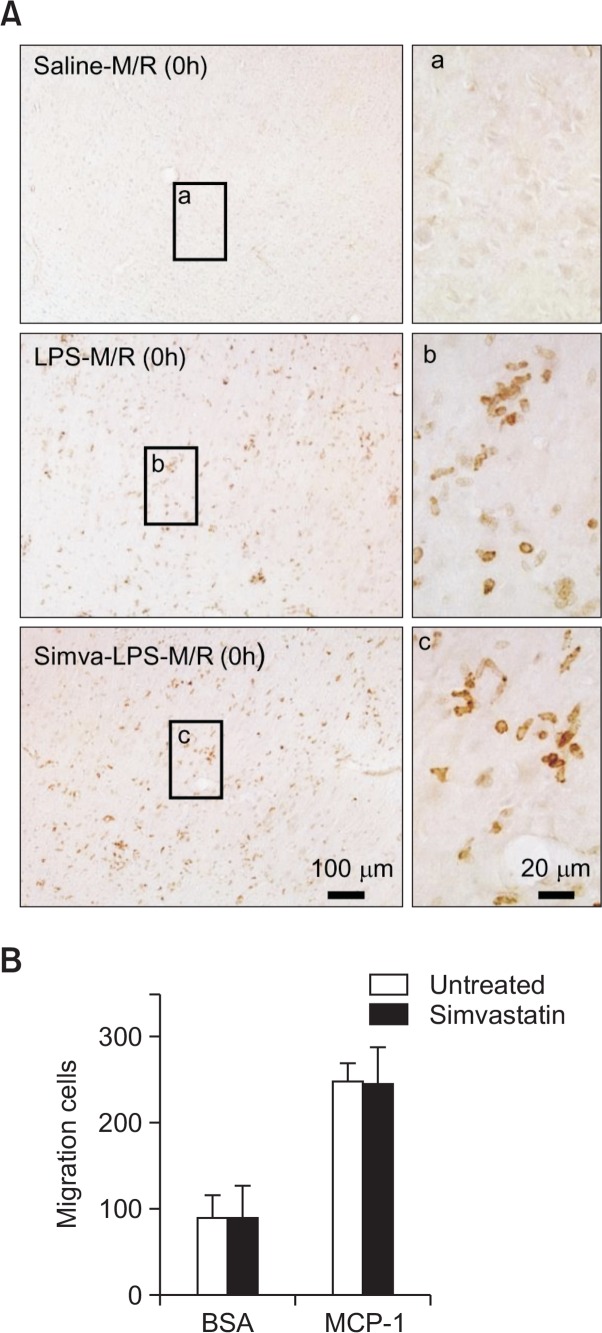
We previously reported that LPS microinjection markedly recruited and activated isolectin B4-positive monocytes and microglia, of which NO production accelerated cerebral ischemic injury (Lee et al., 2005). In the present study, however, we found that treatment with simvastatin did not interfere with the recruitment of isolectin B4 immunopositive cells into the ischemic lesion of LPS-treated rat brain (Fig. 4). In vitro chemotaxis assay also showed that simvastatin did not alter the MCP-1-induced migration of microglia (Fig. 4). Similar results were also obtained using isolated peripheral monocytes (data not shown).
Fig. 4.
No inhibition of infiltration or migration of isolectin B4-positive cells by simvastatin. (A) Rats were treated with simvastatin, saline or LPS, as described in Fig. 1. Rat brain was exposed to MCAO for 2 h, and isolectin B4-positive cells were visualized. Microphotographs are representative from 8 independent experiments. (B) Chemotaxis assay. Microglia were treated with simvastatin (10 μM) for 3 d before and during chemotaxis assay. Cells were then added onto the upper chamber. Lower chambers contain bovine serum albumin (BSA, 1 mg/ml) as a control or MCP-1 (20 ng/ml). n=5.
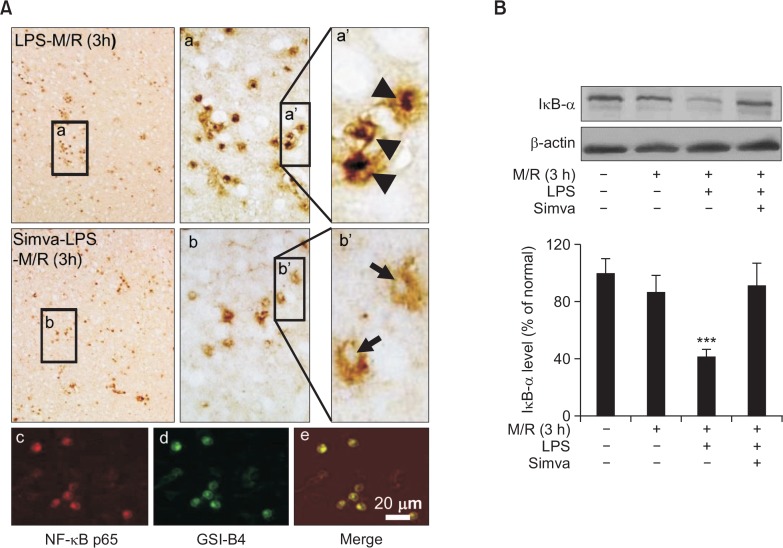
Blockade of NF-κB activity by simvastatin
In various cell types, NF-κB has been closely associated with the expression of iNOS, COX-2 and IL-1β (Tak and Firestein, 2001). Therefore, we further investigated whether those expressions inhibited by simvastatin were due to inhibition of NF-κB activity. Since the gel-mobility shift assay does not provide any information on the type of cells, we assessed the activity of NF-κB by using immunohistochemical techniques. NF-κB was found to be translocated into the nucleus after 2-h MCAO in LPS-microinjected rat brains (Fig. 5A, a, a′), and the immunoreactivity of NF-κB p65-subunit were co-localized with isolectin B4 immunoreactivity (Fig. 5A, c–e). Treatment of rats with simvastatin inhibited the nuclear translocation of NF-κB (Fig. 5A, b, b′). Western blot analyses showed that the level of IκB was significantly decreased by ischemia/reperfusion in LPS-microinjected rat brains (Fig. 5B). Simvastatin reversed the decreased level of IκB to the control level (Fig. 5B).
Fig. 5.
Blockade of NF-κB activity by simvastatin. Rats were treated with simvastatin, saline or LPS, as described in Fig. 1. (A) a and a′ Rat brain was exposed to MCAO for 2 h and reperfusion for 3 h. As marked by arrow heads, translocation of NF-κB p65 subunit into nucleus was markedly observed in round-shaped cells 1 d after LPS injection. (A) b and b′) Pre-treatment with simvastatin inhibited the nuclear translocation of NF-κB p65 subunit. (A) c–e NF-κB p65-positive cells (c, red) are co-localized with isolectin-B4-positive cells (d, green). Microphotographs are representatives from 8 independent experiments. (B) Western blot analysis. IκB proteins were markedly decreased in brains 1 d after LPS injection and the decreased level of IκB proteins was significantly recovered by pre-treatment with simvastatin. Each bar represents mean ± S.D. of 8 rats. ***p<0.001, compared between indicated groups.
DISCUSSION
The present study clearly shows that simvastatin reduces ischemic brain damage in rats subjected to transient ischemic insult with preceding inflammatory responses. Microinjection of LPS into rat corpus callosum 1 day prior to MCAO/reperfusion accelerated cerebral ischemic injury, as previously reported (Lee et al., 2005; Kang et al., 2012). Although some researchers report that LPS pretreatment 48 to 72 hours prior to MCAO reduces ischemic brain damage (Rosenzweig et al., 2004; Furuya et al., 2005; Vartanian et al., 2011), the discrepancy could be due to the timing of LPS administration and the method of its administration. While other researchers injected LPS through intraperitoneal (Rosenzweig et al., 2004), intravenous (Furuya et al., 2005) or subcutaneous method (Vartanian et al., 2011), we injected LPS into corpus callosum of brain. As previously reported (Lee et al., 2005; Kang et al., 2012), intracorpus callosum injection of LPS recruited a large number of amoeboid cells throughout the entire ipsilateral hemisphere (Lee et al., 2005). Although some of these amoeboid cells may be activated microglia, which are morphologically transformed to amoeboid form, most of them are thought to be macrophages/monocytes recruited from the periphery. The present study showed that simvastatin did not inhibit the infiltration/migration of microglia/monocytes into the ischemic lesion.
Activated microglia/monocytes release various kinds of proinflammatory mediators such as NO, prostaglandins and IL-1β and modulate the degree of ischemic tissue damage. Previous reports evidenced that inhibition of iNOS, COX-2 and IL-1β expressions reduced the ischemic brain injury (Gong et al., 2014; Xia et al., 2015). Our results showed that simvastatin could ameliorate LPS-accelerated ischemic brain injury at least in part through inhibition of iNOS, COX-2 and IL-1β expressions in activated microglia/monocytes. Simvastatin strongly decreased the expression of iNOS mRNA and proteins enhanced by ischemia in LPS-microinjected rat brains. The present study also showed that the expression of COX-2 was highly increased in LPS-injected rat brains, and further enhanced by ischemia. Simvastatin largely suppressed the enhanced expression of COX-2. Our present findings are supported by the previous reports showing that statins inhibit the expression of COX-2 in various cell types such as endothelial cells (Massaro et al., 2010) and human monocytic cell lines (Gómez-Hernández et al., 2006; Habib et al., 2007). In the CNS, microglial cells have been reported as the main source of IL-1β in response to LPS (Facci et al., 2014). Although the precise role of IL-1β in cerebral ischemic injury remains to be elucidated, the possible detrimental role of IL-1β in cerebral ischemic injury could be analogized by the reduction of infarct volume in animals treated with IL-1 receptor antagonists (Pradillo et al., 2012).
Activation of NF-κB is well associated with up-regulation of iNOS, COX-2 and IL-1β (Tak and Firestein, 2001). In addition, suppression of NF-κB activation can reduce damage in stroke (Harari and Liao, 2010). In resting microglia or macrophages, NF-κB is present in the cytoplasm as a complex with its inhibitory protein IκB, which blocks the translocation of NF-κB into nucleus. Stimulation by LPS induces the phosphorylation and subsequent degradation of IκB, leading to the release of NF-κB from NF-κB/IκB complexes (Griscavage et al., 1996). NF-κB is then translocated into the nucleus where it regulates the transcription of various genes for inflammatory mediators (Pahl, 1999). Wu et al. (Wu et al., 2013) reported that statins prevent the activation of NF-κB by inhibition of the IκB degradation. This report is concordant with our findings. Our present in vivo results demonstrated that simvastatin might reduce the iNOS, COX-2, IL-1β expression via inhibition of LPS-induced IκB degradation and resultant down-regulation of nuclear translocation of NF-κB.
In summary, simvastatin possesses anti-inflammatory activities including suppressed expression of iNOS, COX-2 and IL-1β. Furthermore, simvastatin inhibited the nuclear translocation of NF-κB by decreasing the degradation of IκB. Based on the present findings, simvastatin pretreatment may play a beneficial role in improving the prognosis and outcome of preceding infection- or inflammation-related stroke.
Acknowledgments
This study was supported by a grant from the Basic Science Research Program (#NRF-2015R1A2A01004202) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning, Korea.
REFERENCES
- Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol. 2011;22:165–170. doi: 10.1097/MOL.0b013e3283453e41. [DOI] [PubMed] [Google Scholar]
- Chróinín DN, Asplund K, Asberg S, Callaly E, Cuadrado-Godia E, Díez-Tejedor E, Di Napoli M, Engelter ST, Furie KL, Giannopoulos S. Statin therapy and outcome after ischemic stroke systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–456. doi: 10.1161/STROKEAHA.112.668277. [DOI] [PubMed] [Google Scholar]
- Churchward MA, Todd KG. Statin treatment affects cytokine release and phagocytic activity in primary cultured microglia through two separable mechanisms. Mol. Brain. 2014;7:85. doi: 10.1186/s13041-014-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colivicchi F, Bassi A, Santini M, Caltagirone C. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38:2652–2657. doi: 10.1161/STROKEAHA.107.487017. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/00004647-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Facci L, Barbierato M, Marinelli C, Argentini C, Skaper SD, Giusti P. Toll-like receptors 2, -3 and -4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1beta release. Sci Rep. 2014;4:6824. doi: 10.1038/srep06824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. Infectious causes of stroke. Lancet Infect Dis. 2014;14:869–880. doi: 10.1016/S1473-3099(14)70755-8. [DOI] [PubMed] [Google Scholar]
- Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. J Neurosurg. 2005;103:715–723. doi: 10.3171/jns.2005.103.4.0715. [DOI] [PubMed] [Google Scholar]
- Giannopoulos S, Katsanos AH, Tsivgoulis G, Marshall RS. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab. 2012;32:1973–1976. doi: 10.1038/jcbfm.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Hernández A, Sánchez-Galán E, Martín-Ventura JL, Vidal C, Blanco-Colio LM, Ortego M, Vega M, Serrano J, Ortega L, Hernández G. Atorvastatin reduces the expression of prostaglandin E2 receptors in human carotid atherosclerotic plaques and monocytic cells: potential implications for plaque stabilization. J Cardiovasc Pharmacol. 2006;47:60–69. doi: 10.1097/01.fjc.0000194252.38683.68. [DOI] [PubMed] [Google Scholar]
- Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L, Yin L, Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PloS One. 2014;9:e89450. doi: 10.1371/journal.pone.0089450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscavage JM, Wilk S, Ignarro LJ. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:3308–3312. doi: 10.1073/pnas.93.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A, Shamseddeen I, Nasrallah MS, Antoun TA, Nemer G, Bertoglio J, Badreddine R, Badr KF. Modulation of COX-2 expression by statins in human monocytic cells. FASEB J. 2007;21:1665–1674. doi: 10.1096/fj.06-6766com. [DOI] [PubMed] [Google Scholar]
- Harari OA, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo T, Broman J, Haapaniemi E, Kaste M, Tatlisumak T, Putaala J. Preceding and poststroke infections in young adults with first-ever ischemic stroke: effect on short-term and long-term outcomes. Stroke. 2013;44:3331–3337. doi: 10.1161/STROKEAHA.113.002108. [DOI] [PubMed] [Google Scholar]
- Ji K, Yang MS, Jeong HK, Min KJ, Kang SH, Jou I, Joe EH. Resident microglia die and infiltrated neutrophils and monocytes become major inflammatory cells in lipopolysaccharide-injected brain. Glia. 2007;55:1577–1588. doi: 10.1002/glia.20571. [DOI] [PubMed] [Google Scholar]
- Kang GH, Yan BC, Cho GS, Kim WK, Lee CH, Cho JH, Kim M, Kang IJ, Won MH, Lee JC. Neuroprotective effect of fucoidin on lipopolysaccharide accelerated cerebral ischemic injury through inhibition of cytokine expression and neutrophil infiltration. J Neurol Sci. 2012;318:25–30. doi: 10.1016/j.jns.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Katchanov J, Heuschmann PU, Endres M, Weber JR. Cerebral infarction in bacterial meningitis: predictive factors and outcome. J Neurol. 2010;257:716–720. doi: 10.1007/s00415-009-5395-9. [DOI] [PubMed] [Google Scholar]
- Lee JC, Cho GS, Kim HJ, Lim JH, Oh YK, Nam W, Chung JH, Kim WK. Accelerated cerebral ischemic injury by activated macrophages/microglia after lipopolysaccharide microinjection into rat corpus callosum. Glia. 2005;50:168–181. doi: 10.1002/glia.20164. [DOI] [PubMed] [Google Scholar]
- Li F, Irie K, Anwer MS, Fisher M. Delayed triphenyltetrazolium chloride staining remains useful for evaluating cerebral infarct volume in a rat stroke model. J Cereb Blood Flow Metab. 1997;17:1132–1135. doi: 10.1097/00004647-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Massaro M, Zampolli A, Scoditti E, Carluccio MA, Storelli C, Distante A, De Caterina R. Statins inhibit cyclooxygenase-2 and matrix metalloproteinase-9 in human endothelial cells: anti-angiogenic actions possibly contributing to plaque stability. Cardiovasc Res. 2010;86:311–320. doi: 10.1093/cvr/cvp375. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Denes A, Greenhalgh AD, Boutin H, Drake C, McColl BW, Barton E, Proctor SD, Russell JC, Rothwell NJ. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab. 2012;32:1810–1819. doi: 10.1038/jcbfm.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian KB, Stevens SL, Marsh BJ, Williams-Karnesky R, Lessov NS, Stenzel-Poore MP. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J. Neuroinflammation. 2011;8:140. doi: 10.1186/1742-2094-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Tian S, Zhou H, Wu Y. Statins protect human endothelial cells from TNF-induced inflammation via ERK5 activation. Biochem Pharmacol. 2013;85:1753–1760. doi: 10.1016/j.bcp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Xia CY, Zhang S, Gao Y, Wang ZZ, Chen NH. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int Immunopharmacol. 2015;25:377–382. doi: 10.1016/j.intimp.2015.02.019. [DOI] [PubMed] [Google Scholar]