Abstract
The emergence and use of synthetic cannabinoids have greatly increased in recent years. These substances are easily dispensed over the internet and on the streets. Some synthetic cannabinoids were shown to have abuse liability and were subsequently regulated by authorities. However, there are compounds that are still not regulated probably due to the lack of abuse liability studies. In the present study, we assessed the abuse liability of three synthetic cannabinoids, namely JWH-030, JWH-175, and JWH-176. The abuse liability of these drugs was evaluated in two of the most widely used animal models for assessing the abuse potential of drugs, the conditioned place preference (CPP) and self-administration (SA) test. In addition, the open-field test was utilized to assess the effects of repeated (7 days) treatment and abrupt cessation of these drugs on the psychomotor activity of animals. Results showed that JWH-175 (0.5 mg/kg), but not JWH-030 or JWH-176 at any dose, significantly decreased the locomotor activity of mice. This alteration in locomotor activity was only evident during acute exposure to the drug and was not observed during repeated treatment and abstinence. Similarly, only JWH-175 (0.1 mg/kg) produced significant CPP in rats. On the other hand, none of the drugs tested was self-administered by rats. Taken together, the present results indicate that JWH-175, but not JWH-030 and JWH-176, may have abuse potential. More importantly, our findings indicate the complex psychopharmacological effects of synthetic cannabinoids and the need to closely monitor the production, dispensation, and use of these substances.
Keywords: Cannabis, Synthetic cannabinoids, JWH, Abuse, Self-administration, Conditioned place preference
INTRODUCTION
Cannabis is one of the most widely-used recreational drugs in the world because of its ability to induce relaxing and euphoric effects (US Department of Health and Human Services. 2011, Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. Substance Abuse and Mental Health Services Administration: Rockville, MD, USA). The psychological effects of cannabis are mainly attributed to the actions of its principal psychoactive component Δ-9 tetrahydrocannabinol (THC) on the cannabinoid receptors in the brain (Golovko, 2011). Since the discovery of THC, hundreds of novel analogs have been synthesized and used as therapeutic agents or as tools to enhance understanding of the endocannabinoid system (Tai and Fantegrossi, 2014). Unfortunately, in recent years, these synthetic cannabinoids have also been used as recreational drugs of abuse (Auwärter et al., 2009). This occurrence can be attributed to the drug’s ability to produce psychoactive effects similar to that induced by cannabis (Gurney et al., 2014).
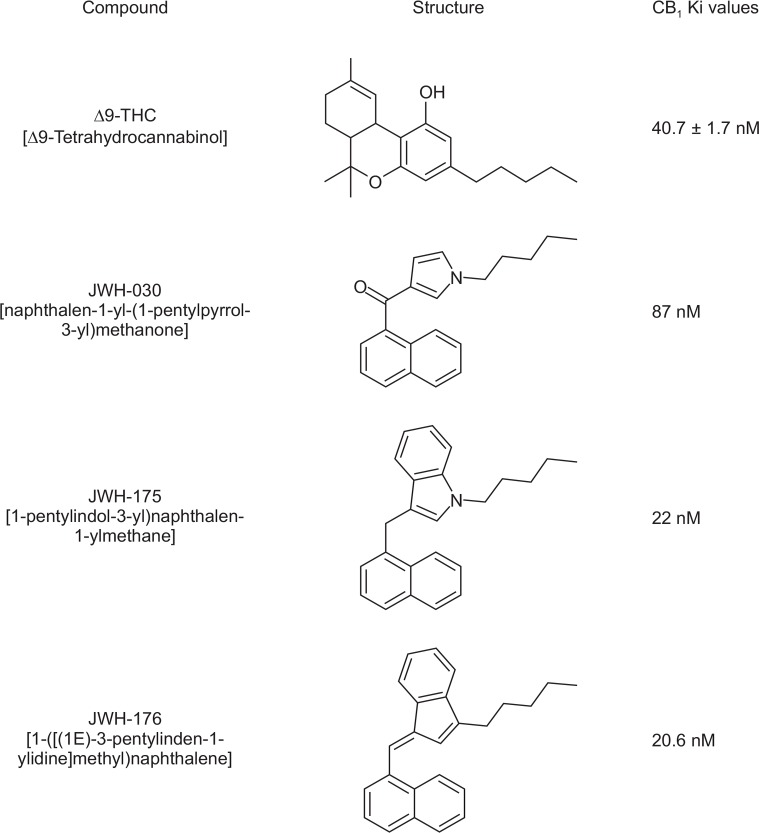
Accordingly, it has been reported the use of synthetic cannabinoids as a recreational drug is on the rise (Fattore and Fratta, 2011). This could be directly related to the fact that these substances are easily distributed and purchased from the internet. Authorities have just started studying and monitoring these substances and, as a result, some of these compounds have already been regulated (Auwärter et al., 2009; Fattore and Fratta, 2011). However, a number of synthetic cannabinoids remain uncontrolled, probably due to the lack of scientific evidence that these substances have abuse or addictive potential. Examples of these are the new synthetic cannabinoid agonists JWH-030 [naphthalen-1-yl-(1-pentylpyrrol-3-yl)methanone], JWH-175 [(1-pentylindol-3-yl) naphthalen-1-ylmethane], and JWH-176 [1-([(1E)-3-pentylinden-1-ylidine]methyl)naphthalene] (Fantegrossi et al., 2014; Presley et al., 2013). As it can be observed in Fig. 1, these synthetic cannabinoids have a high affinity for the cannabinoid CB1 receptors, with JWH-175 and JWH-176 being more potent than THC itself (Huffman and Padgett, 2005). Based on the latter observations, it would be important to evaluate the abuse potential of these synthetic cannabinoids.
Fig. 1.
Chemical structures and receptor affinities (CB1) of Δ9-THC, JWH-030, JWH-175, and JWH-176.
Thus, the goal of the present study was to assess the abuse potential of JWH-030, JWH-175, and JWH-176. Towards this goal, two of the most widely used animal models for assessing the abuse potential of drugs were employed: the conditioned place preference (CPP) and intravenous self-administration (SA) test. The CPP test would give us information on the rewarding effects of the drugs while the SA test would allow us to measure the reinforcing effects of those drugs (Tzschentke, 2007). In addition, the open-field test was utilized to assess the effects of repeated drug treatment and abrupt cessation on the locomotor activity of animals. The locomotor activity during the treatment and abstinence period can provide valuable insights into the addictive profile of drugs (Valjent et al., 2010; de la Peña et al., 2012).
MATERIALS AND METHODS
Animals
Male ICR mice (22 to 27 grams) and Sprague-Dawley (SD) rats (200–300 grams) were obtained from Hanlim Animal Laboratory (Hwasung, Korea). They were housed in groups (for open-filed and CPP test) or individually (for SA test) in a temperature- (22 ± 2°C) and humidity-controlled (55 ± 5%) animal room on a 12/12H light/dark (07:00–19:00H light) schedule. Food and water were freely accessible, except during the initial lever-training sessions of the SA test. Animals were allowed to acclimatize to the laboratory setting for seven days before the commencement of any experiment. Eight to ten animals were used for each treatment group. Animal treatment and maintenance were carried out in accordance with the Principles of Laboratory Animal care (NIH Publication No. 85-23 revised 1985) and the Animal Care and Use Guidelines of Sahmyook University, Korea.
Drugs
JWH-030, JWH-175, and JWH-176 were purchased from Cayman Chemicals (USA). Drugs were suspended in 10% dimethyl sulfoxide (DMSO) and diluted with 0.9% sterile saline solution just before the experiments. Drugs were given intraperitoneally (i.p) (OFT and CPP test) or intravenously (SA test).
Open-field test
Mice were individually placed in the center of a square Plexiglass container with a field measuring 42×42×42 cm. They were allowed to explore the area freely and were given a 2-minute habituation period followed by 10 minutes of behavioral recordings. A computer system (Ethovision, Noldus, Netherlands) recorded the total distance moved (cm) and movement duration (seconds) of each subject. Tests were performed a day before the start of the treatment (baseline; T0), during the first (T1), third (T3), and seventh day (T7) of drug treatment (i.p.), and the first (W1), third (W3), and seventh day (W7) of abstinence.
Conditioned place preference test
Experiments were performed in an apparatus (MED-CPP- 3013-2, Med Associates, Georgia, VT, USA) with two large compartments (17.4×12.7×12.7 cm3). Each compartment had distinct visual and tactile cues, such that one compartment was black with a stainless steel grid floor (3.2 mm diameter rods placed 7.9 mm apart) while the other compartment was white with 6.352 mm stainless steel mesh floor. Each compartment has a Plexiglas cover and an illuminating light. A guillotine door provided access to both compartments. Movement and position of animals in the apparatus were detected by infrared beams and were analyzed, quantified, and recorded by a computer program. The CPP test was composed of three phases namely (1) habituation and pre-conditioning, (2) conditioning, and (3) post-conditioning. For the first phase, mice were allowed free access to both compartments for 20 minutes per day. On the third day, the time spent on each side was recorded (pre-conditioning). The data from the pre-conditioning phase was used to separate animals into groups with approximately equal time spent in each compartment. Mice that spent over 840 sec in one compartment were excluded from the test. The conditioning phase followed wherein subjects received JWH-030 (0.05, 0.1, 0.5 mg/kg), JWH-175 (0.01, 0.05, 0.1 mg/kg) and JWH-176 (0.01, 0.05, 0.1) and were confined to a randomly designated compartment for 45 min. On alternate days (days 4, 6, 8, 10), they were given the vehicle (1% DMSO, 10 ml/kg, s.c.). Immediately after the last conditioning day, the post-conditioning phase followed where animals were drug-free and allowed access to both compartments, similar to the pre-conditioning phase.
Self-administration test
The SA test was performed as previously described (Botanas et al., 2015) with minor modifications. Briefly, the test was performed in standard operant chamber (Coulbourn Instruments, Allentown, Pennsylvania, USA) equipped with a food pellet dispenser, two 4.5 cm wide response levers, a stimulus light positioned 6 cm above each lever, and a house light. A motor-driven syringe pump (Coulbourn, USA) delivered the drug at a rate of 0.01ml/sec through a tubing system that was connected to the catheter in the animals. A software package (Graphic State Notation, Coulbourn) controlled the experimental parameters and data collection.
Briefly, rats were first trained to press a lever for a contingent food reward for three days, 30 minutes/day, on a continuous schedule of reinforcement. Rats that acquired at least 100 pellets from the last session were chosen and prepared for surgery. Surgical and post-surgical techniques were described in detail in our previous studies (de la Peña et al., 2012). Rats were given three days of recovery period before the start of the actual SA sessions. After the recovery period, rats were subjected to a 2-hour per day, seven days, SA test. During these sessions, both levers were present and a response on the left lever would initiate a sequence of event: activation of the infusion pump and delivery of 0.1 ml drug with illumination of the stimulus light above the left lever. Stimulus light remained lit for an additional 20 seconds after the end of the infusion (time-out period). During time-out periods, responses were recorded but did not have any programmed consequences. As a control for general activity, presses on the right lever (inactive lever) were recorded but not reinforced. In all of these conditions, rats were only allowed to obtain a maximum of 30 infusions per session (to prevent probable intoxication) although lever responses were still recorded until the end of each session. A catheter patency test was performed by infusing catheter with 0.1 ml of thiopental sodium (10 mg/kg) a day before and on the last day of the self-administration test. Rats that did not lose muscle tone within 3–5 seconds were excluded from the experiment.
Data analysis
All results are presented as means and standard error of the mean (S.E.M.). The locomotor activity was expressed as the distanced moved (cm) and movement duration (sec) of the animals during drug treatment and abstinence. Results were analyzed using two-way repeated measures analysis of variance (ANOVA) with drugs as the between-subject factor and days as the within-subject factor. Bonferroni’s posttest was used for further analysis. CPP data were expressed as the difference in the time spent in the drug- or saline-paired (control group) compartment during the post- and preconditioning phases. One-way ANOVA was employed to determine the effects of drug followed by Dunnett’s post-test to compare each group to the control group. In the SA test, the number of responses both in the active (left) and inactive (right) levers and the number of infusions obtained were presented. Two-way repeated measures ANOVA was employed to determine variations in lever responses, day or interaction between the two factors. If significant results were obtained, posthoc comparisons were made using Bonferroni’s test. The accepted level of significance was set at p<0.05. All statistical analyses were conducted using GraphPad Prism Version 4.01 software (CA, USA).
RESULTS
Locomotor activity
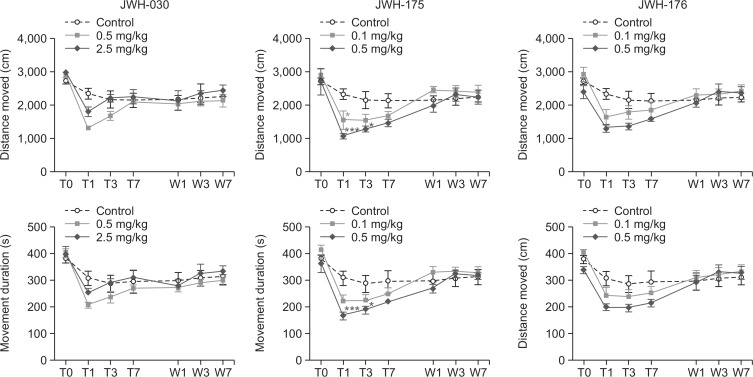
Fig. 2 shows the distance moved (cm) and movement duration (sec) of the mice in the open-field arena during the period of drug treatment and abstinence. Mice treated with JWH-030 showed significant effects of days (distance moved [F (6, 162)=15.67, p<0.001] and movement duration [F (6, 162)=19.89, p<0.001]) but not of the drug (distance moved [F (2, 162)=1.735, p>0.05] and movement duration [F (2, 162)=0.8595, p>0.05]). Similarly, mice treated with JWH-176 also showed significant effects of days (distance moved [F (6, 162)=18.86, p<0.001] movement duration [F (6, 162)=21.92, p<0.001]) but not of the drug (distance moved [F (2, 162)=2.072, p>0.05] movement duration [F (2, 162)=1.078, p>0.05]). On the other hand, mice treated with JWH-175 showed significant effects of days [F (6, 162)=18.78, p<0.001] and of drugs [F (2, 162)=3.521, p<0.05] and interaction between these two factors [F (6, 162)=2.999, p<0.001] but only in the distance moved. Post-hoc analysis revealed that mice treated with 0.1mg/kg of JWH -175 showed decreased distance moved on treatment day 1(p<0.05), as well as those treated with 0.5 mg/kg on treatment days 1 (p<0.001) and 3 (p<0.05).
Fig. 2.
The effects of JWH-030, JWH-175, and JWH-176 on the locomotor activity of mice before the start of the treatment (baseline, T0), on the first (T1), third (T3), and seventh day (T7) of drug treatment (i.p.) and during the first (W1), third (W3), and seventh day (W7) of abstinence. Each point represents the mean ± S.E.M. of the distanced moved (cm) and movement duration (s). n=10 mice per group. *p<0.05, ***p<0.001 significantly different from the control group (Bonferroni’s posttest).
Conditioned place preference test
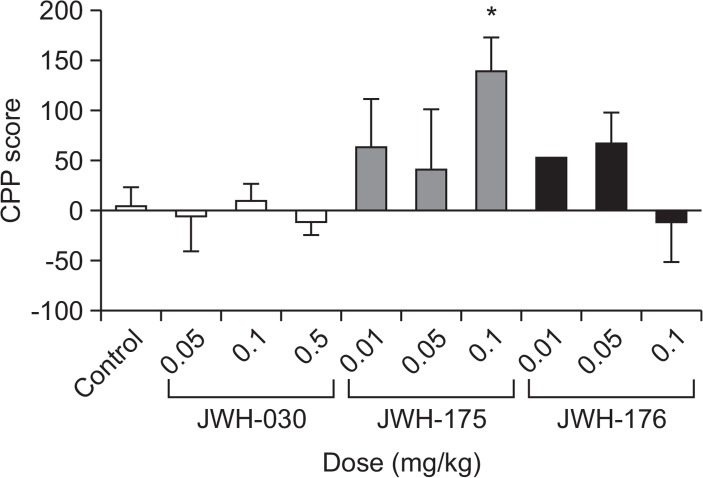
Fig. 3 illustrates the difference between the post and pre-conditioning scores of the saline-, JWH-030-, JWH-175-, JWH-176- treated mice. One-way ANOVA showed a significant difference between the experimental groups [F (9,64)=2.406, p<0.05]. Dunnet’s posttest revealed that only mice treated with JWH-175 at 0.1 mg/kg (q=3.261, p<0.05) showed CPP toward the drug.
Fig. 3.
The effects of JWH-030, JWH-175, and JWH-176 on the conditioned place preference test in mice. Each bar represents the mean ± SEM of the time spent in the drug-paired compartment (A), or the difference in the time spent in the drug-paired or saline-paired side during the post- and preconditioning phases (B) n=6–10 animals per group. *p<0.05 significantly different from the control group (Dunnett’s post test).
Self-administration test
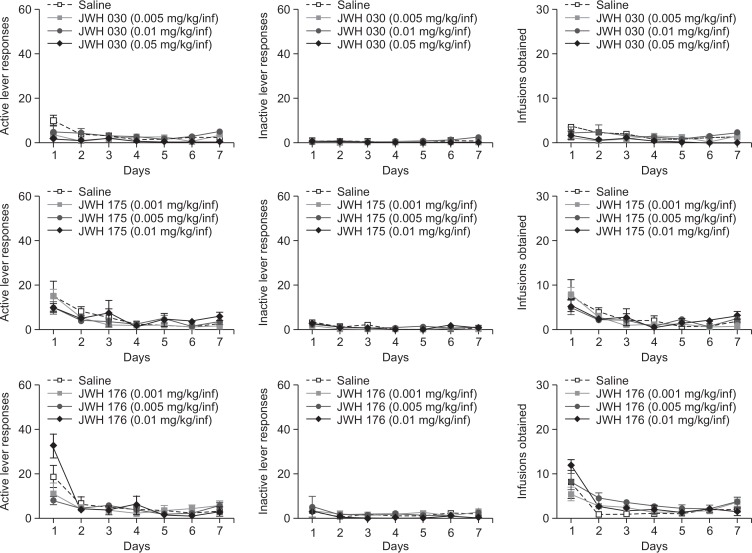
Fig. 4 shows the number active and inactive lever responses made and infusion obtained of saline-, JWH-030-, JWH-175-, JWH-176- treated rats during the 2h/day, 7-day SA sessions under the FR1 schedule. In the active lever responses, two-way repeated measures ANOVA demonstrated that a significant variation in days [F (6, 180)=6.066, p<0.001], drugs [F (3, 180)=3.431, p<0.05] and interaction between the two factors [F (18, 180)=2.432, p<0.01] from the JWH-030 group, albeit on the first day of SA only. Rats self-administering JWH-176 displayed a significant difference in days [F (6, 168)=24.81, p<0.001] but not in drugs [F (3, 168)=0.9096, p>0.05]. In a similar manner, a significant variation in days [F (6, 120)=13.34, p<0.001] but not in drugs [F (3, 120)=0.2867, p>0.05] was also observed in rats self-administering JWH-175. On the other hand, no significant difference in inactive lever responses was found in rats self-administering JWH-176 [F (3, 168)=1.049, p>0.05], JWH-175 [F 3, 120)=1.083, p>0.05], and JWH 030 [F (3, 180)=2.519, p>0.05].
Fig. 4.
The effects of JWH-030, JWH-175, and JWH-176 self-administration test in rats. Active and inactive lever responses made, and infusions obtained during the 2-h, seven days SA sessions under the FR 1 schedule. Values are mean ± S.E.M. n=6–10 animals per group.
For the infusions obtained by rats self-administering JWH-030, a significant effect of days [F (6, 180)=4.039, p<0.001] but not of drugs [F (3, 180)=2.624, p>0.05] was found. A significant effect of days [F (6, 120)=16.05, p<0.001] but not of drugs [F (3, 120)=0.1841, p>0.05] was also observed from JWH-175 group. Similarly, there was also a significant effect of days [F (6, 168)=22.75, p<0.001] but not of drugs [F (3, 168)=1.359, p>0.05] for the rats self-administering JWH-176.
DISCUSSION
The main goal of the present study was to investigate the abuse or addictive potential of the three new synthetic cannabinoids, JWH-030, JWH-175, and JWH-176. The results showed that, among the drugs tested, only JWH -175 reduced the locomotor activity and generated CPP in mice. On the other hand, none of the synthetic cannabinoids was self-administered by rats.
The decrease in locomotor activity observed in mice treated with JWH-175 corroborates with previous studies showing that agonists of the cannabinoid receptor causes hypomotility (Crawley et al., 1993; Brents et al., 2011; Uchiyama et al., 2012). In fact, THC has also been shown to decrease locomotor activity in rodents (Sañudo-Peña et al., 2000; Drews et al., 2005). The decreased locomotor activity induced by cannabinoid agonists has been attributed to the dense presence of CB1 receptors in the cerebellum and the basal ganglia, brain regions that are important in regulating motor activity (Del Arco et al., 1998). Moreover, this decreased locomotor activity has also been associated with the relaxing effects experienced by cannabis users/abusers (Huestis, 2002). Contrastingly, a study has also reported a conflicting result (i.e. increase locomotor activity with cannabinoid agonist treatment) (Drews et al., 2005). This study indicates that synthetic cannabinoid agonists, despite their similarity in molecular target and chemical structure, may have differential effects on locomotor activity. This explanation may also apply to the present results wherein, of the three closely related synthetic cannabinoids, only one induced significant effects on locomotor activity.
The results of the open-field test also show that the effects of the tested synthetic cannabinoids are transient. In addition, it is also evident that abrupt cessation of these drugs, following a 7-day treatment period, did not significantly altered the locomotor activity. Given that changes in locomotor activity may reflect the dependence-causing ability of a drug (Valjent et al., 2010), the present results suggest that JWH-030, JWH-175, and JWH-176 do not have or may have very minimal dependence potential. It could also be interpreted that the open-field test may not have been sensitive enough to detect the dependence potential of the drugs. This limitation can only be resolved by performing additional and more sensitive experiments. Nonetheless, the results of our open-field test can be used as a reference for future studies.
As aforementioned, in recent years, synthetic cannabinoids has been used as recreational drugs due to their ability to produce psychoactive or rewarding effects similar to those of cannabis (Tai and Fantegrossi, 2014). Several studies have reported that these effects are believed to be due to the capacity of synthetic cannabinoids to stimulate the brain dopamine reward pathway, in a similar fashion to other addictive drugs (Solinas et al., 2007). For instance, WIN 55212-2, a synthetic cannabinoid agonist, increased dopamine release in the nucleus accumbens which consequentially produced CPP and SA in rodents (Chaperon et al., 1998; Fadda et al., 2006; Golovko, 2011). In agreement with these studies, our findings showed that JWH-175, at the highest dose tested, induced CPP in mice.
On the contrary, JWH-030 and JWH-176 failed to produce significant CPP. The exact reason for this discrepancy is rather unclear. Previous CPP studies on THC and other synthetic cannabinoid agonists have also yielded conflicting results (McGregor et al., 1996; Valjent and Maldonado, 2000; Schramm-Sapyta et al., 2007). It could be possible that these conflicting results may have been caused by the biphasic effects of cannabinoid receptor targeting drugs (Braida et al., 2004). It is known that cannabinoid CB1 receptor agonists produce euphoric effects; however, these drugs have also been reported to induce dysphoric effects in humans (Cheer et al., 2000; Ghozland et al., 2002). Taking this into consideration, it could be speculated that JWH-030 and JWH-175 failed to induce CPP because it was canceled out by their underlying dysphoric effects. In line with this reasoning, it could also be possible that the significant CPP observed for JWH-175 may not be a manifestation of rewarding effects but merely an adaptation to the initially non-preferred compartment and/or a result of the anxiolytic-like effect of this type of drug (Rey et al., 2012). All of these explanations remain to be hypothetical and need to be validated by further studies. Regardless, our results show that JWH-175 is capable of producing CPP in mice.
On the other hand, none of the synthetic cannabinoids was self-administered by rats. Establishing intravenous self-administration of cannabinoid agonist in animal models has proven to be difficult (Martellotta et al., 1998; Maldonado and de Fonseca, 2002; Solinas et al., 2007). In fact, numerous studies have shown that THC was not self-administered [see (Maldonado, 2002) for review], and that positive result was only observed in experiment performed in non-human primates (Tanda et al., 2000; Justinova et al., 2003) or that THC was directly delivered to the ventral tegmental area (Braida et al., 2004). These results highlight the complexity of the psychopharmacological effects of cannabinoid agonists, and the need for doing parallel experiments (CPP and SA) in assessing the abuse potential of drugs. Although the results of the SA test suggest that none of the tested substances have reinforcing effects, the result of the CPP test, where mice showed “liking” for JWH-175, cannot be discounted. Thus, it would be quite rationale to imply that JWH-175 may have abuse potential. On the other hand, the negative results with JWH-030 and JWH-176 in CPP and SA tests suggest that these drugs would likely have a low potential for abuse.
In summary, the present study showed that JWH-175 decreased locomotor activity in mice, while JWH-030 and JWH176 did not. Furthermore, mice treated with JWH-175 develop significant CPP toward the drug. In contrast, JWH-030 and JWH-175 did not induce in CPP in mice. In the SA test, none of the synthetic cannabinoids was self-administered by rats. Taken together, these results demonstrate the complexity of the psychopharmacological properties of synthetic cannabinoids and its dissociative effects on animal models of drug addiction. Nevertheless, the present study advocates careful monitoring and prompt regulation of these synthetic cannabinoids and its analogs.
Acknowledgments
The authors are grateful to the Ministry of Food and Drug Safety (MFDS) of Korea (14182MFDS979) for their financial assistance.
REFERENCES
- Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- Botanas CJ, de la Peña JB, dela Peña IJ, Tampus R, Yoon R, Kim HJ, Lee YS, Jang CG, Cheong JH. Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: Evidence of its abuse potential. Pharmacol Biochem Behav. 2015;133:31–36. doi: 10.1016/j.pbb.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Braida D, Iosuè S, Pegorini S, Sala M. Δ 9-Tetrahydro-cannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaperon F, Soubrié P, Puech AJ, Thiébot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology. 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Cheer J, Kendall D, Marsden C. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology. 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav. 1993;46:967–972. doi: 10.1016/0091-3057(93)90230-Q. [DOI] [PubMed] [Google Scholar]
- de la Peña JBI, Lee HC, Ike C, Woo TS, Yoon SY, Lee HL, Han JS, Lee JI, Cho YJ, Shin CY. Rewarding and reinforcing effects of the NMDA receptor antagonist-benzodiazepine combination, zoletil®: Difference between acute and repeated exposure. Behav Brain Res. 2012;233:434–442. doi: 10.1016/j.bbr.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Del Arco I, Martí JL, Gorriti MA, Navarro M. Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis. 1998;5:483–501. doi: 10.1006/nbdi.1998.0217. [DOI] [PubMed] [Google Scholar]
- Drews E, Schneider M, Koch M. Effects of the cannabinoid receptor agonist WIN 55,212-2 on operant behavior and locomotor activity in rats. Pharmacol Biochem Behav. 2005;80:145–150. doi: 10.1016/j.pbb.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, Fratta W. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17:1629–1632. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ 9-THC: Mechanism underlying greater toxicity? Life Sci. 2014;97:45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fratta W. Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by μ-opioid and κ-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovko A. Cannabinoids: Neurochemistry and neurobiology. Biol Bull Rev. 2011;1:526–535. doi: 10.1134/S2079086411060028. [DOI] [Google Scholar]
- Gurney SM, Scott K, Kacinko S, Presley B, Logan B. Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Sci Rev. 2014;26:54–78. [PubMed] [Google Scholar]
- Huestis MA. Cannabis(Marijuana)- effects on human behavior and performance. Forensic Sci Rev. 2002;14:15–60. [PubMed] [Google Scholar]
- Huffman JW, Padgett LW. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 2005;12:1395–1411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95:153–164. doi: 10.1016/S0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Maldonado R, de Fonseca FR. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–3331. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martellotta M, Cossu G, Fattore L, Gessa G, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55, 212-2 in drug-naive mice. Neuroscience. 1998;85:327–330. doi: 10.1016/S0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav. 1996;53:657–664. doi: 10.1016/0091-3057(95)02066-7. [DOI] [PubMed] [Google Scholar]
- Presley B, Jansen-Varnum S, Logan B. Analysis of synthetic cannabinoids in botanical material: a review of analytical methods and findings. Forensic Sci Rev. 2013;25:27–46. [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–274. doi: 10.1016/S0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology. 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res. 2007;56:393–405. doi: 10.1016/j.phrs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S, Fantegrossi WE. Synthetic cannabinoids: pharmacology, behavioral effects, and abuse potential. Curr Addict Rep. 2014;1:129–136. doi: 10.1007/s40429-014-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Review on CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama N, Kikura-Hanajiri R, Matsumoto N, Huang Z-L, Goda Y, Urade Y. Effects of synthetic cannabinoids on electroencephalogram power spectra in rats. Forensic Sci Int. 2012;215:179–183. doi: 10.1016/j.forsciint.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Herve D, Girault J-A. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology. 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]