Abstract
Skin aging is the most readily observable process involved in human aging. Ultraviolet B (UVB) radiation causes photo-oxidation via generation of reactive oxygen species (ROS), thereby damaging the nucleus and cytoplasm of skin cells and ultimately leading to cell death. Recent studies have shown that high levels of solar UVB irradiation induce the synthesis of matrix metalloproteinases (MMPs) in skin fibroblasts, causing photo-aging and tumor progression. The MMP family is involved in the breakdown of extracellular matrix in normal physiological processes such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes such as arthritis and metastasis. We investigated the effect of diphlorethohydroxycarmalol (DPHC) against damage induced by UVB radiation in human skin keratinocytes. In UVB-irradiated cells, DPHC significantly reduced expression of MMP mRNA and protein, as well as activation of MMPs. Furthermore, DPHC reduced phosphorylation of ERK and JNK, which act upstream of c-Fos and c-Jun, respectively; consequently, DPHC inhibited the expression of c-Fos and c-Jun, which are key components of activator protein-1 (AP-1, up-regulator of MMPs). Additionally, DPHC abolished the DNA-binding activity of AP-1, and thereby prevented AP-1-mediated transcriptional activation. These data demonstrate that by inactivating ERK and JNK, DPHC inhibits induction of MMPs triggered by UVB radiation.
Keywords: Diphlorethohydroxycarmalol, Human skin keratinocytes, Matrix metalloproteinases, Photo-aging, Ultraviolet B
INTRODUCTION
The sun emits ultraviolet (UV) radiation in the UVA (320–400 nm), UVB (280–320 nm), and UVC (200–280 nm) wavelengths. The Earth’s ozone layer blocks 97–99% of this UV radiation. Due to the ongoing depletion of the ozone layer, elevated levels of UV wave are penetrating the atmosphere and reaching the Earth’s surface, leading to serious environmental problems. Overexposure to UVB radiation causes sunburn and some forms of skin cancer. However, the most deadly effect of UVB on cells is the result of indirect DNA damage mediated by free radicals and oxidative stress; consistent with this, 92% of all melanomas lack the signature mutations of direct UV damage (Davies et al., 2002).
UV radiation can damage collagen fibers, thereby accelerating skin aging. In particular, UVB destroys vitamin A in the skin, which may cause further damage (Berne et al., 1984). In keratinocytes, UVB irradiation upregulates the expression of MMP-1, further contributing to premature skin aging (Kim et al., 2010). MMP-1 also known as interstitial collagenase is one of the matrix metalloproteinases (MMPs) belongs to collagenases group, which plays important roles in physiological processes including embryonic development, morphogenesis, reproduction, and tissue resorption and remodeling (Brinckerhoff and Matrisian, 2002). MMP-1, also known as interstitial collagenase, initiates cleavage of fibrillar collagen (type I and III in skin) at a single site within its central triple helix (Fisher et al., 2002), contributing to the degradation of the extracellular matrix; thus, MMP-1 contributes to the etiology of many age-related degenerative diseases (Kähäri and Saarialho-Kere, 1997; Jacob, 2003). In many cancers, aberrant expression of MMP-1 is associated with both severe disease and poor patient outcome. Moreover, MMP-1 plays a prominent role in the proteolytic release and activation of growth factors, cytokines, and signaling peptides that modulate the senescent microenvironment. Further, it has been reported that MMP-2 and MMP-9, belong to gelatinases group of MMPs are also can be expressed at elevated levels at oxidative stress (Reel et al., 2009). The function of these gelatinases are to degrading type IV collagen, the most abundant component of the basement membrane which is important for maintaining tissue organization, providing structural support for cells, and influencing cell signaling and polarity. Basement membrane decomposition is a key step for the metastatic progression of most cancers (Mook et al., 2004).
The regulatory mechanisms that control senescence-associated MMP-1 expression are unknown. Previously, we hypothesized that anti-oxidants play an important role in regulating MMP-1 expression (Kang et al., 2008a,b). Redox activation of c-Jun-N-Terminal Kinase (JNK) is responsible for the age-dependent increases in MMP-1 expression, and this redox-sensitive signaling pathway controls activation of the AP-1 transcription factor and the chromatin remodeling events that lead to upregulation of MMP-1 (Dasgupta et al., 2010). Oxidative stress also stimulates extracellular signal-regulated kinase (ERK), another important regulator of MMP expression. Inhibition of ERK abrogates Ras- and serum-induced stimulation of the MMP-1 promoter, indicating that ERK plays an essential role in the transcriptional regulation of MMP-1 (Frost et al., 1994). Furthermore, overexpression of dominant-negative MEK blocks insulin-mediated induction of a reporter construct containing the activator protein (AP)-1 element from the MMP-1 promoter (Westermarck and Kähäri, 1999). These studies suggest that the ERK/AP-1 pathway is the major activator of MMP-1 expression. Moreover, ERK and JNK, which belong to the mitogen-activated protein kinase (MAPK) family, are associated with expression of MMP-1 in clinical material (Davidson et al., 2003).
Antioxidants can effectively attenuate UV-induced cell damage and skin photoaging by suppressing cell apoptosis and expression of MMP-1 via MAPK signaling pathways (Ren et al., 2010). Therefore, antioxidants might be useful as new anti-aging agents for photo-damaged skin (Jo et al., 2012). Our previous studies showed that the antioxidant properties of diphlorethohydroxycarmalol (DPHC, Fig. 1) can reduce production of MMP-1 by inhibiting ERK and AP-1 (Kang et al., 2008b). This compound protects cells against damage cause by UVB radiation, as demonstrated the fact that DPHC treatment decreases the length of the damaged DNA tail in a comet assay and prevents morphological changes in fibroblasts (Heo et al., 2010). DPHC may inhibit α-glucosidase and α-amylase activities and alleviate postprandial hyperglycemia in streptozotocin-induced diabetic mice (Heo et al., 2009). Moreover, DPHC exerts antioxidant effects against H2O2-mediated damage, and treatment with this compound represents a potential therapeutic modality for several diseases associated with oxidative stress (Heo and Jeon, 2009). However, the protective effect of DPHC in terms of MMPs and UVB-associated senescence has not been thoroughly studied. Hence, in this study we focused on the ability of DPHC to protect against UVB-induced cellular senescence caused by MMPs activation, and the possible underlying mechanisms involved. A fundamental understanding of the mechanisms by which various antioxidant compounds block MMPs production is essential for the design of safe and effective therapeutic strategies for the treatment of many age-associated degenerative diseases.
Fig. 1.
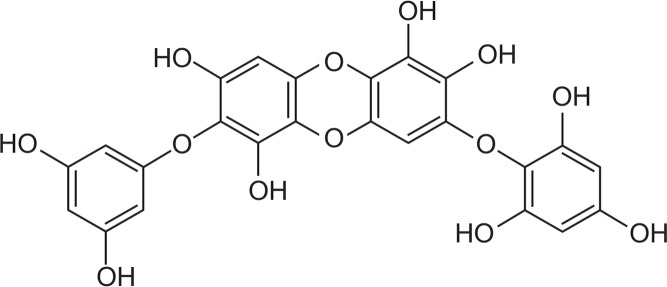
The structure of Diphlorethohydroxycarmalol.
MATERIALS AND METHODS
Reagents
DPHC was dissolved in dimethyl sulfoxide (DMSO) and final concentration in control or DPHC treatment did not exceed the 0.05%. Primary MMP-1 antibody was purchased from Epitomics, Inc. (Burlingame, CA, USA). Primary antibodies against MMP-2 and -9 were purchased from Abcam (Cambridge, MA, USA). Primary antibodies against ERK 2, phospho ERK 1/2, mitogen-activated protein kinase kinase (MEK) 1, phospho MEK 1, JNK 1/2, phospho JNK 1/2, stress-activated protein kinase/extracellular signal-regulated kinase (SEK) 1, phospho SEK 1, c-Fos, c-Jun and phospho c-Jun were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibody against actin was from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and reagents were of analytical grade.
Cell culture
Human HaCaT keratinocytes (Amore Pacific, Gyeonggi-do, Republic of Korea) were maintained at 37°C in an incubator with a humidified atmosphere of 5% CO2/95% air. The cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B.
Drug treatment and UVB irradiation
HaCaT cells were seeded at 1×105 cells/ml, allowed to adhere to the culture plate, and then treated with DPHC. After 1 h, the cells were exposed to ultraviolet B (UVB) at 150 mJ/cm2. The UVB source was a CL-1000M UV Crosslinker (UVP, Upland, CA, USA), which delivered UVB energy at wavelengths ranging from 280 to 320 nm (peak intensity, 302 nm).
Reverse transcriptase polymerase chain reaction
Total RNA was isolated from cells using easy-BLUE (Intron Biotechnology, Daegeon, Republic of Korea). The polymerase chain reaction (PCR) conditions for MMP-1 and the housekeeping gene GAPDH were as follows: 35 cycles of 94°C for 45 sec, 52°C for 1 min, and 72°C for 1 min. Primer pairs (Bionics, Seoul, Republic of Korea) were as follows (forward and reverse, respectively): MMP-1, 5′-GGAGGAAATCTTGC-TCAT-3′ and 5′-CTCAGAAAGAGCAGCATC-3′; GAPDH, 5′-AA - GGTCGGAGTCAACGGATTT-3′ and 5′-GCAGTGAGGGTCTC- TC TCCT-3′. The amplified products were resolved by 1% agarose gel electrophoresis, stained with ethidium bromide, and photographed under UV illumination.
Immunoblot analysis
The cells were harvested, washed twice with PBS, lysed on ice for 10 min in 100 μl PRO-PREPTM protein extraction solution (Intron Biotechnology), and centrifuged at 13,000 rpm for 5 min. The resultant supernatants were collected, and the protein concentrations were determined. Aliquots of the lysates (20 μg of protein) were boiled for 5 min and electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels. The resolved proteins were transferred onto nitrocellulose membranes, which were then incubated with primary antibodies. Next, the membranes were incubated with secondary immunoglobulin G-horseradish peroxidase conjugates (Pierce, Rockford, IL, USA). Protein bands were detected using an enhanced chemiluminescence Western blotting detection kit (Amersham Pharmacia Biotech, Buckinghamshire, UK), and then exposed to X-ray film.
Determination of MMP-1 activity
Secreted active MMP-1 was measured using the Human Active MMP-1 Fluorokine® E Kit (R&D Systems, Minneapolis, MN, USA). HaCaT ells were treated with 20 μM of DPHC for 1 h, after the 150 mJ/cm2 of UVB exposure and the samples were incubated for 1 day. Culture medium was subjected to centrifugation at 1,000×g for 5 min, and then 150 μl of culture supernatants was mixed with 100 μl of assay diluent buffer in 96-well enzyme-linked immunosorbent assay (ELISA) plates. The plates were shaken for 3 h at room temperature, and then unbound material was washed off. Subsequently, 200 μl of activation reagent (0.5 M APMA in DMSO) was added to each well for pro-MMP-1 activation. The plates were incubated for 2 h at 37°C in a humidified environment. After washing, 200 μl of fluorogenic substrate was added. After another 20 h at 37°C, fluorescence was measured using FLUOstar Optima Microplate Reader (BMG Labtech, Cary, NC, USA) with the excitation wavelength set to 320 nm and the emission wavelength set to 405 nm (Tsareva et al., 2007).
Determination of MMP-2 and MMP-9 activities
MMP-2 and MMP-9 enzymatic activities in serum free culture medium were determined by SDS-PAGE gelatin zymography. Gelatin zymography was performed using the Novex In-gel Zymography system (Invitrogen), according to the manufacturer’s instructions. Protein concentrations were analyzed with a Bio-Rad (Hercules, CA, USA) system. Equal amounts of medium for each sample were mixed with 2 × sample buffer and loaded on a Novex 10% Zymogram Gelatin Gel for electrophoresis. After electrophoresis, gels were followed by incubation with Zymogram Renaturing Buffer, and then with Zymogram Developing Buffer. After an overnight reaction, the gel was stained with SimplyBlue Safestain (Invitrogen). After staining, the gels were destained appropriately. Gelatinolytic activity was visualized as a transparent band against a blue background. Gelatinolytic bands were measured densitometrically with an image analyzer (Epson GT-9500 scanner).
Chromatin immunoprecipitation (ChIP) analysis
ChIP assays were performed using the SimpleChIPTM Enzymatic Chromatin IP Kit (Cell Signaling Technology, Danvers, MA, USA), with slight modifications to the manufacturer’s protocol. Cells were cross-linked by the addition of 1% formaldehyde. Chromatin was prepared and digested with nuclease for 12 min at 37°C. c-Jun antibody, normal rabbit IgG and histone H3 (D2B12) XPTM antibody were added to the chromatin digests, which were incubated with constant rotation overnight at 4°C. ChIP-grade protein G magnetic beads were added to capture the immunoprecipitated complexes. The beads were washed, and the immunoprecipitates were eluted with ChIP elution buffer. Cross-links were reversed by incubation of the eluent at 65°C for 30 min, followed by addition of proteinase K and incubation at 65°C for an additional 2 h. The immunoprecipitated DNA fragments were then purified on spin columns. DNA recovered from the immunoprecipitated complex was subjected to 35 cycles of PCR. The primers for the MMP-1 gene promoter were as follows: forward, 5′-CCT CTT GCT GCT CCA ATA TC-3′; reverse, 5′-TCT GCT AGG AGT CAC CAT TTC-3′ (Goffin et al., 2010). The PCR products were separated on 2% agarose gels, and DNA bands were visualized using ethidium bromide staining and Image Quant 350 (GE Healthcare, Wankesha, WI, USA).
Transient transfection and AP-1 luciferase assay
Cells were transiently transfected with a plasmid harboring the AP-1 promoter and the Renilla promoter using LipofectamineTM 2000 (Invitrogen, Eugene, OR, USA). Following overnight transfection, the cells were treated with 20 μM of DPHC, incubated for 1 h, and then exposed to UVB at 150 mJ/cm2. After 24 h, the cells were washed twice with PBS and lysed with passive lysis buffer (Promega, Madison, WI, USA). Following vortex mixing and centrifugation at 12,000×g for 30 sec at 4°C, the supernatant was stored at −70°C. For the luciferase assay, 20 μl of cell extract was mixed with 100 μl of the luciferase assay substrate reagent at room temperature, and the mixture was placed in an illuminometer to measure the light produced. Next, 100 μl of Renilla substrate reagent was added at room temperature, and the light produced was again measured using the illuminometer. AP-1 luciferase activities were calculated by dividing luciferase light production by Renilla light production.
Statistical analysis
All measurements were performed in triplicate, and all values are represented as means ± standard error. The results were subjected to an analysis of variance (ANOVA) using the Tukey test; p<0.05 was considered to represent a significant difference.
RESULTS
DPHC down-regulates UVB-induced MMP-1 expression and activation
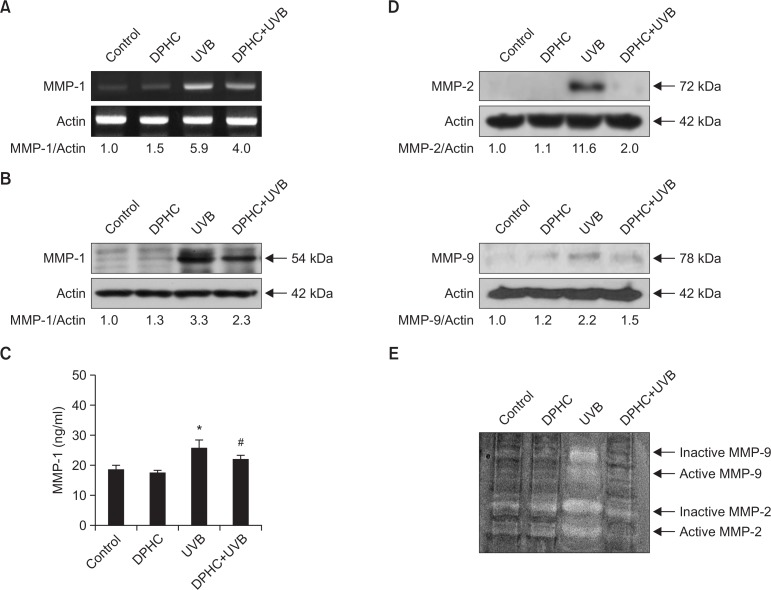
UVB irradiation of skin fibroblasts induces generation of reactive oxygen species (ROS), which promote transcription and expression of MMP-1, causing skin photoaging and skin tumor progression (Brenneisen et al., 1997). UVB irradiation markedly increased the MMP-1 mRNA levels, as demonstrated by RT-PCR analysis; DPHC pretreatment decreased the level of MMP-1 transcript induced by 150 mJ/cm2 of UVB irradiation (Fig. 2A). Consistent with the RT-PCR results, immunoblots confirmed that DPHC decreased the induction of MMP-1 protein expression by UVB irradiation (Fig. 2B). Moreover, DPHC significantly decreased the amount of active MMP-1 (Fig. 2C). Further western blot analysis revealed that DPHC treatment diminished the expression of both MMP-2 and MMP-9 (Fig. 2D). Using gelatin zymography, activation of MMP-2 and MMP-9 enzymes were measured in UVB irradiation-exposed cells. Zymography analysis revealed the presence of inactive and active forms of MMP-2 and MMP-9 in UVB irradiation-exposed cells (Fig. 2E). However, DPHC pre-treated cells showed reduction of inactive and active forms of MMP-2 and MMP-9 (Fig. 2E). These data indicate that DPHC significantly decreased activation of MMP enzymes.
Fig. 2.
The effects of DPHC on UVB-induced MMPs transcription, expression, and activation. (A) MMP-1 mRNA levels were measured by reverse-transcription (RT) PCR analysis. Cells were treated with 20 μM of DPHC for 1 h, and then irradiated with 150 mJ/cm2 UVB. After UVB irradiation, cells were incubated for 24 h in a humidified incubator containing 5% CO2 at 37°C, and the cells were then harvested for RT-PCR. (B, D) Cell lysates were electrophoresed, and MMP-1, -2 and -9 protein expression of was detected by immunoblotting. (C) Active MMP-1 was quantitated in culture media supernatants. Cells were incubated in serum-free media to eliminate interference from MMP-1 in serum. *Significantly different from control cells (p<0.05); #significantly different from UVB-irradiated cells (p<0.05). (E) Inactive and active MMP-2 and -9 were assessed by using gelatin zymography. Top two bands indicate MMP-9 gelatinase and lower two bands indicate MMP-2 gelatinase.
DPHC reduces the ERK and JNK activation in response to UVB irradiation
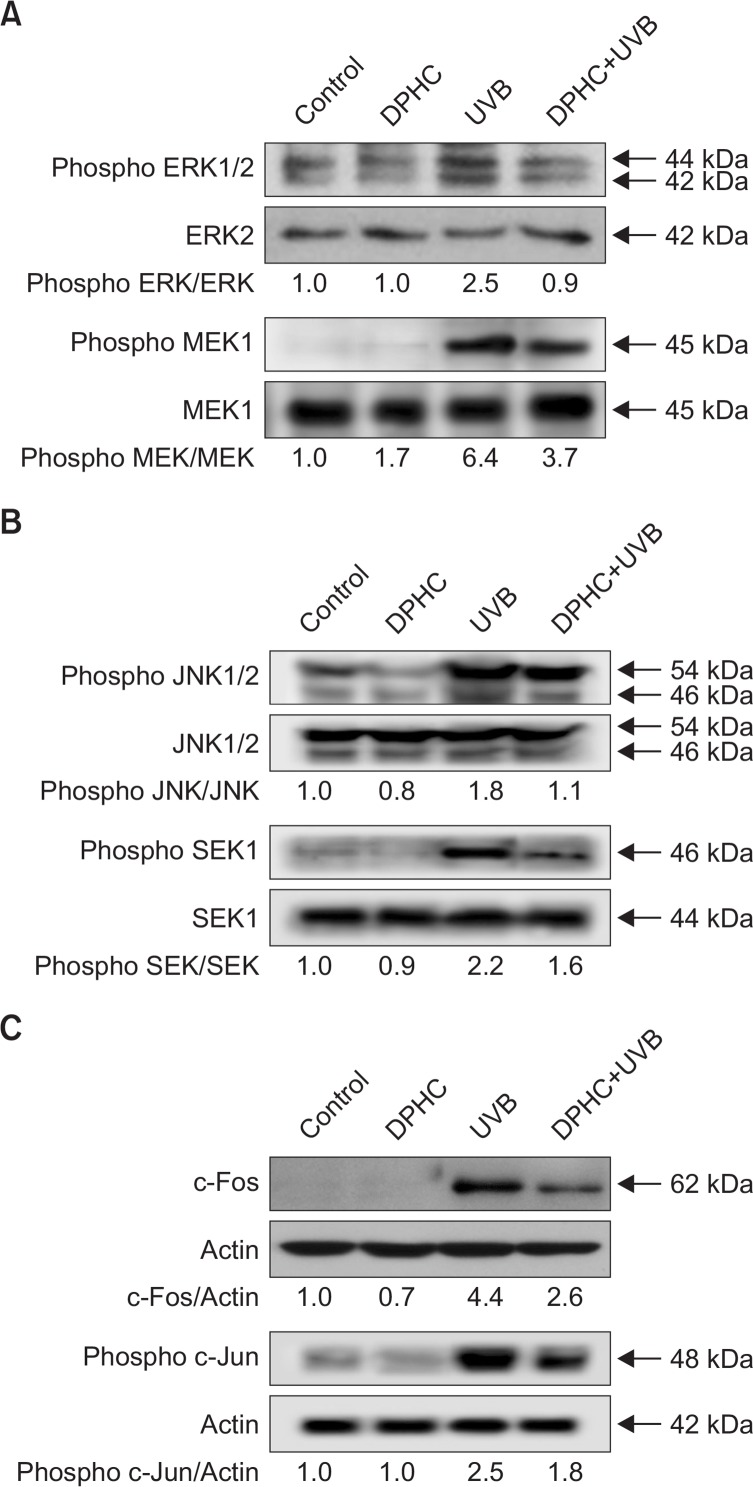
The AP-1 site plays a critical role in the transcriptional activation of MMP promoters (Crawford and Matrisian, 1996; Benbow and Brinckerhoff, 1997). AP-1, a heterodimeric protein consisting of proteins belonging to the Fos and Jun families, is a transcription factor, capable of binding to 5′-TGAGTCA-3′ DNA elements (Farrell et al., 1989; Glover and Harrison, 1995). The Fos and Jun families are regulated by MAPKs, especially ERK and JNK (Cano et al., 1994; Murphy et al., 2002; Bogoyevitch et al., 2010). As shown in Fig. 3A and B, DPHC markedly suppressed the activation of ERK and JNK by UVB irradiation. DPHC also reduced MEK1 and SEK1 expressions. Consequently, UVB-induced activation of c-Fos and phospho-c-Jun was attenuated by DPHC (Fig. 3C). These results suggested that UVB induction of AP-1 components, which are controlled by ERK and JNK activation, are attenuated by DPHC treatment.
Fig. 3.
Effects of DPHC on c-Fos and c-Jun expression via activation of ERK and JNK signal transduction. (A) Cell lysates were electrophoresed and immunoblotted using anti-phospho ERK1/2, anti-ERK2, anti-phospho MEK1, and MEK1 antibodies. (B) Cell lysates were electrophoresed and immunoblotted using anti-phospho JNK1/2, anti-JNK1/2, anti-phospho SEK1, and SEK1 antibodies. (C) Cell lysates were electrophoresed and immunoblotted using c-Fos and phospho c-Jun antibody, respectively.
Suppression of AP-1 transcriptional activity by DPHC treatment
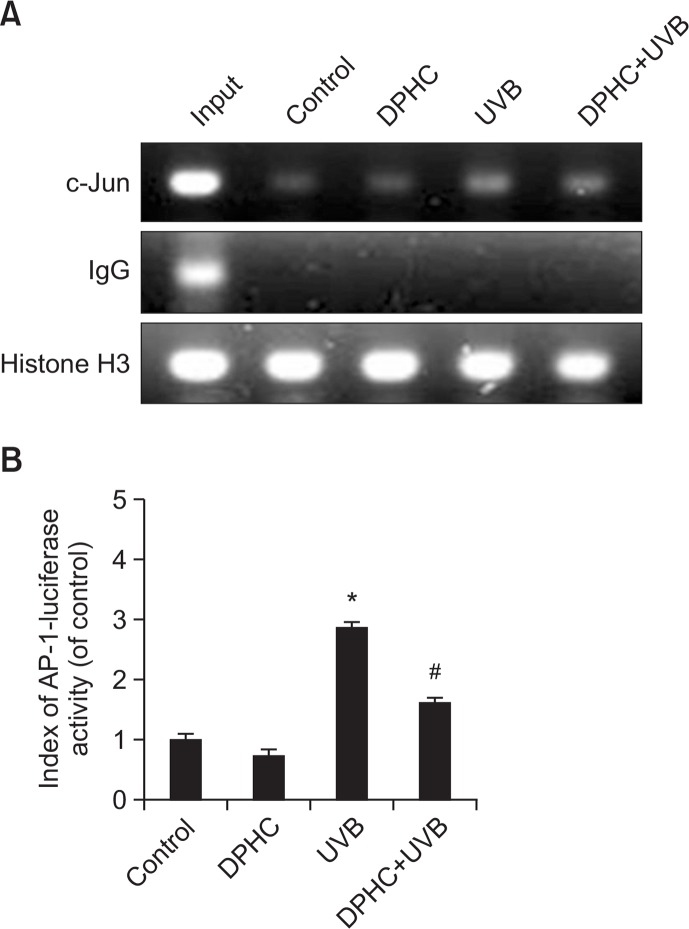
Next, we performed a ChIP assay to investigate the effect of DPHC on AP-1 transcriptional activity. The results revealed that AP-1 transcriptional activity was higher in UVB-irradiated cells than in controls (Fig. 4A). However, DPHC pretreatment significantly attenuated AP-1 transcriptional activity in UVB-exposed HaCaT cells. In addition, we assessed AP-1 transcriptional activity using the luciferase reporter assay. Consistent with the ChIP assay data, the reporter assay showed that UVB-induced elevation of AP-1 transcriptional activity was significantly downregulated by DPHC pretreatment. These investigations suggested that DPHC reduced UVB induction of MMP-1 by suppressing AP-1 transcriptional activity (Fig. 4B).
Fig. 4.
Effects of DPHC on AP-1 transcriptional activity. (A) Cellular extracts were prepared from HaCaT cells treated with 20 μM of DPHC for 1 h, and then irradiated with 150 mJ/cm2 UVB. After UVB irradiation, cells were incubated for 24 h, ChIP assay was performed to asses AP-1 binding to the MMP-1 promoter (B) Cells were transfected with plasmid containing the AP-1 binding site/luciferase construct. After transfection, cells were treated 20 μM of DPHC for 1 h, irradiated with 150 mJ/cm2 UVB dose, and incubated for 24 h. *Significantly different from control cells (p<0.05); #significantly different from UVB-irradiated cells (p<0.05).
DISCUSSION
Proteins of the MMP family are involved in the breakdown of extracellular matrix (ECM) during multiple normal physiological processes such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes such as arthritis and metastasis. In cancer metabolism, MMP-1 expression may be useful as a novel marker for hematogenous metastasis of colorectal cancer, and its inhibition may be a strategy for prevention of metastasis (Sunami et al., 2000). As the range of proteolytic targets of MMPs are more complex. MMP inhibitors (MMPIs) are potentially useful for blocking cancer progression, but at the same time could suppress normal tissue function or host defense processes. Therefore in the process of developing MMPIs it is necessary to focus on exact MMPs and their transcription, secretion of the zymogen into the extracellular matrix, and activation of the zymogen (Purcell et al., 2002). Moreover, MMP-1 causes UVB-mediated senescence and cancer (Westermarck and Kähäri, 1999; Sunami et al., 2000; Wenham et al., 2003; Yaar and Gilchrest, 2007). UVB-irradiated keratinocytes, which express elevated levels of IL-1alpha and IL-6, stimulate MMP-1 production by human fibroblasts, suggesting that UVB irradiation modulates MMP-1 production by both direct and indirect mechanisms (Fagot et al., 2002). Additionally, many reports have suggested that ROS causes senescence in the skin via activation of MMP-1 (Wenk et al., 1999; Brenneisen et al., 2002; Kang et al., 2008b). DPHC can alleviate oxidative stress both directly and indirectly (Heo et al., 2010; Heo et al., 2009; Heo and Jeon, 2009). In this study, we showed that DPHC could suppress MMP-1 expression resulting from UVB-induced cell damage and ROS generation (Fig. 2). Moreover, DPHC down-regulated UVB-induced MMP-1 expression and activation in human keratinocytes (Fig. 2). Similarly MMP-2 and MMP-9 are also important in cancer, which are proven to be expressed at higher concentrations at oxidative stress. Western blot and zymography analysis confirmed that UVB-induced over expression of gelatinases were abrogated by DPHC (Fig. 2).
Overall, oxidative conditions promote expression of MMP-1 via activation of the AP-1 transcription factor (Crawford and Matrisian, 1996; Wenk et al., 1999; Chen et al., 2004). AP-1 complexes bind to the promoter regions of the MMP genes and regulate MMP gene expression (Lin et al., 1993). c-Fos and c-Jun, the key components of AP-1, are primarily transcriptionally regulated by phosphorylated MEK/ERK and SEK/JNK, respectively (Kieser et al., 1997; Eliopoulos and Young, 1998; Favata et al., 1998; Wu et al., 2002). Further it has been proven that JNK inhibition prevents UV-induced c-Jun phosphorylation while ERK inhibition prevents UV-induced c-Fos expression in human dermal fibroblasts (Kim et al., 2005). Aligning with these findings our data revealed that DPHC suppressed UVB-induced phosphorylation of MEK/ERK and SEK/JNK (Fig. 3) and reduced UVB-induced AP-1 transcriptional activity in the MMP-1 promoter region (Fig. 4); together, these observations suggest a mechanism by which MMP-1 expression and activation are down-regulated by DPHC treatment.
In summary, the results of this study suggest that DPHC protects HaCaT cells against senescence-associated MMP-1 as well as MMP-2 and MMP-9 induced by UVB irradiation. In HaCaT cells treated with DPHC, activation of ERK and JNK by UVB radiation was dramatically decreased, resulting in lower DNA-binding activity of AP-1 and ultimately to reduced MMPs expression in response to UVB.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-C1ABA001-2011-0021037).
REFERENCES
- Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/S0945-053X(97)90026-3. [DOI] [PubMed] [Google Scholar]
- Berne B, Vahlquist A, Fischer T, Danielson BG, Berne C. UV treatment of uraemic pruritus reduces the vitamin A content of the skin. Eur J Clin Invest. 1984;14:203–206. doi: 10.1111/j.1365-2362.1984.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim. Biophys. Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic Biol Med. 1997;22:515–524. doi: 10.1016/S0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Cano E, Hazzalin CA, Mahadevan LC. Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and-2 are implicated in the induction of c-fos and c-jun. Mol Cell Biol. 1994;14:7352–7362. doi: 10.1128/MCB.14.11.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Chen J, Li D, Zhang X, Mehta JL. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone. Hypertension. 2004;44:655–661. doi: 10.1161/01.HYP.0000144400.49062.6b. [DOI] [PubMed] [Google Scholar]
- Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996;49:20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- Dasgupta J, Kar S, Liu R, Joseph J, Kalyanaraman B, Remington SJ, Chen C, Melendez JA. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. J Cell Physiol. 2010;225:52–62. doi: 10.1002/jcp.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Givant-Horwitz V, Lazarovici P, Risberg B, Nesland J, Trope CG, Schaefer E, Reich R. Matrix metalloproteinases (MMP), EMMPRIN (extracellular matrix metalloproteinase inducer) and mitogen-activated protein kinases (MAPK): co-expression in metastatic serous ovarian carcinoma. Clin. Exp. Metastasis. 2003;20:621–631. doi: 10.1023/A:1027347932543. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Eliopoulos AG, Young LS. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- Fagot D, Asselineau D, Bernerd F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch Dermatol Res. 2002;293:576–583. doi: 10.1007/s00403-001-0271-1. [DOI] [PubMed] [Google Scholar]
- Farrell PJ, Rowe DT, Rooney CM, Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989;8:127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Frost JA, Geppert TD, Cobb MH, Feramisco JR. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JN, Harrison SC. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature. 1995;373:257–261. doi: 10.1038/373257a0. [DOI] [PubMed] [Google Scholar]
- Goffin L, Seguin-Estévez Q, Alvarez M, Reith W, Chizzolini C. Transcriptional regulation of matrix metalloproteinase-1 and collagen 1A2 explains the anti-fibrotic effect exerted by proteasome inhibition in human dermal fibroblasts. Arthritis Res Ther. 2010;12:R73. doi: 10.1186/ar2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo SJ, Jeon YJ. Evaluation of diphlorethohydroxycarmalol isolated from Ishige okamurae for radical scavenging activity and its protective effect against H2O2-induced cell damage. Process Biochem. 2009;44:412–418. doi: 10.1016/j.procbio.2008.12.005. [DOI] [Google Scholar]
- Heo SJ, Hwang JY, Choi JI, Han JS, Kim HJ, Jeon YJ. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent [alpha]-glucosidase and [alpha]-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2009;615:252–256. doi: 10.1016/j.ejphar.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Heo SJ, Ko SC, Kang SM, Cha SH, Lee SH, Kang DH, Jung WK, Affan A, Oh C, Jeon YJ. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem Toxicol. 2010;48:1355–1361. doi: 10.1016/j.fct.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/S0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Jo WS, Yang KM, Park HS, Kim GY, Nam BH, Jeong MH, Choi YJ. Effect of microalgal extracts of tetraselmis suecica against UVB-induced photoaging in human skin fibroblasts. Toxicol Res. 2012;28:241–248. doi: 10.5487/TR.2012.28.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, Kim BJ, Park JW, Kim HS, Kim DH, Hyun JW. Protective effect of irisolidone, a metabolite of kakkalide, against hydrogen peroxide induced cell damage via antioxidant effect. Bioorg Med Chem. 2008a;16:1133–1141. doi: 10.1016/j.bmc.2007.10.085. [DOI] [PubMed] [Google Scholar]
- Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, Lee K, Kim BJ, Shin T, Park JW, Lee NH, Yoo BS, Hyun JW. Inhibitory effects of triphlorethol-A on MMP-1 induced by oxidative stress in human keratinocytes via ERK and AP-1 inhibition. J Toxicol Environ Health A. 2008b;71:992–999. doi: 10.1080/01932690801934653. [DOI] [PubMed] [Google Scholar]
- Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ryu HC, Kim JH. Low-dose UVB irradiation stimulates matrix metalloproteinase-1 expression via a BLT2-linked pathway in HaCaT cells. Exp Mol Med. 2010;42:833–841. doi: 10.3858/emm.2010.42.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Shin CM, Park CH, Kim KH, Cho KH, Eun HC, Chung JH. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J Lipid Res. 2005;46:1712–1720. doi: 10.1194/jlr.M500105-JLR200. [DOI] [PubMed] [Google Scholar]
- Lin CW, Georgescu HI, Evans CH. The role of AP-1 in matrix metalloproteinase gene expression. Agents Actions. 1993;39:215–218. doi: 10.1007/BF01972770. [DOI] [PubMed] [Google Scholar]
- Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim. Biophys. Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- Purcell WT, Rudek MA, Hidalgo M. Development of matrix metalloproteinase inhibitors in cancer therapy. Hematol Oncol Clin North Am. 2002;16:1189–1227. doi: 10.1016/S0889-8588(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Reel B, Oktay G, Ozkal S, Islekel H, Ozer E, Ozsarlak-Sozer G, Cavdar Z, Akhisaroglu ST, Kerry Z. MMP-2 and MMP-9 alteration in response to collaring in rabbits: the effects of endothelin receptor antagonism. J Cardiovasc Pharmacol Ther. 2009;14:292–301. doi: 10.1177/1074248409343690. [DOI] [PubMed] [Google Scholar]
- Ren SW, Li J, Wang W, Guan HS. Protective effects of kappa-ca3000+CP against ultraviolet-induced damage in HaCaT and MEF cells. J Photochem Photobiol B. 2010;101:22–30. doi: 10.1016/j.jphotobiol.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Sunami E, Tsuno N, Osada T, Saito S, Kitayama J, Tomozawa S, Tsuruo T, Shibata Y, Muto T, Nagawa H. MMP-1 is a prognostic marker for hematogenous metastasis of colorectal cancer. Oncologist. 2000;5:108–114. doi: 10.1634/theoncologist.5-2-108. [DOI] [PubMed] [Google Scholar]
- Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schutz A, Kovacic B, Friedrich K. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia. 2007;9:279–291. doi: 10.1593/neo.06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenham R, Calingaert B, Ali S, McClean K, Whitaker R, Bentley R, Lancaster J, Schildkraut J, Marks J, Berchuck A. Matrix metalloproteinase-1 gene promoter polymorphism and risk of ovarian cancer. J Soc Gynecol Investig. 2003;10:381–387. doi: 10.1016/S1071-5576(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Wenk J, Brenneisen P, Wlaschek M, Poswig A, Briviba K, Oberley TD, Scharffetter-Kochanek K. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloprotease-1. J Biol Chem. 1999;274:25869–25876. doi: 10.1074/jbc.274.36.25869. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Kähäri VM. Regulation of matrix metal-loproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- Wu HM, Wen HC, Lin WW. Proteasome inhibitors stimulate interleukin-8 expression via Ras and apoptosis signal-regulating kinase-dependent extracellular signal-related kinase and c-Jun N-terminal kinase activation. Am J Respir Cell Mol Biol. 2002;27:234–243. doi: 10.1165/ajrcmb.27.2.4792. [DOI] [PubMed] [Google Scholar]
- Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]