Abstract
Consumption of herbal tea [flower buds of Cleistocalyx operculatus (Roxb.) Merr. et Perry (Myrtaceae)] is associated with health beneficial effects against multiple diseases including diabetes, asthma, and inflammatory bowel disease. Emerging evidences have reported that High mobility group box 1 (HMGB1) is considered as a key “late” proinflammatory factor by its unique secretion pattern in aforementioned diseases. Dimethyl cardamonin (2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone, DMC) is a major ingredient of C. operculatus flower buds. In this study, the anti-inflammatory effects of DMC and its underlying molecular mechanisms were investigated on lipopolysaccharide (LPS)-induced macrophages. DMC notably suppressed the mRNA expressions of TNF-α, IL-1β, IL-6, and HMGB1, and also markedly decreased their productions in a time- and dose-dependent manner. Intriguingly, DMC could notably reduce LPS-stimulated HMGB1 secretion and its nucleo-cytoplasmic translocation. Furthermore, DMC dose-dependently inhibited the activation of phosphatidylinositol 3-kinase (PI3K), phosphoinositide-dependent kinase 1 (PDK1), and protein kinase C alpha (PKCα). All these data demonstrated that DMC had anti-inflammatory effects through reducing both early (TNF-α, IL-1β, and IL-6) and late (HMGB1) cytokines expressions via interfering with the PI3K-PDK1-PKCα signaling pathway.
Keywords: Dimethyl cardamonin, Inflammatory mediators, HMGB1, PI3K, PKCα
INTRODUCTION
Inflammatory processes are considered as a series of complicated host defense responses. The purpose of inflammation is to repress vulnera or renovate organic tissues functions (Hirahara et al., 2013). Delayed and unchecked inflammation would cause the etiopathogenesis of a series of chronic diseases, such as asthma, inflammatory bowel disease, arthritis and septic shock syndrome (Kim et al., 2010). Immune macrophages are considered as the most special all important cells with boosting various inflammatory processes. They act as a key part in releasing “early” and “late” inflammation cytokines after stimulation (Valledor et al., 2010; DeGeus and Vervelde, 2013; Cheng et al., 2014). Major “early inflammatory mediators” are tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and so on (Charles et al., 1994; Edward et al., 1995; Pruitt et al., 1995; Abraham et al., 1998). Emerging evidences have demonstrated that High mobility group box 1 (HMGB1) acts as a critical part in inflammatory diseases and plays as a key “late-phase” mediator. Indeed, HMGB1 which is considered as an all important late inflammatory mediator can boost a series of early inflammation cytokines during the inflammatory processes (Wang et al., 1999; Czura et al., 2004; Yang et al., 2005). Hence, repressing HMGB1 proinflammatory activities and its secretion would be an impactful way to ameliorate acute and chronic inflammatory diseases.
Recently, HMGB1 which is identified as a great absorbing intranuclear protein, can be rapidly and passively secreted through necrotic cells or actively released via immune cells in response to proinflammatory induction mediators (Oozawa et al., 2008; Lakhan et al., 2009; Yang et al., 2012; Gong et al., 2014). When HMGB1 is elevated, acetylated and phosphorylated HMGB1 will transfer from nucleus to cytoplasm during inflammation process (Bonaldi et al., 2003; Youn et al., 2006; Venereau et al., 2012; Zhou et al., 2014). Several researchers have suggested that phosphatidylinositol 3-kinase (PI3K), phosphoinositide dependent kinase 1 (PDK1), and classical protein kinase C (cPKCs: PKCα, PKCβ, and PKCγ) signaling pathways could play a role in concerting to dominate HMGB1 release independent of MAPK and NF-κB. Moreover, PI3K-PDK1-Akt downstream target cPKCs are considered as effector kinases of HMGB1 modification secretion (Oh et al., 2009; Oh et al., 2011). Thus, intensive repression of HMGB1 modification secretion is an efficient therapeutic strategy in treating inflammatory diseases.
Cleistocalyx operculatus (Roxb.) Merr. et Perry (Myrtaceae) is identified as an edible plant (Dung et al., 2008). Dried flower buds of C. operculatus are commonly used as an important ingredient for herbal tea and herbal products in the tropical countries (Mai et al., 2010). Flower bud of C. operculatus contains chalcone, flavanone, essential oils, triterpene acids, and sterols. DMC is identified as an all important active constituent of C. operculatus (Fig. 1A) (Ye et al., 2004). DMC has many health beneficial effects including hepatoprotective activities (Yu et al., 2011), anti-tumor (Ye et al., 2005a; Ye et al., 2005b), anti-virus (Dao et al., 2010), anti-microbial (Salem and Werbovetz, 2005), anti-oxidant (Su et al., 2011; Martinez et al., 2012), anti-diarrheal (Amor et al., 2005; Ghayur et al., 2006), anti-drug efflux (Huang et al., 2011; Huang et al., 2012), and anti-diabetic (Hu et al., 2012; Hu et al., 2014a; Hu et al., 2014b). However, the anti-inflammatory effects of DMC along with the underlying mechanisms remain unclear. In the present study, we evaluated the anti-inflammatory effects of DMC and its molecular mechanism on LPS-induced macrophages.
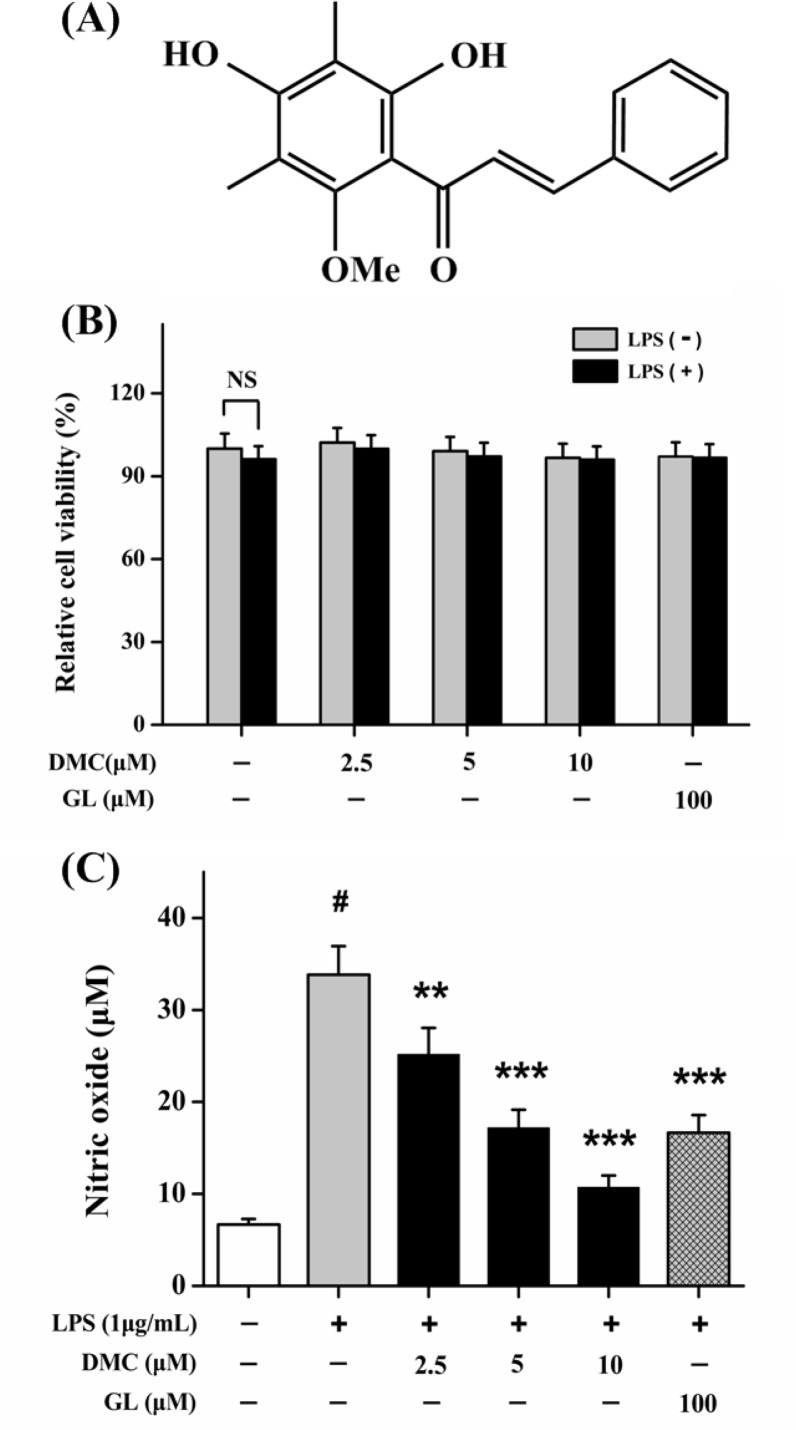
Fig. 1.
(A) Chemical structure of dimethyl cardamonin (2′,4′-dihydroxy-6′-methoxy- 3′,5′-dimethylchalcone, DMC). (B) Effects of DMC on cell viability. (C) Effects of DMC on NO production in LPS-induced RAW 264.7 cells. The concentrations of nitrite were measured via Griess reagent. Treatment with Glycyrrhizin (GL, 100 μM) acted as positive control group. Each value represents the mean ± SD of triplicate experiments. NS, non significant. #p<0.001 as compared with control group. **p<0.01 and ***p<0.001 as compared with LPS-induced group only.
MATERIALS AND METHODS
Materials
DMC (purity is 99%) is come from dried C. operculatus flower buds in our lab (Ye et al., 2004). Mouse anti-HMGB1 antibody and Fluorescein-5-isothiocyanate conjugated immunoglobulin G were obtained from Invitrogen (Carlsbad, CA, USA). PKC, PDK1, PI3K, phospho-PKC (p-PKC), p-PKCα, p-PKCβI, p-PKCβII, p-PKCγ, p-PDK1, p-PI3K, Histone H3.1, β-actin, and Alkaline phosphatase labeled secondary antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). TNF-α, IL-1β, IL-6, and HMGB1 were evaluated via ELISA Kits from R&D Systems (Minneapolis, MN, USA). Glycyrrhizin (GL), wortmannin (PI3K inhibitor, Wt), and LPS (Escherichia coli 0111: B4) were purchased from Sigma Aldrich Company (St. Louis, MO, USA). Cell Counting Kit-8 Assay Kit (CCK-8) was obtained from Nanjing Jiancheng Company (NJ, China).
Cell culture and stimulation
RAW 264.7 cells were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Roswell Park Memorial Institute 1640 (RPMI-1640) with Fetal calf serum (FCS, 10%), streptomycin (100 μm/mL), and penicillin (100 U/mL) at 37°C with 5% CO2. The viability of the RAW 264.7 cells was measured via CCK-8 Assay Kit following the manufacture’s specification. After culturing for 12 h, cells were replaced with fresh medium (RPMI-1640 with 0.25% FCS) with DMC (2.5, 5, and 10 μM) for 2 h, then were stimulated with LPS (1 μg/mL) for 24 h. RNA extracts, proteins, and supernatants were stored in −80°C refrigerator. Treatment with GL (100 μM) was used as a positive control.
Cytokines measurement
For the NO assay, cells were pretreated with DMC for 2 h and then stimulated with LPS (1 μg/mL) for 24 h. The supernatants were collected at the designed time points. NO production was examined via Griess reaction method (Huang et al., 2011). The amounts of the cytokines in the cell culture supernatants including TNF-α, IL-1β, IL-6, and HMGB1 were respectively measured via ELISA Kits following the manufacture’s specification.
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR)
RNA extraction and first-strand cDNA synthesis were performed according to the manufacture’s specification (RT-PCR core kit, Roche, Mannheim, Germany). The primer sequences used for analyzing of TNF-α, IL-1β, IL-6, HMGB1, and β-actin expressions were as follows: TNF-α, 5′-GTAGCCCACGTCG-TAGCAAA-3′ and 5′-C-CCTTCTCCAGCTGGAAGAC-3′; IL-1β, 5′-TCATTGTGGCTGTGGAGAAG-3′ and 5′-CTATGTCC CG-ACCATTGCTG-3′; IL-6, 5′-GGATACCACCCACAACAGAC-3′ and 5′-TTGCCGAGTAGACCTCATAG-3′; HMGB1, 5′-ATGGGC-AAAGGAG-ATCCTAA-3′ and 5′-CTCTGTAGGCAGGAATATCC-3′; β-actin, 5′-TGCTGTCCC-TGTATGCCTCT-3′ and 5′-TGATGT-CACGCACGATTTCC-3′. The PCR productions were measured via electrophoresis, and then were examined by staining ethidium bromide with ultraviolet light illumination. The relative band intensities were analysed via densitometer. The dates were normalized by β-actin.
Immunofluorescence staining
RAW 264.7 cells were cultured in monolayer with glass coverslip. Cells were pretreated with DMC for 2 h before LPS (1 μg/mL) stimulation. Cell samples were fixed by 4% formaldehyde for 0.5 h. Samples were permeabilized by Triton X-100 for 15 min. Cells were incubated with anti-HMGB1 primary antibody and Fluorescein-5- isothiocyanate conjugated secondary antibody. Finally, samples were stained with DAPI for 15 min. Fluorescence was examined by the laser scanning confocal microscopy (Olympus Inc., PA, USA).
Western blot analysis
Cells were lysed with lysing buffer in ice condition. To obtain cytosolic and nuclear proteins, extracts were respectively collected following the manufacture’s specification (Protein Extraction Kit, Pierce Biotechnology Inc., Nepean, Canada). Protein samples were separated via SDS-PAGE, and then were electroblotted to PVDF (Millipore, Bedford, MA, USA). After blocking with 5% non-fat milk, membranes were probed with primary antibodies (1:1000) for p-PKC, p-PKCα, p-PKCβI, p-PKCβII, p-PKCγ, PDK1, p-PDK1, PI3K, p-PI3K, HMGB1, Histone H3.1, and β-actin at 4°C overnight. After three washing, membranes were probed with AP labeled secondary antibody. Membranes were detected by BCIP/NBT. Finally, the relative band intensity was analysed via software Quantity One v4.62 (Bio-Rad, Inc., Berkeley, CA, USA).
Statistical analysis
The data were represented as the mean ± SD of triplicate experiments. The results were performed via one-way ANOVA followed by Tukey’s test. Differences of p<0.05 were taken as statistical significant.
RESULTS
DMC suppresses NO production in LPS-induced macrophages
As shown in Fig. 1B, the cytotoxic effect analysis suggested that DMC at the tested concentrations had low cytotoxicity on RAW 264.7 cells with or without LPS. To evaluate whether DMC could efficiently suppress inflammation, we detected Nitric oxide (NO) production by Griess reaction method in LPS-stimulated RAW 264.7 cells. As shown in Fig. 1C, pretreatment with DMC could dose-dependently suppress LPS- stimulated NO production, and the most inhibition was at concentration of 10 μM (p<0.001). Effect of DMC (5 μM) could be comparable to positive reference GL (100 μM). These results indicated that DMC had low cytotoxicity and could suppress LPS-stimulated NO production.
Effect of DMC on productions of early inflammatory cytokines (TNF-α, IL-1β, and IL-6)
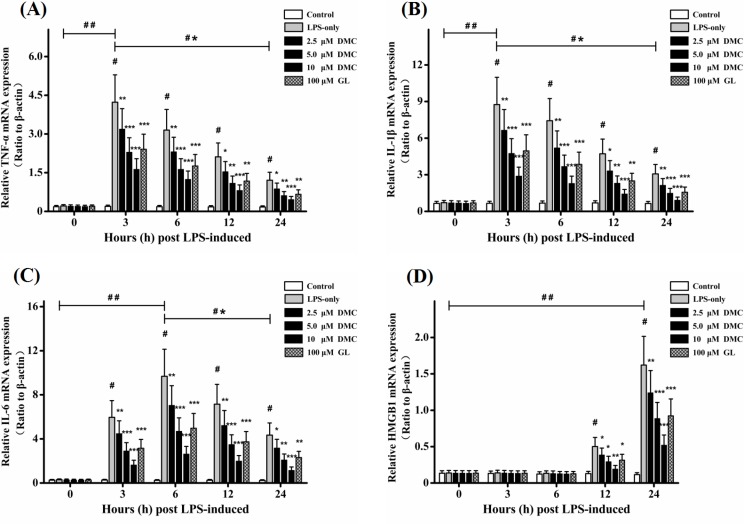
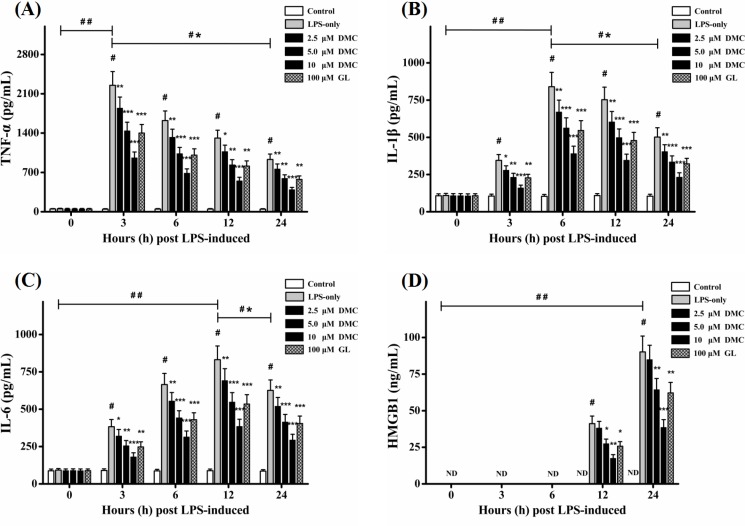
As shown in Fig. 2A–C, the mRNA expressions of TNF-α, IL-1β, and IL-6 were significantly (p<0.001) elevated after LPS stimulation and expressed mostly at 3h and 6h, respectively. Pretreatment with DMC significantly (p<0.05) suppressed their expressions, and the most inhibition was at concentration of 10 μM (p<0.001). To further confirm observations above, TNF-α, IL-1β, and IL-6 productions were examined by ELISA basing on designed time points. As shown in Fig. 3A–C, the data indicated that the levels of these cytokines (p<0.001) were significantly evoked after LPS stimulation and expressed mostly at 3 h, 6 h, and 12 h, respectively. Pretreatment with DMC significantly (p<0.05) decreased the levels of them in a time- and dose-dependent manner, and the most inhibition was at concentration of 10 μM (p<0.001). Effect of DMC (5 μM) could be comparable to positive reference GL (100 μM). These data demonstrated that pretreatment with DMC could suppress the productions of early inflammatory cytokines including TNF-α, IL-1β, and IL-6.
Fig. 2.
Effects of DMC on the expressions of TNF-α, IL-1β, IL-6, and HMGB1 mRNA in LPS-induced RAW 264.7 cells. Levels of TNF-α (A), IL-1β (B), IL-6 (C), and HMGB1 (D) mRNA expressions were measured by RT-PCR. Treatment with Glycyrrhizin (GL, 100 μM) acted as positive control group. β-actin acted as loading control. The bar graph showing semi-quantitative densitometric analysis summarizes the fold change of TNF-α, IL-1β, IL-6, and HMGB1 expressions in each group. Each value represents the mean ± SD of triplicate experiments. #p<0.001 as compared with control group. *p<0.05, **p<0.01 and ***p<0.001 as compared with LPS-induced group only. ##p<0.001 as the maximum value compared with control group. *#p<0.001 as the maximum value compared with the value at 24 h.
Fig. 3.
Effects of DMC on the release of TNF-α, IL-1β, IL-6, and HMGB1 in LPS-induced RAW 264.7 cells. Levels of TNF-α (A), IL-1β (B), IL-6 (C), and HMGB1 (D) were determined by ELISA. Treatment with Glycyrrhizin (GL, 100 μM) acted as positive control group. Each value represents the mean ± SD of triplicate experiments. #p<0.001 as compared with control group. *p<0.05, **p<0.01 and ***p<0.001 as compared with LPS-induced group only. ##p<0.001 as the maximum value compared with control group. *#p<0.001 as the maximum value compared with the value at 24 h.
Effect of DMC on expressions of late inflammatory mediator (HMGB1)
HMGB1 was considered as a key late inflammatory mediator and booster during the inflammatory processes (Czura et al., 2004; Yang et al., 2005). On one hand, HMGB1 mRNA expression was mostly evoked at 24 h with LPS stimulation (p<0.001). Pretreatment with DMC significantly (p<0.05) attenuated its expression in a time- and dose-dependent manner, and the most inhibition was at concentration of 10 μM (p<0.001) (Fig. 2D). On the other hand, pretreatment with DMC significantly (p<0.05) decreased HMGB1 secretion (Fig. 3D) which was consistent with the changes of HMGB1 mRNA expression. Effect of DMC (5 μM) could be comparable to positive reference GL (100 μM). Together these results suggested that DMC exhibited anti-inflammatory effects partly via suppressing HMGB1 secretion.
DMC inhibits LPS-stimulated HMGB1 secretion by suppressing its migration from nucleus to cytoplasm
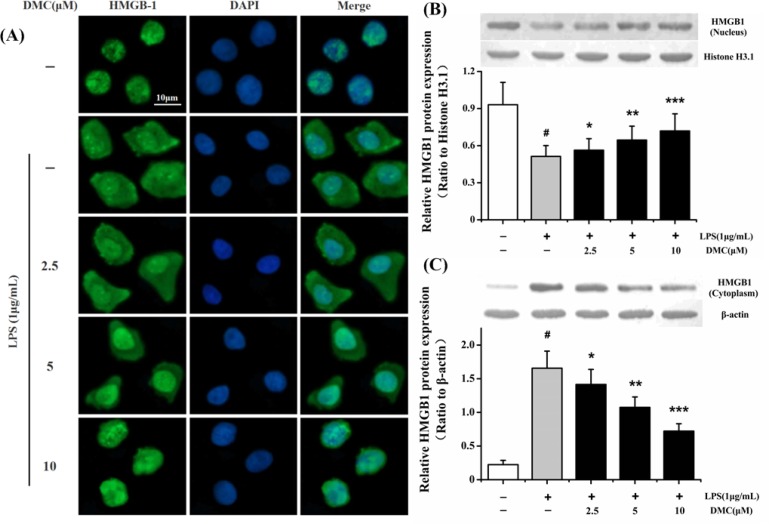
To further evaluate whether DMC could suppress the migration of HMGB1 from nucleus to cytoplasm, the subcellular localization of HMGB1 was examined by laser scanning confocal microscopy. As shown in Fig. 4A, HMGB1 was predominantly distributed in nucleus during normal circumstance. LPS could markedly trigger HMGB1 migration from nucleus to cytoplasm. Intriguingly, pretreatment with DMC could obviously suppress HMGB1 nucleo-cytoplasmic translocation. The concentration of DMC (10 μM) absolutely suppressed HMGB1 cytoplasmic migration. To further confirm our detections, nuclear and cytoplasmic proteins were respectively collected and analysed with Western blotting. As shown in Fig. 4, LPS alone notably (p<0.001) stimulated HMGB1 translocation from nucleus to cytoplasm. On one hand, pretreatment with the tested concentrations of DMC could markedly (p<0.05) cumulate the HMGB1 level in the nucleus (Fig. 4B). On the other hand, DMC could notably (p<0.05) decrease the HMGB1 level in the cytoplasm (Fig. 4C). The most inhibition was at concentration of 10 μM (p<0.001). These results demonstrated that DMC could suppress HMGB1 migration from nucleus to cytoplasm and inhibit the expression of HMGB1.
Fig. 4.
Inhibitory effect of DMC on the translocation of HMGB1 from nucleus to cytoplasm in LPS-induced RAW 264.7 cells. The cells were pretreated with DMC for 2 h before LPS (1 μg/mL) treatment for 24 h. (A) HMGB1 nucleo-cytoplasmic translocation was assayed by laser scanning confocal microscopy. HMGB1 was mainly localized in nucleus of control group (first row). HMGB1 was distributed in both nuclear and cytoplasmic of LPS-induced RAW 264.7 cells (second row). Pretreatment with DMC markedly inhibited LPS stimulated intracellular HMGB1 movement (third to fifth rows). Nucleus (blue, DAPI staining), HMGB1 (green). Bar: 10 μm. The nuclear and cytoplasmic HMGB1 proteins were measured via Western blot technique. (B) Histone H3.1 protein acted as loading control of nuclear HMGB1 protein. (C) β-actin protein acted as loading control of cytoplasmic HMGB1 protein. The bar graph showing semi-quantitative densitometric analysis summarizes the fold change of HMGB1 expression in each group. Each value represents the mean ± SD of triplicate experiments. #p<0.001 as compared with control group. *p<0.05, **p<0.01 and ***p<0.001 as compared with LPS-induced group only.
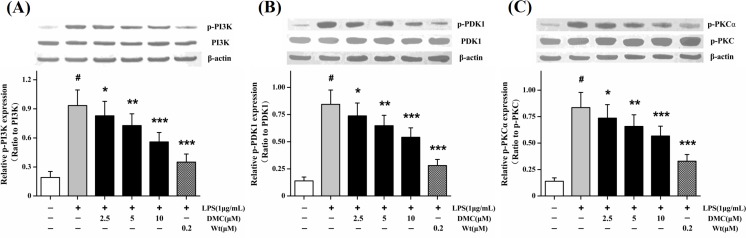
DMC down-regulates LPS-stimulated PI3K-PDK1-PKCα signal pathway
Some studies suggested that PI3K-PDK1 signal pathway probably played a role in concerting to dominate HMGB1 release (Youn and Shin, 2006). As shown in Fig. 5A, LPS alone markedly (p<0.001) stimulated PI3K phosphorylation. Pretreatment with DMC could dose-dependently decrease the phosphorylation level of PI3K. The most inhibition was at concentration of 10 μM (p<0.001). The effect of DMC on the phosphorylation of PDK1 was evaluated since PI3K downstream kinase was PDK1. Consistently, pretreatment with DMC could markedly (p<0.05) decrease the phosphorylation level of PDK1, and the most inhibition was at concentration of 10 μM (p<0.001) (Fig. 5B). Several studies have reported that the PI3K-PDK1-cPKC signal pathway is involved in HMGB1 release (Oh et al., 2009). Intriguingly, pretreatment with DMC could suppress dose-dependently PKCα phosphorylation (p<0.001) (Fig. 5C), but not of PKCβI, PKCβII, and PKCγ phosphorylation (data not shown). Together these data indicated that DMC could down-regulate PI3K-PDK1-PKCα signal pathway and PKCα played a key role in this signaling cascades.
Fig. 5.
Effects of DMC on PI3K-PDK1-PKCα signaling pathway in LPS-induced RAW 264.7 cells. The cells were pretreated with DMC for 2 h before LPS (1 μg/mL) treatment for 1 h. Levels of p-PI3K (A), p-PDK1 (B), and p-PKCα (C) were detected by Western blot technique. Wortmannin (PI3K inhibitor, Wt) was taken as a control. The bar graph showing semi-quantitative densitometric analysis summarizes the fold change of total PI3K, PDK1, and p-PKC expressions in each group. Each value represents the mean ± SD of triplicate experiments. #p<0.001 as compared with control group. *p<0.05, **p<0.01 and ***p<0.001 as compared with LPS-induced group only.
DISCUSSION
Natural viable substances that effectively and strongly inhibit inflammatory booster have been considered as valuable candidates for the treatment of inflammations. The dried flower buds of C. operculatus has been used as anti-inflammatory materials and herbal drink in tropical countries (Mai et al., 2010). In the present study, we demonstrated that DMC from C. operculatus flower buds could reduce the secretion and productions of both early (TNF-α, IL-1β, and IL-6) and late (HMGB1) cytokines in a time- and dose-dependent manner. HMGB1 is delayed relative to aforementioned classical early cytokines, and is also regarded as a critical “late-phase” mediator of inflammation (Wang et al., 1999). But, the anti-inflammatory effects of DMC along with the underlying mechanisms remain unclear. Hence, we deeply evaluated the interrelation between DMC and HMGB1 and found that DMC exhibited anti-inflammatory effects via interfering with the PI3K-PDK1-PKCα signaling pathway.
Inflammation is known as a series of harmful and common diseases. During several inflammation stimuli, HMGB1 can be rapidly and passively secreted through necrotic cells or actively released via immune cells in response to proinflammatory induction mediators (Oozawa et al., 2008; Lakhan et al., 2009; Gong et al., 2014). During the time of HMGB1 elevating, it will migrate from nucleus to cytoplasm in the inflammation process. The transport of HMGB1 to the cytoplasm could induce the productions of early inflammatory cytokines such as TNF-α, IL-1β, and IL-6 (Andersson et al., 2000). Secreted HMGB1 binding to Toll-like receptor 2/4 (TLR2/4) and the receptor for advanced glycation end products (RAGE) could exacerbate the inflammatory response (Youn and Shin, 2006). On one hand, DMC could markedly suppress HMGB1 migration from nucleus to cytoplasm. On the other hand, DMC could notably inhibit the production of HMGB1. These findings suggested that DMC exhibited anti-inflammatory effects via inhibiting the expression of HMGB1.
Some articles have reported that PI3K-PDK1-cPKCs signaling pathways could play a role in concerting to dominate HMGB1 release independent of MAPK and NF-κB. Moreover, cPKCs are considered as effector kinases of HMGB1 modification secretion (Oh et al., 2009; Oh et al., 2011). The findings showed that pretreatment with DMC notably reduced PI3K and PDK1 phosphorylation. Intriguingly, tested concentrations of DMC could dose-dependently suppress LPS-stimulated PKCα phosphorylation, but not all of p-cPKCs (including p-PKCβI, p-PKCβII, and p-PKCγ). Therefore, all data supported the hypothesis which DMC could suppress the secretion of HMGB1 via down-regulating PI3K-PDK1-PKCα signal pathway and PKCα played a key role in this signaling cascades.
In summary, our results demonstrated that DMC from C. operculatus flower buds exhibited anti-inflammatory effects through reducing the release and expressions of both early (TNF-α, IL-1β, and IL-6) and late (HMGB1) cytokines. The mechanisms were involved in suppressing HMGB1 secretion, blocking nucleo-cytoplasmic HMGB1 translocation, and interfering with the PI3K-PDK1-PKCα signaling pathway.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (WF1113010) and partially supported by the National High Technology Research and Development Program of China (2013AA092901) and the National Special Fund for State Key Laboratory of Bioreactor Engineering (2060204).
Footnotes
CONFLICTS OF INTEREST
The authors have no competing financial conflict of interest.
REFERENCES
- Abraham E, Anzueto A, Gutierrez G, Tessler S, Pedro GS, Wunderink R, Nogare AD, Nasraway S, Berman S, Cooney R, Levy H, Baughrnan R, Rurnbak M, Light RB, Poole L, Allred R, Constant J, Pennington J, Porter S. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet. 1998;351:929–933. doi: 10.1016/S0140-6736(05)60602-2. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang HC, Palmblad K, Aveberger AC, Bloom O, Helena EH, Janson A, Kokkola R, Zhang MA, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor EC, Villasenor IM, Ghayur MN, Gilani AH, Choudhary MI. Spasmolytic flavonoids from Syzygium samarangense (Blume) Merr. & L.M Perry. Z. Naturforsch. C. 2005;60:67–71. doi: 10.1515/znc-2005-1-213. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Huang C, Ma TT, Bian EB, He Y, Zhang L, Li J. SOCS1 hypermethylation mediated by DNMT1 is associated with lipopolysaccharide- induced inflammatory cytokines in macrophages. Toxicol Lett. 2014;225:488–497. doi: 10.1016/j.toxlet.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Charles F, Jean-Francois AD, Steven O, John P, Robert B, Gus S, Thomas I, Eric R, Marc S. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome, results from a randomized, double-blind, placebo-controlled trial. J Am Med Assoc. 1994;271:1836–1843. doi: 10.1001/jama.1994.03510470040032. [DOI] [PubMed] [Google Scholar]
- Czura CJ, Yang H, Amella CA, Tracey KJ. HMGB1 in the immunology of sepsis (Not septic shock) and arthritis. Adv Immunol. 2004;84:181–200. doi: 10.1016/S0065-2776(04)84005-7. [DOI] [PubMed] [Google Scholar]
- Dung NT, Kim JM, Kang SC. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food Chem Toxicol. 2008;46:3632–3639. doi: 10.1016/j.fct.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Dao TT, Tung BT, Nguyen PH, Thuong PT, Yoo SS, Kim EH, Kim SK, Oh WK. C-methylated flavonoids from Cleistocalyx operculatus and their inhibitory effects on novel influenza a (H1N1) neuraminidase. J Nat Prod. 2010;73:1636–1642. doi: 10.1021/np1002753. [DOI] [PubMed] [Google Scholar]
- De-Geus ED, Vervelde L. Regulation of macrophage and dendritic cell function by pathogens and through immunomodulation in the avian mucosa. Dev Comp Immunol. 2013;41:341–351. doi: 10.1016/j.dci.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Edward A, Richard W, Henry S, Trish P, Stanley N, Howard L, Roger B, Richard W, Robert B, Randy A. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome, a randomized controlled, double-blind, multicenter clinical trial. J Am Med Assoc. 1995;273:934–941. doi: 10.1001/jama.1995.03520360048038. [DOI] [PubMed] [Google Scholar]
- Ghayur MN, Gilani AH, Khan A, Amor EC, Villasenor IM, Choudhary MI. Presence of calcium antagonist activity explains the use of Syzygium samarangense in diarrhoea. Phytother Res. 2006;20:49–52. doi: 10.1002/ptr.1801. [DOI] [PubMed] [Google Scholar]
- Gong G, Xiang L, Yuan LB, Hu L, Wu W, Cai L, Yin L, Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014;9:89450–89460. doi: 10.1371/journal.pone.0089450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Poholek A, Vahedi G, Laurence A, Kanno Y, Milner JD, O’Shea JJ. Mechanisms underlying helper T-cell plasticity, implications for immune-mediated disease. J Allergy Clin Immunol. 2013;131:1276–1287. doi: 10.1016/j.jaci.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YC, Luo YD, Li L, Joshi MK, Lu YH. In vitro investigation of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone for glycemic control. J Agric Food Chem. 2012;60:10683–10688. doi: 10.1021/jf303078r. [DOI] [PubMed] [Google Scholar]
- Hu YC, Zhang Z, Shi WG, Mi TY, Zhou LX, Huang N, Hoptroff M, Lu YH. 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone promoted glucose uptake and imposed a paradoxical effect on adipocyte differentiation in 3T3-L1 cells. J Agric Food Chem. 2014a;62:1898–1904. doi: 10.1021/jf405368q. [DOI] [PubMed] [Google Scholar]
- Hu YC, Hao DM, Zhou LX, Zhang Z, Huang N, Hoptroff M, Lu YH. 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone protects the impaired insulin secretion induced by glucotoxicity in pancreatic β-Cells. J Agric Food Chem. 2014b;62:1602–1608. doi: 10.1021/jf405365d. [DOI] [PubMed] [Google Scholar]
- Huang HY, Niu JL, Zhao LM, Lu YH. Reversal effect of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone on multi-drug resistance in resistant human hepatocellular carcinoma cell line BEL-7402/5-FU. Phytomedicine. 2011;18:1086–1092. doi: 10.1016/j.phymed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Huang HY, Niu JL, Lu YH. Multidrug resistance reversal effect of DMC derived from buds of Cleistocalyx operculatus in human hepatocellular tumor xenograft model. J Sci Food Agric. 2012;92:135–140. doi: 10.1002/jsfa.4551. [DOI] [PubMed] [Google Scholar]
- Kim KN, Heo SJ, Yoon WJ, Kang SM, Ahn G, Yi TH, Jeon YJ. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol. 2010;649:369–375. doi: 10.1016/j.ejphar.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke, therapeutic approaches. J Transl Med. 2009;7:97–108. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Conde E, Moure A, Dominguez H, Estevez RJ. Protective effect against oxygen reactive species and skin fibroblast stimulation of Couroupita guianensis leaf extracts. Nat Prod Res. 2012;26:314–322. doi: 10.1080/14786411003752094. [DOI] [PubMed] [Google Scholar]
- Mai TT, Yamaguchi K, Yamanaka M, Lam NT, Otsuka Y, Chuyen NV. Protective and anticataract effects of the aqueous extract of Cleistocalyx operculatus flower buds on β-Cells of streptozotocin-diabetic rats. J Agric Food Chem. 2010;58:4162–4168. doi: 10.1021/jf904304w. [DOI] [PubMed] [Google Scholar]
- Oozawa S, Mori S, Kanke T, Takahashi H, Liu KY, Tomono Y, Asanuma M, Miyazaki I, Nishibori M, Sano SJ. Effects of HMGB1 on ischemia-reperfusion injury in the rat heart. Circ J. 2008;72:1178–1184. doi: 10.1253/circj.72.1178. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, Shin JS. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol. 2009;182:5800–5809. doi: 10.4049/jimmunol.0801873. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Youn JH, Min HJ, Kim DH, Lee SS, Choi IH, Shin JS. CKD712, (S)-1-(α-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, inhibits the lipopolysaccharide-stimulated secretion of HMGB1 by inhibiting PI3K and classical protein kinase C. Int Immunopharmacol. 2011;11:1160–1165. doi: 10.1016/j.intimp.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Pruitt JH, Copel EM, Moldawer LL. Interleukin-1 and interleukin-1 antagonism in sepsis, systemic inflammatory response syndrome, and septic shock. Shock. 1995;3:235–251. doi: 10.1097/00024382-199504000-00001. [DOI] [PubMed] [Google Scholar]
- Su MY, Huang HY, Li L, Lu YH. Protective effects of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone to PC12 cells against cytotoxicity induced by hydrogen peroxide. J Agric Food Chem. 2011;59:521–527. doi: 10.1021/jf104408d. [DOI] [PubMed] [Google Scholar]
- Salem MM, Werbovetz KA. Antiprotozoal compounds from Psorothamnus polydenius. J Nat Prod. 2005;68:108–111. doi: 10.1021/np049682k. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Comalada M, Santamaria-Babi LF, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation, aquestion of balance. Adv Immunol. 2010;108:1–20. doi: 10.1016/B978-0-12-380995-7.00001-X. [DOI] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, Marchis FD, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Bloom O, Zhang MH, Vishnubhakat JM, Ombrellino M, Che JT, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Ye CL, Lu YH, Wei DZ. Flavonoids from Cleistocalyx operculatus. Phytochemistry. 2004;65:445–447. doi: 10.1016/j.phytochem.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ye CL, Liu JW, Wei DZ, Lu YH, Qian F. In vivo anti-tumor activity by 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone in a solid human carcinoma xenograft model. Cancer Chemother Pharmacol. 2005a;56:70–74. doi: 10.1007/s00280-004-0975-y. [DOI] [PubMed] [Google Scholar]
- Ye CL, Qian F, Wei DZ, Lu YH, Liu JW. Induction of apoptosis in K562 human leukemia cells by 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone. Leuk Res. 2005b;29:887–892. doi: 10.1016/j.leukres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson L, Tracey KJ, Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of High Mobility Group Box-1 (HMGB1) Mol Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yu WG, Qian J, Lu YH. Hepatoprotective effects 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone on CCl4-induced acute liver injury in mice. J Agric Food Chem. 2011;59:12821–12829. doi: 10.1021/jf2042032. [DOI] [PubMed] [Google Scholar]
- Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang HC, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukocyte Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- Zhou HT, Ji XM, Wu Y, Xuan J, Qi ZL, Shen L, Lan L, Li Q, Yin ZM, Li ZJ, Zhao ZH. A dual-role of Gu-4 in suppressing HMGB1 secretion and blocking HMGB1 pro-inflammatory activity during inflammation. PLoS One. 2014;9:89634–89645. doi: 10.1371/journal.pone.0089634. [DOI] [PMC free article] [PubMed] [Google Scholar]