Abstract
Several theories emphasize that aging is closely related to oxidative stress and disease. The formation of excess ROS can lead to DNA damage and the acceleration of aging. Vigna angularis is one of the important medicinal plants in Korea. We isolated vitexin from V. angularis and elucidated the lifespan-extending effect of vitexin using the Caenorhabditis elegans model system. Vitexin showed potent lifespan extensive activity and it elevated the survival rates of nematodes against the stressful environments including heat and oxidative conditions. In addition, our results showed that vitexin was able to elevate antioxidant enzyme activities of worms and reduce intracellular ROS accumulation in a dose-dependent manner. These studies demonstrated that the increased stress tolerance of vitexin-mediated nematode could be attributed to increased expressions of stress resistance proteins such as superoxide dismutase (SOD-3) and heat shock protein (HSP-16.2). In this work, we also studied whether vitexin-mediated longevity activity was associated with aging-related factors such as progeny, food intake, growth and movement. The data revealed that these factors were not affected by vitexin treatment except movement. Vitexin treatment improved the body movement of aged nematode, suggesting vitexin affects healthspan as well as lifespan of nematode. These results suggest that vitexin might be a probable candidate which could extend the human lifespan.
Keywords: Vigna angularis, Vitexin, Caenorhabditis elegans, Lifespan extension, Stress tolerance
INTRODUCTION
Aging is an inevitable natural process accompanied by a progressive accumulation of damage in all constituent macromolecules such as nucleic acids, lipids and proteins (Chondrogianni et al., 2015). Although the determined mechanisms of aging process are not completely identified, increasing new evidences suggest that aging is considerably associated with reactive oxygen species (ROS) (Si and Liu, 2014). ROS including superoxide radical, hydrogen peroxide and hydroxyl free radical, cause oxidative damage to DNA and other macromolecules in the cell (Chen et al., 2013). Oxidative stress caused by ROS can lead to oxidation of biomolecules such as protein, DNA and biomembranes which is assumed to be the major cause factor of aging. There are so many previous works dealt with the correlation between antioxidants and aging (Kimoto-Kinoshita et al., 1999; Sinha et al., 2010; Gruber et al., 2013) Therefore, drugs with antioxidant properties can be promising candidates for the various aging-related diseases.
Caenorhabditis elegans is a small soil nematode which offers several advantages to study aging and the related pathways, because it has a rapid reproduction rate and a short life span, furthermore the major signaling pathways that regulate longevity and stress resistance in mammals are well conserved in the nematode (Feng et al., 2015; Su and Wink, 2015). And its genome is completely sequenced, displaying great homology to human (Chondrogianni et al., 2015). So, the worm C. elegans has become a popular model organism to explore the potential anti-aging and stress resistance properties of natural compounds due to its features of ease of maintenance, short life cycle, and the availability of various transgenic strains (Feng et al., 2015).
In the course of searching for compounds which are likely to prolong the life of human from plants by using the C. elegans model system, a methanolic extract of Vigna angularis (Leguminosae) was found to show significant longevity activity. This plant has been used as one of the traditional medicines for its diuretic and detoxification activities to treat edema, constipation and diabetes (Yao et al., 2011; Jiang et al., 2014). Previous phytochemical reports on this plant dealt with the several phenolic compounds, saponins and furanylmethyl glycosides (Ariga and Asao, 1981; Kitagawa et al., 1983; Jiang et al., 2014). Earlier pharmacological studies on this plan showed that it has various bioactivities such as anti-inflammatory, anti-oxidant, anti-hypotensive, hypoglycemic and hepatoprotective effects (Han et al., 2004; Itoh et al., 2009; Mukai and Sato, 2009; Yumiko et al., 2009; Jiang et al., 2014). Subsequent activity-guided chromatography of the methanolic extract of V. angularis led to the isolation of compound 1, vitexin (Fig. 1).
Fig. 1.
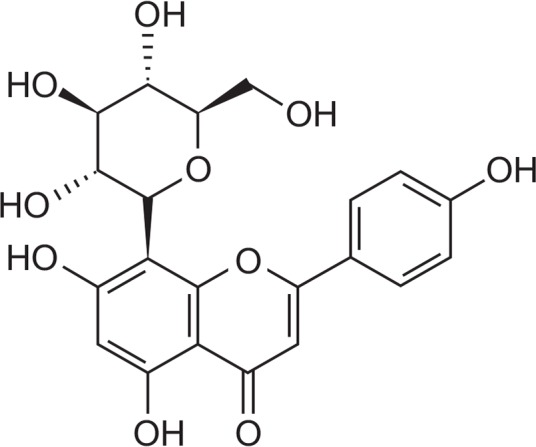
Structure of vitexin.
The aim of this work was to study the effect of vitexin on the lifespan and stress tolerance including thermal and oxidative stress in C. elegans. Moreover, antioxidant capacities of vitexin were analyzed by measuring intracellular ROS level and antioxidant enzyme activities of nematodes. Then, to investigate whether vitexin-mediated increased stress tolerance was due to the regulation of stress-response genes, we quantified SOD-3 and HSP-16.2 expressions using transgenic strains including CF1553 and CL2070, respectively. It was also checked up whether vitexin could give effects on aging parameters such as reproduction, pharyngeal pumping, body length, and movement.
MATERIALS AND METHODS
General
NMR spectra were determined on a JEOL JMN-EX 400 spectrometer (Tokyo, Japan). Sephadex LH-20 was used for column chromatography (GE Healthcare, Uppsala, Sweden). The absorbance was determined using microplate reader (ELISA, Sunrise, Grödig, Austria). TLC was carried out on Merck (Darmstadt, Germany) precoated silica gel F254 plates and silica gel for column chromatography was Kiesel gel 60 (230–400 mesh, Merck, Darmstadt, Germany). Spots were detected under UV and by spraying with 10% H2SO4 in ethanol followed by heating for 3 min. Selected peptone and yeast extracts were obtained from BD bioscience (Sparks, USA). Agar, 2′,7′-dichlorodihydrofluoroscein diacetate, juglone, catalase, xanthine, xanthine oxidase, and nitroblue tetrazolium were purchased from Sigma (St. Louis, USA).
Plant materials, extraction and isolation
The seeds of V. angularis were obtained from National Institute of Crop Science, Gyeongsangbuk-do of Korea in 2013. A voucher specimen was deposited in the herbarium of the College of Pharmacy, Woosuk University (WSU-13-008). The seeds (720 g) were extracted three times with methanol at 50°C, and then the extracts were combined and evaporated in vacuo at 50°C. The methanolic extract (84 g) was successively fractionated as n-hexane (650 mg), methylene chloride (11.27 g), ethyl acetate (1.2 g), n-butanol (14.49 g) and H2O soluble fractions. Ethyl acetate soluble fraction showed the most potent lifespan extension activity in V. angularis (data are not shown). Sephadex LH-20 column of ethyl acetate soluble extract gave five fractions (EA1–EA5) using methanol as a mobile phase. Fraction EA3 (120 mg) was chromatographed by RP Lobar-A column (MeOH-H2O, 40:60) to give five subfractions (EA31–EA33). Subfraction EA31 (71 mg) was chromatographed by silica gel column (CHCl3-MeOH-H2O, 35:10:1) and purified by Sephadex LH-20 column (MeOH) to give compound 1 (31.1 mg).
Vitexin (1)
1H-NMR (400 MHz, DMSO-d6) δ : 8.02 (2H, d, J=8.1 Hz, H-2′, 6′), 6.89 (2H, d, J=8.1 Hz, H-3′, 5′), 6.77 (1H, s, H-3), 6.26 (1H, s, H-6), 4.70 (1H, d, J=9.4 Hz, H-1″). 13C-NMR (100 MHz, DMSO-d6) δ : 181.8 (C-4), 163.9 (C-2), 163.1 (C-7), 161.0 (C-4′), 160.2 (C-5), 155.9 (C-9), 128.7 (C-2′, 6′), 121.6 (C-1′), 115.8 (C-3′, 5′), 104.5 (C-8), 103.5 (C-10), 102.4 (C-3), 98.3 (C-6), 81.8 (C-5″), 78.6 (C-3″), 73.6 (C-1″), 70.9 (C-2″), 70.4 (C-4″), 61.2 (C-6″). Structure characterization of compound 1 was carried out by interpretation of their spectral data comparison with the data reported in the literature.
C. elegans strains and maintenance
Bristol N2 (wild-type) and Escherichia coli OP50 were kindly provided by Dr. Myon-Hee Lee (East Carolina University, NC, USA). The worms were grown at 20°C on nematode growth medium (NGM) agar plate with E. coli OP50 as described previously (Brenner. 1974). To prepare plates supplemented with compound 1, the stock solution in DMSO was inserted into autoclaved NGM plates (at 50°C). A final DMSO concentration of 0.2% (v/v) was maintained under all conditions.
Lifespan assay
The lifespan assays were performed using wild-type at least 3 times independently at 20°C. To obtain age-synchronized nematodes, eggs were transferred to NGM plate in the absence or presence of sample after embryo isolation. Test worms were considered dead when they failed to respond to prodding with the tip of a platinum wire (Lithgow et al. 1995). The worms were transferred to fresh NGM plate every 2 days.
Assessment of stress resistance
The age-synchronized N2 worms were bred on NGM agar plates with or without various concentrations of sample. For the heat tolerance assay the adult day 4 worms were transferred to fresh plates and then incubated at 36°C. The survival rate was scored over 20 h as previously described (Lee et al. 2005). Oxidative stress tolerance was assessed as described previously with minor modification (Mekheimer et al. 2012). In brief, the adult day 7 worms were subjected to plate containing 1 mM of juglone liquid culture and then survivals were recorded over 16 h.
Measurement of antioxidant enzyme activities
To assess enzymatic activity, the worm homogenates were prepared. Briefly, the wild-type worms were harvested from plate with M9 buffer on the adult day 5 and washed 3 times. Then, the collected worms were suspended in homogenization buffer (10 mM Tris-HCl, 150 mM NaCl, 0.1 mM EDTA, pH 7.5) and homogenized on ice. SOD activity was measured spectrophotometrically analyzing the decolorization of formazan using enzymatic reaction between xanthine and xanthine oxidase. The reaction mixture contained 5 μL of worm homogenates and 120 μL of 1.6 mM xanthine, 0.48 mM nitrobluetetrazolium (NBT) in 10 mM phosphate buffer (pH 8.0). After pre-incubation at room temperature for 5 minutes, the reaction was initiated by adding 100 μL of xanthine oxidase (0.05 U/ml) and incubation at 37°C for 20 min. The reaction was stopped by adding 275 μL of 69 mM SDS, and the absorbance at 570 nm was measured. Catalase activity was calculated by spectrophotometry as previously described (Aebi, 1984). Briefly, the prepared homogenates were mixed with the 25 mM H2O2 and after 5 min incubation, absorbance was determined at 240 nm. Catalase activity was expressed in U/mg protein (1 unit will decompose 1.0 μM of H2O2 per min at pH 7.0 at 25°C). The enzymes activities were expressed as a percentage of the scavenged amount per control.
Analysis of intracellular ROS
Intracellular ROS in the nematodes was measured using molecular probe 2′,7′-dichlorodihydrofluoroscein diacetate (H2DCF-DA). Equal number of wild-type worms was incubated in the absence or presence of sample. On the 4th day of adulthood, animals were exposed to 96-well plate containing 50 μM juglone liquid culture for 2 h. Subsequently, 4 worms were transferred into the wells of a 96-well plate containing 50 μL of M9 buffer. Immediately after addition of 50 μL of 25 μM H2DCF-DA solution resulting in a final concentration 12.5 μM, basal fluorescence was quantified in a microplate fluorescence reader at excitation 485 nm and emission 535 nm.
Fluorescence microscopy and visualization
The age-synchronized transgenic nematodes including CF1553 containing a SOD-3::GFP reporter and CL2070 containing HSP-16.2::GFP reporter were maintained in the presence or absence of sample. Prior to microscopy observation, CL2070 mutants were received heat shock at 36°C for 2 h and allowed to recover at 20°C for 2 h. On the 3rd days of adulthood, both transgenic worms were anesthetized with sodium azide (2%) and mounted on 2% agarose pad. The GFP fluorescence of GFP-expressing populations was directly observed under a fluorescence microscope (Nikon Eclipse Ni-u, Japan). To determine the protein expression levels, photographs of the transgenic worms were taken and assayed using Image J software. All experiments were done in triplicate.
Measurement of aging-related factors and locomotion
The age-synchronized N2 worms were bred on NGM agar plates with or without sample. On the 4th day of adulthood, single worms were transferred to fresh plate followed by pharynx contractions and body movements of animals were counted under an inverted microscope for 1 min. For the growth alteration assay, photographs were taken of adult day 4 worms, and the body length of each animal was analyzed by the Nikon software (Nikon, Japan). Reproduction assay was conducted as follows. N2 worms were raised from embryo as in the lifespan assay. L4 larvae were individually transferred to the fresh plate every day to distinguish the parent from the progeny. The progeny was counted at the L2 or L3 stage. On the 7th day of adulthood, single worms were transferred to fresh plate followed by body movements were recorded under an inverted microscope for 10 seconds. The body movements of animals were analyzed by Nikon image software. All tests was completed in triplicate.
Data analysis
The data from the lifespan assay and stress resistance assays were plotted using Kaplan-Meier analysis and statistical significance was analyzed by log-rank test. Other data were presented as mean ± or standard error of the mean, as indicated. Statistical significance of differences between the control and treated groups were analyzed by one-way analysis of variance (ANOVA).
RESULTS
Effects of vitexin on the lifespan of C. elegans
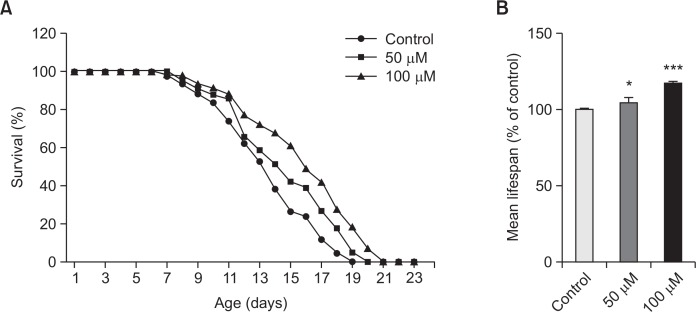
In order to determine the lifespan extension properties of vitexin, lifespan assays were performed with wild-type N2 worms. We found a concentration-dependent effect of vitexin on longevity (Fig. 2A). In addition, there was a significant increase (17.2% at 100 μM of vitexin, p<0.001) in the estimated mean life of vitexin-treated worms compared to control worms (Fig. 2B, Table 1). The mean life duration was 13.5±0.5 days for control worms, and the mean life duration of vitexin for the worms fed at 100 μM were 15.8±0.5 days.
Fig. 2.
Effects of vitexin on the lifespan of wild-type N2 nematodes. Worms were grown in the NGM agar plate at 20°C in the absence or presence of vitexin. The number of worms used per each lifespan assay experiment was 41–43 and three independent experiments were repeated (N=3). (A) The mortality of each group was determined by daily counting of surviving and dead animals. (B) The mean lifespan of the N2 worms was calculated from the survival curves. Statistical difference between the curves was analyzed by log-rank test. Error bars represent the standard error of mean (S.E.M.). Differences compared to the control were considered significant at *p<0.05 and ***p<0.001 by one-way ANOVA.
Table 1.
Effects of vitexin on the lifespan of C. elegans
| Treatment | Mean Lifespan (day) | Maximum lifespan (day) | Change in mean lifespan (%) | Log-rank test |
|---|---|---|---|---|
| Control | 13.5 ± 0.5 | 19 | - | - |
| 50 μM | 14.6 ± 0.4 | 20 | 8.0 | p<0.05* |
| 100 μ M | 15.8 ± 0.5 | 21 | 17.2 | p<0.001*** |
Mean lifespan presented as mean ± S.E.M data. Change in mean lifespan compared with control group (%). Statistical significance of the difference between survival curves was determined by log-rank test using the Kaplan-Meier survival analysis. Differences compared to the control were considered significant at *p<0.05 and***p<0.001.
Effects of vitexin on the stress tolerance of C. elegans
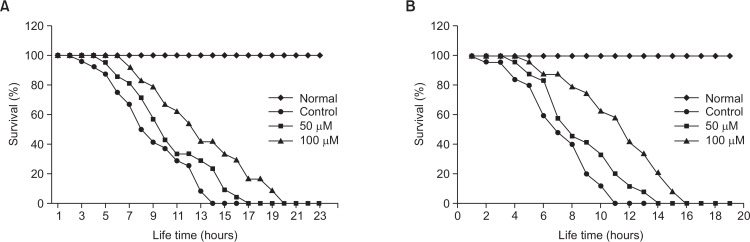
We determined the effect of vitexin on two different kinds of stress conditions including thermal and oxidative stress using wild-type N2 worms. As shown in Fig. 3, we could observe that vitexin exposure induced significant increases in thermo-tolerance, and consequently elevated survival rate of worms. Further, vitexin exposure extended the maximum lifespan of worms by 45.8% (100 μM, p<0.001, Fig. 3A, Table 2). Moreover, it was found that vitexin-treated N2 worms lived longer than control worms under oxidative stress conditions induced by 1 mM juglone in a concentration-dependent manner (100 μM, p<0.001, Fig. 3B, Table 2).
Fig. 3.
Effects of vitexin on the stress tolerance of wild-type N2 nematodes. (A) To assess thermal tolerance, worms were incubated at 36°C and then their viability was scored. (B) For the oxidative stress assays, worms were transferred to 96-well plate containing 1 mM of juglone liquid culture, and then their viability was scored. Statistical difference between the curves was analyzed by log-rank test. All experiments were done in triplicates.
Table 2.
Effects of vitexin on the stress tolerance of C. elegans
| Stress condition | Treatment | Mean lifespan (h) | Maximum lifespan (h) | Change in mean lifespan (%) | Log-rank test |
|---|---|---|---|---|---|
| 36°C thermal tolerance | Control | 9.0 ± 0.6 | 15 | - | - |
| 50 μM | 10.6 ± 0.7 | 17 | 17.4 | p=0.063 | |
| 100 μM | 13.2 ± 0.8 | 20 | 45.8 | p<0.001*** | |
| 1 mM Juglone | Control | 7.3 ± 0.4 | 11 | - | - |
| 50 μM | 8.8 ± 0.5 | 14 | 20.5 | p<0.05* | |
| 100 μM | 11.5 ± 0.6 | 16 | 56.2 | p<0.001*** |
Mean lifespan presented as mean ± S.E.M data. Change in mean lifespan compared with control group (%). Statistical significance of the difference between survival curves was determined by log-rank test using the Kaplan-Meier survival analysis. Differences compared to the control were considered significant at * p<0.05 and ***p<0.001.
Effects of vitexin on the antioxidant enzyme activities and intracellular ROS levels
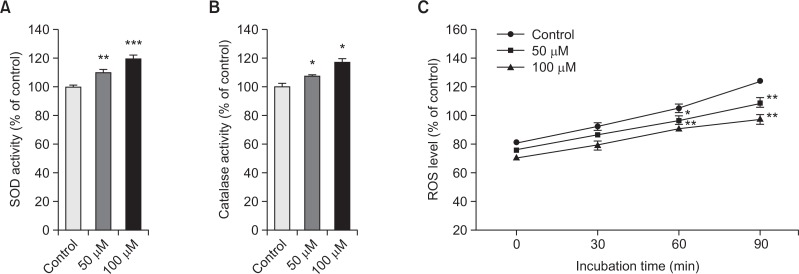
To verify the possible mechanism of vitexin-mediated lifespan extension and elevated stress resistance of nematodes, activities of stress resistance proteins were investigated. The superoxide dismutase (SOD) and catalase enzymatic activities were measured spectrophotometricaly using prepared worm homogenates. Our results showed that vitexin was able to elevate SOD and catalase activities of worms significantly (p<0.05 and p<0.001; Fig. 4A, 4B). We further quantified intracellular ROS levels of vitexin-treated worms compared with untreated controls. Fig. 4C shows that vitexin-fed worms effectively reduced the production of ROS by 14.6% (100 μM, p<0.01) compared with solvent-treated control worms.
Fig. 4.
Effects of vitexin on the stress resistance proteins of wild type N2 nematodes. (A) The enzymatic reaction of xanthine with xanthine oxidase was used to generate O2•− and the SOD activity was estimated spectrophotometrically through formazan formation by NBT reduction. SOD activity was expressed as a percentage of the scavenged amount per control. (B) Catalase activity was calculated from the concentration of residual H2O2, as determined by a spectrophotometric method. Catalase activity was expressed in U/mg protein. (C) Intracellular ROS accumulation was quantified spectrometrically at excitation 485 nm and emission 535 nm. Plates were read every 30 min for 2 h. Data are expressed as the mean ± S.E.M. of three independent experiments (N=3). Differences compared to the control were considered significant at *p<0.05, **p<0.01 and ***p<0.001 by one-way ANOVA.
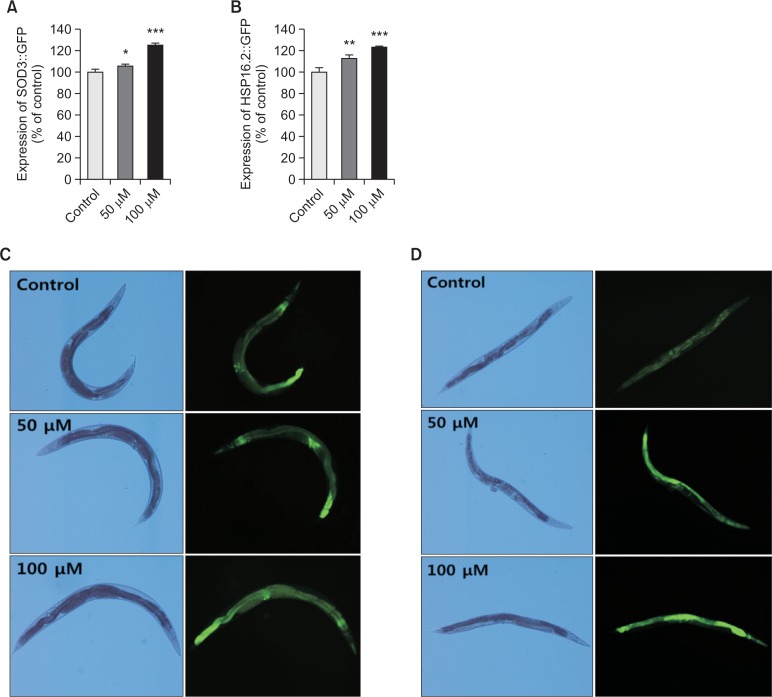
Effects of vitexin on the SOD-3 and HSP-16.2 expressions in transgenic nematodes
To investigate whether vitexin-mediated increased stress tolerance was due to regulation of stress-response genes, we quantified SOD-3 and HSP-16.2 expressions using transgenic strains including CF1553 and CL2070, respectively. Our data shows that vitexin-treated CF1553 worms exhibited significantly higher SOD-3::GFP intensity (25.4% at 100 μM, p<0.001), compared with untreated control worms (Fig. 5A, 5C). The CL2070 worms containing HSP-16.2::GFP reporter gene were received heat shock at 36°C for 2 h and allowed to recover at 20°C for 2 h, followed by quantifying fluorescence intensity. This heat shock-induced HSP-16.2::GFP expression was further up-regulated by 100 μM of vitexin about 23.5% (p<0.001, Fig. 5B, 5D).
Fig. 5.
Effects of vitexin on the expression of SOD-3 and HSP-16.2 was determined using transgenic nematodes. Mean GFP intensity of CF1553 (A) and CL2070 (B) mutants were represented as mean ± S.E.M. of values from 18 to 26 animals per each experiment. The GFP intensity was quantified using Image software by determining average pixel intensity. Images of SOD-3::GFP (C) and HSP-16.2::GFP (D) expressions of CF1553 worms in the presence or absence of vitexin. Data are expressed as the mean ± standard deviation of three independent experiments (N=3). Differences compared to the control were considered significant at *p<0.05, **p<0.01 and ***p<0.001 by one-way ANOVA.
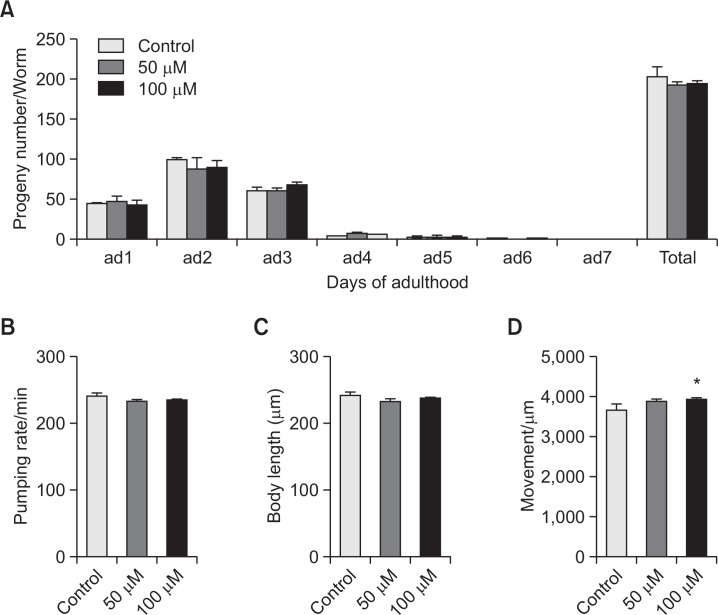
Effects of vitexin on the aging-related factors of C. elegans
In order to verify the possible mechanism of vitexin on the lifespan of nematodes, we observed vitexin-induced change in parameters of aging-related factors such as progeny, pharyngeal pumping, and body length. We did not find any significant statistical changes between vitexin-fed worms and control worms on the reproduction rate, food intake, and body length (Fig. 6A, 6B, 6C). These results demonstrate that the alteration of those aging-related factors is not responsible for vitexin-mediated lifespan extension in C. elegans.
Fig. 6.
Effects of vitexin on the various aging-related factors of wild-type N2 nematodes. (A) Daily and total reproductive outputs were counted. The progeny was counted at the L2 or L3 stage. (B) On the 4th days of adulthood, the pharyngeal pumping rates were measured. (C) For the growth alteration assay, photographs were taken of worms and the body length of each animal was analyzed. (D) The body movements were counted under a dissecting microscope for 10 seconds. Data are expressed as the mean ± S.E.M. of three independent experiments (N=3). Differences compared to the control were considered significant at *p<0.05 by one-way ANOVA.
Effects of vitexin on the locomotory activities in C. elegans
To estimate the healthspan of worms, we measured the body movement of nematodes after vitexin treatment. As can be seen in Fig. 6D, vitexin exposure induced slightly increase in body movement of worms. Data shows that vitexin enhanced the travel range of worms per 10 seconds about 7.5% at 100 μM (p<0.05, Fig. 6D).
DISCUSSION
The average human lifespan has increased with the development of economy and medicine. Naturally, people are interested in lifespan extension. In this work, we isolated vitexin from the seed of V. angularis and elucidated its longevity activity in C. elegans. Vitexin was reported by the isolation from several plants (Quercia et al., 1978, Palme et al., 1994, Khole et al., 2014). Several early studies have revealed that vitexin has been shown to various pharmacological properties including antiviral (Li et al., 2002), anti-depressant-like (Can et al., 2013), α-glucosidase inhibitory (Choo et al., 2012), antinociceptive (Özkay and Can, 2013) and anti-diabetic activities (Choi et al., 2014).
To verify the effect of vitexin on the longevity, we carried out lifespan assay with wild-type N2 worm, C. elegans under normal culture condition. The C. elegans model system offers various useful methods for aging-related research because it has diverse excellences such as short lifespan, ease of handling, rapid generation and a large number of mutant strains (Guarente and Kenyon, 2000; Chondrogianni et al., 2015).
We found that vitexin treatment considerably enhanced the lifespan of nematode in a concentration-dependent manner. In addition, vitexin considerably increased the survival rate of N2 worms under both of heat stress and juglone-induced oxidative stress conditions. Since there is a clear correlation between lifespan-extension and the stress tolerance (Kenyon, 2010), resistant ability against stress condition may positively affect vitexin-mediated prolonged lifespan. In this study, vitexin-treated worms exhibited significant increase in survival rate under thermal stress condition, compared to control worms, suggesting that the vitexin-treatment enhanced thermo-tolerance. Furthermore, the result of juglone-induced oxidative stress assay showed that vitexin-treated worms survived longer than the control as well. These results indicate that the lifespan extending ability of vitexin is quite possibly associated with increased stress tolerance. Heat shock proteins (HSPs) are expressed under heat stress condition (Swindell, 2009), so we tried to reveal possible involvement of HSP-16.2 in the vitexin-mediated stress resistance. The HSP-16.2 family of C. elegans is expressed under thermal stress conditions, so they can role as stress-sensitive reporters to assess longevity (Strayer et al., 2003). In this study, GFP-fused transgenic strain CL2070 was used to measure HSP-16.2 expression level. We found that HSP-16.2 expression induced by heat shock was significantly elevated in the worms treated with vitexin, compared with control worms, suggesting the vitexin-mediated longevity and enhanced stress resistance against thermal stress of vitexin may be explained by this property.
The ROS accumulation is largely regulated by a complicated antioxidant defense system such as SOD, catalase and glutathione peroxidase (Finkel and Holbrook, 2000). To know how vitexin controls oxidative stress, we analyzed the antioxidant enzymes such as SOD and catalase activities using nematode homogenates. Our results showed that both enzyme activities were significantly up-regulated by vitexin, suggesting attenuation of hydroxyl radical levels resulted in diminished oxidative stress. As mentioned above, vitexin treatment provided lifespan extension under oxidative stress condition induced by juglone. It was also found that SOD activity was considerably up-regulated in the vitexin-treated worms compared with the control worms. To verify whether this enhanced enzyme activity was due to direct scavenging of ROS or altered protein expression, further quantification of SOD expression was performed using GFP-fused transgenic strain CF1553. The results exhibited that vitexin-fed worms showed higher GFP intensity compared with the control worms, indicated that vitexin treatment increased SOD expression. In addition, intracellular ROS levels were also considerably reduced by vitexin treatment. These results suggest that antioxidant properties of vitexin might be partially attributed to extended lifespan and increased survival rate of worms under stress conditions.
Reductions in aging-related factors like reproduction, food intake and body size are closely related to longevity (Mörck and Pilon, 2006; Surco-Laos et al., 2012), we further investigated whether vitexin affects aging-related factors. The results revealed that there were no significant variation in the number of progeny, body length and pharyngeal pumping between vitexin-fed worms and control worms. These results present evidence that vitexin extends lifespan in C. elegans independent of altering aging-related factors. Furthermore, to know vitexin’s influence on the functional aging, locomotion assay was conducted. Vitexin slightly up-regulated the body movement of N2 worms suggesting that vitexin might provide a beneficial effects on healthspan to some extent as well as lifespan.
Several flavonoid compounds like quercetin, kaempferol, naringenin and myricetin were reported their lifespan extending properties in C. elegans (Grünz et al., 2012). As a flavone C-glycoside, vitexin has been isolated from several medicinal and other plants. Plant extracts containing vitexin were reported to possess antioxidant, anti-inflammatory and antinociceptive activities (Borqhi et al., 2013). Previous study also revealed that vitexin has several aging-related effects such as antioxidant, antitumor and inflammatory pain inhibitory activities as well as protecting effect against ischemia/reperfusion injury via modulating mitogen-activated protein kinase and apoptosis signaling in mice (Kim et al., 2005).
In conclusion, in this work, vitexin showed lifespan extension of C. elegans through its antioxidant potential and regulating stress resistance protein. Therefore, the seed of V. angularis and vitexin could be useful for longevity in human, but yet, since these are the preliminary data, the question about if vitexin provide positive or negative effect against aging in human is still unknown and it should be studied furthermore.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ0092182015)” Rural Development Administration, Republic of Korea.
REFERENCES
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ariga T, Asao Y. Isolation, identification and organoleptic astringency of dimeric proanthocyanidins occurring in Azuki beans. Agric Biol Chem. 1981;45:2709–2712. doi: 10.1271/bbb1961.45.2709. [DOI] [Google Scholar]
- Borghi SM, Carvalho TT, Staurengo-Ferrari L, Hohmann MS, Pinge-Filho P, Casagrande R, Verri WA., Jr Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J Nat Prod. 2013;76:1141–1149. doi: 10.1021/np400222v. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can ÖD, Özkay ÜD, Üçel Uİ. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur J Pharmacol. 2013;699:250–257. doi: 10.1016/j.ejphar.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Chen W, Sudji IR, Wang E, Joubert E, van Wyk BE, Wink M. Ameliorative effect of aspalathin from rooibos (Aspalathus linearis) on acute oxidative stress in Caenorhabditis elegans. Phytomedicine. 2013;20:380–386. doi: 10.1016/j.phymed.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Choi JS, Islam Md N, Ali Md Y, Kim Eon J, Kim MY, Jung HA. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem Toxicol. 2014;64:27–33. doi: 10.1016/j.fct.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Chondrogianni N, Voutetakis K, Kapetanou M, Delitsikou V, Papaevgeniou N, Sakellari M, Lefaki M, Filippopoulou K, Gonos ES. Proteasome activation: An innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res Rev. 2015;23:37–55. doi: 10.1016/j.arr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Choo CY, Sulong NY, Man F, Wong TW. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J Ethnopharmacol. 2012;142:776–781. doi: 10.1016/j.jep.2012.05.062. [DOI] [PubMed] [Google Scholar]
- Feng S, Cheng H, Xu Z, Shen S, Yuan M, Liu J, Ding C. Thermal stress resistance and aging effects of Panax notoginseng polysaccharides on Caenorhabditis elegans. Int J Biol Macromol. 2015;81:188–194. doi: 10.1016/j.ijbiomac.2015.07.057. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Gruber J, Fong S, Chen C-B, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell Barry. Mitochondria-targeted antioxidants and metabolic modulators as harmacological interventions to slow ageing. Biotech Adv. 2013;31:563–592. doi: 10.1016/j.biotechadv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Grünz G, Haas K, Soukup S, Klingenspor M, Kulling SE, Daniel H, Spanier B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech Ageing Dev. 2012;133:1–10. doi: 10.1016/j.mad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–62. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Han KH, Fukushima M, Ohba K, Shimada K, Sekikawa M, Chiji H, Lee CH, Nakano M. Hepatoprotective effects of the water extract from adzuki bean hulls on acetaminophen-induced damage in rat liver. J Nutr Sci Vitaminol. 2004;50:380–383. doi: 10.3177/jnsv.50.380. [DOI] [PubMed] [Google Scholar]
- Itoh T, Kobayashi M, Horio F, Furuichi Y. Hypoglycemic effect of hot-water extract of adzuki (Vigna angularis) in spontaneously diabetic KK-A(y) mice. Nutrition. 2009;25:134–141. doi: 10.1016/j.nut.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zeng KW, David B, Massiot G. Constituents of Vigna angularis and their in vitro anti-inflammatory activity. Phytochemistry. 2014;107:111–118. doi: 10.1016/j.phytochem.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Khole S, Chatterjee S, Variyar P, Sharma A, Devasagayam TPA, Ghaskadbi S. Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J. Funct. Foods. 2014;6:270–279. doi: 10.1016/j.jff.2013.10.016. [DOI] [Google Scholar]
- Kim JH, Lee BC, Kim JH, Sim GS, Lee DH, Lee KE, Yun YP, Pyo HB. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch Pharm Res. 2005;28:195–202. doi: 10.1007/BF02977715. [DOI] [PubMed] [Google Scholar]
- Kimoto-Kinoshita S, Nishida S, Tomura TT. Age-related change of antioxidant capacities in the cerebral cortex and hippocampus of stroke-prone spontaneously hypertensive rats. Neurosci Lett. 1999;273:41–44. doi: 10.1016/S0304-3940(99)00623-0. [DOI] [PubMed] [Google Scholar]
- Kitagawa I, Wang HK, Saito M, Yoshikawa M. Saponin and sapogenol. XXXI. Chemical constituents of the seeds of Vigna angularis (Willd.) Ohwi et Ohashi, (1) Triterpenoidal sapogenols and 3-furanmethanol β-D-glucopyranoside. Chem Pharm Bull. 1983;31:664–673. doi: 10.1248/cpb.31.664. [DOI] [Google Scholar]
- Lee EY, Shim YH, Chitwood DJ, Hwang SB, Lee J, Paik YK. Cholesterol-producing transgenic Caenorhabditis elegans lives longer due to newly acquired enhanced stress resistance. Biochem Biophys Res Commun. 2005;328:929–936. doi: 10.1016/j.bbrc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Li YL, Ma SC, Yang YT, Ye SM, But PPH. Antiviral activities of flavonoids and organic acid from Trollius chinensis Bunge. J Ethnopharmacol. 2002;79:365–368. doi: 10.1016/S0378-8741(01)00410-X. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekheimer RA, Sayed AA, Ahmed EA. Novel 1,2,4-triazolo[1,5-a]pyridines and their fused ring systems attenuate oxidative stress and prolong lifespan of Caenorhabiditis elegans. J Med Chem. 2012;55:4169–4177. doi: 10.1021/jm2014315. [DOI] [PubMed] [Google Scholar]
- Mörck C, Pilon M. C. elegans feeding defective mutants have shorter body lengths and increased autophagy. BMC Dev Biol. 2006;6:39. doi: 10.1186/1471-213X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y, Sato S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr Metab Cardiovasc Dis. 2009;19:491–497. doi: 10.1016/j.numecd.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Özkay ÜD, Can ÖD. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharmacol Biochem Behav. 2013;109:23–30. doi: 10.1016/j.pbb.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Palme E, Bilia AR, De Feo V, Morelli I. Flavonoid glycosides from Cotoneaster thymaefolia. Phytochemistry. 1994;35:1381–1382. doi: 10.1016/S0031-9422(06)80133-0. [DOI] [Google Scholar]
- Quercia V, Turchetto L, Pierini N, Cuozzo V, Percaccio G. Identification and determination of vitexin and isovitexin in Passiflora incarnata extracts. J. Chromatogr. A. 1978;161:396–402. doi: 10.1016/S0021-9673(01)85261-4. [DOI] [Google Scholar]
- Si H, Liu DJ. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J Nutr Biochem. 2014;25:581–591. doi: 10.1016/j.jnutbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Saha A, Basu S, Pal K, Chakrabarti S. Aging and antioxidants modulate rat brain levels of homocysteine and dehydroepiandrosterone sulphate (DHEA-S): Implications in the pathogenesis of Alzheimer’s disease. Neurosci Lett. 2010;483:123–126. doi: 10.1016/j.neulet.2010.07.075. [DOI] [PubMed] [Google Scholar]
- Strayer A, Wu Z, Christen Y, Link CD, Luo Y. Expression of the small heat-shock protein Hsp16-2 in Caenorhabditis elegans is suppressed by Ginkgo biloba extract EGb 761. FASEB J. 2003;17:2305–2307. doi: 10.1096/fj.03-0376fje. [DOI] [PubMed] [Google Scholar]
- Su S, Wink M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry. 2015;117:340–350. doi: 10.1016/j.phytochem.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Surco-Laos F, Dueñas M, González-Manzano S, Cabello J, Santos-Buelga C, González-Paramás AM. Influence of catechins and their methylated metabolites on lifespan and resistance to oxidative and thermal stress of Caenorhabditis elegans and epicatechin uptake. Food Res Int. 2012;46:514–521. doi: 10.1016/j.foodres.2011.10.014. [DOI] [Google Scholar]
- Swindell WR. Heat shock proteins in long-lived worms and mice with insulin/insulin-like signaling mutations. Aging (Albany NY) 2009;1:573–577. doi: 10.18632/aging.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Cheng X, Wang L, Wang S, Ren G. A determination of potential alpha-glucosidase inhibitors from Azuki Beans (Vigna angularis) Int J Mol Sci. 2011;12:6445–6451. doi: 10.3390/ijms12106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumiko H, Tomomi M, Motonori F, Kazuo T, Yoshiteru I. Constituents and anti-oxidative activity of a hot-water extract of Adzuki (Vigna angularis) beans. J Jpn Soc Nutr Food Sci. 2009;62:3–11. doi: 10.4327/jsnfs.62.3. [DOI] [Google Scholar]